Characterization of Influenza D Virus in Danish Calves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples, Clinical Examination, and Herd Descriptions
2.2. RNA Extraction
2.3. Real-Time RT-PCR
2.4. Sanger Sequencing
2.5. Whole-Genome Sequencing
2.6. Consensus Sequence Generation
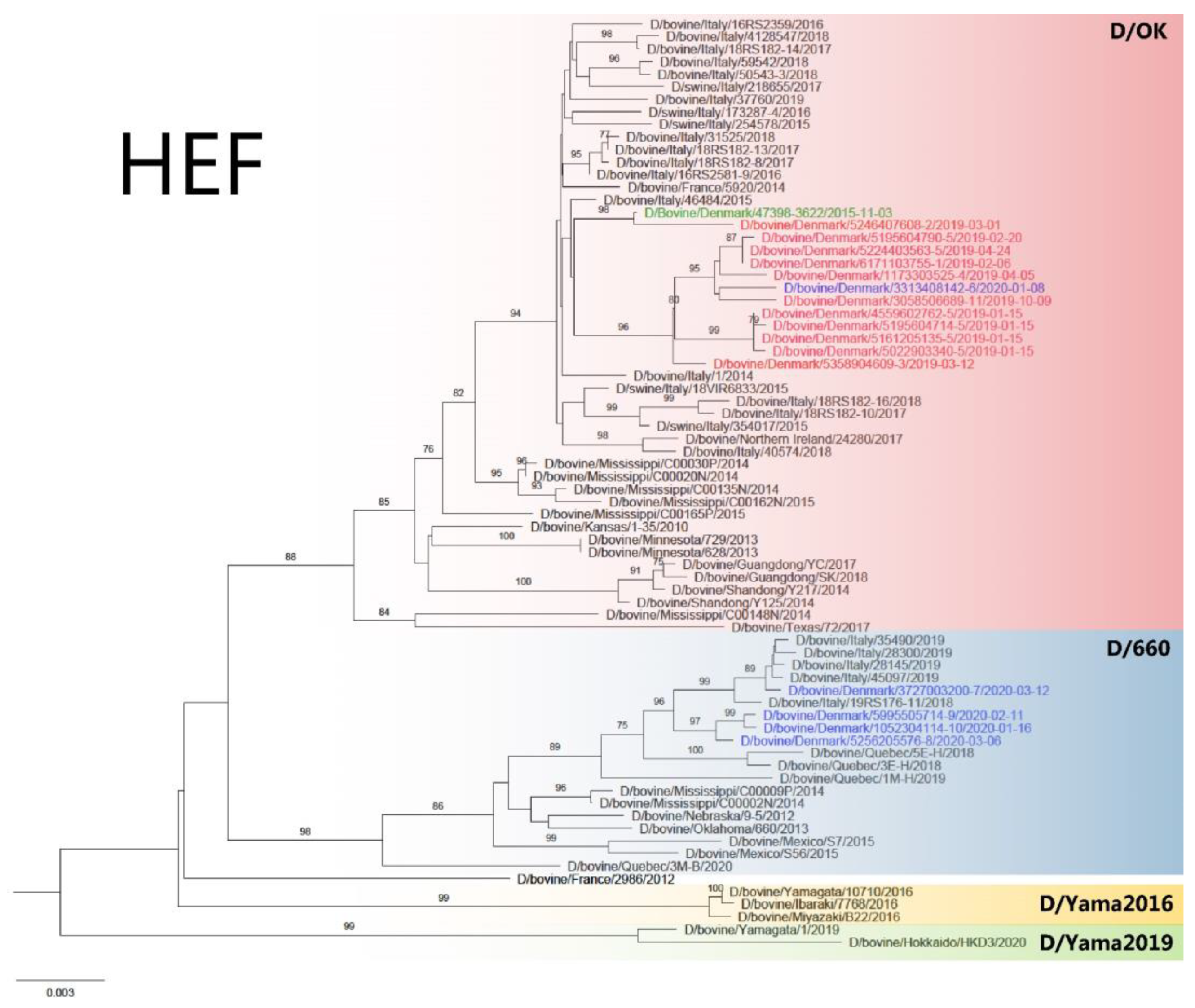
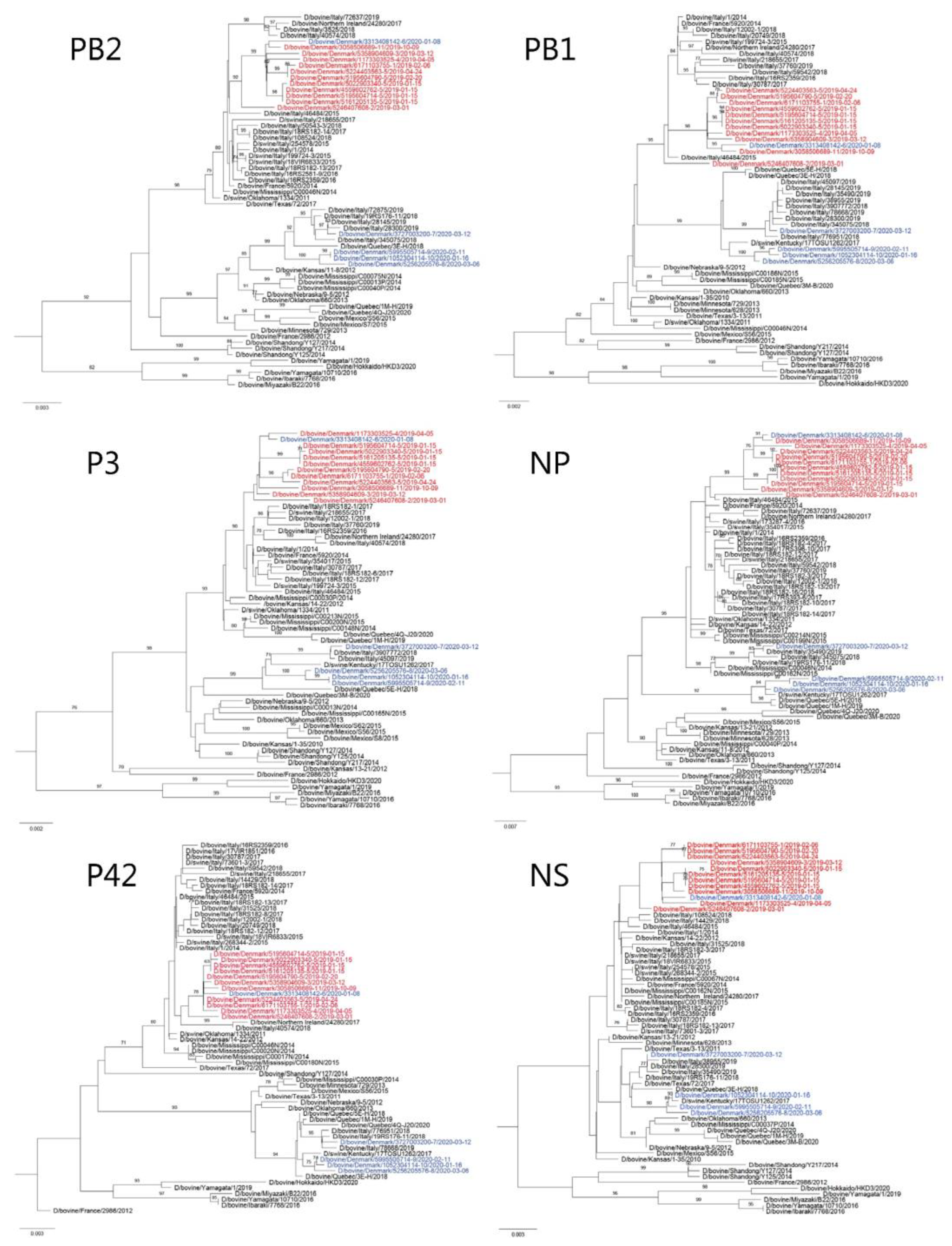
2.7. Maximum Likelihood Phylogeny
2.8. Prediction of Glycosylation Sites
3. Results
3.1. Clinical Signs
3.2. WGS and Phylogenetic Analysis
3.3. Prediction of Glycosylation Sites
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hause, B.M.; Collin, E.A.; Liu, R.; Huang, B.; Sheng, Z.; Lu, W.; Wang, D.; Nelson, E.A.; Li, F. Characterization of a novel influenza virus in cattle and swine: Proposal for a new genus in the Orthomyxoviridae family. mBio 2014, 5, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hause, B.M.; Ducatez, M.; Collin, E.A.; Ran, Z.; Liu, R.; Sheng, Z.; Armien, A.; Kaplan, B.; Chakravarty, S.; Hoppe, A.D.; et al. Isolation of a Novel Swine Influenza Virus from Oklahoma in 2011 Which Is Distantly Related to Human Influenza C Viruses. PLoS Pathog. 2013, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asha, K.; Kumar, B. Emerging Influenza D Virus Threat: What We Know so Far! J. Clin. Med. 2019, 8, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Ferguson, L.; Smith, D.R.; Woolums, A.R.; Epperson, W.B.; Wan, X.F. Serological evidence for high prevalence of Influenza D Viruses in Cattle, Nebraska, United States, 2003–2004. Virology 2017, 501, 88–91. [Google Scholar] [CrossRef]
- Oliva, J.; Eichenbaum, A.; Belin, J.; Gaudino, M.; Guillotin, J.; Alzieu, J.P.; Nicollet, P.; Brugidou, R.; Gueneau, E.; Michel, E.; et al. Serological Evidence of Influenza D Virus Circulation Among Cattle and Small Ruminants in France. Viruses 2019, 11, 516. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, L.; Luo, K.; Olivier, A.K.; Cunningham, F.L.; Blackmon, S.; Hanson-Dorr, K.; Sun, H.; Baroch, J.; Lutman, M.W.; Quade, B.; et al. Influenza D Virus Infection in Feral Swine Populations, United States. Emerg. Infect. Dis. 2018, 24, 1020–1028. [Google Scholar] [CrossRef] [Green Version]
- Nedland, H.; Wollman, J.; Sreenivasan, C.; Quast, M.; Singrey, A.; Fawcett, L.; Christopher-Hennings, J.; Nelson, E.; Kaushik, R.S.; Wang, D.; et al. Serological evidence for the co-circulation of two lineages of influenza D viruses in equine populations of the Midwest United States. Zoonoses Public Heal. 2018, 65, e148–e154. [Google Scholar] [CrossRef]
- Salem, E.; Cook, E.A.J.; Lbacha, H.A.; Oliva, J.; Awoume, F.; Aplogan, G.L.; Hymann, E.C.; Muloi, D.; Deem, S.L.; Alali, S.; et al. Serologic Evidence for Influenza C and D Virus among Ruminants and Camelids, Africa, 1991–2015. Emerg. Infect. Dis. 2017, 23, 1556–1559. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, L.; Olivier, A.K.; Genova, S.; Epperson, W.B.; Smith, D.R.; Schneider, L.; Barton, K.; McCuan, K.; Webby, R.J.; Wan, X.-F. Pathogenesis of Influenza D Virus in Cattle. J. Virol. 2016, 90, 5636–5642. [Google Scholar] [CrossRef] [Green Version]
- Mitra, N.; Cernicchiaro, N.; Torres, S.; Li, F.; Hause, B.M. Metagenomic characterization of the virome associated with bovine respiratory disease in feedlot cattle identified novel viruses and suggests an etiologic role for influenza D virus. J. Gen. Virol. 2016, 97, 1771–1784. [Google Scholar] [CrossRef]
- Ferguson, L.; Eckard, L.; Epperson, W.B.; Long, L.P.; Smith, D.; Huston, C.; Genova, S.; Webby, R.; Wan, X.F. Influenza D virus infection in Mississippi beef cattle. Virology 2015, 486, 28–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.M.; Wang, S.C.; Peng, C.; Yu, J.M.; Zhuang, Q.Y.; Hou, G.Y.; Liu, S.; Li, J.P.; Chen, J.M. Identification of a potential novel type of influenza virus in Bovine in China. Virus Genes 2014, 49, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Endoh, M.; Kobayashi, T.; Takenaka-Uema, A.; Chambers, J.K.; Uchida, K.; Nishihara, M.; Hause, B.; Horimoto, T. Influenza D Virus Infection in Herd of Cattle, Japan. Emerg. Infect. Dis. 2016, 22, 1517–1519. [Google Scholar] [CrossRef]
- Ducatez, M.F.; Pelletier, C.; Meyer, G. Influenza d virus in cattle, France, 2011–2014. Emerg. Infect. Dis. 2015, 21, 368–371. [Google Scholar] [CrossRef]
- Chiapponi, C.; Faccini, S.; De Mattia, A.; Baioni, L.; Barbieri, I.; Rosignoli, C.; Nigrelli, A.; Foni, E. Detection of influenza D virus among swine and cattle, Italy. Emerg. Infect. Dis. 2016, 22, 352–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flynn, O.; Gallagher, C.; Mooney, J.; Irvine, C.; Ducatez, M.; Hause, B.; McGrath, G.; Ryan, E. Influenza D virus in cattle, Ireland. Emerg. Infect. Dis. 2018, 24, 389–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dane, H.; Duffy, C.; Guelbenzu, M.; Hause, B.; Fee, S.; Forster, F.; McMenamy, M.J.; Lemon, K. Detection of influenza D virus in bovine respiratory disease samples, UK. Transbound. Emerg. Dis. 2019, 66, 2184–2187. [Google Scholar] [CrossRef]
- Snoeck, C.J.; Oliva, J.; Pauly, M.; Losch, S.; Wildschutz, F.; Muller, C.P.; Hübschen, J.M.; Ducatez, M.F. Influenza D virus circulation in cattle and swine, Luxembourg, 2012–2016. Emerg. Infect. Dis. 2018, 24, 1388–1389. [Google Scholar] [CrossRef] [Green Version]
- Ng, T.F.F.; Kondov, N.O.; Deng, X.; Van Eenennaam, A.; Neibergs, H.L.; Delwart, E. A Metagenomics and Case-Control Study to Identify Viruses Associated with Bovine Respiratory Disease. J. Virol. 2015, 89, 5340–5349. [Google Scholar] [CrossRef] [Green Version]
- Ellis, J.A. Update on viral pathogenesis in BRD. Anim. Health Res. Rev. 2009, 10, 149–153. [Google Scholar] [CrossRef]
- Fulton, R.W. Bovine respiratory disease research (1983–2009). Anim. Health Res. Rev. 2009, 10, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.; Chengappa, M.M.; Kuszak, J.; McVey, D.S. Bacterial pathogens of the bovine respiratory disease complex. Vet. Clin. Food Anim. 2010, 26, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Moreno, A.; Snoeck, C.J.; Zohari, S.; Saegerman, C.; O’Donovan, T.; Ryan, E.; Zanni, I.; Foni, E.; Sausy, A.; et al. Emerging Influenza D virus infection in European livestock as determined in serology studies: Are we underestimating its spread over the continent? Transbound. Emerg. Dis. 2020, 68, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Salem, E.; Hägglund, S.; Cassard, H.; Corre, T.; Näslund, K.; Foret, C.; Gauthier, D.; Pinard, A.; Delverdier, M.; Zohari, S.; et al. Pathogenesis, Host Innate Immune Response, and Aerosol Transmission of Influenza D Virus in Cattle. J. Virol. 2019, 93, e01853-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hause, B.M.; Huntimer, L.; Falkenberg, S.; Henningson, J.; Lechtenberg, K.; Halbur, T. An inactivated influenza D virus vaccine partially protects cattle from respiratory disease caused by homologous challenge. Vet. Microbiol. 2017, 199, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Mazzetto, E.; Bortolami, A.; Fusaro, A.; Mazzacan, E.; Maniero, S.; Vascellari, M.; Beato, M.S.; Schiavon, E.; Chiapponi, C.; Terregino, C.; et al. Replication of Influenza D Viruses of Bovine and Swine Origin in Ovine Respiratory Explants and Their Attachment to the Respiratory Tract of Bovine, Sheep, Goat, Horse, and Swine. Front. Microbiol. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, C.; Thomas, M.; Sheng, Z.; Hause, B.M.; Collin, E.A.; Knudsen, D.E.B.; Pillatzki, A.; Nelson, E.; Wang, D.; Kaushik, R.S.; et al. Replication and Transmission of the Novel Bovine Influenza D Virus in a Guinea Pig Model. J. Virol. 2015, 89, 11990–12001. [Google Scholar] [CrossRef] [Green Version]
- White, S.K.; Ma, W.; McDaniel, C.J.; Gray, G.C.; Lednicky, J.A. Serologic evidence of exposure to influenza D virus among persons with occupational contact with cattle. J. Clin. Virol. 2016, 81, 31–33. [Google Scholar] [CrossRef]
- Trombetta, C.M.; Marchi, S.; Manini, I.; Kistner, O.; Li, F.; Piu, P.; Manenti, A.; Biuso, F.; Sreenivasan, C.; Druce, J.; et al. Influenza D virus: Serological Evidence in the Italian Population from 2005 to 2017. Viruses 2020, 12, 30. [Google Scholar] [CrossRef] [Green Version]
- Holwerda, M.; Kelly, J.; Laloli, L.; Stürmer, I.; Portmann, J.; Stalder, H.; Dijkman, R. Determining the replication kinetics and cellular tropism of influenza D virus on primary well-differentiated human airway epithelial cells. Viruses 2019, 11, 377. [Google Scholar] [CrossRef] [Green Version]
- Murakami, S.; Sato, R.; Ishida, H.; Katayama, M.; Takenaka-Uema, A.; Horimoto, T. Influenza d virus of new phylogenetic lineage, Japan. Emerg. Infect. Dis. 2020, 26, 168–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collin, E.A.; Sheng, Z.; Lang, Y.; Ma, W.; Hause, B.M.; Li, F. Cocirculation of Two Distinct Genetic and Antigenic Lineages of Proposed Influenza D Virus in Cattle. J. Virol. 2015, 89, 1036–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiapponi, C.; Faccini, S.; Fusaro, A.; Moreno, A.; Prosperi, A.; Merenda, M.; Baioni, L.; Gabbi, V.; Rosignoli, C.; Alborali, G.L.; et al. Detection of a new genetic cluster of influenza D virus in Italian cattle. Viruses 2019, 11, 1110. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Yu, J.; Hause, B.M.; Park, J.Y.; Sreenivasan, C.; Uprety, T.; Sheng, Z.; Wang, D.; Li, F. Emergence of new phylogenetic lineage of Influenza D virus with broad antigenicity in California, United States. Emerg. Microbes Infect. 2021, 10, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Fertner, M.; Toft, N.; Læssøe, H.; Boklund, A. A register-based study of the antimicrobial usage in Danish veal calves and young bulls. Prev. Vet. Med. 2016, 131, 41–47. [Google Scholar] [CrossRef]
- Goecke, N.B.; Nielsen, B.H.; Petersen, M.B.; Larsen, L.E. Design of a High-Throughput Real-Time PCR System for Detection of Bovine Respiratory and Enteric Pathogens. Front. Vet. Sci. 2021, 8, 1–15. [Google Scholar] [CrossRef]
- Love, W.J.; Lehenbauer, T.W.; Kass, P.H.; Van Eenennaam, A.L.; Aly, S.S. Development of a novel clinical scoring systemfor on-farmdiagnosis of bovine respiratory disease in pre-weaned dairy calves. PeerJ 2014, 2, 1–25. [Google Scholar] [CrossRef] [Green Version]
- McGuirk, S.M. Disease Management of Dairy Calves and Heifers. Vet. Clin. Food Anim. 2008, 24, 139–153. [Google Scholar] [CrossRef]
- Ryt-Hansen, P.; Larsen, I.; Kristensen, C.S.; Krog, J.S.; Larsen, L.E. Limited impact of influenza A virus vaccination of piglets in an enzootic infected sow herd. Res. Vet. Sci. 2019, 127, 47–56. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree version 1.4.4. 2018. Available online: http://tree.bio.ed.ac.uk/software/figtree/.
- He, W.T.; Lu, M.; Xing, G.; Shao, Y.; Zhang, M.; Yang, Y.; Li, X.; Zhang, L.; Li, G.; Cao, Z.; et al. Emergence and adaptive evolution of influenza D virus. Microb. Pathog. 2021, 160, 1–9. [Google Scholar] [CrossRef]
| Strain | Herd ID | Date of Sampling | Production Type | Source | Sex | Age | Temperature (℃) | Cough * | Nasal Discharge ■ | Eye Discharge ■ | Lineage HEF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| D/bovine/Denmark/6171103755-1/2019 | 1 | 06-02-2019 | Dairy | NS | Heifer | 3 m | 38.5 | 0 | 2 | 1 | D/OK |
| D/bovine/Denmark/5246407608-2/2019 | 2 | 01-03-2019 | Veal | NS | Bull | 2 waa | 39.9 | 0 | 2 | 0 | D/OK |
| D/bovine/Denmark/5358904609-3/2019 | 3 | 12-03-2019 | Veal | NS | Bull | 3 m | 39.0 | 0 | 2 | 0 | D/OK |
| D/bovine/Denmark/1173303525-4/2019 | 4 | 05-04-2019 | Veal | NS | Bull | 3 m | 38.9 | 0 | 2 | 0 | D/OK |
| D/bovine/Denmark/4559602762-5/2019 | 5 | 15-01-2019 | Veal | NS | Bull | 2 waa | 38.7 | 0 | 2 | 0 | D/OK |
| D/bovine/Denmark/5022903340-5/2019 | 5 | 15-01-2019 | Veal | NS | Bull | 2 waa | 38.9 | 0 | 1 | 1 | D/OK |
| D/bovine/Denmark/5161205135-5/2019 | 5 | 15-01-2019 | Veal | NS | Bull | 2 waa | 38.5 | 0 | 1 | 1 | D/OK |
| D/bovine/Denmark/5195604714-5/2019 | 5 | 15-01-2019 | Veal | NS | Bull | 3 m | 39.3 | 0 | 2 | 0 | D/OK |
| D/bovine/Denmark/5195604790-5/2019 | 5 | 20-02-2019 | Veal | NS | Bull | 2 waa | 39.1 | 0 | 2 | 1 | D/OK |
| D/bovine/Denmark/5224403563-5/2019 | 5 | 24-04-2019 | Veal | NS | Bull | 3 m | 38.3 | 0 | 1 | 0 | D/OK |
| D/bovine/Denmark/3313408142-6/2020 | 6 | 08-01-2020 | Veal | NS | Bull | 3 m | 39.0 | 3 | 2 | 2 | D/OK |
| D/bovine/Denmark/3727003200-7/2020 | 7 | 12-03-2020 | Veal | NS | Bull | 2 waa | 40.5 | 0 | 2 | 2 | D/660 |
| D/bovine/Denmark/5256205576-8/2020 | 8 | 06-03-2020 | Veal | NS | Heifer | 3 m | 39.6 | 3 | 2 | 2 | D/660 |
| D/bovine/Denmark/5995505714-9/2020 | 9 | 11-02-2020 | Veal | NS | Bull | 3 m | 39.5 | 0 | 2 | 2 | D/660 |
| D/bovine/Denmark/1052304114-10/2020 | 10 | 16-01-2020 | Veal | NS | Bull | 3 m | 39.2 | 2 | 2 | 0 | D/660 |
| D/bovine/Denmark/3058506689-11/2019 | 11 | 09-10-2019 | Veal | NS | Bull | 3 m | 39.7 | 0 | 2 | 0 | D/OK |
| D/bovine/Denmark/4070604622-12/2019 | 12 | 16-01-2019 | Veal | NS | Bull | 3 m | 39.5 | 0 | 1 | 1 | - |
| D/bovine/Denmark/47398-3622/2015 | 13 | 03-11-2015 | Veal | BAL | nd | nd | nd | nd | nd | nd | D/OK |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goecke, N.B.; Liang, Y.; Otten, N.D.; Hjulsager, C.K.; Larsen, L.E. Characterization of Influenza D Virus in Danish Calves. Viruses 2022, 14, 423. https://doi.org/10.3390/v14020423
Goecke NB, Liang Y, Otten ND, Hjulsager CK, Larsen LE. Characterization of Influenza D Virus in Danish Calves. Viruses. 2022; 14(2):423. https://doi.org/10.3390/v14020423
Chicago/Turabian StyleGoecke, Nicole B., Yuan Liang, Nina D. Otten, Charlotte K. Hjulsager, and Lars E. Larsen. 2022. "Characterization of Influenza D Virus in Danish Calves" Viruses 14, no. 2: 423. https://doi.org/10.3390/v14020423
APA StyleGoecke, N. B., Liang, Y., Otten, N. D., Hjulsager, C. K., & Larsen, L. E. (2022). Characterization of Influenza D Virus in Danish Calves. Viruses, 14(2), 423. https://doi.org/10.3390/v14020423






