Phage–Bacteria Interactions in Potential Applications of Bacteriophage vB_EfaS-271 against Enterococcus faecalis
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria, Media, and Growth Conditions
2.2. Propagation of Phage vB_EfaS-271
2.3. Phage Titration
2.4. Determination of Number of Colony Forming Units
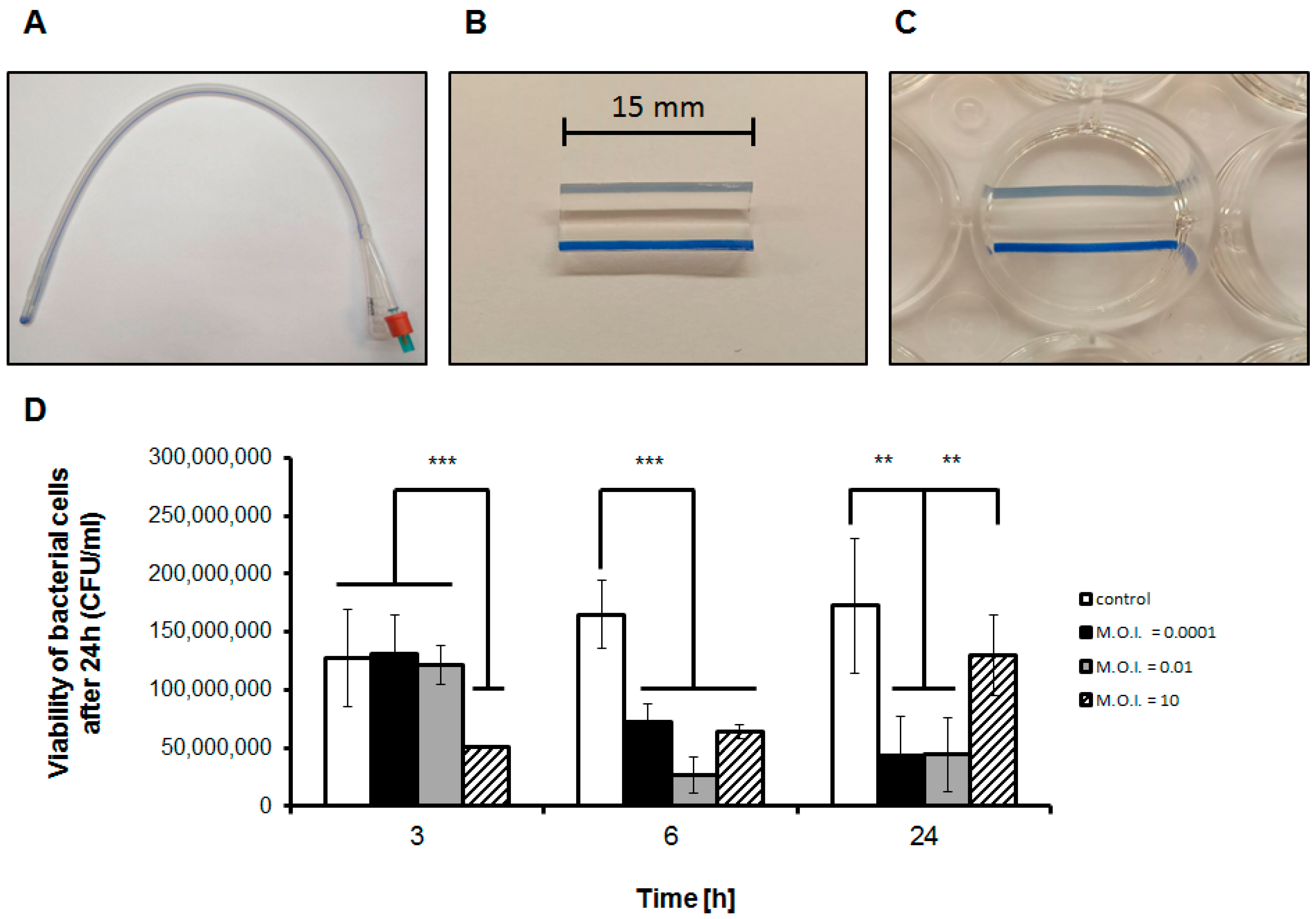
2.5. Formation and Assessment of Biofilm Formed on Catheters
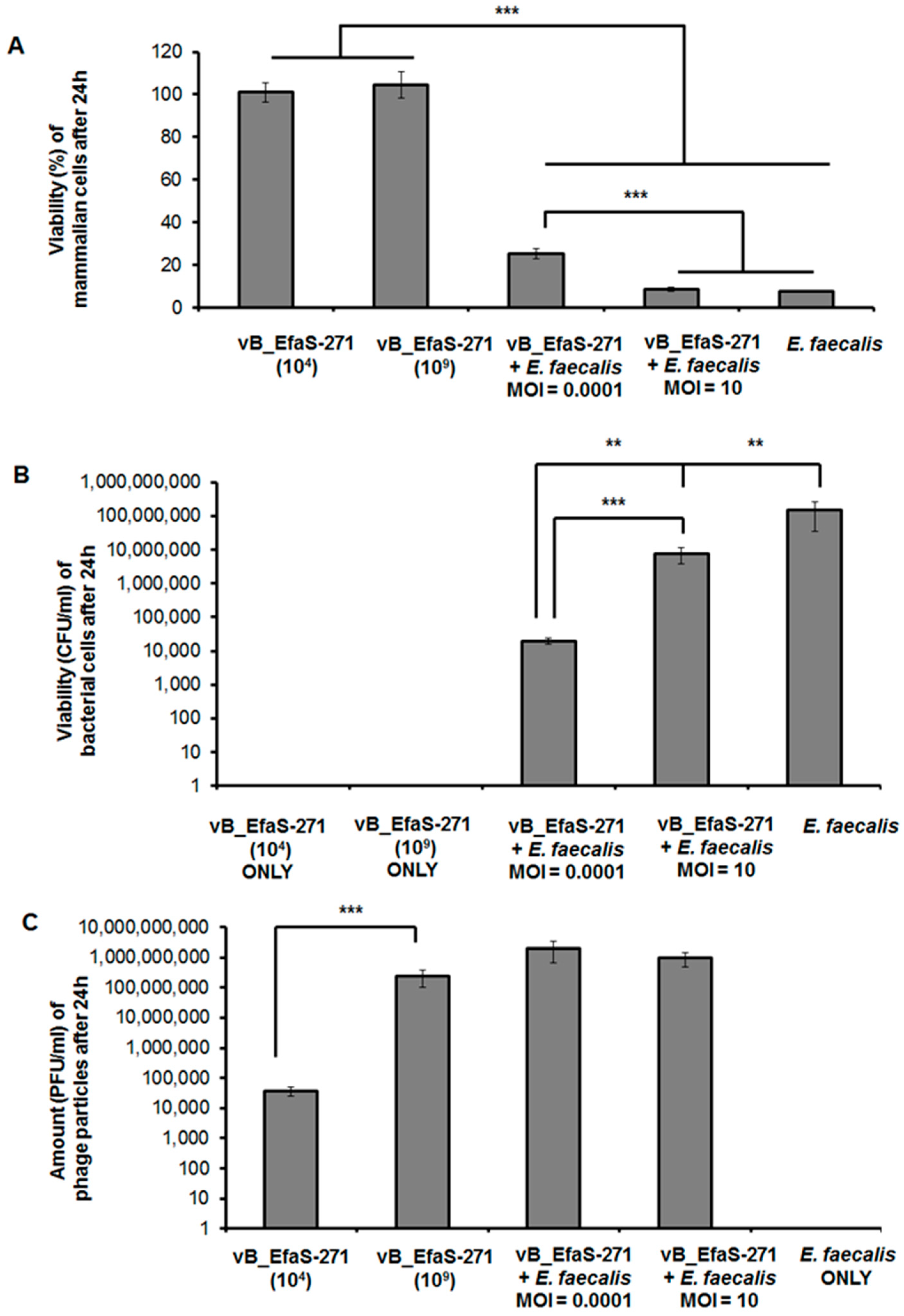
2.6. Assessment of vB_EfaS-271 Phage Cytotoxicity
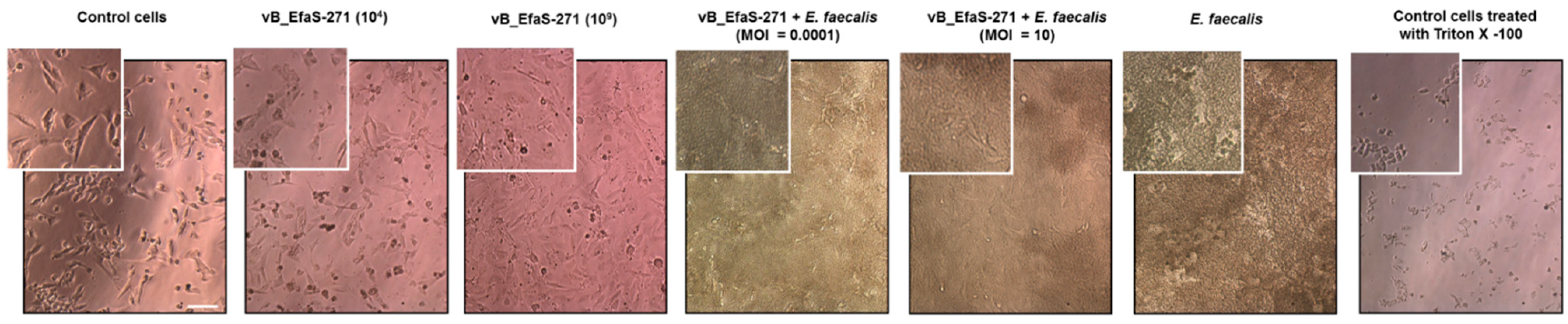
2.7. Microscopic Analyses
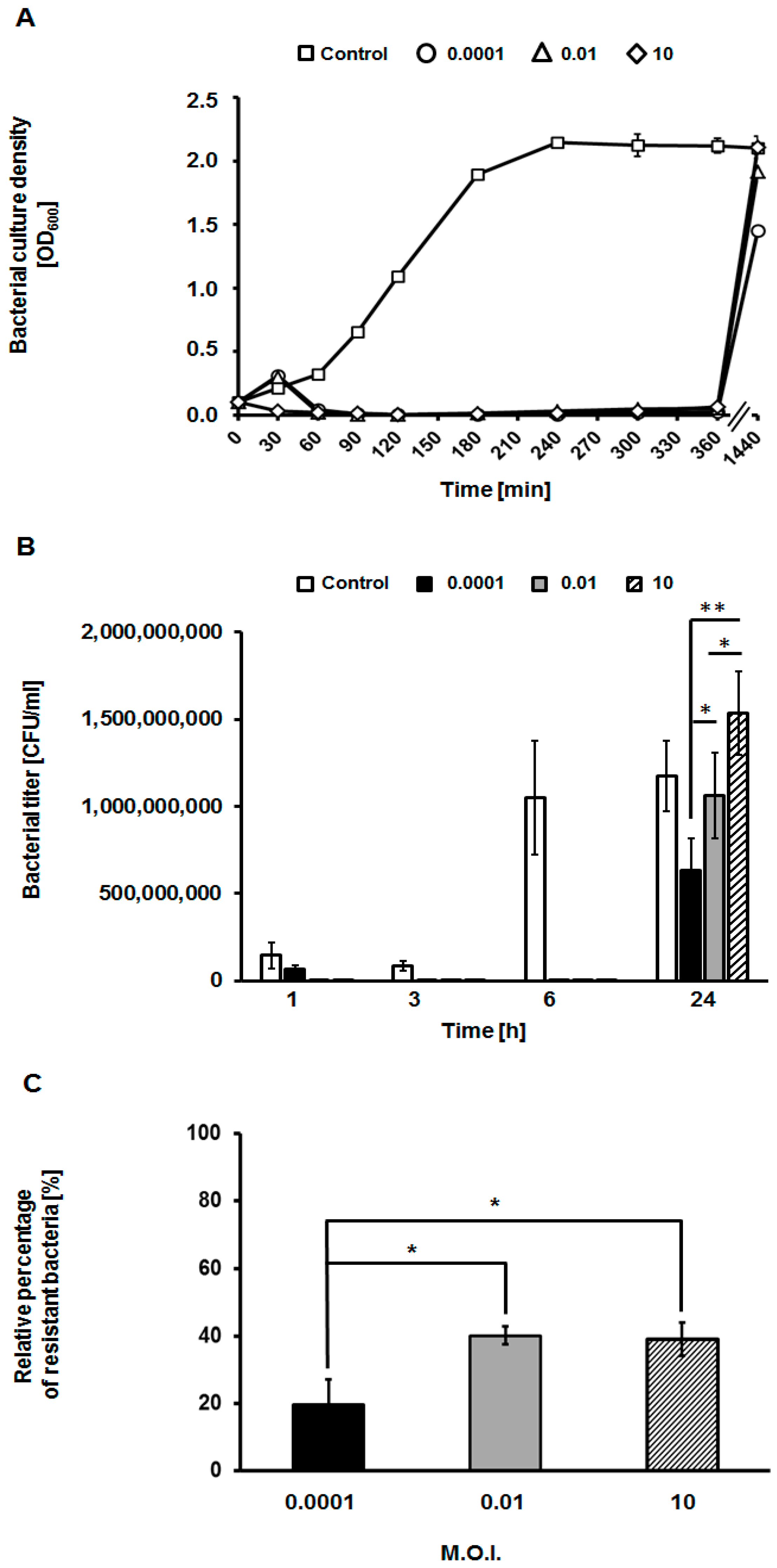
2.8. Assessment of Appearance of Phage-Resistant Bacteria
2.9. Statistical Analysis
3. Results
3.1. Efficiency of Phage Treatment of Biofilms Formed on Catheters
3.2. Assessment of Toxicity of vB_EfaS-271 Phage Particles to Mammalian Cells and Effects of This Phage on E. faecalis Co-Cultured with These Cells
3.3. Appearance of Phage-Resistant Bacteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ho, C.; Lau, A.; Cimon, K.; Farrah, K.; Gardam, M. Screening, isolation, and decolonization strategies for vancomycin-resistant enterococci or extended spectrum beta-lactamase producing organisms: A systematic review of the clinical evidence and health services impact. CADTH Technol. Overv. 2013, 3, e3202. [Google Scholar] [PubMed]
- Jabbari Shiadeh, S.M.; Pormohammad, A.; Hashemi, A.; Lak, P. Global prevalence of antibiotic resistance in blood-isolated Enterococcus faecalis and Enterococcus faecium: A systematic review and meta-analysis. Infect Drug Resist. 2019, 12, 2713–2725. [Google Scholar] [CrossRef] [PubMed]
- Olawale, K.O.; Fadiora, S.O.; Taiwo, S.S. Prevalence of hospital acquired enterococci infections in two primary-care hospitals in Osogbo, Southwestern Nigeria. Afr. J. Infect. Dis. 2011, 5, 2. [Google Scholar] [CrossRef]
- McBride, S.; Upton, A.; Roberts, S. Clinical characteristics and outcomes of patients with vancomycin-susceptible Enterococcus faecalis and Enterococcus faecium bacteraemia—A five-year retrospective review. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Vergis, E.N.; Hayden, M.K.; Chow, J.W.; Snydman, D.R.; Zervos, M.J.; Linden, P.K.; Wagener, M.M.; Schmitt, B.; Muder, R.R. Determinants of vancomycin resistance and mortality rates in Enterococcal bacteremia: A prospective multicenter study. Ann. Intern. Med. 2001, 135, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, L.M.; Fritsche, T.R.; Moet, G.J.; Biedenbach, D.J.; Jones, R.N. Antimicrobial resistance and molecular epidemiology of vancomycin-resistant enterococci from North America and Europe: A report from the SENTRY antimicrobial surveillance program. Diagn. Microbiol. Infect. Dis. 2007, 58, 163–170. [Google Scholar] [CrossRef]
- Hidron, A.I.; Edwards, J.R.; Patel, J.; Horan, T.C.; Sievert, D.M.; Pollock, D.A.; Fridkin, S.K. National Healthcare Safety Network Team; Participating National Healthcare Safety Network Facilitiess. Antimicrobial-resistant pathogens associated with healthcare-associated infections: Annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect. Control Hosp. Epidemiol. 2008, 29, 996–1011. [Google Scholar] [CrossRef]
- Reik, R.; Tenover, F.C.; Klein, E.; McDonald, L.C. The burden of vancomycin-resistant enterococcal infections in US hospitals, 2003 to 2004. Diagn. Microbiol. Infect. Dis. 2008, 62, 81–85. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [PubMed]
- van Harten, R.M.; Willems, R.J.; Martin, N.I.; Hendrickx, A.P. Multidrug-resistant enterococcal infections: New compounds, novel antimicrobial therapies? Trends Microbiol. 2017, 25, 467–479. [Google Scholar] [CrossRef]
- Chlebicki, M.P.; Kurup, A. Vancomycin-resistant Enterococcus: A review from a Singapore perspective. Ann. Acad. Med. Singap. 2008, 37, 861–869. [Google Scholar]
- Raza, T.; Ullah, S.R.; Mehmood, K.; Andleeb, S. Vancomycin resistant enterococci: A brief review. J. Pak. Med. Assoc. 2018, 68, 768–772. [Google Scholar] [PubMed]
- Tan, C.A.Z.; Antypas, H.; Kline, K.A. Overcoming the challenge of establishing biofilms in vivo: A roadmap for enterococci. Curr. Opin. Microbiol. 2020, 53, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Górski, A.; Międzybrodzki, R.; Węgrzyn, G.; Jończyk-Matysiak, E.; Borysowski, J.; Weber-Dąbrowska, B. Phage therapy: Current status and perspectives. Med. Res. Rev. 2020, 40, 459–463. [Google Scholar] [CrossRef]
- Górski, A.; Międzybrodzki, R.; Łobocka, M.; Głowacka-Rutkowska, A.; Bednarek, A.; Borysowski, J.; Jończyk-Matysiak, E.; Łusiak-Szelachowska, M.; Weber-Dąbrowska, B.; Bagińska, N.; et al. Phage therapy: What have we learned? Viruses 2018, 10, 288. [Google Scholar] [CrossRef]
- Bolocan, A.S.; Upadrasta, A.; Bettio, P.H.D.A.; Clooney, A.G.; Draper, L.A.; Ross, R.P.; Hill, C. Evaluationof phage therapy in the context of Enterococcus faecalis and its associated diseases. Viruses 2019, 11, 366. [Google Scholar] [CrossRef]
- Stevens, R.H.; Zhang, H.; Hsiao, C.; Kachlany, S.; Tinoco, E.M.; DePew, J.; Fouts, D.E. Structural proteins of Enterococcus faecalis bacteriophage ϕEf11. Bacteriophage 2016, 6, e1251381. [Google Scholar] [CrossRef]
- Tinoco, J.M.; Buttaro, B.; Zhang, H.; Liss, N.; Sassone, L.; Stevens, R. Effect of a genetically engineered bacteriophage on Enterococcus faecalis biofilms. Arch Oral Biol. 2016, 71, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Tinoco, J.M.; Liss, N.; Zhang, H.; Nissan, R.; Gordon, W.; Tinoco, E.; Sassone, L.; Stevens, R. Antibacterial effect of genetically-engineered bacteriophage ϕEf11/ϕFL1C(Δ36)P(nisA) on dentin infected with antibiotic-resistant Enterococcus faecalis. Arch Oral Biol. 2017, 82, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Liang, J.; Zhang, Y.; Hu, L.; Gong, P.; Cai, R.; Zhang, L.; Zhang, H.; Ge, J.; Ji, Y.; et al. The bacteriophage EF-P29 efficiently protects against lethal vancomycin-resistant Enterococcus faecalis and alleviates gut microbiota imbalance in a murine bacteremia model. Front. Microbiol. 2017, 8, 837. [Google Scholar] [CrossRef]
- Gelman, D.; Beyth, S.; Lerer, V.; Adler, K.; Poradosu-Cohen, R.; Coppenhagen-Glazer, S.; Hazan, R. Combined bacteriophages and antibiotics as an efficient therapy against VRE Enterococcus faecalis in a mouse model. Res. Microbiol. 2018, 169, 531–539. [Google Scholar] [CrossRef]
- Shlezinger, M.; Friedman, M.; Houri-Haddad, Y.; Hazan, R.; Beyth, N. Phages in a thermoreversible sustained-release formulation targeting E. faecalis in vitro and in vivo. PLoS ONE 2019, 14, e0219599. [Google Scholar] [CrossRef]
- Kishimoto, T.; Ishida, W.; Fukuda, K.; Nakajima, I.; Suzuki, T.; Uchiyama, J.; Matsuzaki, S.; Todokoro, D.; Daibata, M.; Fukushima, A. Therapeutic effects of intravitreously administered bacteriophage in a mouse model of endophthalmitis caused by vancomycin-sensitive or -resistant Enterococcus faecalis. Antimicrob. Agents Chemother. 2019, 63, e01088-19. [Google Scholar] [CrossRef]
- Duan, Y.; Llorente, C.; Lang, S.; Brandl, K.; Chu, H.; Jiang, L.; White, R.C.; Clarke, T.H.; Nguyen, K.; Torralba, M.; et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 2019, 575, 505–511. [Google Scholar] [CrossRef]
- Çolakoğlu, M.; Xue, J.; Trajkovski, M. Bacteriophage prevents alcoholic liver disease. Cell 2020, 180, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Topka-Bielecka, G.; Bloch, S.; Nejman-Faleńczyk, B.; Grabski, M.; Jurczak-Kurek, A.; Górniak, M.; Dydecka, A.; Necel, A.; Węgrzyn, G.; Węgrzyn, A. Characterization of the bacteriophage vB_EfaS-271 infecting Enterococcus faecalis. Int. J. Mol. Sci. 2020, 21, 6345. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, D.; Maciąg-Dorszyńska, M.; Bogucka, K.; Szalewska-Pałasz, A.; Herman-Antosiewicz, A. Various modes of action of dietary phytochemicals, sulforaphane and phenethyl isothiocyanate, on pathogenic bacteria. Sci. Rep. 2019, 9, 13677. [Google Scholar] [CrossRef] [PubMed]
- Jurczak-Kurek, A.; Gąsior, T.; Nejman-Faleńczyk, B.; Bloch, S.; Dydecka, A.; Topka, G.; Necel, A.; Jakubowska-Deredas, M.; Narajczyk, M.; Richert, M.; et al. Biodiversity of bacteriophages: Morphological and biological properties of a large group of phages isolated from urban sewage. Sci. Rep. 2016, 6, 34338. [Google Scholar] [CrossRef] [PubMed]
- Colomer-Winter, C.; Lemos, J.A.; Flores-Mireles, A.L. Biofilm assays on fibrinogen-coated silicone catheters and 96-well polystyrene plates. Bio-protocol 2019, 9, e3196. [Google Scholar] [CrossRef] [PubMed]
- Kart, D.; Kustimur, A.S.; Sağıroğlu, M.; Kalkancı, A. Evaluation of antimicrobial durability and anti-biofilm effects in urinary catheters against Enterococcus faecalis clinical isolates and reference strains. Balkan Med. J. 2017, 34, 546–552. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topka-Bielecka, G.; Nejman-Faleńczyk, B.; Bloch, S.; Dydecka, A.; Necel, A.; Węgrzyn, A.; Węgrzyn, G. Phage–Bacteria Interactions in Potential Applications of Bacteriophage vB_EfaS-271 against Enterococcus faecalis. Viruses 2021, 13, 318. https://doi.org/10.3390/v13020318
Topka-Bielecka G, Nejman-Faleńczyk B, Bloch S, Dydecka A, Necel A, Węgrzyn A, Węgrzyn G. Phage–Bacteria Interactions in Potential Applications of Bacteriophage vB_EfaS-271 against Enterococcus faecalis. Viruses. 2021; 13(2):318. https://doi.org/10.3390/v13020318
Chicago/Turabian StyleTopka-Bielecka, Gracja, Bożena Nejman-Faleńczyk, Sylwia Bloch, Aleksandra Dydecka, Agnieszka Necel, Alicja Węgrzyn, and Grzegorz Węgrzyn. 2021. "Phage–Bacteria Interactions in Potential Applications of Bacteriophage vB_EfaS-271 against Enterococcus faecalis" Viruses 13, no. 2: 318. https://doi.org/10.3390/v13020318
APA StyleTopka-Bielecka, G., Nejman-Faleńczyk, B., Bloch, S., Dydecka, A., Necel, A., Węgrzyn, A., & Węgrzyn, G. (2021). Phage–Bacteria Interactions in Potential Applications of Bacteriophage vB_EfaS-271 against Enterococcus faecalis. Viruses, 13(2), 318. https://doi.org/10.3390/v13020318







