Norovirus Genetic Diversity in Children under Five Years Old with Acute Diarrhea in Mozambique (2014–2015)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Stool Sample Collection
2.2. Laboratory Testing
2.2.1. Detection and Molecular Characterization
2.2.2. Phylogenetic Analysis
2.3. Data Management and Analyses
2.4. Ethical Approval
3. Results
3.1. Norovirus Prevalence
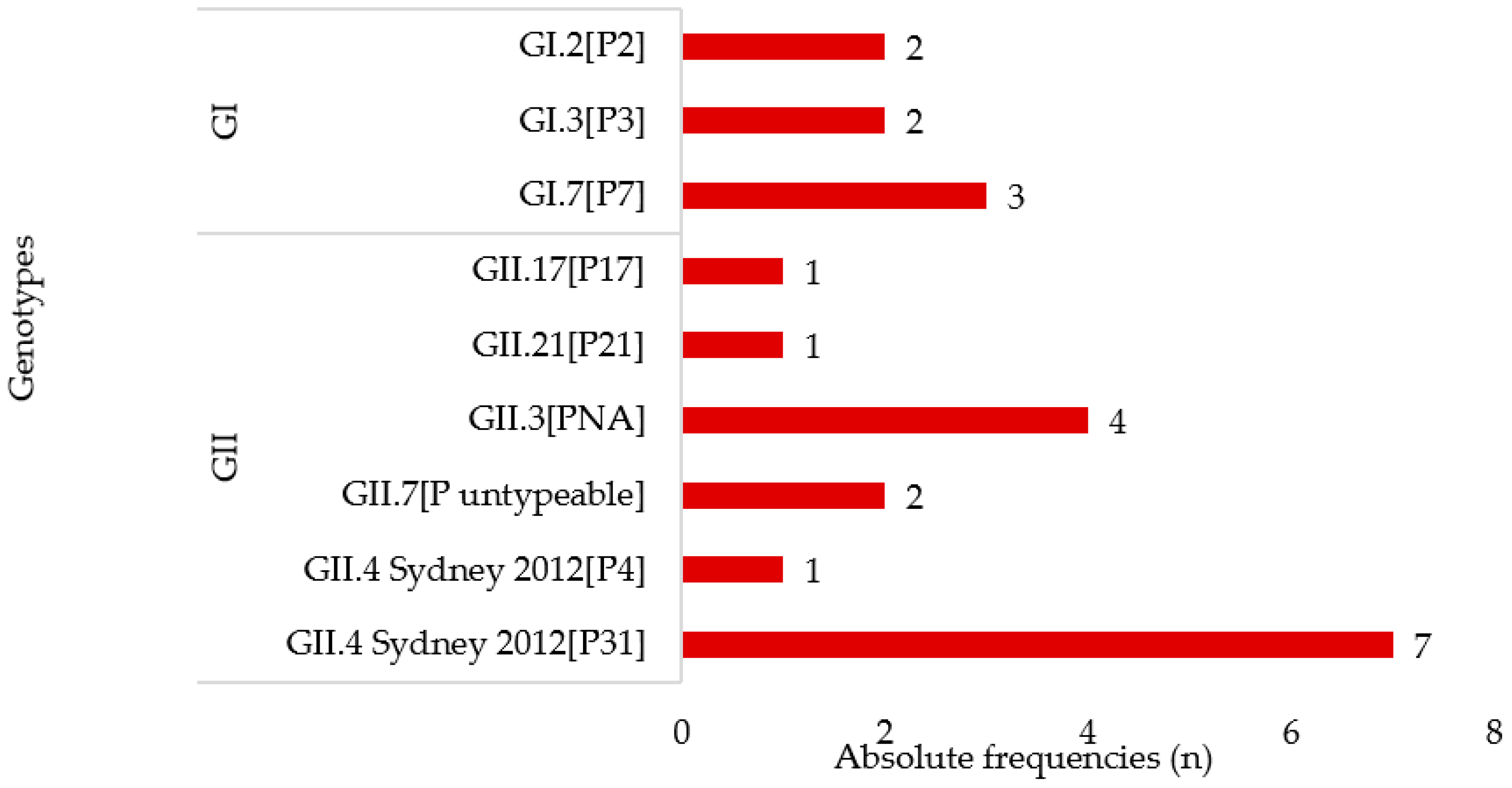
3.2. Norovirus Genotyping
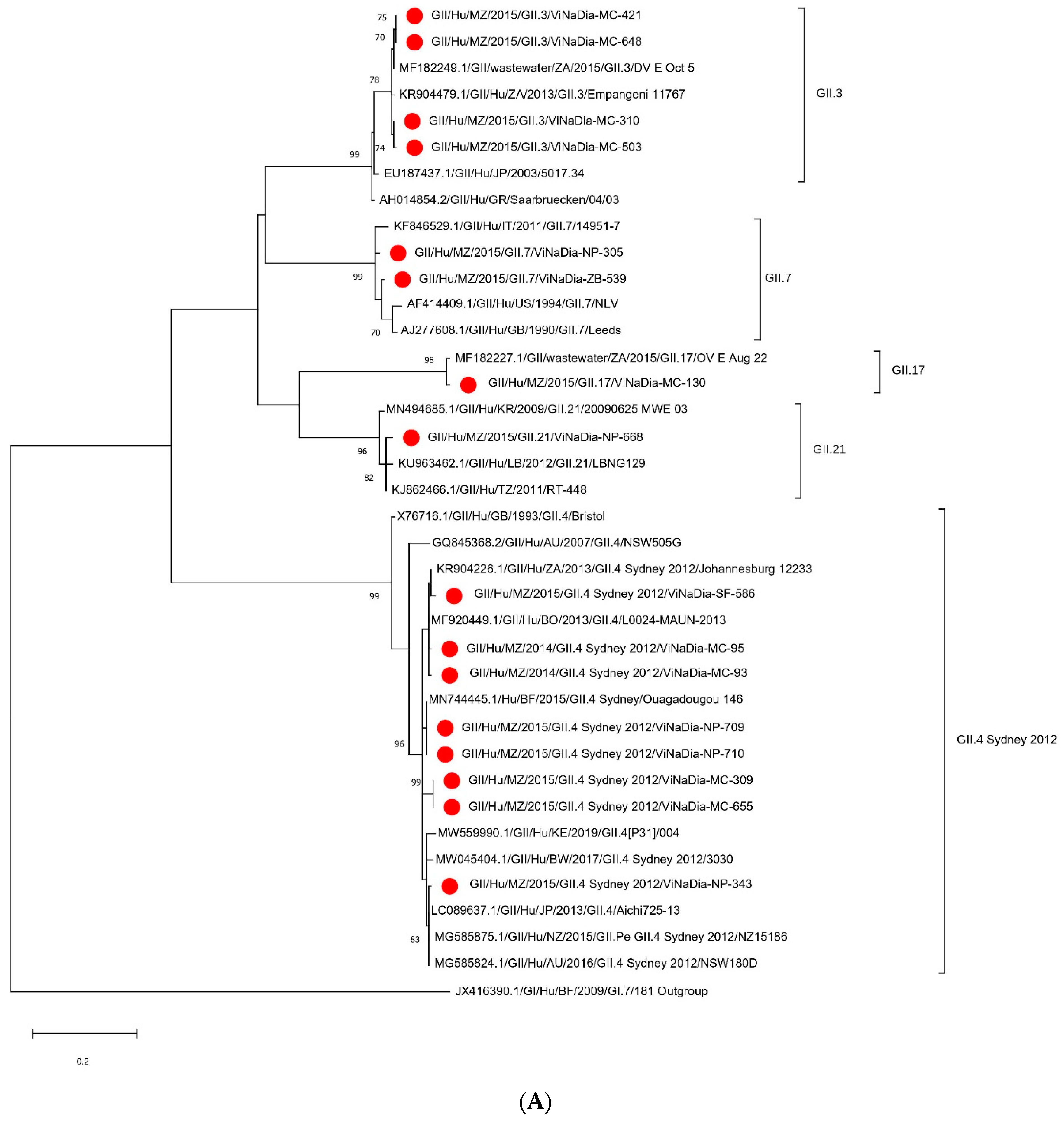
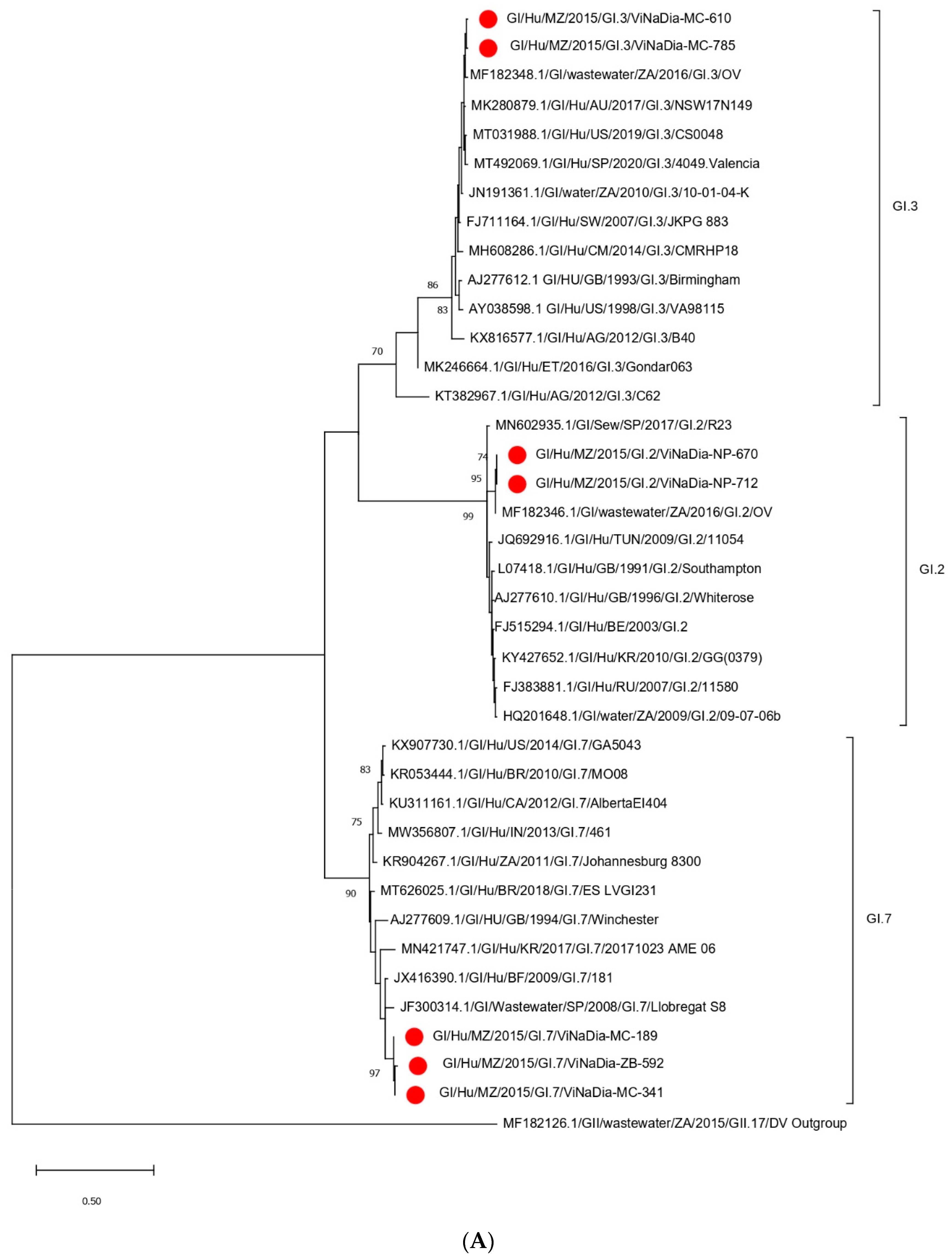
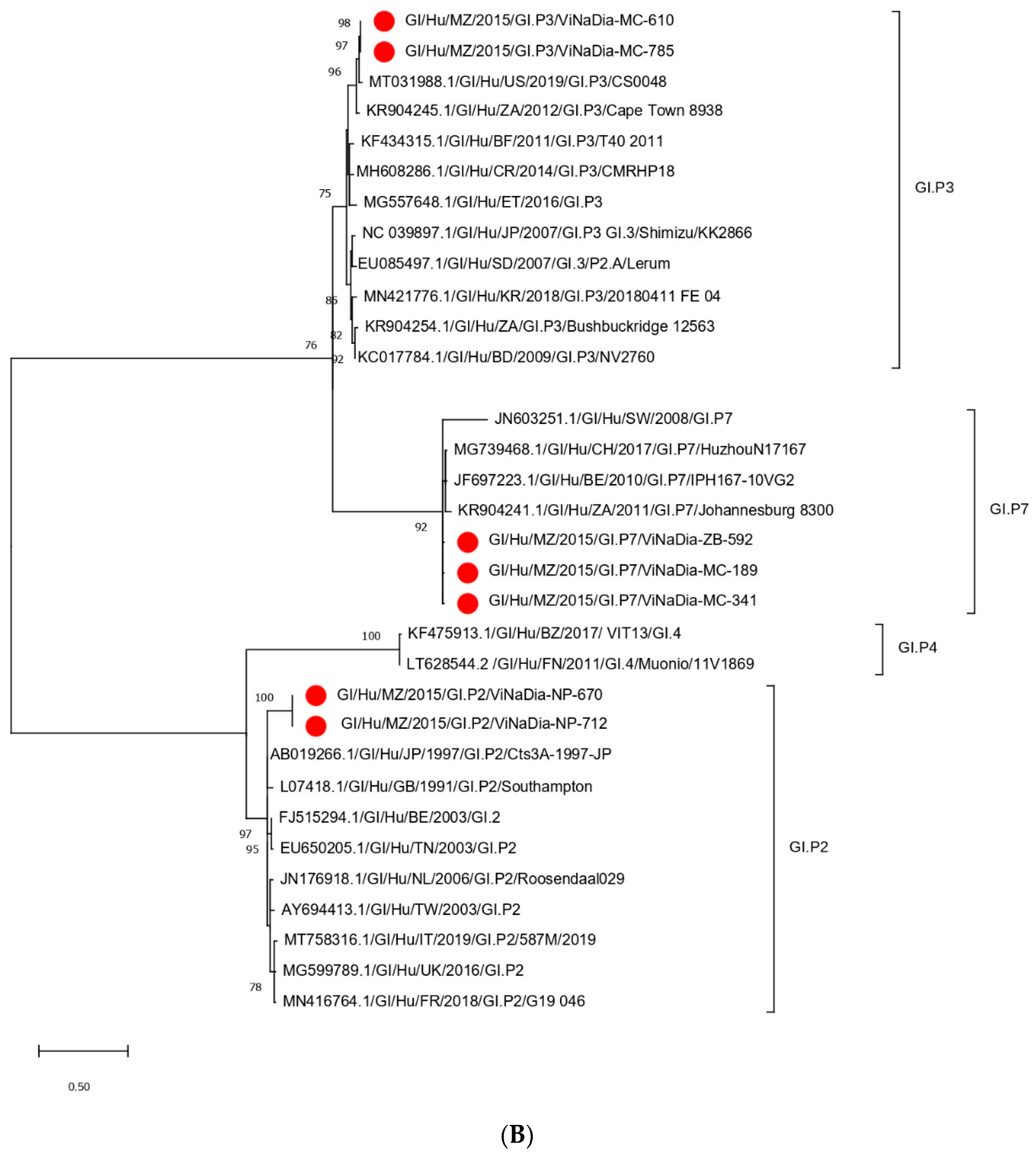
3.3. Phylogenetic Analyses
3.3.1. VP1 Gene
3.3.2. RdRp Gene
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Operario, D.J.; Platts-Mills, J.A.; Nadan, S.; Page, N.; Seheri, M.; Mphahlele, J.; Praharaj, I.; Kang, G.; Araujo, I.T.; Leite, J.P.G.; et al. Etiology of Severe Acute Watery Diarrhea in Children in the Global Rotavirus Surveillance Network Using Quantitative Polymerase Chain Reaction. J. Infect. Dis. 2017, 216, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M.; Widdowson, M.-A.; Glass, R.I.; Akazawa, K.; Vinjé, J.; Parashar, U.D. Systematic Literature Review of Role of Noroviruses in Sporadic Gastroenteritis. Emerg. Infect. Dis. 2008, 14, 1224–1231. [Google Scholar] [CrossRef]
- Nguyen, G.T.; Phan, K.; Teng, I.; Pu, J.; Watanabe, T. A Systematic Review and Meta-Analysis of the Prevalence of Norovirus in Cases of Gastroenteritis in Developing Countries. Medicine 2017, 96, e8139. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Hall, A.J.; Robinson, A.E.; Verhoef, L.; Premkumar, P.; Parashar, U.D.; Koopmans, M.; Lopman, B.A. Global Prevalence of Norovirus in Cases of Gastroenteritis: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2014, 14, 725–730. [Google Scholar] [CrossRef]
- Chhabra, P.; de Graaf, M.; Parra, G.I.; Chan, M.C.-W.; Green, K.; Martella, V.; Wang, Q.; White, P.A.; Katayama, K.; Vennema, H.; et al. Updated Classification of Norovirus Genogroups and Genotypes. J. Gen. Virol. 2019, 100, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.A.; Stark, K.; Mockenhaupt, F.P.; Reither, K.; Weitzel, T.; Ignatius, R.; Saad, E.; Seidu-Korkor, A.; Bienzle, U.; Schreier, E. Molecular Characterization of Enteric Viral Agents from Children in Northern Region of Ghana. J. Med. Virol. 2008, 80, 1790–1798. [Google Scholar] [CrossRef]
- Ballard, S.-B.; Reaves, E.J.; Luna, C.G.; Silva, M.E.; Rocha, C.; Heitzinger, K.; Saito, M.; Apaza, S.; Espetia, S.; Blazes, D.L.; et al. Epidemiology and Genetic Characterization of Noroviruses among Adults in an Endemic Setting, Peruvian Amazon Basin, 2004–2011. PLoS ONE 2015, 10, e0131646. [Google Scholar] [CrossRef]
- Victoria, M.; Carvalho-Costa, F.A.; Heinemann, M.B.; Leite, J.P.; Miagostovich, M. Prevalence and Molecular Epidemiology of Noroviruses in Hospitalized Children with Acute Gastroenteritis in Rio de Janeiro, Brazil, 2004. Pediatr. Infect. Dis. J. 2007, 26, 602–606. [Google Scholar] [CrossRef]
- Ferreira, M.S.R.; Victoria, M.; Carvalho-Costa, F.A.; Vieira, C.B.; Xavier, M.P.T.P.; Fioretti, J.M.; Andrade, J.; Volotão, E.M.; Rocha, M.; Leite, J.P.G.; et al. Surveillance of Norovirus Infections in the State of Rio De Janeiro, Brazil 2005–2008. J. Med. Virol. 2010, 82, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Mans, J.; Armah, G.E.; Steele, A.D.; Taylor, M.B. Norovirus Epidemiology in Africa: A Review. PLoS ONE 2016, 11, e0146280. [Google Scholar] [CrossRef] [Green Version]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and Aetiology of Diarrhoeal Disease in Infants and Young Children in Developing Countries (the Global Enteric Multicenter Study, GEMS): A Prospective, Case-Control Study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Nhampossa, T.; Mandomando, I.; Acacio, S.; Quintó, L.; Vubil, D.; Ruiz, J.; Nhalungo, D.; Sacoor, C.; Nhabanga, A.; Nhacolo, A.; et al. Diarrheal Disease in Rural Mozambique: Burden, Risk Factors and Etiology of Diarrheal Disease among Children Aged 0–59 Months Seeking Care at Health Facilities. PLoS ONE 2015, 10, e0119824. [Google Scholar] [CrossRef]
- de Deus, N.; Chilaúle, J.J.; Cassocera, M.; Bambo, M.; Langa, J.S.; Sitoe, E.; Chissaque, A.; Anapakala, E.; Sambo, J.; Guimarães, E.L.; et al. Early Impact of Rotavirus Vaccination in Children Less than Five Years of Age in Mozambique. Vaccine 2018, 36, 7205–7209. [Google Scholar] [CrossRef] [PubMed]
- João, E.D.; Munlela, B.; Chissaque, A.; Chilaúle, J.; Langa, J.; Augusto, O.; Boene, S.S.; Anapakala, E.; Sambo, J.; Guimarães, E.; et al. Molecular Epidemiology of Rotavirus A Strains Pre- and Post-Vaccine (Rotarix®) Introduction in Mozambique, 2012–2019: Emergence of Genotypes G3P[4] and G3P[8]. Pathogens 2020, 9, 671. [Google Scholar] [CrossRef]
- Beuret, C.; Kohler, D.; Baumgartner, A.; Lüthi, T.M. Norwalk-Like Virus Sequences in Mineral Waters: One-Year Monitoring of Three Brands. Appl. Environ. Microbiol. 2002, 68, 1925–1931. [Google Scholar] [CrossRef]
- da Silva, A.K.; Le Saux, J.-C.; Parnaudeau, S.; Pommepuy, M.; Elimelech, M.; Le Guyader, F.S. Evaluation of Removal of Noroviruses during Wastewater Treatment, Using Real-Time Reverse Transcription-PCR: Different Behaviors of Genogroups I and II. Appl. Environ. Microbiol. 2007, 73, 7891–7897. [Google Scholar] [CrossRef]
- Loisy, F.; Atmar, R.L.; Guillon, P.; Cann, P.L.; Pommepuy, M.; Guyader, F.S.L. Real-Time RT-PCR for Norovirus Screening in Shellfish. J. Virol. Methods 2005, 123, 1–7. [Google Scholar] [CrossRef]
- Vennema, H.; de Bruin, E.; Koopmans, M. Rational Optimization of Generic Primers Used for Norwalk-like Virus Detection by Reverse Transcriptase Polymerase Chain Reaction. J. Clin. Virol. 2002, 25, 233–235. [Google Scholar] [CrossRef]
- Kojima, S.; Kageyama, T.; Fukushi, S.; Hoshino, F.B.; Shinohara, M.; Uchida, K.; Natori, K.; Takeda, N.; Katayama, K. Genogroup-Specific PCR Primers for Detection of Norwalk-like Viruses. J. Virol. Methods 2002, 100, 107–114. [Google Scholar] [CrossRef]
- Mabasa, V.V.; Meno, K.D.; Taylor, M.B.; Mans, J. Environmental Surveillance for Noroviruses in Selected South African Wastewaters 2015–2016: Emergence of the Novel GII.17. Food Environ. Virol. 2018, 10, 16–28. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Dove, W.; Cunliffe, N.A.; Gondwe, J.S.; Broadhead, R.L.; Molyneux, M.E.; Nakagomi, O.; Hart, C.A. Detection and Characterization of Human Caliciviruses in Hospitalized Children with Acute Gastroenteritis in Blantyre, Malawi. J. Med. Virol. 2005, 77, 522–527. [Google Scholar] [CrossRef]
- Trainor, E.; Lopman, B.; Iturriza-Gomara, M.; Dove, W.; Ngwira, B.; Nakagomi, O.; Nakagomi, T.; Parashar, U.; Cunliffe, N. Detection and Molecular Characterisation of Noroviruses in Hospitalised Children in Malawi, 1997–2007: Norovirus Infections in Malawian Children. J. Med. Virol. 2013, 85, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Nordgren, J. Host Genetic Factors Affect Susceptibility to Norovirus Infections in Burkina Faso. PLoS ONE 2013, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Mattison, K.; Sebunya, T.K.; Shukla, A.; Noliwe, L.N.; Bidawid, S. Molecular Detection and Characterization of Noroviruses from Children in Botswana. J. Med. Virol. 2010, 82, 321–324. [Google Scholar] [CrossRef]
- Howard, L.M.; Mwape, I.; Siwingwa, M.; Simuyandi, M.; Guffey, M.B.; Stringer, J.S.A.; Chi, B.H.; Edwards, K.M.; Chilengi, R. Norovirus Infections in Young Children in Lusaka Province, Zambia: Clinical Characteristics and Molecular Epidemiology. BMC Infect. Dis. 2017, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Mans, J.; Murray, T.Y.; Taylor, M.B. Novel Norovirus Recombinants Detected in South Africa. Virol. J. 2014, 11, 168. [Google Scholar] [CrossRef]
- Vinjé, J. Advances in Laboratory Methods for Detection and Typing of Norovirus. J. Clin. Microbiol. 2015, 53, 373–381. [Google Scholar] [CrossRef]
- Cannon, J.L.; Barclay, L.; Collins, N.R.; Wikswo, M.E.; Castro, C.J.; Magaña, L.C.; Gregoricus, N.; Marine, R.L.; Chhabra, P.; Vinjé, J. Genetic and Epidemiologic Trends of Norovirus Outbreaks in the United States from 2013 to 2016 Demonstrated Emergence of Novel GII.4 Recombinant Viruses. J. Clin. Microbiol. 2017, 55, 14. [Google Scholar] [CrossRef]
- Cannon, J.L.; Bonifacio, J.; Bucardo, F.; Buesa, J.; Bruggink, L.; Chan, M.C.-W.; Fumian, T.M.; Giri, S.; Gonzalez, M.D.; Hewitt, J.; et al. Global Trends in Norovirus Genotype Distribution among Children with Acute Gastroenteritis. Emerg. Infect. Dis. 2021, 27, 8. [Google Scholar] [CrossRef]
- Patrício, G. A Migração Internacional e o Processo de Desenvolvimento na Região Norte de Moçambique: Estudo de caso da Província de Nampula; Universidade de Lisboa: Lisbon, Portugal, 2015. [Google Scholar]

| Target | Norovirus | Primer | Sequence (5′–3′) * | Polarity | Location |
|---|---|---|---|---|---|
| Region A | Noroviruses | JV12Y [18] | ATA CCA CTA TGA TGC AGA YTA | + | 4279–4299 ‡ |
| JV13I [18] | TCA TCA TCA CCA TAG AAI GAG | − | 4585–4605 ‡ | ||
| Region B | GI | Mon 432 [15] | TGG ACI CGY GGI CCY AAY CA | + | |
| Mon 434 [15] | GAA SCG CAT CCA RCG GAA CAT | − | |||
| GII | Mon 431 [15] | TGG ACI AGR GGI CCY AAY CA | + | ||
| Mon 433 [15] | GAA YCT CAT CCA YCT GAA CAT | − | |||
| Region C | GI | QNIF4 [16] | CGC TGG ATG CGN TTC CAT | + | 5291–5308 † |
| G1SKF [19] | CTG CCC GAA TTY GTA AAT GA | + | 5342–5361 † | ||
| G1SKR [19] | CCA ACC CAR CCA TTR TAC A | − | 5653–5671 † | ||
| GII | QNIF2 [17] | ATG TTC AGR TGG ATG AGR TTC TCW GA | + | 5012–5037 ‡ | |
| G2SKF [19] | CNT GGG AGG GCG ATC GCA A | + | 5046–5064 ‡ | ||
| G2SKR [19] | CCR CCN GCA TRH CCR TTR TAC AT | − | 5367–5389 ‡ |
| Characteristic | Number in Category/ Total Number of Samples (%) | Number of NoV-Positive Samples (%) | p-Value |
|---|---|---|---|
| Total Enrolled | 204 | 29 (14.2) | |
| Sex | 0.034 a | ||
| Male | 118/204 (57.8) | 22/118 (18.6) | |
| Female | 86/204 (42.2) | 7/86 (8.1) | |
| Age group (months) | 0.292 b | ||
| 0–11 | 100/204 (49.1) | 16/100 (16.0) | |
| 12–23 | 78/204 (38.2) | 12/78 (15.4) | |
| 24–59 | 26/204 (12.7) | 1/26 (3.8) | |
| Province | 0.400 b | ||
| Maputo city | 116/204 (56.7) | 18/116 (15.5) | |
| Sofala | 5/204 (2.5) | 1/5 (20.0) | |
| Zambézia | 8/204 (3.9) | 2/8 (25.0) | |
| Nampula | 75/204 (36.8) | 8/75 (10.7) | |
| Vomiting | 0.986 a | ||
| Yes | 133/202 (65.8) | 19/133 (14.3) | |
| No | 69/202 (34.2) | 10/69 (14.5) | |
| Unknown/missing | 2 | 0 | |
| Fever | 0.308 a | ||
| Yes | 60/196 (30.6) | 6/60 (10.0) | |
| No | 136/196 (69.4) | 21/136 (15.4) | |
| Unknown/missing | 8 | 2 | |
| HIV Status | 0.735 b | ||
| Positive | 17/149 (11.4) | 3/17 (17.6) | |
| Negative | 134/149 (88.6) | 21/134 (15.7) | |
| Unknown/missing | 53 | 5 | |
| Source of drinking water | 0.496 a | ||
| Tap | 113/197 (57.4) | 14/113 (12.4) | |
| Public tap | 40/197 (20.3) | 8/40 (20.0) | |
| Well | 43/197 (21.8) | 6/43 (14.0) | |
| Purchased/bottled water | 1/197 (0.5) | 0 | |
| Unknown/missing | 7 | 1 | |
| Diarrhea episodes | 0.916 b | ||
| 3 | 81/204 (39.7) | 12/81 (14.8) | |
| 4–6 | 101/204 (49.5) | 15/101 (14.8) | |
| ≥7 | 22/204 (10.8) | 2/22 (9.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chilaúle, J.J.; Munlela, B.; Mans, J.; Mabasa, V.V.; Marques, S.; Bauhofer, A.F.L.; Jane, G.; Anapakala, E.; Oliveira, F.; Cossa-Moiane, I.; et al. Norovirus Genetic Diversity in Children under Five Years Old with Acute Diarrhea in Mozambique (2014–2015). Viruses 2022, 14, 2001. https://doi.org/10.3390/v14092001
Chilaúle JJ, Munlela B, Mans J, Mabasa VV, Marques S, Bauhofer AFL, Jane G, Anapakala E, Oliveira F, Cossa-Moiane I, et al. Norovirus Genetic Diversity in Children under Five Years Old with Acute Diarrhea in Mozambique (2014–2015). Viruses. 2022; 14(9):2001. https://doi.org/10.3390/v14092001
Chicago/Turabian StyleChilaúle, Jorfélia J., Benilde Munlela, Janet Mans, Victor V. Mabasa, Selma Marques, Adilson Fernando Loforte Bauhofer, Graziela Jane, Elda Anapakala, Fernanda Oliveira, Idalécia Cossa-Moiane, and et al. 2022. "Norovirus Genetic Diversity in Children under Five Years Old with Acute Diarrhea in Mozambique (2014–2015)" Viruses 14, no. 9: 2001. https://doi.org/10.3390/v14092001
APA StyleChilaúle, J. J., Munlela, B., Mans, J., Mabasa, V. V., Marques, S., Bauhofer, A. F. L., Jane, G., Anapakala, E., Oliveira, F., Cossa-Moiane, I., Guimarães, E., Sambo, J., Bero, D. M., Chissaque, A., Deus, N. d., & Taylor, M. B. (2022). Norovirus Genetic Diversity in Children under Five Years Old with Acute Diarrhea in Mozambique (2014–2015). Viruses, 14(9), 2001. https://doi.org/10.3390/v14092001






