Abstract
Schools have been a point of attention during the pandemic, and their closure one of the mitigating measures taken. A better understanding of the dynamics of the transmission of SARS-CoV-2 in elementary education is essential to advise decisionmakers. We conducted an uncontrolled non-interventional prospective study in Belgian French-speaking schools to describe the role of attending asymptomatic children and school staff in the spread of COVID-19 and to estimate the transmission to others. Each participant from selected schools was tested for SARS-CoV-2 using a polymerase chain reaction (PCR) analysis on saliva sample, on a weekly basis, during six consecutive visits. In accordance with recommendations in force at the time, symptomatic individuals were excluded from school, but per the study protocol, being that participants were blinded to PCR results, asymptomatic participants were maintained at school. Among 11 selected schools, 932 pupils and 242 school staff were included between January and May 2021. Overall, 6449 saliva samples were collected, of which 44 came back positive. Most positive samples came from isolated cases. We observed that asymptomatic positive children remaining at school did not lead to increasing numbers of cases or clusters. However, we conducted our study during a period of low prevalence in Belgium. It would be interesting to conduct the same analysis during a high prevalence period.
1. Introduction
Schools have been the subject of many controversies during the COVID-19 pandemic. Local infection prevention and control (IPC) measures differed between countries. While some countries chose for a zero-COVID strategy from the beginning [1], others (such as many European countries) took public health measures aiming at controlled circulation of the virus. Those countries had to find the right balance between the competing risks for society of hospitals, and especially intensive care units, under pressure; the risks related to the lockdown of several sectors (e.g., culture); and that of a more genuine focus on the wellbeing of children by keeping schools open [2].
In some parts of the world, on the contrary, school closure was chosen as a major strategy to slow down the epidemic. Most reviews show, however, the major downside of this mitigation measure [3], because it dramatically impacts education and both the physical and mental health of millions of children. Many therefore concluded that schools should be the last institutions to close [4,5].
Schools are complex, open, and interconnected systems with the outside world and its communities. By consequence, the dynamic of transmission of SARS-CoV-2 within schools is highly dependent on several factors, such as: virus incidence in the community [6], circulating virus variants (i.e., variants of concern), household transmission [7,8], transport use [9], population density [10], traveling, environmental factors (e.g., temperature), participation in extracurricular activities, social determinants of participants (e.g., ethnicity) [11] and the application of mitigations measures such as mask use (by teachers and/or pupils) [12], ventilation [13] and vaccination coverage [14].
Both our knowledge and the dynamics themselves of SARS-CoV-2 transmission between children and from children to adults have evolved during the crisis, especially with the emergence of new variants. Although children were initially considered as super-spreaders in similitude to influenza epidemics [15], accumulating data suggest that they did not play a major role early in the pandemic [16]. However, a study performed in a primary school during the second wave in Belgium shows a similar pattern of transmission (i) between children and (ii) between children and teachers or employees, suggesting that transmission from children might have been higher at later stages than suspected [17].
The general objective of this study is to describe the dynamics of transmission of SARS-CoV-2 in elementary education in the French community (named Fédération Wallonie-Bruxelles (FWB)), Belgium, during winter and spring 2021.
2. Materials and Methods
An uncontrolled non-interventional prospective study, named DYNAtracs, DYNAmic of TRAnsmission of Coronavirus in Schools, was conducted between January 2021 and May 2021, in Belgian French-speaking elementary schools (children between 6 and 12 years old) from the FWB. This sub-study is part of a larger study exploring the dynamics of transmission of SARS-CoV-2 in elementary schools and the wellbeing of children during the pandemic. The specific objectives of this sub-study were to describe, during the second (i.e., starting on week 36, 2020) and third (week 7 to week 25, 2021) waves (Figure 1) [18] of the pandemic when the Alpha variant dominated, the role of asymptomatic children attending primary schools in the spread of SARS-CoV-2, and to estimate the incidence of children who were infected through contact with an index case outside of the school environment. In addition, the study was designed to assess the acceptability of a salivary sample taken with a swish/gargle technique in children.
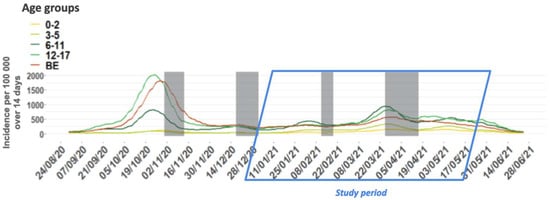
Figure 1.
Fourteen-day incidence of COVID-19 cases per 100,000 persons by age group and for the general population, from 1 September 2020 to 30 June 2021, Belgium. The incidence is represented by the sum of the number of cases in the previous 14 days over the number of people in that age group. “BE” indicates the whole population in Belgium (all age groups). Shaded areas indicate school holiday periods.
2.1. Study Setting and Participants Enrolment
Among Belgian elementary schools from the FWB, we identified a representative sample using purposive sampling according to three surrogate markers: (i) school size, (ii) socio-economic status (SES) of the pupils attending the schools, and (iii) cumulative incidence of SARS-CoV-2 infection in the geographic province of the school during the first wave of the pandemic (spring 2020).
More specifically, we applied the FWB’s definition of a small versus large school, i.e., a school with a lower hosting capacity, or of more than 230 pupils, in order to consider the contacts within the school. A value for SES, measured using the FWB’s official 20-point-scale index for all schools, equal or greater than 13 (upper tertile) was defined as high, and a value lower or equal to 7 (first tertile) was defined as low. A low versus high incidence region was described as a province with a cumulative municipal incidence of less than 5.0/1000 persons versus more than 5.0/1000 persons, on 6th May 2020. Investigators refer to the publicly available Belgian epidemiological reports from Sciensano, the Belgian institute of health [19]. Crossing the three criteria resulted in the definition of eight categories of schools, from which the sample was selected. Schools were included sequentially, and upon acceptance, if an invited school declined, another school meeting the same criteria was invited to participate.
All children registered and attending the participating schools, along with all adults working inside the schools, were then invited to participate through a letter. For each school, investigators provided information about the nature of the study to the children’s legal representatives—parents or guardians—and to school staff, during an online live meeting. A website with details about the study design, study information, informed consent, and videos on how to perform a mouth rinse/gargle specimen was available for all (potential) participants (https://www.sesa.ucl.ac.be/Dynatracs/, accessed on 25 August 2022). The informative letters were translated from French into the most encountered languages in the school communities.
Only children who provided informed consent signed by their legal guardian and staff who signed the consent were included in the study.
2.2. Study Design, Sample Collection and Laboratory Analysis
Pupils and staff included in the study population were followed-up during six weekly visits over subsequent weeks, according to the school calendar. Therefore, school breaks were not included in the study time. The start date differed between schools due to differences in timeline for obtaining consents, school location, and field planning. On the first visit corresponding to the inclusion visit, a blood sample was taken from all participants by finger prick to perform a rapid serological test (Avioq®, Bio-Tech, Shandong China). The CE-labeled Avioq® test is a lateral-flow antibody IgG/IgM test (colloidal gold) that targets the SARS-CoV-2 N-protein. The combined sensitivity for IgM and IgG was 68.8% (CI 95% 60.3–76%) with a specificity of 95.8% (CI 95% 88.5–98.6%) [20]. After collection, serological tests were first read on site by one of three designated study staff and sequentially sent for secondary reading to one single laboratory (Department of Microbiology, Cliniques universitaires Saint-Luc, Brussels) by one experienced microbiologist. During that same first visit, a mouth rinse/gargle specimen for SARS-CoV-2 detection by quantitative real-time reverse transcriptase PCR (RT-qPCR) was also collected. On subsequent weekly visits, all participants provided a mouth rinse/gargle specimen for a total duration of six weeks. Adult participants were invited to self-collect their mouth rinse/gargle specimens while pupils’ specimens were collected under supervision of study staff. All samples were collected on site and in a well-ventilated room or outside depending on the facilities and the meteorological conditions. The supervising study staff was wearing personal protective equipment (PPE) including a FFP2 mask. For each collection, a 5 mL vial of sterile 0.9% saline was squeezed into the participant’s mouth. They were then asked to swish the content for 5 s followed by tilting their heads back and gargling for 5 s. This swish/gargle cycle was repeated two more times and then the saline was expelled into a dedicated device designed by the University of Liège, commercialized by Diagenode (4100 Seraing, Belgium). The sampling kit was equipped with a dosing funnel that permitted the collection of exactly 1.2 mL of saliva, which was subsequently mixed with 2 mL of lysis buffer, inactivating the virus [21]. The self-collected swish/gargle sampling technique was previously evaluated in adults and school-aged children and compared to nasopharyngeal swabbing. It had a better acceptability with a good sensitivity of 97.5% (95% CI 86.8–99.9%) [22].
Participants were asked to not drink or eat within the 1 h preceding sampling. In case of sampling failure, a second attempt was not allowed. Saliva samples were directly dispatched to one of the three participating laboratories, attached to the three French-speaking universities, i.e., Department of Microbiology of Cliniques universitaires Saint-Luc, Federal testing platform COVID-19 of the Université Libre de Bruxelles, and the COVID-19 laboratory of the University of Liège. On reception, all samples were stored at –80 °C while awaiting processing. All RT-qPCR tests were performed according to directions from the laboratory of the University of Liège, which elaborated the collecting device and technique, as described in Saegerman et al. [21]. RT-qPCR results are reported as values of cycle threshold (Ct value, i.e., as defined by Public Health England, a semi-quantitative indicator of the concentration of viral genetic material in a sample [23]). In the study, positive samples were arbitrarily classified into three categories of Ct Values as follows: <25, 25–30, >30.
Phylogenetic analysis was performed on positive saliva samples presenting Ct values < 25 to investigate transmission of virus between participants. Total nucleic acid was extracted using the MagMAX™ Viral/Pathogen II Nucleic Acid Isolation Kit according to the manufacturer instructions (Cat. No. A48383, ThermoFisher Scientific, Waltham, MA, USA). The amplicon-based Illumina COVIDSeq protocol (Illumina Inc., San Diego, CA 92122, USA) in combination with the ARTIC v4 primers pools (https://artic.network/, accessed on 25 August 2022) was used for sequencing according to manufacturer’s instructions. The pooled library was diluted to a final concentration of 100pM for a single read (1 × 150 bp) sequencing on a NextSeq 1000 instrument. Generated fastq files were uploaded on the cloud-based ASP-IDNS®−5 analysis software (SmartGene, 1015 Lausanne, Switzerland). For analysis we used the “SARS-CoV-2 full genome” pipeline version 2.5.0_COV_v0.2. Online Nextclade version 2.3.0 software as a first sequence aligner, allowing comparison to the Wuhan-hu-1/2019 (MN908947) SARS-CoV-2 reference genome and permitting a clade assignment (https://clades.nextstrain.org, accessed on 25 August 2022). FASTA sequences were also submitted to the Pangolin (4.1.1) COVID-19 Lineage Assigner. Phylognentic Tree was generated by submitting the Fasta files to the NGPhylogeny web interface [24]. The workflow included: sequence alignment using the MAFFT software, curation of the sequences with the block mapping and gathering with entropy (BMGE) software, tree generation using the fast distance-based phylogeny inference program FastME 2.0, and tree output formatted with the Newick display. A detailed description of SARS-CoV-2 whole-genome sequences method is available in the Supplementary file S1.
All participants as well as study staff were blinded to RT-qPCR results, until the end of the study, as per protocol. Therefore, participation in the study and PCR test results had no impact on school attendance. During the study period, school attendance was, however, subject to health measures dictated by the Belgian government for all participants. These mitigation measures changed over time. At the time of the study, sanitary measures in elementary schools were as follows: frequent hand hygiene, mask wearing for adult staff when in close contact with pupils or other staff, quarantine measures for symptomatic individuals and high-risk contacts until PCR results from samples taken by the individual’s healthcare provider, and finally, closure of classes if two pupils or the teacher had confirmed SARS-CoV-2 cases. Therefore, no symptomatic individuals should have been tested in this study.
In addition, we cross-referenced positive PCR results with the cases reported to the school health promotion department (SHPD) in the participating schools. During the pandemic, the SHPD was designated to carry out surveillance of COVID-19 cases in schools and perform contact tracing in agreement with the regional public health units. SHPD reported the weekly numbers of pupils and staff with confirmed SARS-CoV-2 infections and collected data on the suspected source of infection (index case inside or outside the school) and counted probable secondary cases, i.e., having been infected in the school environment.
2.3. Statistical Analysis
Because this was a pilot study, no formal sample size determination was performed. Categorical variables are described by counts and percentages and continuous variables by means and standard deviations or median and interquartile ranges (IQR) for non-normal distributions. Data were analyzed using STATA software (version 14.1 StataCorp LP, College Station, TX, USA).
The protocol was approved by the Ethical Committee of the Cliniques universitaires Saint-Luc, UCLouvain (approval number 2020/16NOV/552, approved on the 20/11/20) and was registered on clinicaltrial.gov (Number NCT05046470) and on ISRCTN (Number ISRCTN16837012).
3. Results
We conducted this study between 14 January 2021 and 18 May 2021.
All schools were selected according to the above-mentioned criteria. Among eight schools that were invited to participate, two schools were excluded due to a high rate of refusal from either the staff or both the pupils and staff. Subsequently, five new schools were invited, resulting in a total of 11 included schools out of 13. The geographical distribution of the 11 schools included is depicted in Figure 2.
Figure 2.
Geographical distribution of primary schools included in the study.
In total, 932 children and 242 school staff were included and completed the study, which corresponds to an overall participation rate of 37.5% and 54.7%, respectively (Figure 3, Table 1). Participation varied between schools, ranging from 10.4% to 71.1% and from 20.0% to 100% in children and school staff, respectively (Table 1).
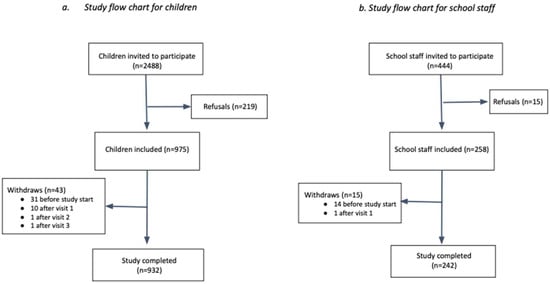
Figure 3.
Study flowchart.

Table 1.
Description of schools, participation rate, seropositivity rate at inclusion and number of positive SARS-CoV-2 PCR tests.
The different schools were included sequentially over time. Six schools participated from January week 2 2021 to March week 10 2021, including one week of school break. Four schools started in February on week 8 of 2021 and ended in April on week 17 of 2021, with a 4-week period of school break, and finally one school participated from week 11 March 2021 to May week 20 2021 (Figure 4).
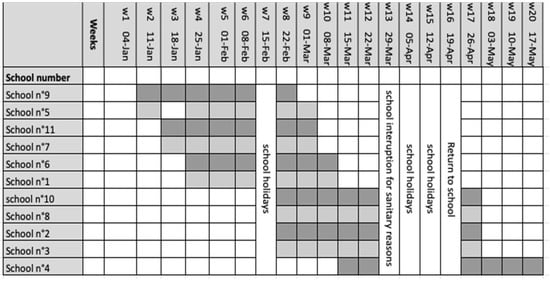
Figure 4.
Study timeline. Sequentially inclusion of schools in the study. The weeks mentioned correspond to the calendar weeks of the year 2021. Shaded areas indicate the weeks of school visits. School n°5 was closed (optional school holiday) on the day of the study staff’s visit in week 3.
Among 1162 available serological tests (6 missing and 6 invalid) performed once on study inclusion, 191 children and 61 staff tested seropositive, which corresponds to a positivity rate of 20.7% (95% CI 18.2–23.4) and 25.4% (95% CI 20.5–31.5), respectively, in children and school staff (Table 1) for whom a valid result is available.
Over the whole study period which included six weekly visits to each of the schools, a total of 6449 saliva samples were obtained; 5226 from children and 1217 from school staff, which corresponds to an average of 5.6 tests per child and 5.0 tests per adult. Some samples are missing due to absenteeism of participants on the collection date or due to invalid samples (swallowing or wrong execution) (Table 2). During the entire study period, 44 (0.7%) SARS-CoV-2 PCR tests were positive, 29 in children and 6 in school staff, which corresponds to a positivity rate of 3.11% in children and 2.46% in school staff. Of these children, nine had a positive test for two consecutive weeks (Table 3). Among all PCR-positive tested participants, 27/29 children (93.1%) and 2/6 (33.3%) school staff had a negative serological test at the beginning of the study. In four schools, we did not detect any positive PCR tests, and in 8 out of 11 schools, there were no positive PCR tests in school staff.

Table 2.
Summary table of SARS-CoV-2 PCR results for each school.

Table 3.
Description of positive SARS-CoV-2 PCR cases in the study population by week of the study.
In school n°8, four cases were detected concomitantly in the same class (first grade) and had a positive PCR SARS-CoV-2 for 2 weeks during visits 3 and 4. In the same school, three other positive cases were detected in the same class (4th grade), on visit 2. No school staff member participating in the study tested positive in this school during the study period (Table 3). In school n°7, two cases were also detected in the same 4th grade class. All the other positive cases were isolated.
Cross-referencing the data from our study with information from the SHPD, there was no detectable increase in cases during the study period or in a 2-week time span after the end of the study period in all schools except in school n°2 (Figure 5). In school n°2, the SHPD declared a total of 36 cases between week 9 and week 18 of year 2021; 32 pupils and 4 school staff members (Figure 5, supplementary data Table S1). Among the 32 pupils detected, 14 who were considered as index cases for secondary cases within the school were themselves infected outside the school area, and 18 were considered as secondary cases, i.e., having been infected themselves in the school environment. The four school staff members who tested positive are considered as index cases. Due to the large number of cases detected in the school, first-grade classes were closed during weeks 11 and 12, the second grade on week 12, as well as one class of the third grade.
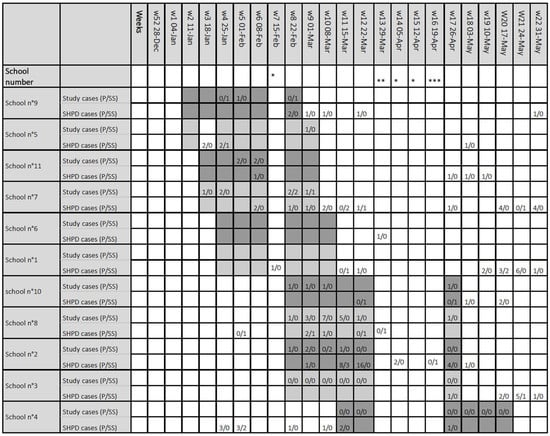
Figure 5.
Reported cases of positive SARS-CoV-2 PCR during the study period detected by the study samples and reported by health promotion department from each school. SARS-CoV-2 cases detected by the study DynaTracs (first line) and reported by the school health promotion department (second line). Distinction was made between pupils (p) and adults school staff (SS). The weeks mentioned correspond to the calendar weeks of the year 2021. Shaded areas indicate the weeks of school visits. * School holidays, ** School Interruption for sanitary reasons, *** Return to school. p: pupils, SS: school staff, SHPD: school health promotion department.
Next-generation-sequencing analyses of the positive PCR results could be performed in 10 positive PCR with a Ct of less than 25. Unfortunately, only 57% of the genome analyzed was common to all 10 samples, allowing phylogenetic analyses to be performed on a very fragmented genome. The results obtained under these conditions suggested that the ten strains differ, as shown on Figure 6. Only two viruses seemed close, but participants came from different schools.
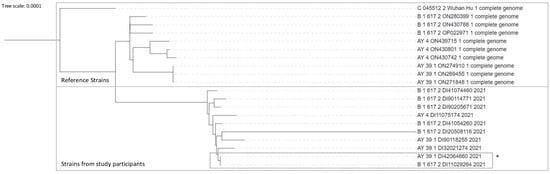
Figure 6.
Phylogenetic tree comparing reference strains including Wuhan strain and strains from 10 participants. The upper part of the diagram represents the results from reference strains. The shaded part of the diagram represents results from the study samples. * Two strains with similarities, - participants coming from different schools.
4. Discussion
A better understanding of SARS-CoV-2 transmission in schools is of the utmost importance to guide future decisionmakers on school closures and educational disruptions, actions that have implications far beyond physical health. As mentioned above, the role of children in the dynamics of transmission SARS-COV-2 during the different stages of the pandemic and under the different variants of concern remains controversial. Since the circulation of more transmissible variants, it seems clear that children and young adolescents are as prone as older individuals to infection but less likely to develop a symptomatic or severe infection [1,2,3,25]. The question of whether these young populations are naturally less infectious than older age groups is still debated [25]. Moreover, as they experience less symptomatic disease, they can more easily escape testing strategies and are considered as potential silent drivers of the infection [26]. The singular design of this study, being weekly sampling during consecutive weeks with no exclusion of positive but asymptomatic pupils and adult staff is suited to estimate the potential and effective role of asymptomatic cases in transmission within the classes and the schools of elementary education.
Overall, the literature shows that the risk of transmission to and from children in schools seems to be low, especially in elementary education [27,28,29]. Our observational uncontrolled prospective study supports a low secondary transmission in schools. Among 11 selected schools, only 44 (0.7%) repetitively performed SARS-CoV-2 RT-qPCR tests came back positive, in 29 children and 6 school staff. These data are in line with similar observations made in schools. Vogel and colleagues, in Germany, reported that 8 out of 23,905 samples were positive in 17 elementary schools despite high community incidence rates [30]. In the UK, Ladhani and colleagues concluded that SARS-CoV-2 infection rates were low in primary schools following partial and full reopening of schools [31].
Our study detected silent cases, being asymptomatic cases that escaped the detection system in place in Belgium at that time. Indeed, they were not picked up through the School Health Promotion Department (SHPD). Among our positive cases, 20/29 pupils were isolated cases in a class and 2 were isolated cases in the school. There was no increase in the number of cases detected in the following weeks, either. One can argue that not all individuals attending the different schools were participating in the study. However, even if we could have missed secondary cases, data do not support silent transmission in school resulting in apparent ill cases, as no increase in cases was detected by SHPD, up to a 2-week period after the end of the study. Furthermore, we did not observe many clusters, as only one school (school n°8) had two classes with, respectively, four and three pupils who tested positive on the same visit. Inside these classes, the classmates participating in the study did not become infected on the following visits. When we cross-referenced our data with the SHPD, we found that their data are in line with our observation. No school staff from this school had a positive test during the study period. This could have contributed to low secondary transmission, as transmission is more likely to occur if the index case is a teacher rather than a child, other factors being equal [29]. According to these results, we estimate that over-testing of asymptomatic children who have been in contact with confirmed SARS-CoV-2-positive individuals is probably not necessary. Indeed, most cases detected in our study were isolated cases (except for school 8).
The size of the study was not formally calculated but it had enough power to detect a significant transmission rate. Using a purposive sampling, the aim of the study was to find an index case in a class and then at least one new case in the same class at the next visits. With 932 children across 119 classes, the mean number of children per school class was 8. We observed at least one case in 18 school classes before visit 6, and there was no new case in the same class in subsequent visits in 17 out of these 18 classes. Only school class 4B in School 7 had a case at visit 2 followed by a new case at visit 5, one month later (Table 3 and Figure 4). To observe 0 new cases in 7 classmates, 17 times out of 18, and 1 new case in 7 classmates 1 time out of 18 leads to a likelihood over 95% that the transmission probability is lower than 0.009. The size of our study was large enough to detect such a low transmission rate.
Unfortunately, due to low viral loads (low Ct values) and due to technical issues, phylogenetic analyses could be performed only in 10 positive saliva samples and concerned just 57% of the genome, a percentage common to all 10 samples. The results obtained in these limiting conditions suggested that individuals were infected outside the school, but we do not have the power to confirm that.
Additionally, schools seem to reflect the evolution of transmission in society [32]. During our study period, the dominant strain was the variant of concern B.1.1.7 (Alpha), and SARS-CoV-2 circulation in Belgium was low. The positivity rate in our study was 0.7%. In one school, we observed two clusters, in March 2021. These results might simply reflect the multitude of factors involved in the transmission, including the intertwined relationship between community cases and school-acquired cases. Indeed, data from Sciensano, assessing the weekly incidence of SARS-CoV-2 in Belgium at that time, showed an ongoing increase in the number of positive SARS-CoV-2 in the whole country during that period, starting on February the 26th.
In our study protocol, a salivary test was preferred over nasopharyngeal swabbing. This non-invasive method seems better accepted for repeated testing in children, as highlighted by Vogel and colleagues [30]. On site, salivary tests for weekly surveillance were well accepted by participants. Sampling was performed in the setting of a scientific study and, therefore, was supervised by study staff. As the weeks went by, children were used to the method and only a few tests were lost due to swallowing or spitting wrongly outside the container. We propose that this less invasive method should be the norm when testing is performed in the pediatric population.
Our study has limitations. First, the participation rate in schools varied widely between schools and was never exhaustive. Recruitment is always a challenge; however, in the time of the pandemic, sanitary measures further complicated recruitment. Access to information was not uniform in the population, and the virtual communication with the targeted population impeded participation. Second, the phylogenetic analysis was inconclusive due to the limited number of analyzed samples and the limitations of the technique as well as of the obtained results. The lack of this information prevents us from asserting the circulation of the same virus within a class or a school. Third, this study took place during a low incidence period of COVID-19 cases in Belgium, and therefore, of low circulation of the virus. In January 2021, at the beginning of our study, the incidence rate for the last 14 days, in Belgium, was 205 for 100,000 inhabitants, with a positivity rate of 6.7%. In May 2021, at the end of our study period, the incidence was 355 for 100,000 inhabitants, with a positivity rate of 6.3%. In May 2021, the immunization rate for all individuals of more than 18 years of age was 12.4%, and, for the population of more than 65 years old, this was 27.7%. The dynamics of COVID-19 transmission changed quickly after the end of this study, with the appearance of the Delta variant inducing a wave from late July 2021 until early December 2021, followed by the Omicron wave [33]. As consequence, due to the higher transmissibility of those variants, the incidence of COVID-19 in Belgium also increased significantly [19]. During the wave of Fall 2020, the COVID-19 incidence in the school population was considerably lower than in the general population [18,33]. This figure has changed during the wave of Fall 2021 with a recorded incidence in children aged 0–9 years old 5.3 times higher than during the same period 1 year before [33].
5. Conclusions
Globally, in elementary schools, the positivity rate of repetitive weekly PCR sampling for SARS-CoV-2 in asymptomatic individuals was low during our study period. While asymptomatic positive cases were detected, children remaining present in class during the Alpha variant wave of the pandemic did not lead to an increased number of secondary cases or clusters in their class or school. These data strengthen the view that keeping elementary education open at that point was a balanced decision, especially when we counterbalance the detrimental effect of disrupting education and the negative impact on the well-being of children of school closure. However, it would be interesting to conduct the same study during a period of high incidence, or during the circulation of other VOCs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v14102199/s1, Table S1: Weekly reporting of COVID-19 cases by the school health promotion department (SHPD). Supplementary File S1: Detailed description of SARS-CoV-2 whole-genome sequences method.
Author Contributions
J.F., O.C., J.M., N.R., J.R., B.K., L.G., D.V.d.L. and A.R. participated in the protocol conceptualization. F.M., J.R., B.K., H.R.-V., B.B., M.-L.D., C.H., F.B. and L.G. participated in the elaboration of analytical tools and performance of microbiological analyses. J.F., O.C., K.C., M.D.K., F.R., D.V.d.L. and A.R. participated in the collection data. J.F., O.C., K.C., J.M., M.D.K., B.K., H.R.-V., B.B., A.R. and D.V.d.L. interpreted the results. J.F., O.C., J.M. and D.V.d.L. participated in the writing—original draft preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Fédération Wallonie-Bruxelles, 44, Boulevard Léopold II, 1040 Brussels, Belgium. The funding number is subsides-covid19.cfwb.be/D.O.11-A.B.010502-Ex2020. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Cliniques universitaires Saint-Luc, UCLouvain (approval number 2020/16NOV/552, on the 20/11/20), and was registered on clinicaltrial.gov (Number NCT05046470) and on ISRCTN (Number ISRCTN16837012).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
J.F., O.C., K.C., D.V.d.L. and A.R. had full access to all data and took responsibility for the integrity of the data.
Acknowledgments
We thank all those who contributed to the study: the participating schools, school staff members, children, and their families. Special thanks go to the child who participated in the creation of the study information video. We would like to thank Laurent Gillet and Fabrice Bureau from the Immunology-Vaccinology lab of the faculty of Veterinary Medicine, ULiège, who shared with us their technique, contributing to the performance of less invasive tests in children, and for our constructive discussions.
Conflicts of Interest
J.F., O.C., K.C., M.D.K., N.R., F.R., J.R., B.K., H.R.-V., B.B., M.L.D., C.H., A.R. and D.V.d.L. have no conflicts of interest to declare for this study. J.R. became an employee of SmartGene during the course of study data analysis, a company active in bioinformatics services. J.M. was an employee of bioMerieux Canada, Inc until July 2021; this is unrelated to the study. L.G. and F.B. participated in the creation of the saliva collection kit. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Normile, D. ‘Zero COVID’ countries seek exit strategies. Science 2021, 373, 1294–1295. [Google Scholar] [CrossRef]
- Viner, R.M.; Bonell, C.; Drake, L.; Jourdan, D.; Davies, N.; Baltag, V.; Jerrim, J.; Proimos, J.; Darzi, A. Reopening schools during the COVID-19 pandemic: Governments must balance the uncertainty and risks of reopening schools against the clear harms associated with prolonged closure. Arch. Dis. Child. 2021, 106, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Nyhan, K.; Zhou, X.; Zhu, Y.; Castro, D.; Vermund, S.H.; Brault, M. School closures and reopenings during the COVID-19 pandemic: A scoping review protocol. BMJ Open 2022, 12, e054292. [Google Scholar] [CrossRef]
- UNESCO. Education: From Disruption to Recovery. Global Monitoring of School Closures. Available online: https://en.unesco.org/covid19/educationresponse (accessed on 5 August 2022).
- Viner, R.; Bonell, C.; Blakemore, S.J.; Hargreaves, J.; Panovska-Griffiths, J. Schools should still be the last to close and first to open if there were any future lockdown. BMJ 2022, 376, o21. [Google Scholar] [CrossRef]
- Boey, L.; Roelants, M.; Merckx, J.; Hens, N.; Desombere, I.; Duysburgh, E.; Vandermeulen, C. Age-dependent seroprevalence of SARS-CoV-2 antibodies in school-aged children from areas with low and high community transmission. Eur. J. Pediatr. 2022, 181, 571–578. [Google Scholar] [CrossRef]
- Madewell, Z.J.; Yang, Y.; Longini, I.M., Jr.; Halloran, E.; Dean, N. Household Transmission of SARS-CoV-2: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2031756. [Google Scholar] [CrossRef]
- Madewell, Z.J.; Yang, Y.; Longini, I.M., Jr.; Halloran, E.; Dean, N. Factors Associated With Household Transmission of SARS-CoV-2: An Updated Systematic Review and Meta-analysis. JAMA Netw. Open 2021, 4, e2122240. [Google Scholar] [CrossRef]
- Abulhassan, Y.; Davis, G.A. Considerations for the transportation of school aged children amid the Coronavirus pandemic. Transp. Res. Interdiscip. Perspect. 2021, 9, 100290. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.P.; Flaxman, S.; Gallinat, A.S.; Kinosian, S.P.; Stemkovski, M.; Unwin, H.J.T.; Watson, O.J.; Whittaker, C.; Cattarino, L.; Dorigatti, I.; et al. Temperature and population density influence SARS-CoV-2 transmission in the absence of nonpharmaceutical interventions. Proc. Natl. Acad. Sci. USA 2021, 118, 25. [Google Scholar] [CrossRef] [PubMed]
- Upshaw, T.L.; Brown, C.; Smith, R.; Perri, M.; Ziegler, C.; Pinto, A.D. Social determinants of COVID-19 incidence and outcomes: A rapid review. PLoS ONE 2021, 16, e0248336. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Epalza Ibarrondo, C.; Toubiana, J.; Van der Linden, D. Urgent need to develop evidence-based COVID-19 recommendations for primary schools. Arch. Dis. Child. 2021, 106, 1039–1040. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.G.; Ibrahim, A.M. Indoor Air Changes and Potential Implications for SARS-CoV-2 Transmission. JAMA 2021, 325, 2112–2113. [Google Scholar] [CrossRef] [PubMed]
- Giardina, J.; Bilinski, A.; Fitzpatrick, M.C.; Kendall, E.A.; Linas, B.P.; Salomon, J.; Ciaranello, A.L. Model-Estimated Association Between Simulated US Elementary School-Related SARS-CoV-2 Transmission, Mitigation Interventions, and Vaccine Coverage Across Local Incidence Levels. JAMA Netw. Open 2022, 5, e2147827. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.; Vynnycky, E.; Hawker, J.; Olowokure, B.; Mangtani, P. School closures and influenza: Systematic review of epidemiological studies. BMJ Open 2013, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, W.; Dozier, M.; He, Y.; Kirolos, A.; Theodoratou, E. The role of children in the transmission of SARS-CoV-2: Updated rapid review. J. Glob. Health 2020, 10, 021101. [Google Scholar] [CrossRef]
- Meuris, C.; Kremer, C.; Geerinck, A.; Locquet, M.; Bruyère, O.; Defêche, J.; Meex, C.; Hayette, M.P.; Duchene, L.; Dellot, P.; et al. Transmission of SARS-CoV-2 After COVID-19 Screening and Mitigation Measures for Primary School Children Attending School in Liege, Belgium. JAMA Netw. Open 2021, 4, e2128757. [Google Scholar] [CrossRef] [PubMed]
- Proesmans, K.; Bloemen, B.; Hancart, S.; De Bock, F.; Duysburgh, E.; Cornelissen, L.; Klamer, S. SARS-COV-2 Chez Les Enfants et Les Adolescents de 0 à 17 ans en Belgique, Pendant L’année Scolaire 2020–2021. Bruxelles, Belgique: Sciensano; 2021. Numéro de Dépôt Légal: D/2021/14.440/67. Available online: https://covid-19.sciensano.be/sites/default/files/Covid19/COVID-19_THEMATIC%20REPORT_SCHOOLS_SURVEILLANCE.pdf (accessed on 27 September 2022).
- Sciensano. COVID-19—Situation épidémiologique; 2020–2022. Available online: https://covid-19.sciensano.be/sites/default/files/Covid19/COVID-19_Daily%20report_20200506%20-%20FR.pdf (accessed on 30 August 2022).
- Montesinos, I.; Gruson, D.; Kabamba, B.; Dahma, H.; Van den Wijngaert, S.; Reza, S.; Carbone, V.; Vandenberg, O.; Gulbis, B.; Wolff, F.; et al. Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies. J. Clin. Virol. 2020, 128, 104413. [Google Scholar] [CrossRef]
- Saegerman, C.; Diep, A.N.; Renault, V.; Donneau, A.F.; Stamatakis, L.; Coppieters, W.; Michel, F.; Breuer, C.; Dandoy, M.; Ek, O.; et al. A 2-month field cohort study of SARS-CoV-2 in saliva of BNT162b2 vaccinated nursing home workers. Commun. Med. 2022, 2, 1. [Google Scholar] [CrossRef]
- Goldfarb, D.M.; Tilley, P.; Al-Rawahi, G.N.; Srigley, J.A.; Ford, G.; Pedersen, H.; Pabbi, A.; Hannam-Clark, S.; Charles, M.; Dittrick, M.; et al. Self-Collected Saline Gargle Samples as an Alternative to Health Care Worker-Collected Nasopharyngeal Swabs for COVID-19 Diagnosis in Outpatients. J. Clin. Microbiol. 2021, 59, 4. [Google Scholar] [CrossRef]
- Public Health England. Understanding Cycle Threshold (Ct) in SARS-CoV-2 RT-PCR. A Guide for Health Protection Teams. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/926410/Understanding_Cycle_Threshold__Ct__in_SARS-CoV-2_RT-PCR_.pdf (accessed on 21 September 2022).
- Lemoine, F.; Correia, D.; Lefort, V.; Doppelt-Azeroual, O.; Mareuil, F.; Cohen-Boulakia, S.; Gascuel, O. NGPhylogeny.fr: New generation phylogenetic services for non-specialists. Nucleic Acids Res. 2019, 47, W260–W265. [Google Scholar] [CrossRef]
- Götzinger, F.; Strenger, V. The Role of Children and Young People in the Transmission of SARS-CoV-2. Pediatr. Infect. Dis. J. 2022, 41, e172–e174. [Google Scholar] [CrossRef] [PubMed]
- Moghadas, S.M.; Fitzpatrick, M.C.; Shoukat, A.; Zhang, P.; Galvani, A. Simulated Identification of Silent COVID-19 Infections Among Children and Estimated Future Infection Rates With Vaccination. JAMA Netw. Open 2021, 4, e217097. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Li, X.; Dozier, M.; He, Y.; Kirolos, A.; Lang, Z.; Mathews, C.; Siegfried, N.; Theodoratou, E. What is the evidence for transmission of COVID-19 by children in schools? A living systematic review. J. Glob. Health 2020, 10, 021104. [Google Scholar] [CrossRef]
- Spielberger, B.D.; Goerne, T.; Geweniger, A.; Henneke, P.; Roland, E. Intra-Household and close -contact SARS-CoV-2 transmission among children- a systematic review. Front. Pediatr. 2021, 9, 613292. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. COVID-19 in Children and the Role of School Settings in Transmission—Second Update. 8 July 2021. Stockholm: ECDC; 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/children-and-school-settings-covid-19-transmission (accessed on 25 August 2022).
- Vogel, S.; von Both, U.; Nowak, E.; Ludwig, J.; Köhler, A.; Lee, N.; Dick, E.; Rack-Hoch, A.; Wicklein, B.; Neusser, J.; et al. SARS-CoV-2 Saliva Mass Screening in Primary Schools: A 10-Week Sentinel Surveillance Study in Munich, Germany. Diagnostics 2022, 12, 162. [Google Scholar] [CrossRef]
- Ladhani, S.N.; Baawuah, F.; Beckmann, J.; Okike, I.O.; Ahmad, S.; Garstang, J.; Brent, A.J.; Brent, B.; Walker, J.; Andrews, N.; et al. SARS-CoV-2 infection and transmission in primary schools in England in June–December, 2020 (sKIDs): An active, prospective surveillance study. Lancet Child Adolesc. Health 2021, 5, 417–427. [Google Scholar] [CrossRef]
- Ismail, S.A.; Saliba, V.; Bernal, J.L.; Ramsay, M.E.; Ladhani, S.N. SARS-CoV-2 infection and transmission in educational settings: A prospective, cross-sectional analysis of infection clusters and outbreaks in England. Lancet Infect. Dis. 2021, 21, 344–353. [Google Scholar] [CrossRef]
- Natalia, Y.A.; Faes, C.; Molenberghs, G. The COVID-19 wave in Belgium during the Fall of 2020 and its association with higher education. PLoS ONE 2022, 17, e0264516. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).