Incidence, Disease Severity, and Follow-Up of Influenza A/A, A/B, and B/B Virus Dual Infections in Children: A Hospital-Based Digital Surveillance Program
Abstract
1. Introduction
- The incidence of IV dual infections in children based on a hospital-based digital surveillance program with a known denominator of all ILI cases and
- Disease severity, symptoms, and detailed course of illness in children with IV dual infections
2. Materials and Methods
2.1. Institutional Review Board Statement
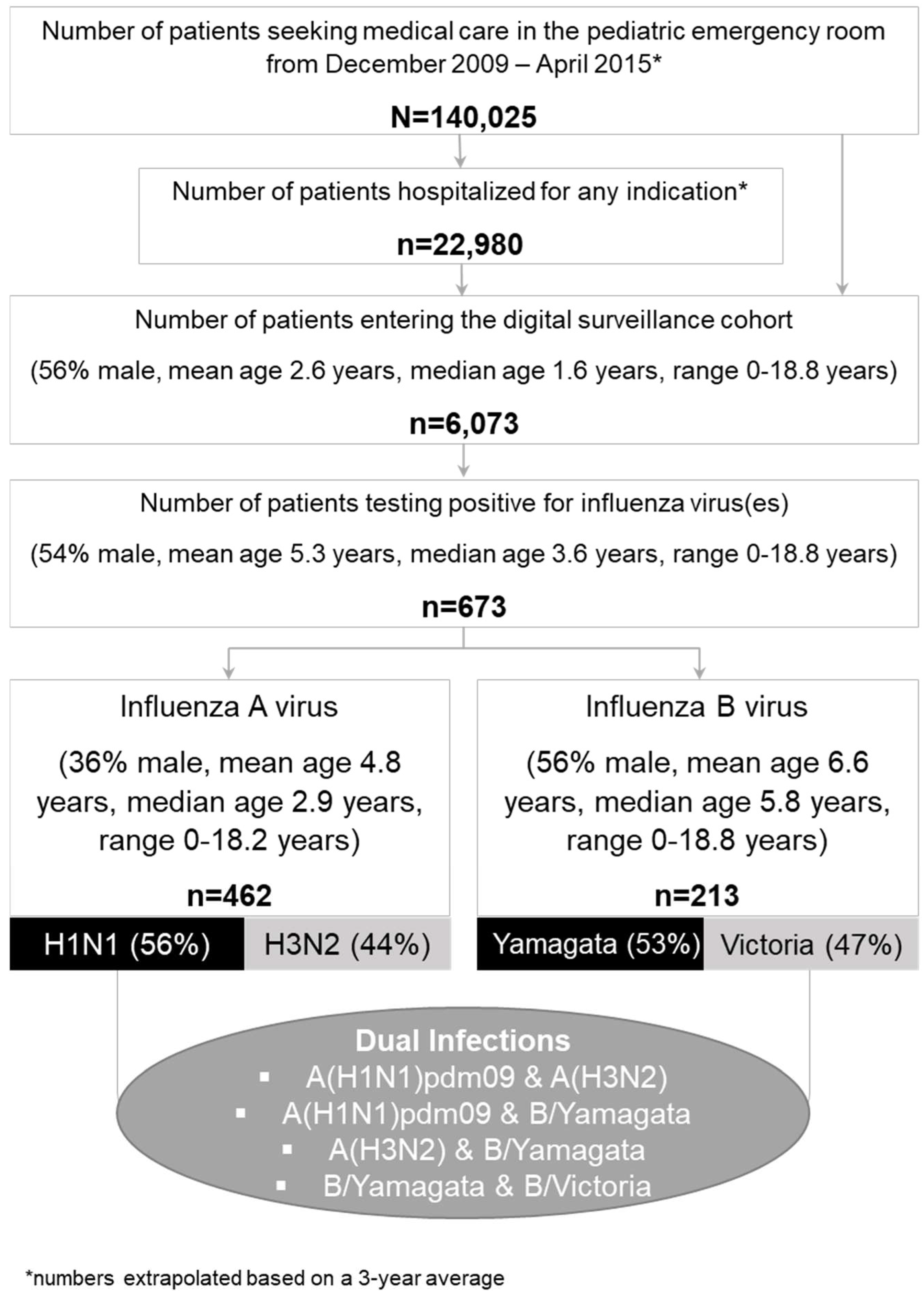
2.2. Patient Cohort
2.3. Assessment of Symptoms and Disease Severity
2.4. Virology Testing
2.5. Statistics
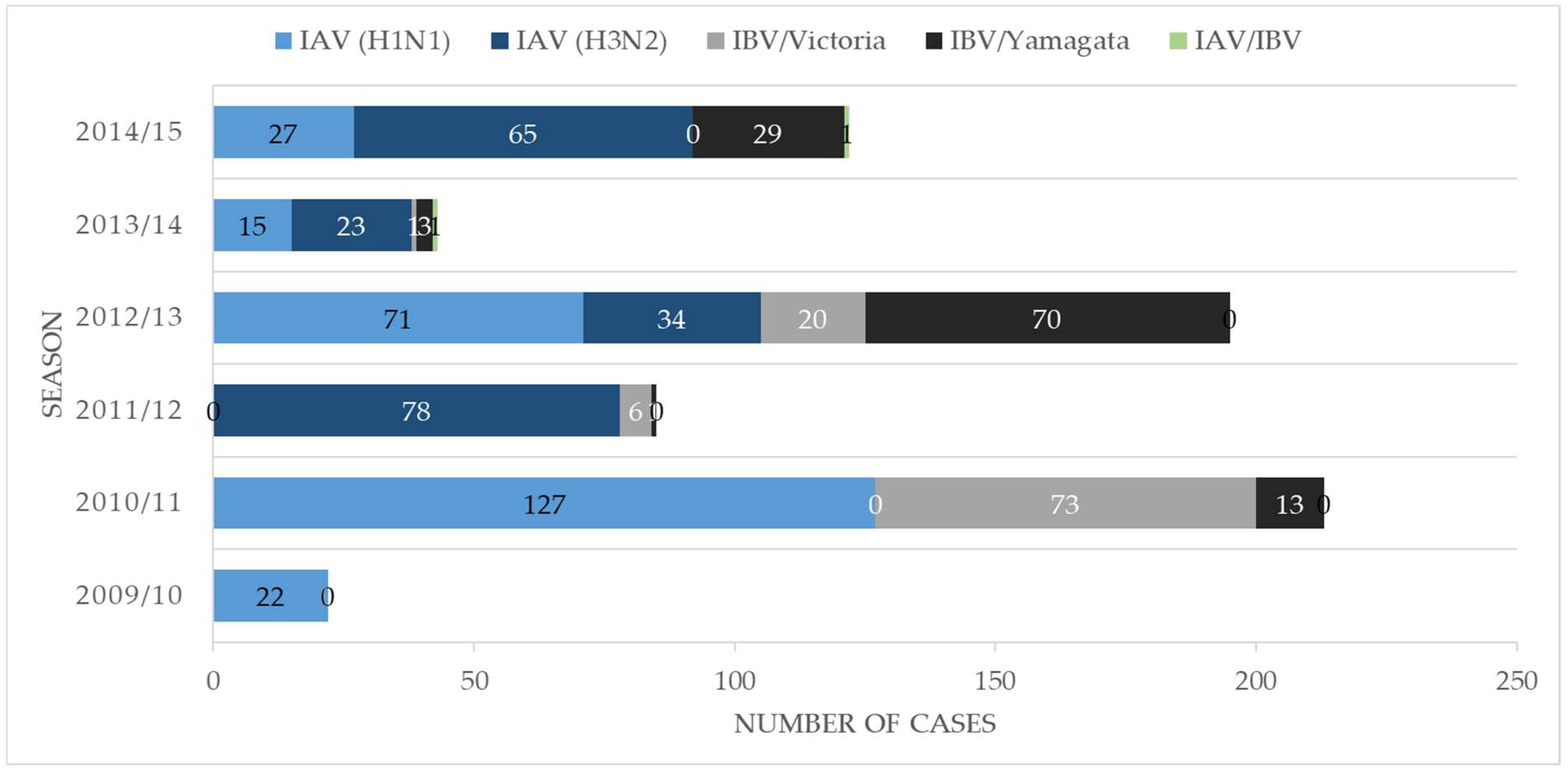
3. Results
3.1. Overview of Patient Characteristics
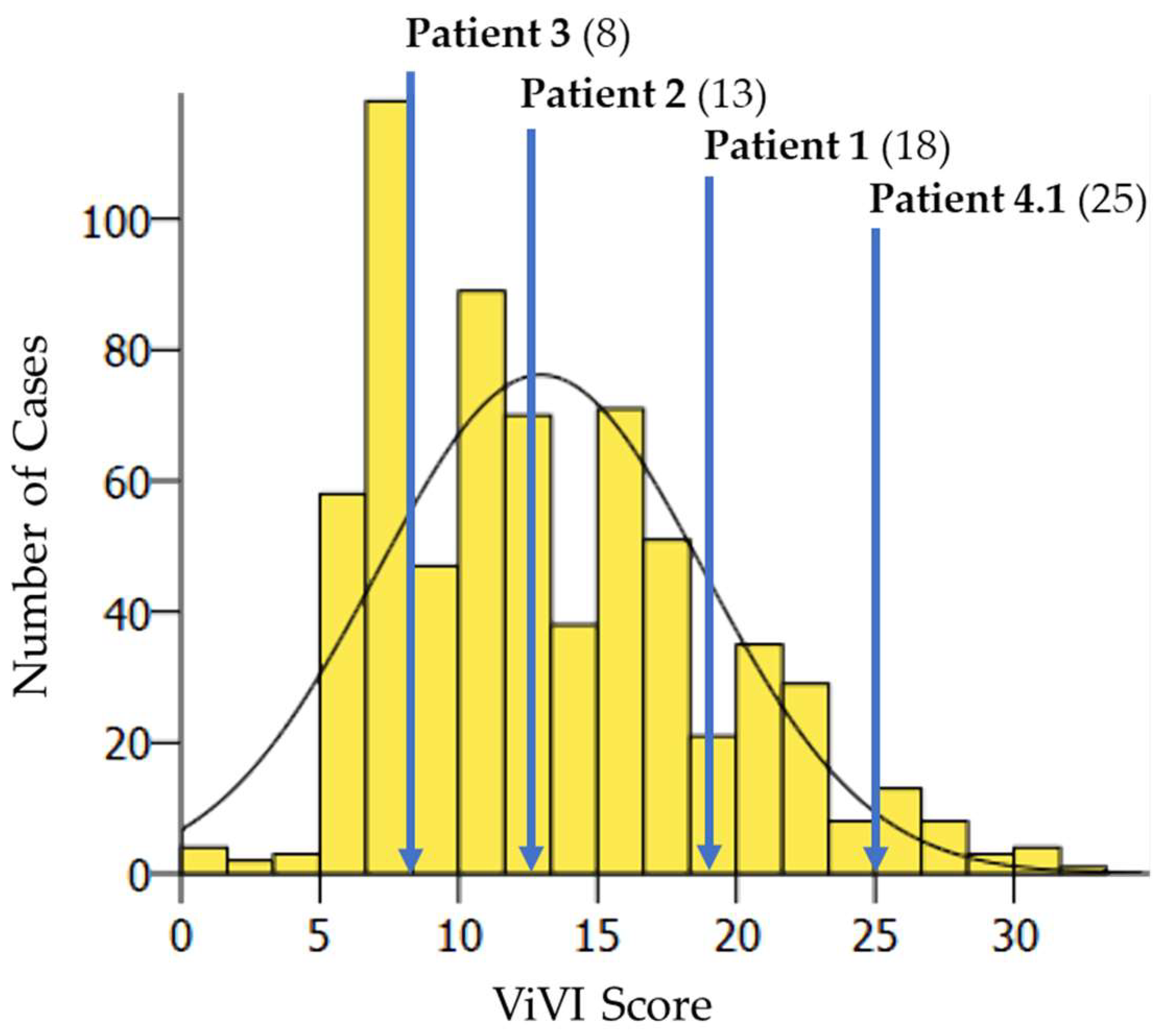
3.2. Detailed Clinical Vignettes—The Dynamics of Disease Severity and Viral Load
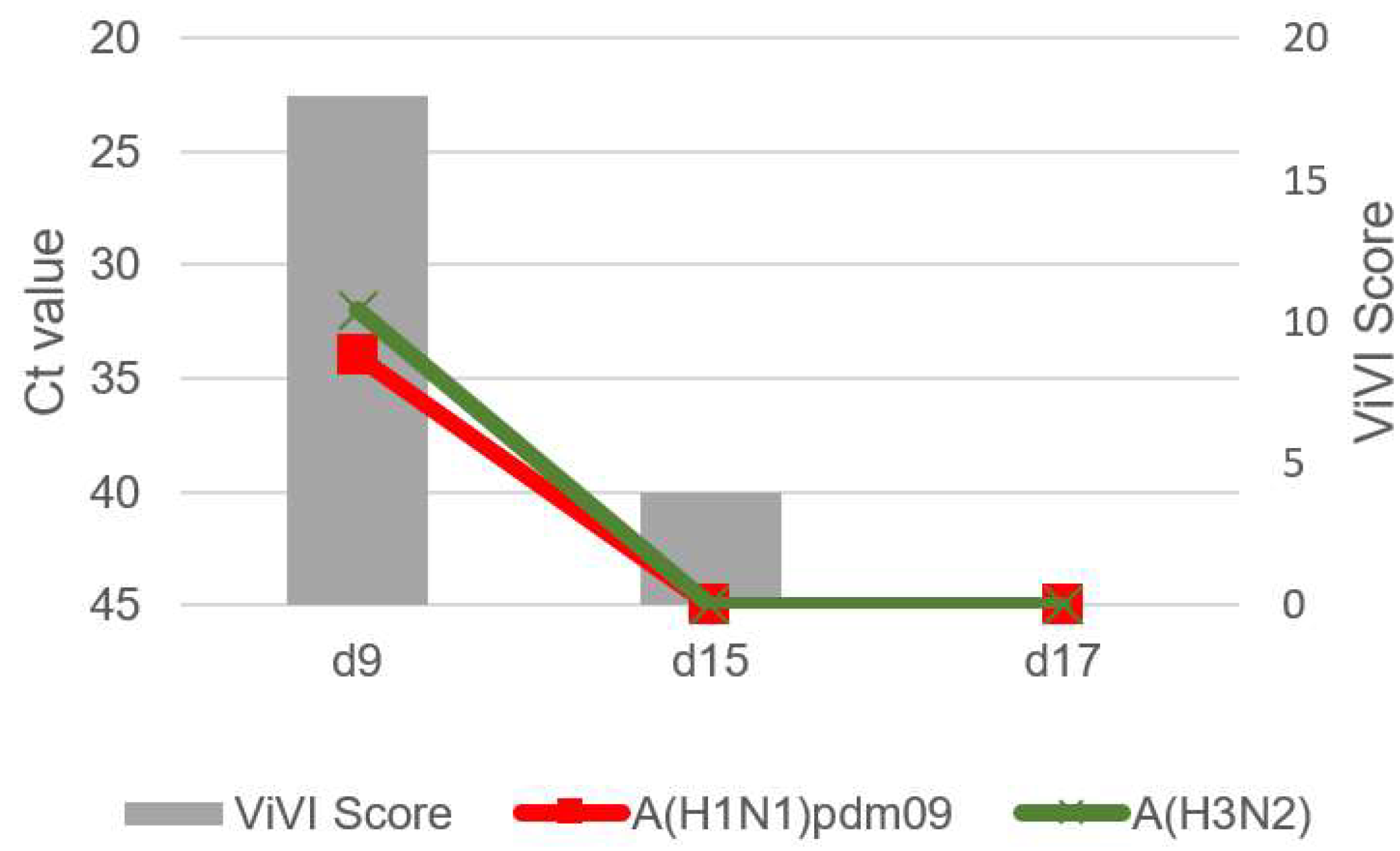
3.3. Patient 1 (Season 2012/2013): A(H1N1)pdm09 & A(H3N2) Dual Infection
3.4. Patient 2 (Season 2012/2013): B/Victoria & B/Yamagata Dual Infection
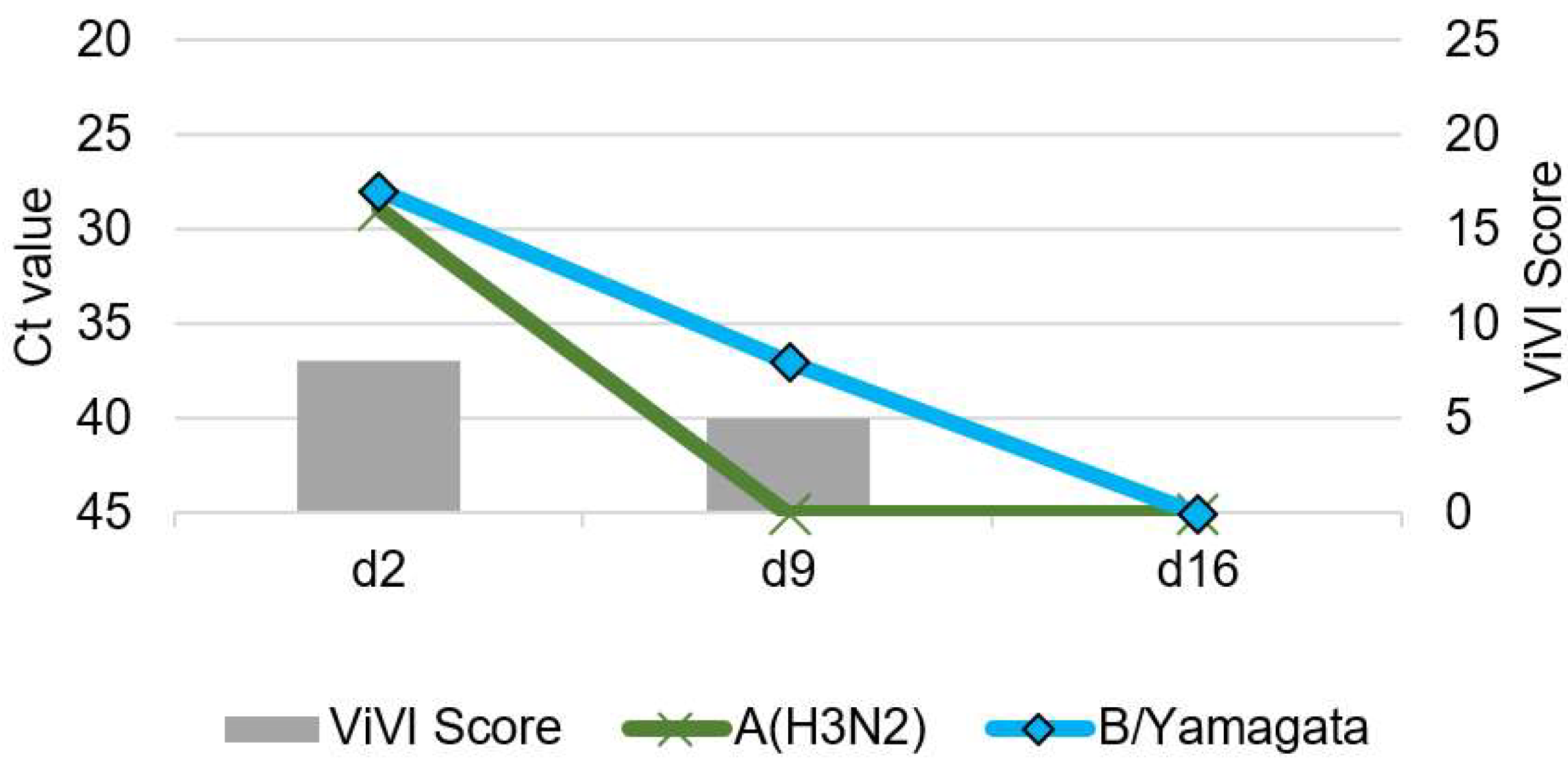
3.5. Patient 3 (Season 2014/2015): A(H3N2) & B/Yamagata Dual Infection
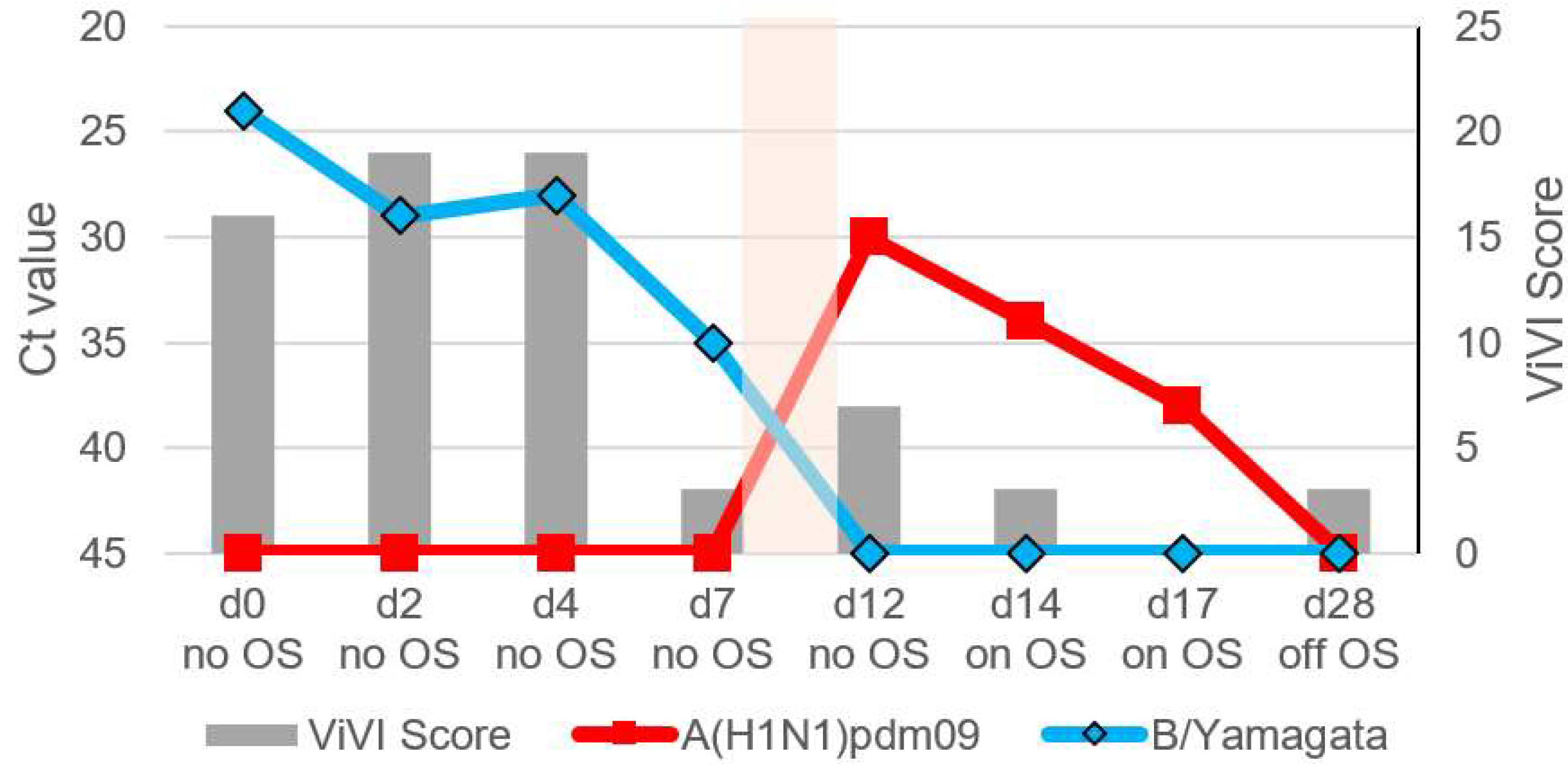
3.6. Patient 4.1 (Season 2012/2013): A(H1N1)pdm09 & B/Yamagata Dual Infection
3.7. Patient 4.2 (Season 2012/2013): B/Yamagata & Subsequent A(H1N1)pdm09 Infection
4. Discussion
- -
- IV dual infections are rare but may exist during influenza seasons with co-circulation of different subtypes and/or lineages.
- -
- We observed complicated and/or prolonged disease with seizures, bronchopneumonia, or biphasic fever in IV dual infections; ViVI Disease Severity Scores were slightly above-average compared to the rest of the cohort.
- -
- During follow-up, ViVI Disease Severity Scores reflected not only the clinical course of illness but also the progression of viral load (as approximated by Ct values), including during rebound.
- -
- Combined digital (mobile health) and virological (PCR) surveillance of influenza and other respiratory viruses—ideally with longitudinal follow-up-will improve our understanding of the clinical impact of viral-viral co-infections in ILI patients.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Li, Y.; O’Brien, K.L.; Madhi, S.A.; Widdowson, M.A.; Byass, P.; Omer, S.B.; Abbas, Q.; Ali, A.; Amu, A.; et al. Global burden of respiratory infections associated with seasonal influenza in children under 5 years in 2018: A systematic review and modelling study. Lancet Glob. Health 2020, 8, e497–e510. [Google Scholar] [CrossRef]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Memoli, M.J.; Athota, R.; Reed, S.; Czajkowski, L.; Bristol, T.; Proudfoot, K.; Hagey, R.; Voell, J.; Fiorentino, C.; Ademposi, A.; et al. The natural history of influenza infection in the severely immunocompromised vs nonimmunocompromised hosts. Clin. Infect. Dis. 2014, 58, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Davison, A.J.; Dempsey, D.M.; Dutilh, B.E.; Garcia, M.L.; et al. Changes to virus taxonomy and to the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2021). Arch. Virol. 2021, 166, 2633–2648. [Google Scholar] [CrossRef] [PubMed]
- Littlefield, K. Evaluating the Zoonotic Potential of Non-Human Influenza D Virus. Master’s Thesis, Johns Hopkins University, Baltimore, MD, USA, 2021. [Google Scholar]
- Trombetta, C.M.; Marchi, S.; Manini, I.; Kistner, O.; Li, F.; Piu, P.; Manenti, A.; Biuso, F.; Sreenivasan, C.; Druce, J.; et al. Influenza D Virus: Serological Evidence in the Italian Population from 2005 to 2017. Viruses 2019, 12, 30. [Google Scholar] [CrossRef]
- Sederdahl, B.K.; Williams, J.V. Epidemiology and Clinical Characteristics of Influenza C Virus. Viruses 2020, 12, 89. [Google Scholar] [CrossRef]
- Nuwarda, R.F.; Alharbi, A.A.; Kayser, V. An Overview of Influenza Viruses and Vaccines. Vaccines 2021, 9, 1032. [Google Scholar] [CrossRef]
- Blyth, C.C.; Webb, S.A.; Kok, J.; Dwyer, D.E.; van Hal, S.J.; Foo, H.; Ginn, A.N.; Kesson, A.M.; Seppelt, I.; Iredell, J.R.; et al. The impact of bacterial and viral coinfection in severe influenza. Influenza Other Respir. Viruses 2013, 7, 168–176. [Google Scholar] [CrossRef]
- Abelenda-Alonso, G.; Rombauts, A.; Gudiol, C.; Meije, Y.; Ortega, L.; Clemente, M.; Ardanuy, C.; Niubo, J.; Carratala, J. Influenza and Bacterial Coinfection in Adults with Community-Acquired Pneumonia Admitted to Conventional Wards: Risk Factors, Clinical Features, and Outcomes. Open Forum Infect. Dis. 2020, 7, ofaa066. [Google Scholar] [CrossRef]
- Tief, F.; Hoppe, C.; Seeber, L.; Obermeier, P.; Chen, X.; Karsch, K.; Muhlhans, S.; Adamou, E.; Conrad, T.; Beresniak, A.; et al. An inception cohort study assessing the role of pneumococcal and other bacterial pathogens in children with influenza and ILI and a clinical decision model for stringent antibiotic use. Antivir. Ther. 2016, 21, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Rath, B.; Conrad, T.; Myles, P.; Alchikh, M.; Ma, X.; Hoppe, C.; Tief, F.; Chen, X.; Obermeier, P.; Kisler, B.; et al. Influenza and other respiratory viruses: Standardizing disease severity in surveillance and clinical trials. Expert Rev. Anti Infect. Ther. 2017, 15, 545–568. [Google Scholar] [CrossRef] [PubMed]
- Mandelia, Y.; Procop, G.W.; Richter, S.S.; Worley, S.; Liu, W.; Esper, F. Dynamics and predisposition of respiratory viral coinfections in children and adults. Clin. Microbiol. Infect. 2021, 27, 631.e1–631.e6. [Google Scholar] [CrossRef]
- Gregianini, T.S.; Varella, I.R.S.; Fisch, P.; Martins, L.G.; Veiga, A.B.G. Dual and Triple Infections With Influenza A and B Viruses: A Case-Control Study in Southern Brazil. J. Infect. Dis. 2019, 220, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.A.; Kasper, M.R.; Yasuda, C.Y.; Savuth, C.; Spiro, D.J.; Halpin, R.; Faix, D.J.; Coon, R.; Putnam, S.D.; Wierzba, T.F.; et al. Dual infection of novel influenza viruses A/H1N1 and A/H3N2 in a cluster of Cambodian patients. Am. J. Trop. Med. Hyg. 2011, 85, 961–963. [Google Scholar] [CrossRef]
- Takao, S.; Hara, M.; Kakuta, O.; Shimazu, Y.; Kuwayama, M.; Fukuda, S.; Miyazaki, K. Eleven cases of coinfection with influenza type A and type B suspected by use of a rapid diagnostic kit and confirmed by RT-PCR and virus isolation. Kansenshogaku Zasshi 2005, 79, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Pando, R.; Drori, Y.; Friedman, N.; Glatman-Freedman, A.; Sefty, H.; Shohat, T.; Mendelson, E.; Hindiyeh, M.; Mandelboim, M. Influenza A(H1N1)pdm 2009 and influenza B virus coinfection in hospitalized and non-hospitalized patients during the 2015–2016 epidemic season in Israel. J. Clin. Virol. 2017, 88, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Z.D.; Tang, F.; Wei, M.T.; Tong, Y.G.; Zhang, L.; Xin, Z.T.; Ma, M.J.; Zhang, X.A.; Liu, L.J.; et al. Mixed infections of pandemic H1N1 and seasonal H3N2 viruses in 1 outbreak. Clin. Infect. Dis. 2010, 50, 1359–1365. [Google Scholar] [CrossRef]
- Tramuto, F.; Maida, C.M.; Magliozzo, F.; Amodio, E.; Vitale, F. Occurrence of a case of influenza A(H1N1)pdm09 and B coinfection during the epidemic season 2012–2013. Infect. Genet. Evol. 2014, 23, 95–98. [Google Scholar] [CrossRef]
- Ward, K.A.; Armstrong, P.; McAnulty, J.M.; Iwasenko, J.M.; Dwyer, D.E. Outbreaks of pandemic (H1N1) 2009 and seasonal influenza A (H3N2) on cruise ship. Emerg. Infect. Dis. 2010, 16, 1731–1737. [Google Scholar] [CrossRef]
- Shimada, S.; Sadamasu, K.; Shinkai, T.; Kakuta, O.; Kikuchi, Y.; Shinohara, M.; Uchida, K.; Doi, R.; Kohmoto, K.; Shimizu, M.; et al. Virological analysis of a case of dual infection by influenza A (H3N2) and B viruses. Jpn. J. Infect. Dis. 2006, 59, 67–68. [Google Scholar]
- Obermeier, P.; Muehlhans, S.; Hoppe, C.; Karsch, K.; Tief, F.; Seeber, L.; Chen, X.; Conrad, T.; Boettcher, S.; Diedrich, S.; et al. Enabling Precision Medicine with Digital Case Classification at the Point-of-Care. EBioMedicine 2016, 4, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Alchikh, M.; Conrad, T.; Hoppe, C.; Ma, X.; Broberg, E.; Penttinen, P.; Reiche, J.; Biere, B.; Schweiger, B.; Rath, B. Are we missing respiratory viral infections in infants and children? Comparison of a hospital-based quality management system with standard of care. Clin. Microbiol. Infect. 2019, 25, 380.e9–380.e16. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pouran Yousef, K.; Duwe, S.; Karsch, K.; Grover, S.; Wahlisch, S.; Obermeier, P.; Tief, F.; Muhlhans, S.; Seeber, L.; et al. Quantitative influenza follow-up testing (QIFT)—A novel biomarker for the monitoring of disease activity at the point-of-care. PLoS ONE 2014, 9, e92500. [Google Scholar] [CrossRef] [PubMed]
- Karsch, K.; Chen, X.; Miera, O.; Peters, B.; Obermeier, P.; Francis, R.C.; Amann, V.; Duwe, S.; Fraaij, P.; Heider, A.; et al. Pharmacokinetics of Oral and Intravenous Oseltamivir Treatment of Severe Influenza B Virus Infection Requiring Organ Replacement Therapy. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 155–164. [Google Scholar] [CrossRef]
- Rath, B. Disease Severity in Influenza and Other Viral Respiratory Infections. In Proceedings of the Options X for the Control of Influenza, Singapore, 28 August–1 September 2019. [Google Scholar]
- Rath, B.; von Kleist, M.; Tief, F.; Karsch, K.; Tuerk, E.; Muehlhans, S.; Louis, F.; Skopnik, H.; Schweiger, B.; Duwe, S. Virus load kinetics and resistance development during oseltamivir treatment in infants and children infected with Influenza A(H1N1) 2009 and Influenza B viruses. Pediatr. Infect. Dis. J. 2012, 31, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Conrad, T.; Alchikh, M.; Reiche, J.; Schweiger, B.; Rath, B. Can we distinguish respiratory viral infections based on clinical features? A prospective pediatric cohort compared to systematic literature review. Rev. Med. Virol. 2018, 28, e1997. [Google Scholar] [CrossRef] [PubMed]
- WHO. Clinical Management of Human Infection with Pandemic (H1N1) 2009: Revised Guidance. Available online: http://www.who.int/csr/resources/publications/swineflu/clinical_management_h1n1.pdf (accessed on 3 March 2022).
- Rath, B.; Maltezou, H.C.; Papaevangelou, V.; Papagrigoriou-Theodoridou, M.A.; Alchikh, M.; Myles, P.; Schweiger, B.; PEDSIDEA Network; Asimaki, H.; Dimopoulou, D.; et al. Partnering for enhanced digital surveillance of influenza-like disease and the effect of antivirals and vaccines (PEDSIDEA). Influenza Other Respir. Viruses 2019, 13, 309–318. [Google Scholar] [CrossRef]
- Schulze, M.; Nitsche, A.; Schweiger, B.; Biere, B. Diagnostic approach for the differentiation of the pandemic influenza A(H1N1)v virus from recent human influenza viruses by real-time PCR. PLoS ONE 2010, 5, e9966. [Google Scholar] [CrossRef][Green Version]
- Biere, B.; Bauer, B.; Schweiger, B. Differentiation of influenza B virus lineages Yamagata and Victoria by real-time PCR. J. Clin. Microbiol. 2010, 48, 1425–1427. [Google Scholar] [CrossRef]
- Toda, S.; Okamoto, R.; Nishida, T.; Nakao, T.; Yoshikawa, M.; Suzuki, E.; Miyamura, S. Isolation of influenza A/H3 and B viruses from an influenza patient: Confirmation of coinfection by two influenza viruses. Jpn. J. Infect. Dis. 2006, 59, 142–143. [Google Scholar]
- Eshaghi, A.; Blair, J.; Burton, L.; Choi, K.W.; De Lima, C.; Duncan, C.; Guyard, C.; Higgins, R.; Lombos, E.; Low, D.E.; et al. Characterization of an influenza A and influenza B coinfection of a patient in a long-term care facility with co-circulating influenza A and influenza B. Int. J. Infect. Dis. 2009, 13, e127–e128. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, F.; Vasquez, V.; de Egea, V.; Catalan, P.; Rodriguez-Sanchez, B.; Bouza, E. Influenza A and B co-infection: A case-control study and review of the literature. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.A.; Muthoka, P.; Emukule, G.O.; Kalani, R.; Njuguna, H.; Waiboci, L.W.; Ahmed, J.A.; Bigogo, G.; Feikin, D.R.; Njenga, M.K.; et al. Results from the first six years of national sentinel surveillance for influenza in Kenya, July 2007–June 2013. PLoS ONE 2014, 9, e98615. [Google Scholar] [CrossRef]
- Falchi, A.; Arena, C.; Andreoletti, L.; Jacques, J.; Leveque, N.; Blanchon, T.; Lina, B.; Turbelin, C.; Dorleans, Y.; Flahault, A.; et al. Dual infections by influenza A/H3N2 and B viruses and by influenza A/H3N2 and A/H1N1 viruses during winter 2007, Corsica Island, France. J. Clin. Virol. 2008, 41, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Rath, B.A.; Seng, S. Partnering for Enhanced Digital Surveillance of Influenza-like Disease and the Effect of Antivirals and Vaccines (PEDSIDEA)—First Results in the United States. Pediatrics 2020, 146, 29. [Google Scholar] [CrossRef]
- Reina, J.; Lopez, C. Co-infection with influenza type A (H1N1)pdm09 and influenza type B in a patient with human immunodeficiency virus infection. Enferm. Infecc. Microbiol. Clin. 2014, 32, 203–204. [Google Scholar] [CrossRef]
- Calistri, A.; Salata, C.; Cosentino, M.; Asnicar, S.; Franchin, E.; Cusinato, R.; Pacenti, M.; Donatelli, I.; Palu, G. Report of two cases of influenza virus A/H1N1v and B coinfection during the 2010/2011 epidemics in the Italian Veneto Region. Virol. J. 2011, 8, 502. [Google Scholar] [CrossRef]
- Almajhdi, F.N.; Ali, G. Report on influenza A and B viruses: Their coinfection in a Saudi leukemia patient. Biomed. Res. Int. 2013, 2013, 290609. [Google Scholar] [CrossRef]
- Buda, S.; Koepke, K.; Prahm, K.; Schweiger, B.; Wedde, M.; Duwe, S.; Buchholz, U.; an der Heiden, M.; Haas, W. Bericht zur Epidemiologie der Influenza in Deutschland Saison 2012/13. Available online: https://influenza.rki.de/saisonberichte/2012.pdf (accessed on 30 January 2022).
- Wu, A.; Mihaylova, V.T.; Landry, M.L.; Foxman, E.F. Interference between rhinovirus and influenza A virus: A clinical data analysis and experimental infection study. Lancet Microbe 2020, 1, e254–e262. [Google Scholar] [CrossRef]
- Guan, M.; Blackmon, S.; Olivier, A.K.; Zhang, X.; Liu, L.; Woolums, A.; Crenshaw, M.A.; Liao, S.F.; Webby, R.; Epperson, W.; et al. Time-Dependent Proinflammatory Responses Shape Virus Interference during Coinfections of Influenza A Virus and Influenza D Virus. Viruses 2022, 14, 224. [Google Scholar] [CrossRef]
- Wong, S.S.; Oshansky, C.M.; Guo, X.J.; Ralston, J.; Wood, T.; Seeds, R.; Newbern, C.; Waite, B.; Reynolds, G.; Widdowson, M.A.; et al. Severe Influenza Is Characterized by Prolonged Immune Activation: Results from the SHIVERS Cohort Study. J. Infect. Dis. 2018, 217, 245–256. [Google Scholar] [CrossRef]
- ECDC European Center for Disease Prevention and Control. Influenza Virus Characterisation, Summary Europe, December 2021. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Influenza-characterisation-report-Dec-2021.pdf (accessed on 2 February 2022).
- Koutsakos, M.; Wheatley, A.K.; Laurie, K.; Kent, S.J.; Rockman, S. Influenza lineage extinction during the COVID-19 pandemic? Nat. Rev. Microbiol. 2021, 19, 741–742. [Google Scholar] [CrossRef]


| Patient | Age in Years/Sex | Influenza Virus Dual Infection | Underlying Condition | Influenza Vaccination | Hospitalization | Chief Complaint and Clinical Hallmarks | Antiviral Treatment | Baseline ViVI Score | Maximum ViVI Score |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 11/F | A(H1N1) pdm09, A(H3N2) | no | no | yes | Suspected appendicitis with mesenteric lymphadenitis, then seizure | no | 18 | 18 |
| 2 | 5/M | B/Yamagata, B/Victoria | no | no | yes | Bloody vomiting, listlessness, prolonged LRTI, biphasic disease severity | no | 13 | 13 |
| 3 | 17/F | A(H3N2), B/Yamagata | Crohn’s disease, immunosuppressive therapy | no | no | Prolonged URTI with fever | no | 8 | 8 |
| 4.1 | 2/M | A(H1N1) pdm09, B/Yamagata | no | no | yes | Listlessness, severe/prolonged LRTI, later biphasic disease severity, wheezing | yes | 25 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obermeier, P.E.; Seeber, L.D.; Alchikh, M.; Schweiger, B.; Rath, B.A. Incidence, Disease Severity, and Follow-Up of Influenza A/A, A/B, and B/B Virus Dual Infections in Children: A Hospital-Based Digital Surveillance Program. Viruses 2022, 14, 603. https://doi.org/10.3390/v14030603
Obermeier PE, Seeber LD, Alchikh M, Schweiger B, Rath BA. Incidence, Disease Severity, and Follow-Up of Influenza A/A, A/B, and B/B Virus Dual Infections in Children: A Hospital-Based Digital Surveillance Program. Viruses. 2022; 14(3):603. https://doi.org/10.3390/v14030603
Chicago/Turabian StyleObermeier, Patrick E., Lea D. Seeber, Maren Alchikh, Brunhilde Schweiger, and Barbara A. Rath. 2022. "Incidence, Disease Severity, and Follow-Up of Influenza A/A, A/B, and B/B Virus Dual Infections in Children: A Hospital-Based Digital Surveillance Program" Viruses 14, no. 3: 603. https://doi.org/10.3390/v14030603
APA StyleObermeier, P. E., Seeber, L. D., Alchikh, M., Schweiger, B., & Rath, B. A. (2022). Incidence, Disease Severity, and Follow-Up of Influenza A/A, A/B, and B/B Virus Dual Infections in Children: A Hospital-Based Digital Surveillance Program. Viruses, 14(3), 603. https://doi.org/10.3390/v14030603






