SARS-CoV-2 Spike and Nucleocapsid Antibody Response in Vaccinated Croatian Healthcare Workers and Infected Hospitalized Patients: A Single Center Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Serum Processing
2.1.1. Healthcare Workers (HCW)
2.1.2. Hospitalized Patients
2.2. Development of Spike and Nucleocapsid ELISA
2.3. Statistics
3. Results
3.1. Development and Validation of ELISA Methods for Screening of Anti-Spike and Anti-Nucleocapsid Antibody Response
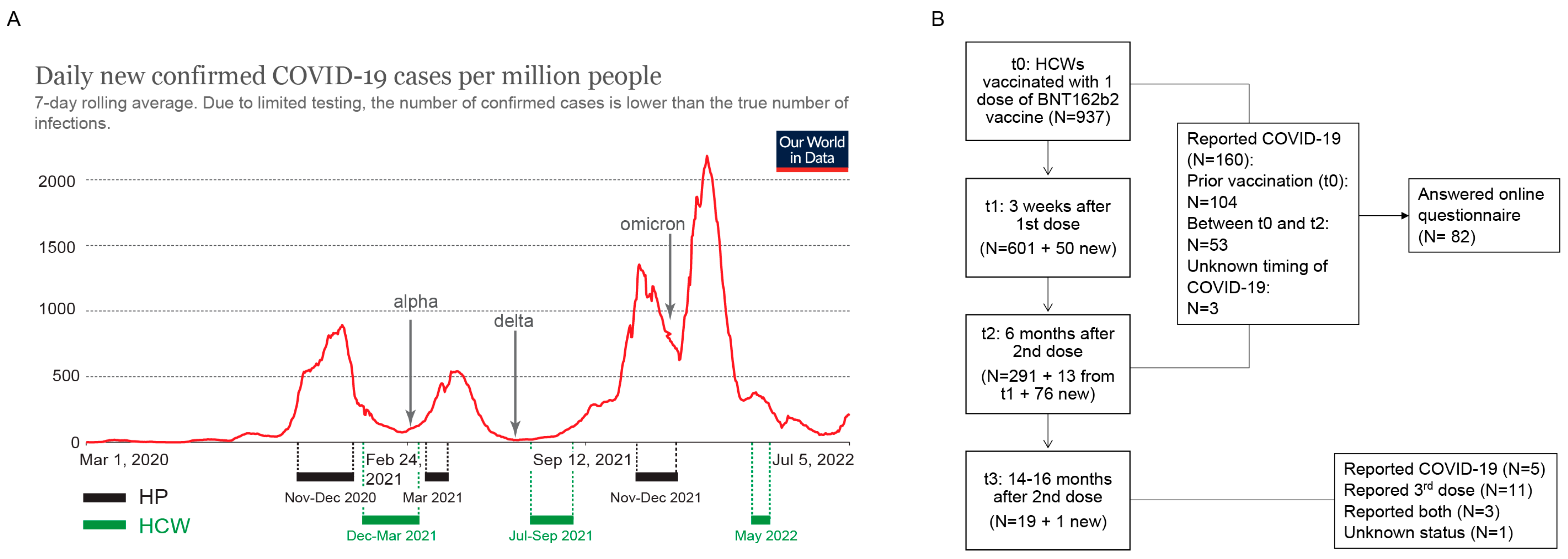
3.2. Demographic and Clinical Characteristics of the HCW Cohort
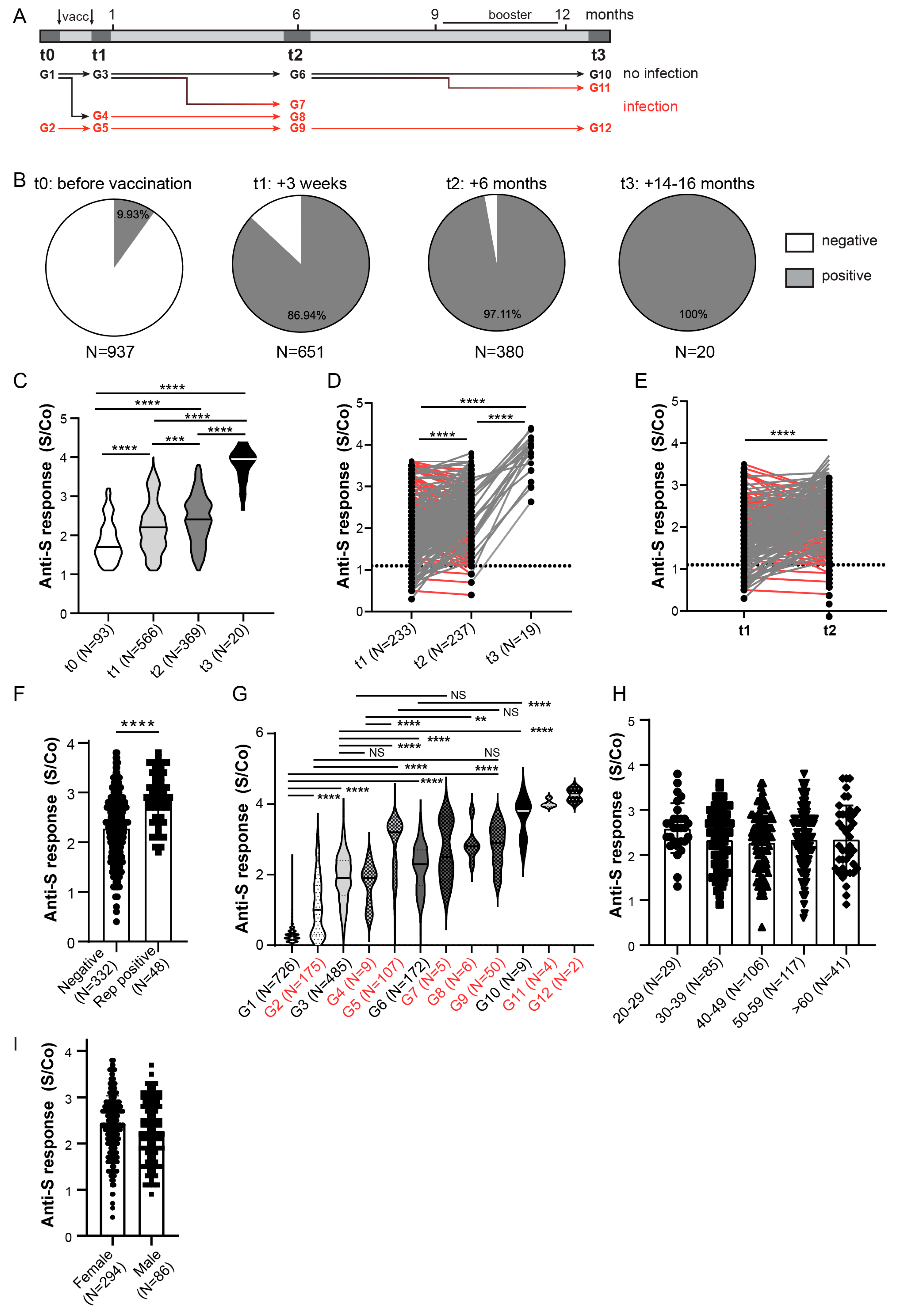
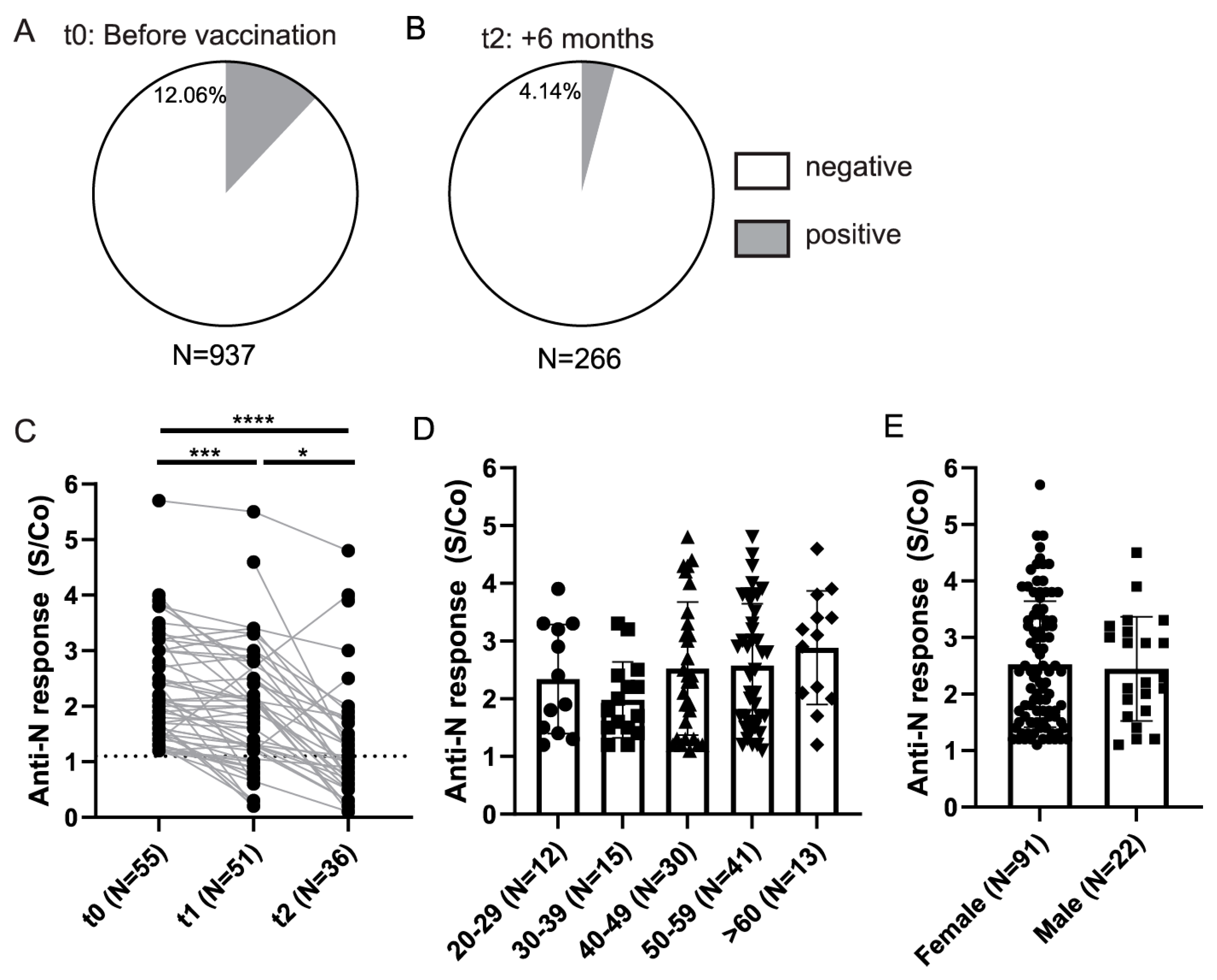
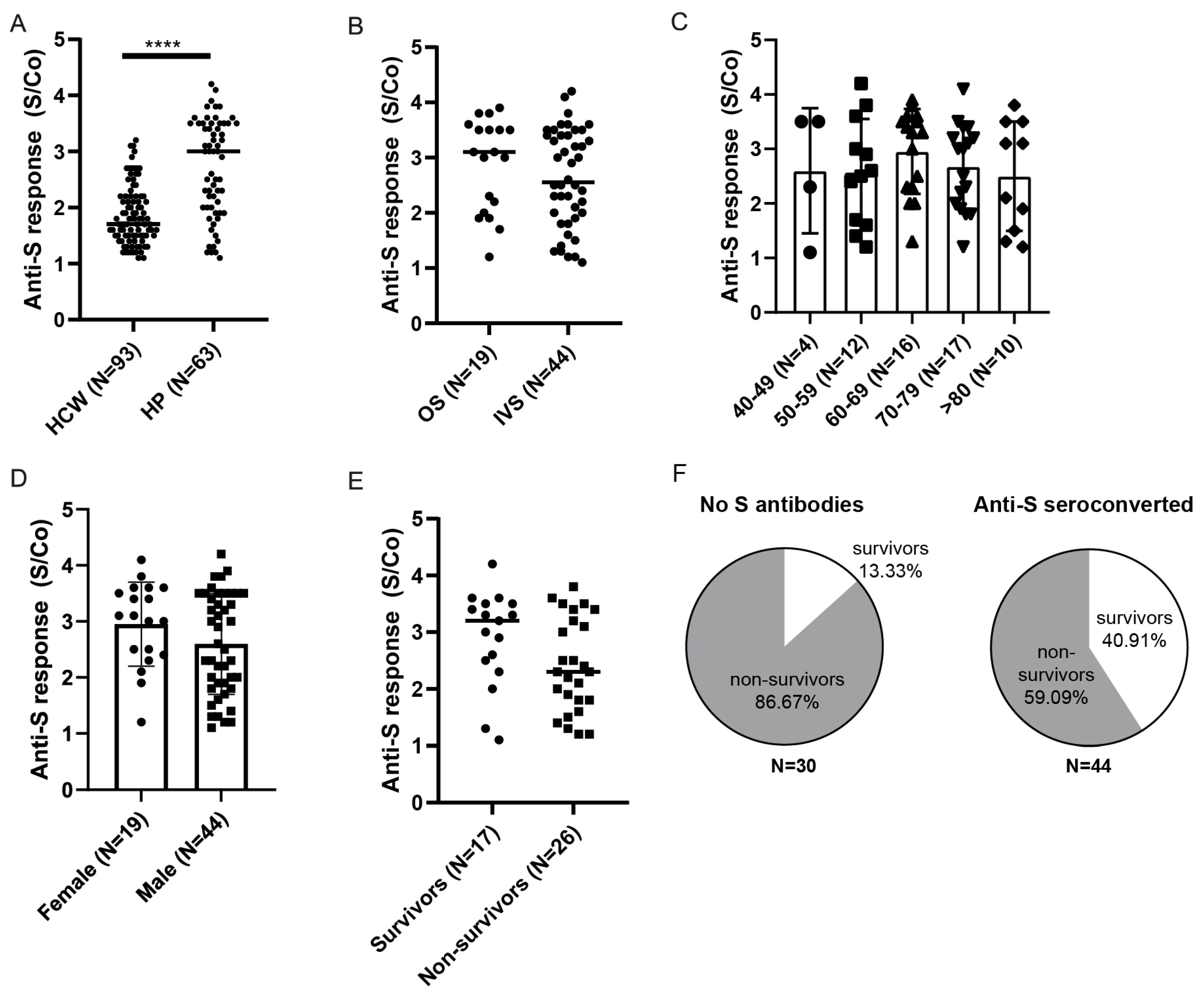
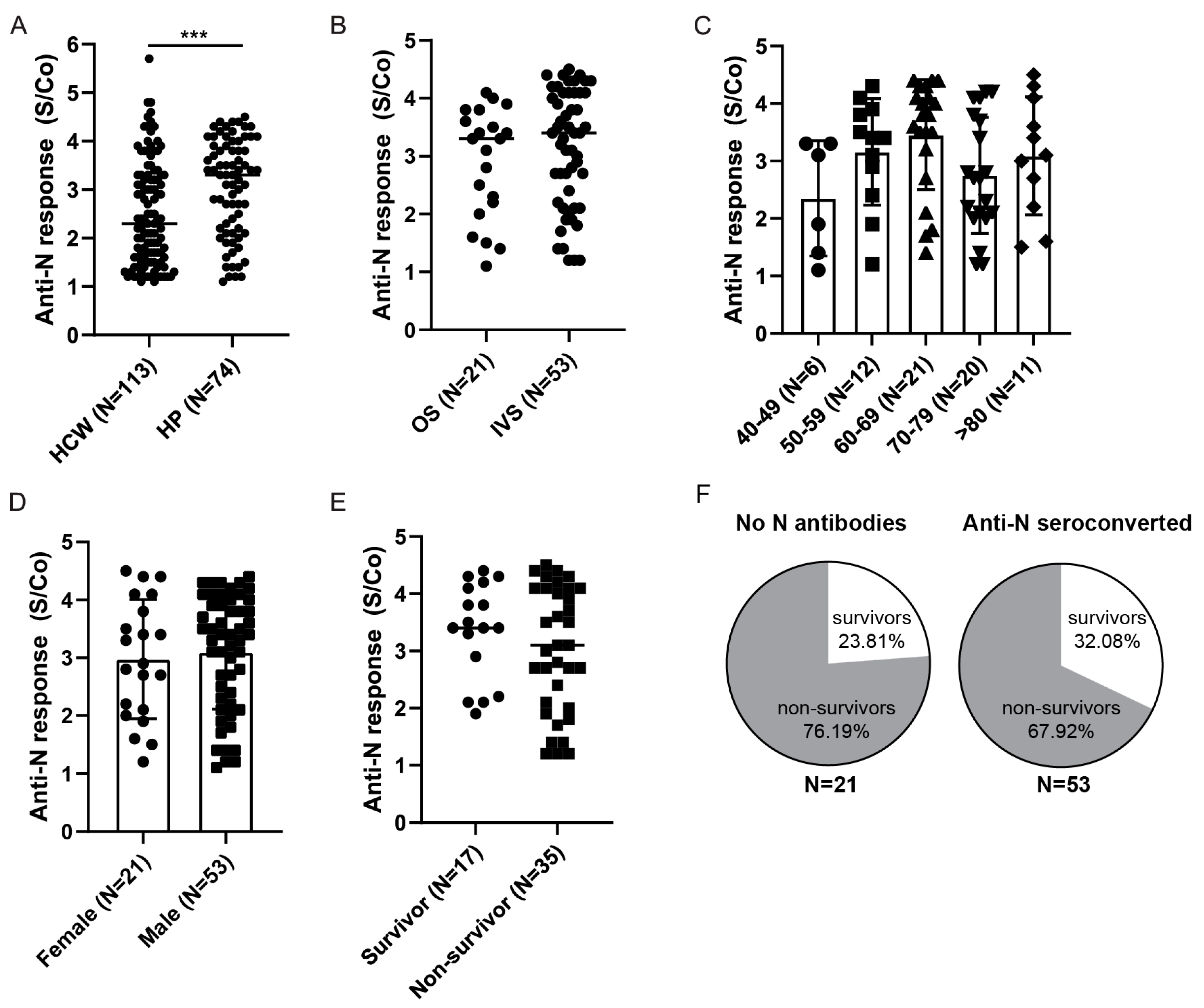
3.3. Longitudinal Assessment of Anti-Spike and Anti-Nucleocapsid Antibody Response in HCWs
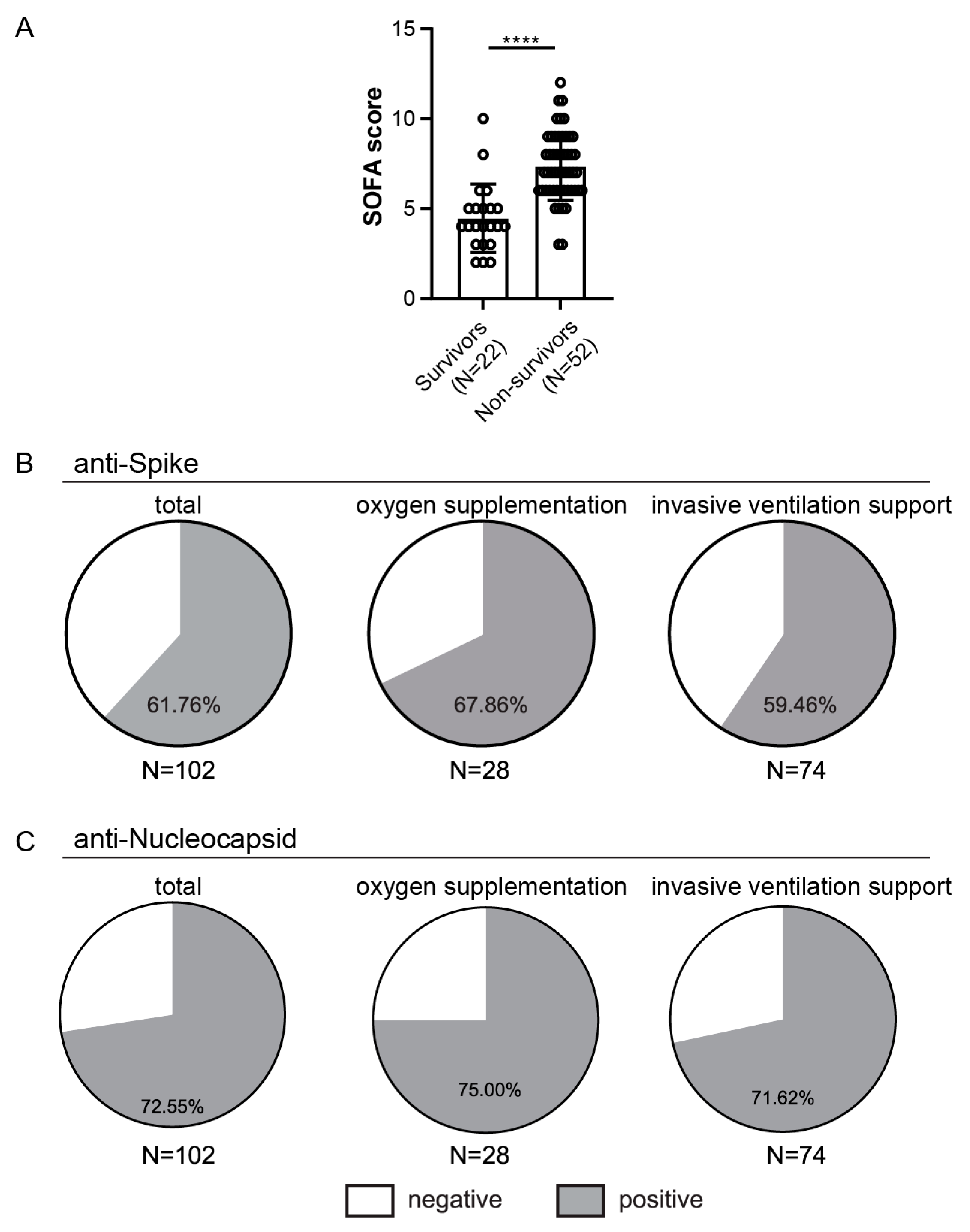
3.4. Demographic and Clinical Characteristics of the Hospitalized Patients’ Population
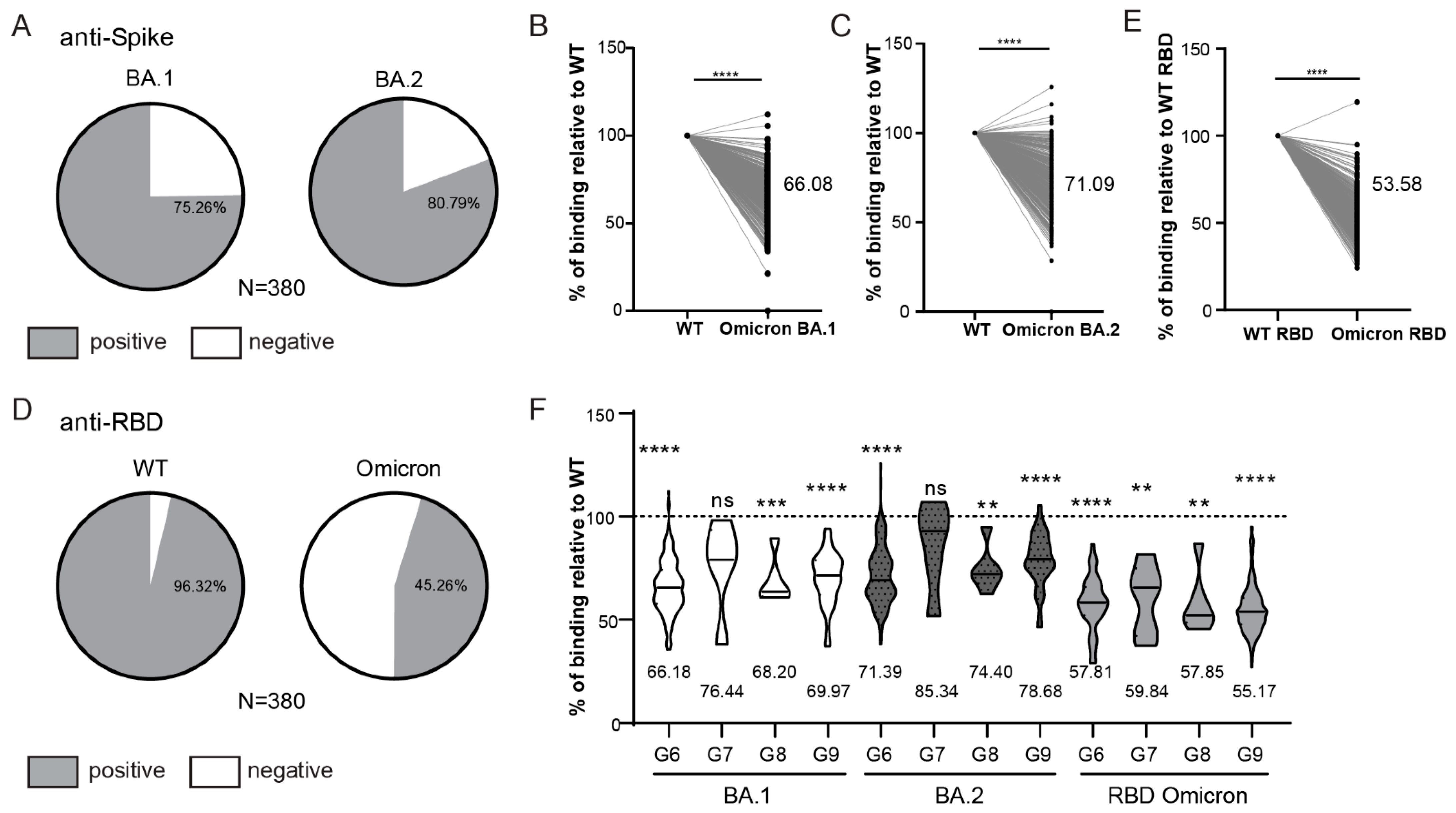
3.5. Anti-Spike and Anti-Nucleocapsid Antibody Response in Hospitalized Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Hannah Ritchie, E.M.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Ortiz-Ospina, E.; Hasell, J.; Macdonald, B.; Beltekian, D.; Roser, M. Coronavirus Pandemic (COVID-19). Available online: https://ourworldindata.org/coronavirus (accessed on 20 June 2022).
- Liu, L.; Iketani, S.; Guo, Y.; Chan, J.F.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Huang, Y.; Nair, M.S.; et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature 2022, 602, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Odak, I.; Schultze-Florey, C.R.; Hammerschmidt, S.I.; Ritter, C.; Willenzon, S.; Friedrichsen, M.; Ravens, I.; Sikora, R.; Bayir, L.M.; Gutierrez Jauregui, R.; et al. Longitudinal Tracking of Immune Responses in COVID-19 Convalescents Reveals Absence of Neutralization Activity against Omicron and Staggered Impairment to Other SARS-CoV-2 Variants of Concern. Front. Immunol. 2022, 13, 863039. [Google Scholar] [CrossRef]
- Youngs, J.; Provine, N.M.; Lim, N.; Sharpe, H.R.; Amini, A.; Chen, Y.L.; Luo, J.; Edmans, M.D.; Zacharopoulou, P.; Chen, W.; et al. Identification of immune correlates of fatal outcomes in critically ill COVID-19 patients. PLoS Pathog. 2021, 17, e1009804. [Google Scholar] [CrossRef]
- Huang, A.T.; Garcia-Carreras, B.; Hitchings, M.D.T.; Yang, B.; Katzelnick, L.C.; Rattigan, S.M.; Borgert, B.A.; Moreno, C.A.; Solomon, B.D.; Trimmer-Smith, L.; et al. A systematic review of antibody mediated immunity to coronaviruses: Kinetics, correlates of protection, and association with severity. Nat. Commun. 2020, 11, 4704. [Google Scholar] [CrossRef]
- Rostami, A.; Sepidarkish, M.; Leeflang, M.M.G.; Riahi, S.M.; Nourollahpour Shiadeh, M.; Esfandyari, S.; Mokdad, A.H.; Hotez, P.J.; Gasser, R.B. SARS-CoV-2 seroprevalence worldwide: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 331–340. [Google Scholar] [CrossRef]
- Wolff, F.; Dahma, H.; Duterme, C.; Van den Wijngaert, S.; Vandenberg, O.; Cotton, F.; Montesinos, I. Monitoring antibody response following SARS-CoV-2 infection: Diagnostic efficiency of 4 automated immunoassays. Diagn. Microbiol. Infect. Dis. 2020, 98, 115140. [Google Scholar] [CrossRef]
- Krajewski, R.; Golebiowska, J.; Makuch, S.; Mazur, G.; Agrawal, S. Update on serologic testing in COVID-19. Clin. Chim. Acta 2020, 510, 746–750. [Google Scholar] [CrossRef]
- Bonanni, P.; Cantón, R.; Gill, D.; Halfon, P.; Liebert, U.G.; Crespo, K.A.N.; Martín, J.J.P.; Trombetta, C.M. The Role of Serology Testing to Strengthen Vaccination Initiatives and Policies for COVID-19 in Europe. COVID 2021, 1, 20–38. [Google Scholar] [CrossRef]
- Kraay, A.N.M.; Nelson, K.N.; Zhao, C.Y.; Demory, D.; Weitz, J.S.; Lopman, B.A. Modeling serological testing to inform relaxation of social distancing for COVID-19 control. Nat. Commun. 2021, 12, 7063. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.D.; Cepinskas, G.; Slessarev, M.; Martin, C.M.; Daley, M.; Patel, M.A.; Miller, M.R.; Patterson, E.K.; O’Gorman, D.B.; Gill, S.E.; et al. Critically Ill COVID-19 Patients Exhibit Anti-SARS-CoV-2 Serological Responses. Pathophysiology 2021, 28, 212–223. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Cubuk, J.; Alston, J.J.; Incicco, J.J.; Singh, S.; Stuchell-Brereton, M.D.; Ward, M.D.; Zimmerman, M.I.; Vithani, N.; Griffith, D.; Wagoner, J.A.; et al. The SARS-CoV-2 nucleocapsid protein is dynamic, disordered, and phase separates with RNA. Nat. Commun. 2021, 12, 1936. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Gao, Y.; Liu, X.; Nie, Z.; Sun, H.; Lin, K.; Peng, H.; Wang, S. Identification and functional analysis of the SARS-COV-2 nucleocapsid protein. BMC Microbiol. 2021, 21, 58. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef]
- Smits, V.A.J.; Hernandez-Carralero, E.; Paz-Cabrera, M.C.; Cabrera, E.; Hernandez-Reyes, Y.; Hernandez-Fernaud, J.R.; Gillespie, D.A.; Salido, E.; Hernandez-Porto, M.; Freire, R. The Nucleocapsid protein triggers the main humoral immune response in COVID-19 patients. Biochem. Biophys. Res. Commun. 2021, 543, 45–49. [Google Scholar] [CrossRef]
- Burbelo, P.D.; Riedo, F.X.; Morishima, C.; Rawlings, S.; Smith, D.; Das, S.; Strich, J.R.; Chertow, D.S.; Davey, R.T.; Cohen, J.I. Sensitivity in Detection of Antibodies to Nucleocapsid and Spike Proteins of Severe Acute Respiratory Syndrome Coronavirus 2 in Patients with Coronavirus Disease 2019. J. Infect. Dis. 2020, 222, 206–213. [Google Scholar] [CrossRef]
- Chukwudozie, O.S.; Chukwuanukwu, R.C.; Iroanya, O.O.; Eze, D.M.; Duru, V.C.; Dele-Alimi, T.O.; Kehinde, B.D.; Bankole, T.T.; Obi, P.C.; Okinedo, E.U. Attenuated Subcomponent Vaccine Design Targeting the SARS-CoV-2 Nucleocapsid Phosphoprotein RNA Binding Domain: In Silico Analysis. J. Immunol. Res. 2020, 2020, 2837670. [Google Scholar] [CrossRef]
- Dangi, T.; Class, J.; Palacio, N.; Richner, J.M.; Penaloza MacMaster, P. Combining spike- and nucleocapsid-based vaccines improves distal control of SARS-CoV-2. Cell Rep. 2021, 36, 109664. [Google Scholar] [CrossRef]
- Silva, E.K.V.B.; Bomfim, C.G.; Barbosa, A.P.; Noda, P.; Noronha, I.L.; Fernandes, B.H.V.; Machado, R.R.G.; Durigon, E.L.; Catanozi, S.; Rodrigues, L.G.; et al. Immunization with SARS-CoV-2 Nucleocapsid protein triggers a pulmonary immune response in rats. PLoS ONE 2022, 17, e0268434. [Google Scholar] [CrossRef] [PubMed]
- Movsisyan, M.; Chopikyan, A.; Kasparova, I.; Hakobjanyan, G.; Carrat, F.; Sukiasyan, M.; Rushanyan, M.; Chalabyan, M.; Shariff, S.; Kantawala, B.; et al. Kinetics of anti-nucleocapsid IgG response in COVID-19 immunocompetent convalescent patients. Sci. Rep. 2022, 12, 12403. [Google Scholar] [CrossRef]
- Demmer, R.T.; Baumgartner, B.; Wiggen, T.D.; Ulrich, A.K.; Strickland, A.J.; Naumchik, B.M.; Bohn, B.; Walsh, S.; Smith, S.; Kline, S.; et al. Identification of Natural SARS-CoV-2 Infection in Seroprevalence Studies among Vaccinated Populations. Mayo Clin. Proc. 2022, 97, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Welch, S.R.; Davies, K.A.; Buczkowski, H.; Hettiarachchi, N.; Green, N.; Arnold, U.; Jones, M.; Hannah, M.J.; Evans, R.; Burton, C.; et al. Analysis of Inactivation of SARS-CoV-2 by Specimen Transport Media, Nucleic Acid Extraction Reagents, Detergents, and Fixatives. J. Clin. Microbiol. 2020, 58, e01713-20. [Google Scholar] [CrossRef]
- Pribanic Matesic, M.; Kucan Brlic, P.; Lenac Rovis, T.; Macak Safranko, Z.; Chaouat, A.E.; Miklic, K.; Malic, S.; Ivankovic, N.; Schubert, M.; Bertoglio, F.; et al. Collection of Monoclonal Antibodies Targeting SARS-CoV-2 Proteins. Viruses 2022, 14, 443. [Google Scholar] [CrossRef]
- Korn, J.; Schackermann, D.; Kirmann, T.; Bertoglio, F.; Steinke, S.; Heisig, J.; Ruschig, M.; Rojas, G.; Langreder, N.; Wenzel, E.V.; et al. Baculovirus-free insect cell expression system for high yield antibody and antigen production. Sci. Rep. 2020, 10, 21393. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, D.; Amanat, F.; Chromikova, V.; Jiang, K.; Strohmeier, S.; Arunkumar, G.A.; Tan, J.; Bhavsar, D.; Capuano, C.; Kirkpatrick, E.; et al. SARS-CoV-2 Seroconversion in Humans: A Detailed Protocol for a Serological Assay, Antigen Production, and Test Setup. Curr. Protoc. Microbiol. 2020, 57, e100. [Google Scholar] [CrossRef]
- Ashorn, P.; Krohn, K. Washing of ELISA plates with running tap water. J. Immunol. Methods 1986, 88, 141–142. [Google Scholar] [CrossRef]
- Terato, K.; Do, C.T.; Cutler, D.; Waritani, T.; Shionoya, H. Preventing intense false positive and negative reactions attributed to the principle of ELISA to re-investigate antibody studies in autoimmune diseases. J. Immunol. Methods 2014, 407, 15–25. [Google Scholar] [CrossRef]
- Waritani, T.; Chang, J.; McKinney, B.; Terato, K. An ELISA protocol to improve the accuracy and reliability of serological antibody assays. MethodsX 2017, 4, 153–165. [Google Scholar] [CrossRef]
- Arapovic, J.; Rajic, B.; Pati, S.; Brizic, I.; Azinovic, I.; Šušak, B.; Ostojic, M.; Tutiš, B.; Raguž, A.B.; Tomic, V.; et al. Cytomegalovirus Seroprevalence and Birth Prevalence of Congenital CMV Infection in Bosnia and Herzegovina: A Single-Center Experience. Pediatr. Infect. Dis. J. 2020, 39, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Siqueira, L.O.; Ferreira, F.A.P.; Sousa, B.G.; Mebus-Antunes, N.C.; Neves-Martins, T.C.; Almeida, F.C.L.; Ferreira, G.C.; Salmon, D.; Wermelinger, L.S.; Da Poian, A.T. On the caveats of a multiplex test for SARS-CoV-2 to detect seroconversion after infection or vaccination. Sci. Rep. 2022, 12, 10366. [Google Scholar] [CrossRef] [PubMed]
- Rostamzadeh, D.; Mortezagholi, S.; Alinejad, M.; Jooya, S.R.; Eskandarian, M.; Metvaei, A.; Vafaei, S.; Aboulghasemi, H.; Younesi, V.; Shabani, M. Serological assay for anti-SARS-CoV-2 antibodies improves sensitivity of diagnosis of COVID-19 patients. Med. Microbiol. Immunol. 2021, 210, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Grzelak, L.; Temmam, S.; Planchais, C.; Demeret, C.; Tondeur, L.; Huon, C.; Guivel-Benhassine, F.; Staropoli, I.; Chazal, M.; Dufloo, J.; et al. A comparison of four serological assays for detecting anti-SARS-CoV-2 antibodies in human serum samples from different populations. Sci. Transl. Med. 2020, 12, eabc3103. [Google Scholar] [CrossRef] [PubMed]
- Ynga-Durand, M.; Maaß, H.; Milošević, M.; Krstanović, F.; Pribanić Matešić, M.; Jonjić, S.; Protić, A.; Brizić, I.; Šustić, A.; Čičin-Šain, L. SARS-CoV-2 Viral Load in the Pulmonary Compartment of Critically Ill COVID-19 Patients Correlates with Viral Serum Load and Fatal Outcomes. Viruses 2022, 14, 1292. [Google Scholar] [CrossRef]
- Brochot, E.; Demey, B.; Touzé, A.; Belouzard, S.; Dubuisson, J.; Schmit, J.-L.; Duverlie, G.; Francois, C.; Castelain, S.; Helle, F. Anti-spike, Anti-nucleocapsid and Neutralizing Antibodies in SARS-CoV-2 Inpatients and Asymptomatic Individuals. Front. Microbiol. 2020, 11, 58425. [Google Scholar] [CrossRef] [PubMed]
- Golec, M.; Fronczek, M.; Zembala-John, J.; Chrapiec, M.; Konka, A.; Wystyrk, K.; Botor, H.; Brzoza, Z.; Kasperczyk, S.; Buldak, R.J. Early and Longitudinal Humoral Response to the SARS-CoV-2 mRNA BNT162b2 Vaccine in Healthcare Workers: Significance of BMI, Adipose Tissue and Muscle Mass on Long-Lasting Post-Vaccinal Immunity. Viruses 2022, 14, 868. [Google Scholar] [CrossRef] [PubMed]
- de Visscher, N.; Holemans, X.; Gillain, A.; Kornreich, A.; Lagasse, R.; Piette, P.; Ventura, M.; Thys, F. SARS-CoV-2 Seroprevalence among Healthcare Workers after the First and Second Pandemic Waves. Viruses 2022, 14, 1535. [Google Scholar] [CrossRef]
- Atzl, M.; Muendlein, A.; Winder, T.; Fraunberger, P.; Brandtner, E.-M.; Geiger, K.; Klausberger, M.; Duerkop, M.; Sprenger, L.; Mutschlechner, B.; et al. SARS-CoV-2 RBD-specific and NP-specific antibody response of healthcare workers in the westernmost Austrian state Vorarlberg: A prospective cohort study. BMJ Open 2022, 12, e052130. [Google Scholar] [CrossRef]
- Vilibic-Cavlek, T.; Stevanovic, V.; Ilic, M.; Barbic, L.; Capak, K.; Tabain, I.; Krleza, J.L.; Ferenc, T.; Hruskar, Z.; Topic, R.Z.; et al. SARS-CoV-2 Seroprevalence and Neutralizing Antibody Response after the First and Second COVID-19 Pandemic Wave in Croatia. Pathogens 2021, 10, 774. [Google Scholar] [CrossRef]
- Naaber, P.; Tserel, L.; Kangro, K.; Sepp, E.; Jürjenson, V.; Adamson, A.; Haljasmägi, L.; Rumm, A.P.; Maruste, R.; Kärner, J.; et al. Dynamics of antibody response to BNT162b2 vaccine after six months: A longitudinal prospective study. Lancet Reg. Health Eur. 2021, 10, 100208. [Google Scholar] [CrossRef] [PubMed]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.; et al. SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. medRxiv 2021. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Shaw, R.H.; Supasa, P.; Liu, C.; Stuart, A.S.; Pollard, A.J.; Liu, X.; Lambe, T.; Crook, D.; Stuart, D.I.; et al. Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. Lancet 2022, 399, 234–236. [Google Scholar] [CrossRef]
- Schubert, M.; Bertoglio, F.; Steinke, S.; Heine, P.A.; Ynga-Durand, M.A.; Maass, H.; Sammartino, J.C.; Cassaniti, I.; Zuo, F.; Du, L.; et al. Human serum from SARS-CoV-2-vaccinated and COVID-19 patients shows reduced binding to the RBD of SARS-CoV-2 Omicron variant. BMC Med. 2022, 20, 102. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Castro Dopico, X.; Ols, S.; Lore, K.; Karlsson Hedestam, G.B. Immunity to SARS-CoV-2 induced by infection or vaccination. J. Intern. Med. 2022, 291, 32–50. [Google Scholar] [CrossRef]
- Carvalho, T.; Krammer, F.; Iwasaki, A. The first 12 months of COVID-19: A timeline of immunological insights. Nat. Rev. Immunol. 2021, 21, 245–256. [Google Scholar] [CrossRef]
- Tomic, A.; Skelly, D.T.; Ogbe, A.; O’Connor, D.; Pace, M.; Adland, E.; Alexander, F.; Ali, M.; Allott, K.; Azim Ansari, M.; et al. Divergent trajectories of antiviral memory after SARS-CoV-2 infection. Nat. Commun. 2022, 13, 1251. [Google Scholar] [CrossRef]
- Coppeta, L.; Ferrari, C.; Somma, G.; Mazza, A.; D’Ancona, U.; Marcuccilli, F.; Grelli, S.; Aurilio, M.T.; Pietroiusti, A.; Magrini, A.; et al. Reduced Titers of Circulating Anti-SARS-CoV-2 Antibodies and Risk of COVID-19 Infection in Healthcare Workers during the Nine Months after Immunization with the BNT162b2 mRNA Vaccine. Vaccines 2022, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Perez-Alos, L.; Armenteros, J.J.A.; Madsen, J.R.; Hansen, C.B.; Jarlhelt, I.; Hamm, S.R.; Heftdal, L.D.; Pries-Heje, M.M.; Moller, D.L.; Fogh, K.; et al. Modeling of waning immunity after SARS-CoV-2 vaccination and influencing factors. Nat. Commun. 2022, 13, 1614. [Google Scholar] [CrossRef] [PubMed]
- Kwok, S.L.; Cheng, S.M.; Leung, J.N.; Leung, K.; Lee, C.K.; Peiris, J.M.; Wu, J.T. Waning antibody levels after COVID-19 vaccination with mRNA Comirnaty and inactivated CoronaVac vaccines in blood donors, Hong Kong, April 2020 to October 2021. Eurosurveillance 2022, 27, 2101197. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Suthar, M.S.; Arunachalam, P.S.; Hu, M.; Reis, N.; Trisal, M.; Raeber, O.; Chinthrajah, S.; Davis-Gardner, M.E.; Manning, K.; Mudvari, P.; et al. Durability of immune responses to the BNT162b2 mRNA vaccine. Med 2022, 3, 25–27. [Google Scholar] [CrossRef]
- Khan, Q.J.; Bivona, C.R.; Martin, G.A.; Zhang, J.; Liu, B.; He, J.; Li, K.H.; Nelson, M.; Williamson, S.; Doolittle, G.C.; et al. Evaluation of the Durability of the Immune Humoral Response to COVID-19 Vaccines in Patients with Cancer Undergoing Treatment or Who Received a Stem Cell Transplant. JAMA Oncol. 2022, 8, 1053–1058. [Google Scholar] [CrossRef]
- Terpos, E.; Karalis, V.; Ntanasis-Stathopoulos, I.; Apostolakou, F.; Gumeni, S.; Gavriatopoulou, M.; Papadopoulos, D.; Malandrakis, P.; Papanagnou, E.-D.; Korompoki, E.; et al. Sustained but Declining Humoral Immunity against SARS-CoV-2 at 9 Months Postvaccination with BNT162b2: A Prospective Evaluation in 309 Healthy Individuals. HemaSphere 2022, 6, e677. [Google Scholar] [CrossRef]
- Tut, G.; Lancaster, T.; Krutikov, M.; Sylla, P.; Bone, D.; Kaur, N.; Spalkova, E.; Bentley, C.; Amin, U.; Jadir, A.T.; et al. Profile of humoral and cellular immune responses to single doses of BNT162b2 or ChAdOx1 nCoV-19 vaccines in residents and staff within residential care homes (VIVALDI): An observational study. Lancet Healthy Longev. 2021, 2, e544–e553. [Google Scholar] [CrossRef]
- Uwamino, Y.; Kurafuji, T.; Takato, K.; Sakai, A.; Tanabe, A.; Noguchi, M.; Yatabe, Y.; Arai, T.; Ohno, A.; Tomita, Y.; et al. Dynamics of antibody titers and cellular immunity among Japanese healthcare workers during the 6 months after receiving two doses of BNT162b2 mRNA vaccine. Vaccine 2022, 40, 4538–4543. [Google Scholar] [CrossRef]
- Amanat, F.; Thapa, M.; Lei, T.; Ahmed, S.M.S.; Adelsberg, D.C.; Carreño, J.M.; Strohmeier, S.; Schmitz, A.J.; Zafar, S.; Zhou, J.Q.; et al. SARS-CoV-2 mRNA vaccination induces functionally diverse antibodies to NTD, RBD, and S2. Cell 2021, 184, 3936–3948.e3910. [Google Scholar] [CrossRef]
- Chansaenroj, J.; Yorsaeng, R.; Posuwan, N.; Puenpa, J.; Wanlapakorn, N.; Sudhinaraset, N.; Sripramote, M.; Chalongviriyalert, P.; Jirajariyavej, S.; Kiatpanabhikul, P.; et al. Long-term specific IgG response to SARS-CoV-2 nucleocapsid protein in recovered COVID-19 patients. Sci. Rep. 2021, 11, 23216. [Google Scholar] [CrossRef]
- Gallais, F.; Gantner, P.; Bruel, T.; Velay, A.; Planas, D.; Wendling, M.J.; Bayer, S.; Solis, M.; Laugel, E.; Reix, N.; et al. Evolution of antibody responses up to 13 months after SARS-CoV-2 infection and risk of reinfection. EBioMedicine 2021, 71, 103561. [Google Scholar] [CrossRef] [PubMed]
- Koerber, N.; Priller, A.; Yazici, S.; Bauer, T.; Cheng, C.-C.; Mijočević, H.; Wintersteller, H.; Jeske, S.; Vogel, E.; Feuerherd, M.; et al. Dynamics of spike-and nucleocapsid specific immunity during long-term follow-up and vaccination of SARS-CoV-2 convalescents. Nat. Commun. 2022, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Löfström, E.; Eringfält, A.; Kötz, A.; Wickbom, F.; Tham, J.; Lingman, M.; Nygren, J.M.; Undén, J. Dynamics of IgG-avidity and antibody levels after COVID-19. J. Clin. Virol. 2021, 144, 104986. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, A.; Shajahan, S.; Harris, K.; Hallam, L.; Hockham, C.; Womersley, K.; Woodward, M.; Sheel, M. Sex and Gender in COVID-19 Vaccine Research: Substantial Evidence Gaps Remain. Front. Glob. Women′s Health 2021, 2, 761511. [Google Scholar] [CrossRef]
- Brady, E.; Nielsen, M.W.; Andersen, J.P.; Oertelt-Prigione, S. Lack of consideration of sex and gender in COVID-19 clinical studies. Nat. Commun. 2021, 12, 4015. [Google Scholar] [CrossRef]
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.P.; Lim, E.Y.; Touizer, E.; Meng, B.; Abdullahi, A.; Baker, S.; Dougan, G.; et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 2021, 596, 417–422. [Google Scholar] [CrossRef]
- Müller, L.; Andrée, M.; Moskorz, W.; Drexler, I.; Walotka, L.; Grothmann, R.; Ptok, J.; Hillebrandt, J.; Ritchie, A.; Rabl, D.; et al. Age-dependent Immune Response to the Biontech/Pfizer BNT162b2 Coronavirus Disease 2019 Vaccination. Clin. Infect. Dis. 2021, 73, 2065–2072. [Google Scholar] [CrossRef]
- Ward, H.; Whitaker, M.; Flower, B.; Tang, S.N.; Atchison, C.; Darzi, A.; Donnelly, C.A.; Cann, A.; Diggle, P.J.; Ashby, D.; et al. Population antibody responses following COVID-19 vaccination in 212,102 individuals. Nat. Commun. 2022, 13, 907. [Google Scholar] [CrossRef]
- Tut, G.; Lancaster, T.; Butler, M.S.; Sylla, P.; Spalkova, E.; Bone, D.; Kaur, N.; Bentley, C.; Amin, U.; Jadir, A.T.; et al. Robust SARS-CoV-2-specific and heterologous immune responses in vaccine-naïve residents of long-term care facilities who survive natural infection. Nat. Aging 2022, 2, 536–547. [Google Scholar] [CrossRef]
- McCartney, P.R. Sex-Based Vaccine Response in the Context of COVID-19. J. Obstet. Gynecol. Neonatal Nurs. 2020, 49, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Bignucolo, A.; Scarabel, L.; Mezzalira, S.; Polesel, J.; Cecchin, E.; Toffoli, G. Sex Disparities in Efficacy in COVID-19 Vaccines: A Systematic Review and Meta-Analysis. Vaccines 2021, 9, 825. [Google Scholar] [CrossRef] [PubMed]
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef]
- Lo Sasso, B.; Agnello, L.; Giglio, R.V.; Gambino, C.M.; Ciaccio, A.M.; Vidali, M.; Ciaccio, M. Longitudinal analysis of anti-SARS-CoV-2 S-RBD IgG antibodies before and after the third dose of the BNT162b2 vaccine. Sci. Rep. 2022, 12, 8679. [Google Scholar] [CrossRef]
- Takahashi, T.; Ellingson, M.K.; Wong, P.; Israelow, B.; Lucas, C.; Klein, J.; Silva, J.; Mao, T.; Oh, J.E.; Tokuyama, M.; et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020, 588, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Alahmad, B.; Al-Shammari, A.A.; Alterki, A.; Hammad, M.; Cherian, P.; Alkhairi, I.; Sindhu, S.; Thanaraj, T.A.; Mohammad, A.; et al. Previous COVID-19 Infection and Antibody Levels after Vaccination. Front. Public Health 2021, 9, 778243. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Xiao, S.; Debes, A.K.; Egbert, E.R.; Caturegli, P.; Colantuoni, E.; Milstone, A.M. Durability of Antibody Levels after Vaccination with mRNA SARS-CoV-2 Vaccine in Individuals with or without Prior Infection. JAMA 2021, 326, 2524–2526. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Sprouse, K.R.; Bowen, J.E.; Joshi, A.; Franko, N.; Navarro, M.J.; Stewart, C.; Cameroni, E.; McCallum, M.; Goecker, E.A.; et al. SARS-CoV-2 breakthrough infections elicit potent, broad, and durable neutralizing antibody responses. Cell 2022, 185, 872–880.e873. [Google Scholar] [CrossRef]
- Andeweg, S.P.; de Gier, B.; Eggink, D.; van den Ende, C.; van Maarseveen, N.; Ali, L.; Vlaemynck, B.; Schepers, R.; Hahné, S.J.M.; Reusken, C.B.E.M.; et al. Protection of COVID-19 vaccination and previous infection against Omicron BA.1, BA.2 and Delta SARS-CoV-2 infections. Nat. Commun. 2022, 13, 4738. [Google Scholar] [CrossRef]
- Altarawneh, H.N.; Chemaitelly, H.; Hasan, M.R.; Ayoub, H.H.; Qassim, S.; AlMukdad, S.; Coyle, P.; Yassine, H.M.; Al-Khatib, H.A.; Benslimane, F.M.; et al. Protection against the Omicron Variant from Previous SARS-CoV-2 Infection. N. Engl. J. Med. 2022, 386, 1288–1290. [Google Scholar] [CrossRef]
- Ibarrondo, F.J.; Fulcher, J.A.; Goodman-Meza, D.; Elliott, J.; Hofmann, C.; Hausner, M.A.; Ferbas, K.G.; Tobin, N.H.; Aldrovandi, G.M.; Yang, O.O. Rapid Decay of Anti-SARS-CoV-2 Antibodies in Persons with Mild COVID-19. N. Engl. J. Med. 2020, 383, 1085–1087. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.S.; Jones, F.K.; Nodoushani, A.; Kelly, M.; Becker, M.; Slater, D.; Mills, R.; Teng, E.; Kamruzzaman, M.; Garcia-Beltran, W.F.; et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci. Immunol. 2020, 5, eabe0367. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.-X.; Tang, X.-J.; Shi, Q.-L.; Li, Q.; Deng, H.-J.; Yuan, J.; Hu, J.-L.; Xu, W.; Zhang, Y.; Lv, F.-J.; et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Zohar, T.; Alter, G. Dissecting antibody-mediated protection against SARS-CoV-2. Nat. Rev. Immunol. 2020, 20, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S. SARS-CoV-2 (COVID-19), viral load and clinical outcomes; lessons learned one year into the pandemic: A systematic review. World J. Crit. Care Med. 2021, 10, 132–150. [Google Scholar] [CrossRef]
- Šušak, B.; Mikulić, V.; Lazarević, A.; Mikulić, I.; Arapovic, J. Sustained seroprevalence of SARS-CoV-2 antibodies one year after infection: One of the first COVID-19 cluster cases in Bosnia and Herzegovina. Bosn. J. Basic Med. Sci. 2022, 22, 147–152. [Google Scholar] [CrossRef]
- Hendriks, J.; Schasfoort, R.; Koerselman, M.; Dannenberg, M.; Cornet, A.D.; Beishuizen, A.; van der Palen, J.; Krabbe, J.; Mulder, A.H.L.; Karperien, M. High Titers of Low Affinity Antibodies in COVID-19 Patients Are Associated with Disease Severity. Front. Immunol. 2022, 13, 867716. [Google Scholar] [CrossRef]
- Havervall, S.; Jernbom Falk, A.; Klingstrom, J.; Ng, H.; Greilert-Norin, N.; Gabrielsson, L.; Salomonsson, A.C.; Isaksson, E.; Rudberg, A.S.; Hellstrom, C.; et al. SARS-CoV-2 induces a durable and antigen specific humoral immunity after asymptomatic to mild COVID-19 infection. PLoS ONE 2022, 17, e0262169. [Google Scholar] [CrossRef]
- Menon, V.; Shariff, M.A.; Perez Gutierrez, V.; Carreño, J.M.; Yu, B.; Jawed, M.; Gossai, M.; Valdez, E.; Pillai, A.; Venugopal, U.; et al. Longitudinal humoral antibody response to SARS-CoV-2 infection among healthcare workers in a New York City hospital. BMJ Open 2021, 11, e051045. [Google Scholar] [CrossRef]
- Griffin, A.J.; O’Donnell, K.L.; Shifflett, K.; Lavik, J.-P.; Russell, P.M.; Zimmerman, M.K.; Relich, R.F.; Marzi, A. Serum from COVID-19 patients early in the pandemic shows limited evidence of cross-neutralization against variants of concern. Sci. Rep. 2022, 12, 3954. [Google Scholar] [CrossRef]
- Yang, L.; Xu, Q.; Yang, B.; Li, J.; Dong, R.; Da, J.; Ye, Z.; Xu, Y.; Zhou, H.; Zhang, X.; et al. IgG antibody titers against SARS-CoV-2 nucleocapsid protein correlate with the severity of COVID-19 patients. BMC Microbiol. 2021, 21, 351. [Google Scholar] [CrossRef]
- Lynch, K.L.; Whitman, J.D.; Lacanienta, N.P.; Beckerdite, E.W.; Kastner, S.A.; Shy, B.R.; Goldgof, G.M.; Levine, A.G.; Bapat, S.P.; Stramer, S.L.; et al. Magnitude and Kinetics of Anti-Severe Acute Respiratory Syndrome Coronavirus 2 Antibody Responses and Their Relationship to Disease Severity. Clin. Infect. Dis. 2021, 72, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.H.; Dharmarajan, S.; Lehtimaki, M.; Kirshner, S.L.; Kozlowski, S. Early antibody responses associated with survival in COVID19 patients. PLoS Pathog. 2021, 17, e1009766. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; Astudillo, M.G.; Yang, D.; Miller, T.E.; Feldman, J.; Hauser, B.M.; Caradonna, T.M.; Clayton, K.L.; Nitido, A.D.; et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell 2021, 184, 476–488.e411. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, J. The Flawed Science of Antibody Testing for SARS-CoV-2 Immunity. JAMA 2021, 326, 1781–1782. [Google Scholar] [CrossRef] [PubMed]
- Shrwani, K.; Sharma, R.; Krishnan, M.; Jones, T.; Mayora-Neto, M.; Cantoni, D.; Temperton, N.J.; Dobson, S.L.; Subramaniam, K.; McNamara, P.S.; et al. Detection of Serum Cross-Reactive Antibodies and Memory Response to SARS-CoV-2 in Prepandemic and Post-COVID-19 Convalescent Samples. J. Infect. Dis. 2021, 224, 1305–1315. [Google Scholar] [CrossRef]
- Dowell, A.C.; Butler, M.S.; Jinks, E.; Tut, G.; Lancaster, T.; Sylla, P.; Begum, J.; Bruton, R.; Pearce, H.; Verma, K.; et al. Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 40–49. [Google Scholar] [CrossRef] [PubMed]
| Time Point | Prior Vaccination | +3 Weeks (after 1st Dose) | +6 Months (after 2 Doses) | +14–16 Months |
|---|---|---|---|---|
| Total number of participants | 937 | 651 (601 *) | 380 (291 *) | 20 (19 *) |
| Self-reported COVID-19 | 104 | nd | 53 | 7 |
| Booster dose | / | / | / | 14 |
| Gender | 77.2% F 22.8% M | 75.3% F 24.7% M | 78.4% F 21.6% M | 70.0% F 30.0% M |
| Age (mean ± SD) | 46 ± 11 | 45 ± 11 | 46 ± 11 | 49 ± 13 |
| Patient Characteristics | Oxygen Supplementation | Invasive Ventilation Support | ||
|---|---|---|---|---|
| n = 102 | n = 28 | n = 74 | ||
| Median age | 68 (40–89) | 71 | 67 | |
| Gender | ||||
| Female | 29 (28%) | 8 (28%) | 21 (28%) | |
| Male | 73 (72%) | 20 (72%) | 53 (72%) | |
| Patients on Invasive Ventilation (IVS) | Survivors | Non-Survivors | |
|---|---|---|---|
| n = 74 | n = 22 (30%) | n = 52 (70%) | |
| Vaccinated | 2/74 | ||
| Average time on IVS (days) | 10 (±9) | 11 (±14) | 10 (±7) |
| Average ICU stay (days) | 13 (±11) | 18 (±17) | 11 (±7) |
| SOFA score at admission | 6 (±2) | 4 (±2) | 7 (±2) |
| Gender | |||
| Female | 21 (28%) | 6 | 15 |
| Male | 53 (72%) | 16 | 37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brlić, P.K.; Pavletić, M.; Lerga, M.; Krstanović, F.; Matešić, M.P.; Miklić, K.; Malić, S.; Mikša, L.; Pajcur, M.; Peruč, D.; et al. SARS-CoV-2 Spike and Nucleocapsid Antibody Response in Vaccinated Croatian Healthcare Workers and Infected Hospitalized Patients: A Single Center Cohort Study. Viruses 2022, 14, 1966. https://doi.org/10.3390/v14091966
Brlić PK, Pavletić M, Lerga M, Krstanović F, Matešić MP, Miklić K, Malić S, Mikša L, Pajcur M, Peruč D, et al. SARS-CoV-2 Spike and Nucleocapsid Antibody Response in Vaccinated Croatian Healthcare Workers and Infected Hospitalized Patients: A Single Center Cohort Study. Viruses. 2022; 14(9):1966. https://doi.org/10.3390/v14091966
Chicago/Turabian StyleBrlić, Paola Kučan, Martina Pavletić, Mate Lerga, Fran Krstanović, Marina Pribanić Matešić, Karmela Miklić, Suzana Malić, Leonarda Mikša, Maja Pajcur, Dolores Peruč, and et al. 2022. "SARS-CoV-2 Spike and Nucleocapsid Antibody Response in Vaccinated Croatian Healthcare Workers and Infected Hospitalized Patients: A Single Center Cohort Study" Viruses 14, no. 9: 1966. https://doi.org/10.3390/v14091966
APA StyleBrlić, P. K., Pavletić, M., Lerga, M., Krstanović, F., Matešić, M. P., Miklić, K., Malić, S., Mikša, L., Pajcur, M., Peruč, D., Schubert, M., Bertoglio, F., Arapović, J., Protić, A., Šustić, A., Milošević, M., Šain, L. Č., Jonjić, S., Lisnić, V. J., & Brizić, I. (2022). SARS-CoV-2 Spike and Nucleocapsid Antibody Response in Vaccinated Croatian Healthcare Workers and Infected Hospitalized Patients: A Single Center Cohort Study. Viruses, 14(9), 1966. https://doi.org/10.3390/v14091966








