The Novel Finding of Dynamic Change in eGFR Up to One Year after End of Treatment in HCV-Infected Patients Receiving Sofosbuvir and Velpatasvir
Abstract
:1. Introduction
2. Material and Methods
2.1. Patient Population
2.2. Study Design
3. Statistical Analysis
4. Results
4.1. Patient Characteristics
4.2. Overall Response to Antiviral Treatment
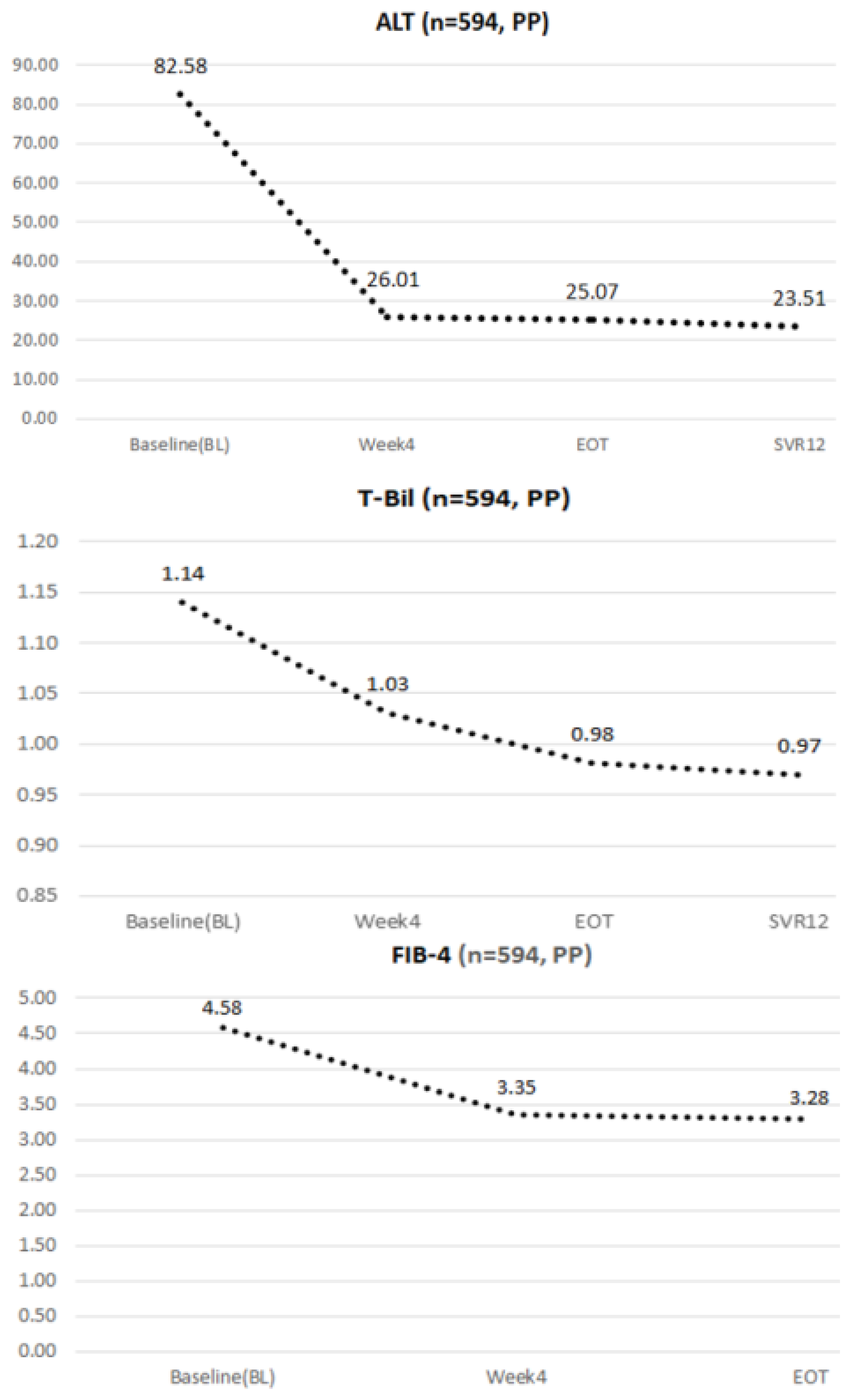
4.3. Dynamic Changes in ALT, Total Bilirubin, and FIB-4 during the Study Period
4.4. The Dynamic Changes in eGFR during the Study Period
4.5. Univariate and Multivariate Analysis of Predictive Factors for the Deterioration of Renal Function
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 161–176. [Google Scholar] [CrossRef] [Green Version]
- Kowdley, K.V.; Gordon, S.C.; Reddy, K.R.; Rossaro, L.; Bernstein, D.E.; Lawitz, E.; Shiffman, M.L.; Schiff, E.; Ghalib, R.; Ryan, M.; et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N. Engl. J. Med. 2014, 370, 1879–1888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, G.R.; Afdhal, N.; Roberts, S.K.; Bräu, N.; Gane, E.J.; Pianko, S.; Lawitz, E.; Thompson, A.; Shiffman, M.L.; Cooper, C.; et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N. Engl. J. Med. 2015, 373, 2608–2617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, J.F.; Ira, M.J.; Hézode, C.; Asselah, T.; Ruane, P.J.; Gruener, N.; Abergel, A.; Mangia, A.; Lai, C.-L.; Chan, H.L.Y.; et al. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N. Engl. J. Med. 2015, 373, 2599–2607. [Google Scholar]
- Curry, M.P.; O’Leary, J.G.; Bzowej, N.; Muir, A.J.; Korenblat, K.M.; Fenkel, J.M.; Reddy, K.R.; Lawitz, E.; Flamm, S.L.; Schiano, T.; et al. Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis. N. Engl. J. Med. 2015, 373, 2618–2628. [Google Scholar] [CrossRef]
- Wyles, D.; Brau, N.; Kottilil, S.; Daar, E.S.; Ruane, P.; Workowski, K.; Luetkemeyer, A.; Adeyemi, O.; Kim, A.Y.; Doehle, B.; et al. Sofosbuvir and Velpatasvir for the Treatment of Hepatitis C Virus in Patients Coinfected With Human Immunodeficiency Virus Type 1: An Open-Label, Phase 3 Study. Clin. Infect. Dis. 2017, 65, 6–12. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.F.; Iio, E.; Jun, D.W.; Ogawa, E.; Toyoda, H.; Hsu, Y.C.; Haga, H.; Iwane, S.; Enomoto, M.; Lee, D.H.; et al. Direct-acting antivirals in East Asian hepatitis C patients: Real-world experience from the REAL-C Consortium. Hepatol. Int. 2019, 13, 587–598. [Google Scholar] [CrossRef]
- Mangia, A.; Piazzolla, V.; Giannelli, A.; Visaggi, E.; Minerva, N.; Palmieri, V.; Carraturo, I.; Potenza, D.; Napoli, N.; Lauletta, G.; et al. SVR12 rates higher than 99% after sofosbuvir/velpatasvir combination in HCV infected patients with F0-F1 fibrosis stage: A real world experience. PLoS ONE 2019, 14, e0215783. [Google Scholar]
- Buggisch, P.; Wursthorn, K.; Stoehr, A.; Atanasov, P.K.; Supiot, R.; Lee, J.; Ting, J.; Petersen, J. Real-world effectiveness and safety of sofosbuvir/velpatasvir and ledipasvir/sofosbuvir hepatitis C treatment in a single centre in Germany. PLoS ONE 2019, 14, e0214795. [Google Scholar] [CrossRef] [PubMed]
- Belperio, P.S.; Shahoumian, T.A.; Loomis, T.P.; Mole, L.A.; Backus, L.I. Real-world effectiveness of daclatasvir plus sofosbuvir and velpatasvir/sofosbuvir in hepatitis C genotype 2 and 3. J. Hepatol. 2019, 70, 15–23. [Google Scholar] [CrossRef]
- Saxena, V.; Koraishy, F.M.; Sise, M.E.; Lim, J.K.; Schmidt, M.; Chung, R.T.; Liapakis, A.; Nelson, D.R.; Fried, M.W.; Terrault, N.A.; et al. HCV-TARGET. Safety and efficacy of sofosbuvir-containing regimens in hepatitis C-infected patients with impaired renal function. Liver Int. 2016, 36, 807–816. [Google Scholar] [CrossRef]
- Butt, A.A.; Ren, Y.; Puenpatom, A.; Arduino, J.M.; Kumar, R.; Abou-Samra, A.-B. Effectiveness, treatment completion and safety of sofosbuvir/ledipasvir and paritaprevir/ritonavir/ombitasvir + dasabuvir in patients with chronic kidney disease: An ERCHIVES study. Aliment. Pharmacol. Ther. 2018, 48, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Mallet, V.; Parlati, L.; Dorval, O.; Kramer, L.; Hernvann, A.; Pichard, A.V.; Guerin, C.; Fontaine, H.; Sogni, P.; Pol, S. Estimated glomerular filtration rate variations and direct acting antivirals treatment for chronic hepatitis C: A retrospective longitudinal study. J. Hepatol. 2018, 68, 22. [Google Scholar] [CrossRef]
- Liu, C.-H.; Lee, M.-H.; Lin, J.-W.; Liu, C.-J.; Su, T.-H.; Tseng, T.-C.; Chen, P.-J.; Chen, D.-S.; Kao, J.-H. Evolution of eGFR in chronic HCV patients receiving sofosbuvir-based or sofosbuvir-free direct-acting antivirals. J. Hepatol. 2020, 72, 839–846. [Google Scholar] [CrossRef]
- Borgia, S.M.; Dearden, J.; Yoshida, E.M.; Shafran, S.D.; Brown, A.; Ben-Ari, Z.; Cramp, M.E.; Cooper, C.; Foxton, M.; Rodriguez, C.F.; et al. Sofosbuvir/velpatasvir for 12 weeks in hepatitis C virus-infected patients with end-stage renal disease undergoing dialysis. J. Hepatol. 2019, 71, 660–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, S.-M.; Tsai, M.-C.; Lin, C.-Y.; Chen, C.-H.; Lu, S.-N.; Hung, C.-H.; Sheen, I.-S.; Chien, R.-N.; Lin, C.-L.; Hu, T.-H.; et al. Serial changes of renal function after directly acting antivirals treatment for chronic hepatitis C: A 1-year follow-up study after treatment. PLoS ONE 2020, 15, e0231102. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Lim, S.G.; Xie, Q.; Văn, K.N.; Piratvisuth, T.; Huang, Y.; Wu, S.; Xu, M.; Tang, H.; Cheng, J.; et al. Sofosbuvir-velpatasvir for treatment of chronic hepatitis C virus infection in Asia: A single-arm, open-label, phase 3 trial. Lancet Gastroenterol. Hepatol. 2019, 4, 127–134. [Google Scholar] [CrossRef]
- Isakov, V.; Chulanov, V.; Abdurakhmanov, D.; Burnevich, E.; Nurmukhametova, E.; Kozhevnikova, G.; Gankina, N.; Zhuravel, S.; Romanova, S.; Hyland, R.H.; et al. Sofosbuvir/velpatasvir for the treatment of HCV: Excellent results from a phase-3, open-label study in Russia and Sweden. Infect. Dis. 2019, 51, 131–139. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Charlton, M.; Curry, M.P.; O’Leary, J.G.; Brown, R.S.; Hunt, S. Patient-reported outcomes with sofosbuvir and velpatasvir with or without RBV for hepatitis C virus-related decompensated cirrhosis: An exploratory analysis from the randomised, open-label ASTRAL-4 phase 3 trial. Lancet Gastroenterol. Hepatol. 2016, 1, 122–132. [Google Scholar] [CrossRef]
- Esteban, R.; Pineda, J.A.; Calleja, J.L.; Casado, M.; Rodríguez, M.; Turnes, J.; Amado, L.E.M.; Morillas, R.M.; Forns, X.; Acevedo, J.M.P.; et al. Efficacy of Sofosbuvir and Velpatasvir, With and Without RBV, in Patients with Hepatitis C Virus Genotype 3 Infection and Cirrhosis. Gastroenterology 2018, 155, 1120–1127. [Google Scholar] [CrossRef] [Green Version]
- Grebely, J.; Dalgard, O.; Conway, B.; Cunningham, E.B.; Bruggmann, P.; Hajarizadeh, B.; Amin, J.; Bruneau, J.; Hellard, M.; Litwin, A.H.; et al. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): An open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol. Hepatol. 2018, 3, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Gane, E.J.; Shiffman, M.L.; Etzkorn, K.; Morelli, G.; Stedman, C.A.M.; Davis, M.N.; Hinestrosa, F.; Dvory-Sobol, H.; Huang, K.C.; Osinusi, A.; et al. Sofosbuvir-velpatasvir with RBV for 24 weeks in hepatitis C virus patients previously treated with a direct-acting antiviral regimen. Hepatology 2017, 66, 1083–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangia, A.; Cenderello, G.; Copetti, M.; Verucchi, G.; Piazzolla, V.; Lorusso, C.; Santoro, R.; Squillante, M.M.; Orlandini, A.; Minisini, R.; et al. SVR12 Higher than 97% in GT3 Cirrhotic Patients with Evidence of Portal Hypertension Treated with SOF/VEL without RBV: A Nation-Wide Cohort Study. Cells 2019, 8, 313. [Google Scholar] [CrossRef] [Green Version]
- Izumi, N.; Takehara, T.; Chayama, K.; Yatsuhashi, H.; Takaguchi, K.; Ide, T.; Kurosaki, M.; Ueno, Y.; Toyoda, H.; Kakizaki, S.; et al. Sofosbuvir-velpatasvir plus RBV in Japanese patients with genotype 1 or 2 hepatitis C who failed direct-acting antivirals. Hepatol. Int. 2018, 12, 356–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takehara, T.; Sakamoto, N.; Nishiguchi, S.; Ikeda, F.; Tatsumi, T.; Ueno, Y.; Yatsuhashi, H.; Takikawa, Y.; Kanda, T.; Sakamoto, M.; et al. Efficacy and safety of sofosbuvir-velpatasvir with or without RBV in HCV-infected Japanese patients with decompensated cirrhosis: An open-label phase 3 trial. J. Gastroenterol. 2019, 54, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dashti-Khavidaki, S.; Khalili, H.; Nasiri-Toosi, M. Potential nephrotoxicity of sofosbuvir-based treatment in patients infected with hepatitis C virus: A review on incidence, type and risk factors. Expert Rev. Clin. Pharmacol. 2018, 11, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Lau, G.K.K.; Wei, L.; Moriyama, M.; Yu, M.-L.; Chuang, W.-L.; Ibrahim, A.; Lesmana, C.R.A.; Sollano, J.; Kumar, M.; et al. APASL clinical practice recommendation: How to treat HCV-infected patients with renal impairment? Hepatol. Int. 2019, 13, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Singer, A.W.; McNabb, B.L.; Osinusi, A.O.; Brainard, D.M.; Littman, M.; de Bruyn, A.R.V.T.; Chokkalingam, A.P. Direct-acting antiviral treatment patterns among hepatitis C patients with advanced chronic kidney disease: A retrospective cohort study. Hepatology 2016, 62, 396A. [Google Scholar]
- Li, M.; Chen, J.; Fang, Z.; Li, Y.; Lin, Q. Sofosbuvir-based regimen is safe and effective for hepatitis C infected patients with stage 4–5 chronic kidney disease: A systematic review and meta-analysis. Virol. J. 2019, 16, 34. [Google Scholar] [CrossRef] [Green Version]

| Factors | Mean (Range) |
|---|---|
| Mean age, years (range) | 63.1 (19–95) |
| Male gender, n (%) | 282 (47.4%) |
| HCV RNA Q | 2,659,919 (=Log 6.4) |
| HCV genotype, n (%) | |
| GT 1 | 226 (38.1%) |
| 1a | 30 (5.1%) |
| 1b | 195 (32.8%) |
| GT 2 | 297 (50.0%) |
| GT 3 | 14 (2.4%) |
| GT 6 | 29 (4.9%) |
| Mixed/unknown | 28 (4.7%) |
| DM, n (%) | 78/339 (23.1%) |
| SOF/VEL + RBV, n (%) | 68 (12.4%) |
| Fibrosis stage, n (%) | |
| Non-cirrhosis | 295 (49.7%) |
| Cirrhosis | 122 (20.5%) |
| Unknown | 177 (29.8%) |
| Cirrhosis registered | 111/371 (29.3%) |
| Compensated | 46 (12.3%) |
| Decompensated | 65 (17.5%) |
| Treatment history, n (%) | |
| PEG-IFN experienced | 39 (7.1%) |
| HBV co-infection | 45 (7.5%) |
| HCC, n (%) | 73 (13.5%) |
| ALT (U/L) | 82.8 (8–2615) |
| AST (U/L) | 74.0 (13–2150) |
| eGFR (mL/min/1.73 m2) | 89.9 (6–256) |
| CKD stage 1 | 277 (46.6%) |
| CKD stage 2 | 245 (41.3%) |
| CKD stage 3 | 65 (10.9%) |
| CKD stage 4 | 3 (0.5%) |
| CKD stage 5 | 4 (0.7%) |
| Total bilirubin (mg/dL) | 1.1 (0.2–27.2) |
| FIB-4, n (%) | |
| <3.25 | 316 (53.2%) |
| ≥3.25 | 275 (46.3%) |
| Unknown | 3 (0.5%) |
| Total (n = 21) | |
|---|---|
| Mean age, years (range) | 68.7 (52–85) |
| Expire date | |
| Before EOT | 4 |
| Between EOT to off-treatment week 12 | 17 |
| HCV genotype, n (%) | |
| GT 1b | 4 (19.0%) |
| GT 2 | 16 (76.2%) |
| GT 6 | 1 (4.8%) |
| SOF/VEL + RBV, n (%) | 6 (28.5%) a |
| Causes of mortality | |
| HCC | 5 (23.9%) |
| Decompensated cirrhosis | 1 (4.8%) |
| Mixed HCC/decompensated cirrhosis | 6 (28.5%) |
| EVB | 2 (9.5%) |
| Others/unknown | 7 (33.3%) b |
| Cirrhosis registered | 14 (66.7%) |
| ALT (U/L) | 116.7 (17–789) |
| AST (U/L) | 197.8 (29–407) |
| eGFR (mL/min/1.73 m2) | 75.64 (37–166) |
| CKD Stage 1 | 5 (23.9%) |
| CKD Stage 2 | 10 (47.6%) |
| CKD Stage 3 | 6 (28.5%) |
| Total bilirubin (mg/dL) | 5.8 (0.5–14.1) |
| Factors | SVR12 (n = 594 by PP *) | ||
|---|---|---|---|
| Overall | 590/594 | (99.3%) | |
| Genotype | 1a | 30/30 | (100%) |
| 1b | 195/195 | (100%) | |
| 2 | 295/297 | (99.3%) | |
| 3 | 13/14 | (92.9%) | |
| 6 | 29/29 | (100%) | |
| Mixed | 27/28 | (96.4%) | |
| Peg-IFN experienced | Naïve | 548/552 | (99.3%) |
| Experienced | 41/41 | (100%) | |
| HBV/HCV co-infection | HBV (+) | 45/45 | (100%) |
| HBV (−) | 540/544 | (99.3%) | |
| FIB-4 | <3.25 | 314/316 | (99.4%) |
| ≥3.25 | 273/275 | (99.3%) | |
| Cirrhosis | Liver cirrhosis | 121/122 | (99.2%) |
| Non-liver cirrhosis | 292/295 | (99.0%) | |
| Unknown | 177/177 | (100%) | |
| +RBV | With RBV | 67/68 | (98.5%) |
| Without RBV | 523/526 | (99.4%) | |
| EOT | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| Variable | Comparison | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Age (years) | ≥60 vs. <60 | 0.715 (0.419–1.221) | 0.219 | ||
| Sex | M vs. F | 0.610 (0.352–1.057) | 0.078 | ||
| Liver cirrhosis | Yes vs. No | 1.262 (0.734–2.172) | 0.400 | ||
| HCC | Yes vs. No | 1.447 (0.737–2.843) | 0.282 | ||
| Diabetes mellitus | Yes vs. No | 1.508 (0.641–3.548) | 0.347 | ||
| Ribavirin | Yes vs. No | 1.373 (0.628–3.000) | 0.427 | ||
| Baseline eGFR | ≥60 vs. <60 | 1.508 (0.731–3.111) | 0.266 | 2.776 (1.106–6.965) | 0.030 |
| Base_FIB-4 | ≥3.25 vs. <3.25 | 1.077 (0.632–1.835) | 0.786 | ||
| HBV | Yes vs. No | 0.681 (0.232–1.999) | 0.485 | ||
| History of PR use | Yes vs. No | 0.866 (0.324–2.315) | 0.774 | ||
| SVR12 | Univariate | Multivariate | |||
| Age (years) | ≥60 vs. <60 | 1.366 (0.745–2.504) | 0.314 | ||
| Sex | M vs. F | 1.526 (0.859–2.712) | 0.149 | ||
| Liver cirrhosis | Yes vs. No | 2.130 (1.199–3.786) | 0.010 | ||
| HCC | Yes vs. No | 1.637 (0.783–3.423) | 0.190 | ||
| Diabetes mellitus | Yes vs. No | 3.009 (1.376–6.578) | 0.006 | 2.548 (1.093–5.940) | 0.030 |
| Ribavirin | Yes vs. No | 3.681 (1.889–7.174) | <0.001 | 4.369 (1.771–10.78) | 0.010 |
| Baseline eGFR | ≥60 vs. <60 | 1.527 (0.682–3.419) | 0.304 | ||
| Base_FIB-4 | ≥3.25 vs. <3.25 | 1.245 (0.696–2.228) | 0.460 | ||
| HBV | Yes vs. No | 0.429 (0.101–1.824) | 0.252 | ||
| History of PR use | Yes vs. No | 0.773 (0.230–2.599) | 0.677 | ||
| SVR24 | Univariate | Multivariate | |||
| Age (years) | ≥60 vs. <60 | 1.026 (0.568–1.852) | 0.933 | ||
| Sex | M vs. F | 1.492 (0.845–2.633) | 0.168 | ||
| Liver cirrhosis | Yes vs. No | 2.753 (1.540–4.920) | 0.001 | ||
| HCC | Yes vs. No | 1.679 (0.856–3.293) | 0.132 | ||
| Diabetes mellitus | Yes vs. No | 2.500 (1.148–5.445) | 0.021 | 2.702 (1.191–6.131) | 0.017 |
| Ribavirin | Yes vs. No | 3.632 (1.902–6.934) | <0.001 | 2.428 (0.981–6.006) | 0.055 |
| Baseline eGFR | ≥60 vs. <60 | 1.150 (0.490–2.702) | 0.748 | ||
| Base_FIB-4 | ≥3.25 vs. <3.25 | 2.124 (1.150–3.922) | 0.016 | 2.699 (1.050–6.935) | 0.039 |
| HBV | Yes vs. No | 0.952 (0.316–2.863) | 0.930 | ||
| History of PR use | Yes vs. No | 0.507 (0.150–1.718) | 0.276 | ||
| SVR48 | Univariate | Multivariate | |||
| Age (years) | ≥60 vs. <60 | 1.298 (0.666–2.532) | 0.444 | ||
| Sex | M vs. F | 1.250 (0.680–2.297) | 0.472 | ||
| Liver cirrhosis | Yes vs. No | 2.192 (1.184–4.059) | 0.013 | ||
| HCC | Yes vs. No | 0.265 (0.043–1.636) | 0.153 | ||
| Diabetes mellitus | Yes vs. No | 2.524 (1.129–5.639) | 0.024 | 2.572 (1.133–5.836) | 0.024 |
| Ribavirin | Yes vs. No | 2.560 (1.235–5.305) | 0.011 | 3.018 (1.156–7.883) | 0.024 |
| Baseline eGFR | ≥60 vs. <60 | 1.000 (0.419–2.385) | 1.000 | ||
| Base_FIB-4 | ≥3.25 vs. <3.25 | 1.910 (1.013–3.601) | 0.045 | ||
| HBV | Yes vs. No | 1.622 (0.573–4.596) | 0.362 | ||
| History of PR use | Yes vs. No | 1.133 (0.370–3.471) | 0.827 | ||
| EOT | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| Variable | Comparison | OR (95%CI) | p-Value | OR (95% CI) | p-Value |
| Age (years) | ≥60 vs. <60 | 0.661 (0.371–1.180) | 0.162 | ||
| Sex | M vs. F | 0.594 (0.325–1.082) | 0.089 | ||
| Liver cirrhosis | Yes vs. No | 1.180 (0.651–2.138) | 0.585 | ||
| HCC | Yes vs. No | 1.432 (0.671–3.057) | 0.353 | ||
| Diabetes mellitus | Yes vs. No | 1.167 (0.400–3.406) | 0.778 | ||
| Ribavirin | Yes vs. No | 1.102 (0.437–2.775) | 0.837 | ||
| Base_FIB-4 | ≥3.25 vs. <3.25 | 1.149 (0.641–2.059) | 0.640 | ||
| HBV | Yes vs. No | 0.601 (0.176–2.050) | 0.416 | ||
| History of PR use | Yes vs. No | 1.003 (0.370–2.716) | 0.996 | ||
| SVR12 | Univariate | Multivariate | |||
| Age (years) | ≥60 vs. <60 | 1.895 (0.943–3.809) | 0.073 | 4.094 (1.161–14.437) | 0.028 |
| Sex | M vs. F | 1.361 (0.717–2.585) | 0.346 | ||
| Liver cirrhosis | Yes vs. No | 2.082 (1.093–3.964) | 0.026 | ||
| HCC | Yes vs. No | 2.178 (0.984–4.823) | 0.055 | ||
| Diabetes mellitus | Yes vs. No | 1.599 (0.595–4.298) | 0.352 | ||
| Ribavirin | Yes vs. No | 4.200 (1.990–8.865) | <0.001 | 4.671 (1.683–12.960) | 0.003 |
| Base_FIB-4 | ≥3.25 vs. <3.25 | 1.628 (0.846–3.130) | 0.144 | ||
| HBV | Yes vs. No | 0.535 (0.124–2.301) | 0.401 | ||
| History of PR use | Yes vs. No | 0.927 (0.272–3.156) | 0.904 | ||
| SVR24 | Univariate | Multivariate | |||
| Age (years) | ≥60 vs. <60 | 1.152 (0.615–2.158) | 0.658 | ||
| Sex | M vs. F | 1.468 (0.799–2.696) | 0.216 | ||
| Liver cirrhosis | Yes vs. No | 2.761 (1.487–5.127) | 0.001 | ||
| HCC | Yes vs. No | 1.792 (0.863–3.722) | 0.118 | ||
| Diabetes mellitus | Yes vs. No | 2.292 (0.956–5.492) | 0.063 | ||
| Ribavirin | Yes vs. No | 5.214 (2.576–10.553) | <0.001 | 5.200 (1.983–13.634) | 0.001 |
| Base_FIB-4 | ≥3.25 vs. <3.25 | 2.088 (1.083–4.022) | 0.028 | ||
| HBV | Yes vs. No | 1.074 (0.351–3.287) | 0.901 | ||
| History of PR use | Yes vs. No | 0.524 (0.153–1.791) | 0.303 | ||
| SVR48 | Univariate | Multivariate | |||
| Age (years) | ≥60 vs. <60 | 1.649 (0.804–3.384) | 0.173 | ||
| Sex | M vs. F | 1.147 (0.594–2.212) | 0.683 | ||
| Liver cirrhosis | Yes vs. No | 1.792 (0.926–3.469) | 0.083 | ||
| HCC | Yes vs. No | 0.785 (0.310–1.984) | 0.608 | ||
| Diabetes mellitus | Yes vs. No | 2.621 (1.068–6.433) | 0.035 | 2.765 (1.104–6.922) | 0.030 |
| Ribavirin | Yes vs. No | 2.396 (1.060–5.415) | 0.036 | 3.143 (1.047–9.435) | 0.041 |
| Base_FIB-4 | ≥3.25 vs. <3.25 | 1.433 (0.735–2.794) | 0.291 | ||
| HBV | Yes vs. No | 2.124 (0.722–6.246) | 0.171 | ||
| History of PR use | Yes vs. No | 1.191 (0.384–3.695) | 0.762 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-K.; Chen, L.-W.; Chang, T.-S.; Tung, S.-Y.; Lin, C.-Y.; Hung, C.-H.; Lu, S.-N.; Lin, C.-L.; Chen, C.-H.; Hsu, C.-W.; et al. The Novel Finding of Dynamic Change in eGFR Up to One Year after End of Treatment in HCV-Infected Patients Receiving Sofosbuvir and Velpatasvir. Viruses 2022, 14, 362. https://doi.org/10.3390/v14020362
Wu C-K, Chen L-W, Chang T-S, Tung S-Y, Lin C-Y, Hung C-H, Lu S-N, Lin C-L, Chen C-H, Hsu C-W, et al. The Novel Finding of Dynamic Change in eGFR Up to One Year after End of Treatment in HCV-Infected Patients Receiving Sofosbuvir and Velpatasvir. Viruses. 2022; 14(2):362. https://doi.org/10.3390/v14020362
Chicago/Turabian StyleWu, Cheng-Kun, Li-Wei Chen, Te-Sheng Chang, Shui-Yi Tung, Chun-Yen Lin, Chao-Hung Hung, Sheng-Nan Lu, Chih-Lang Lin, Chien-Hung Chen, Chao-Wei Hsu, and et al. 2022. "The Novel Finding of Dynamic Change in eGFR Up to One Year after End of Treatment in HCV-Infected Patients Receiving Sofosbuvir and Velpatasvir" Viruses 14, no. 2: 362. https://doi.org/10.3390/v14020362
APA StyleWu, C.-K., Chen, L.-W., Chang, T.-S., Tung, S.-Y., Lin, C.-Y., Hung, C.-H., Lu, S.-N., Lin, C.-L., Chen, C.-H., Hsu, C.-W., Hu, T.-H., & Sheen, I.-S. (2022). The Novel Finding of Dynamic Change in eGFR Up to One Year after End of Treatment in HCV-Infected Patients Receiving Sofosbuvir and Velpatasvir. Viruses, 14(2), 362. https://doi.org/10.3390/v14020362







