Viral Vectors for COVID-19 Vaccine Development
Abstract
1. Introduction
2. Selection of Viral Vector
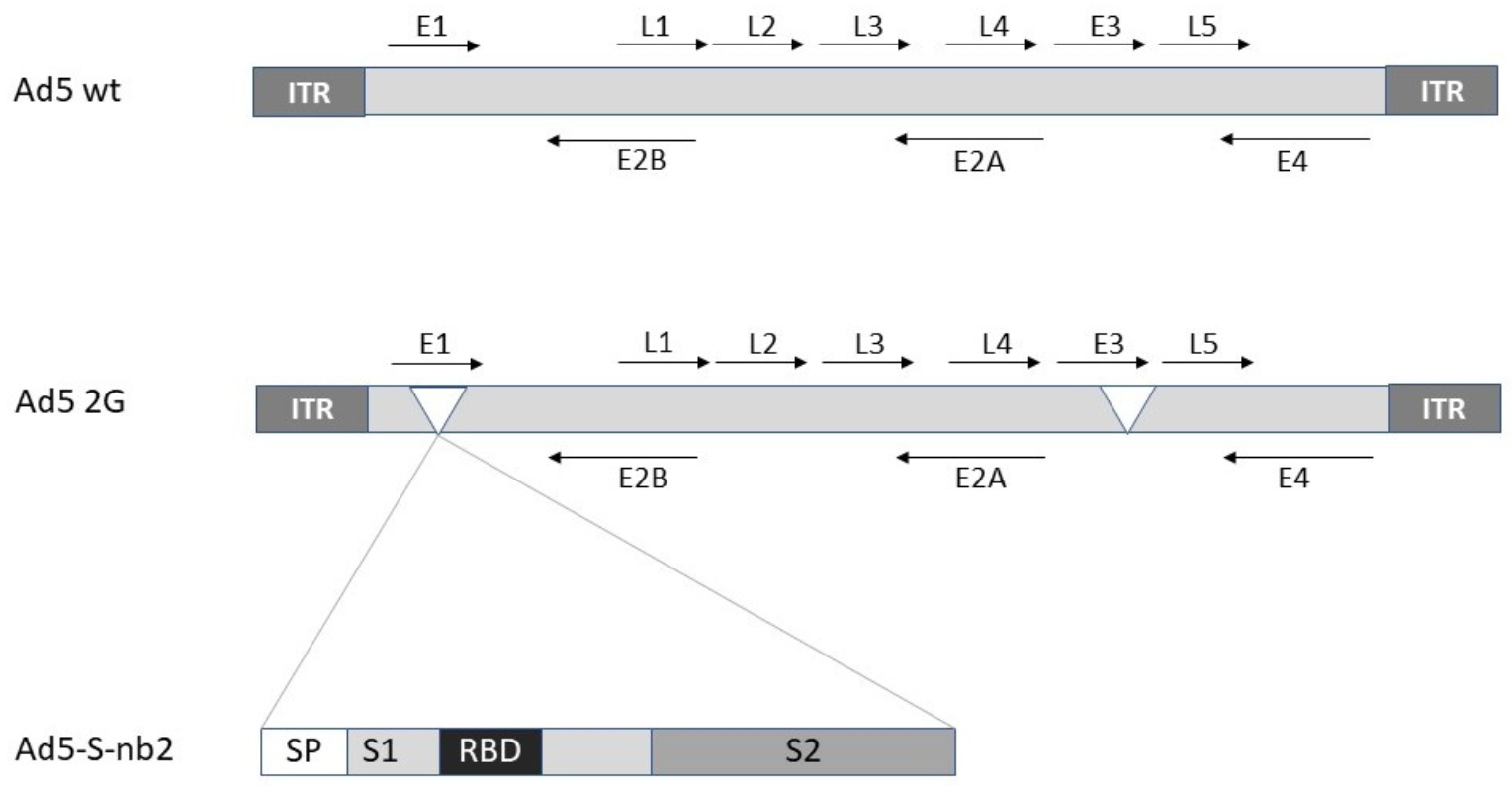
2.1. Adenovirus
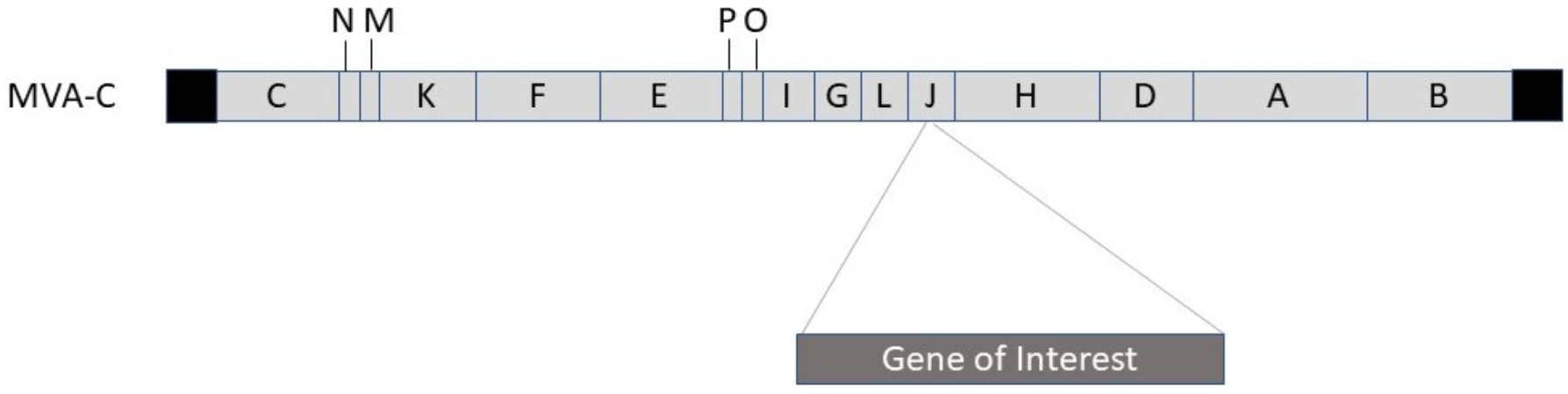
2.2. Poxvirus
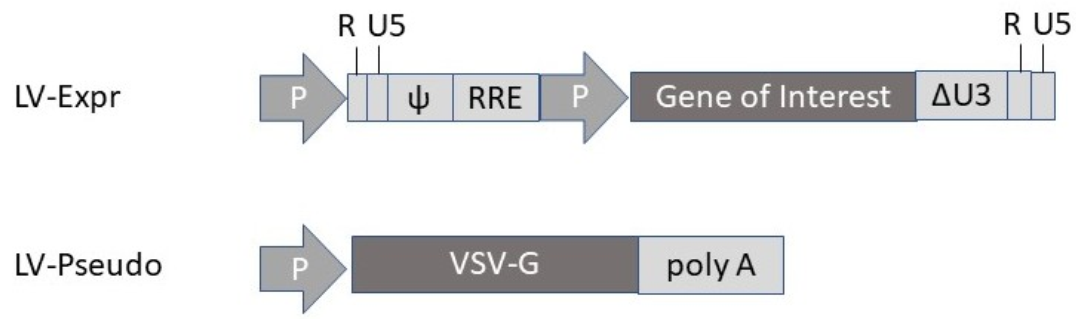
2.3. Lentivirus
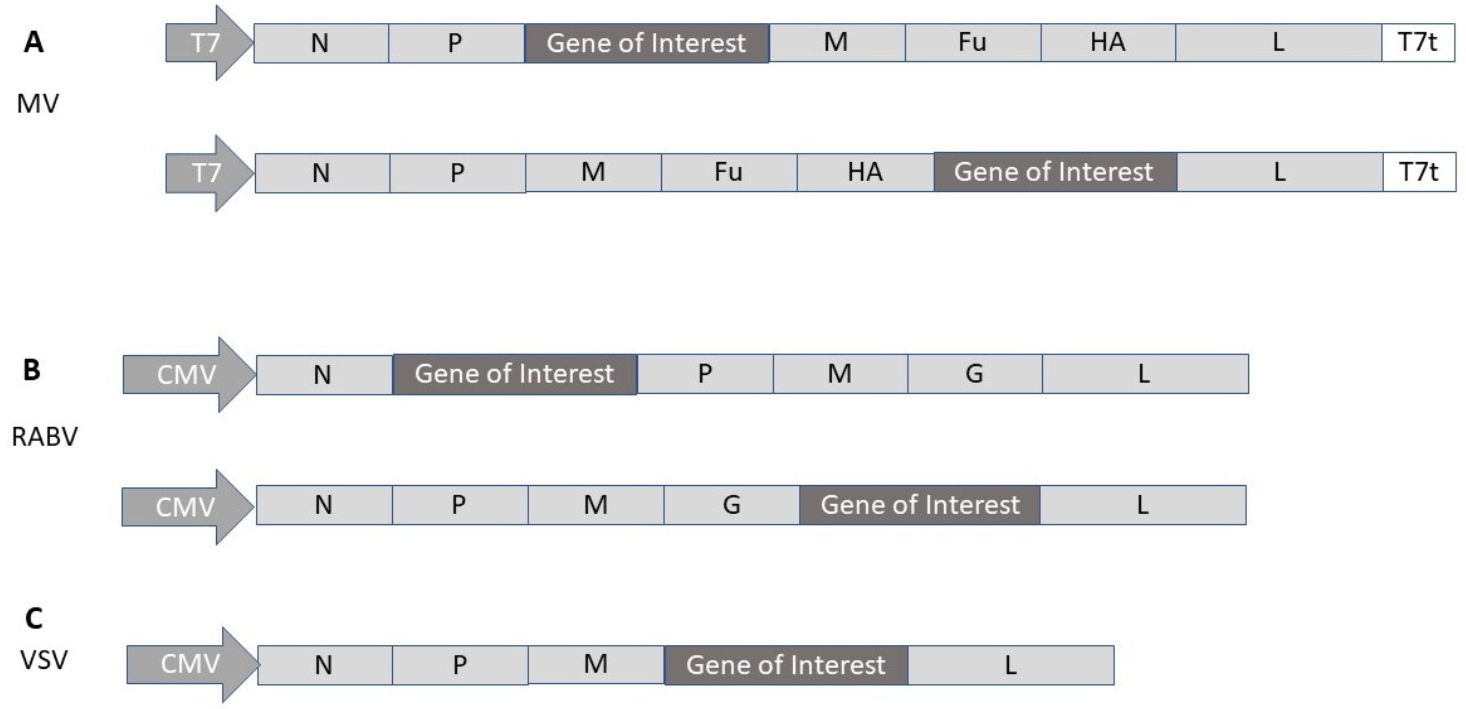
2.4. Measles Virus
2.5. Rhabdovirus
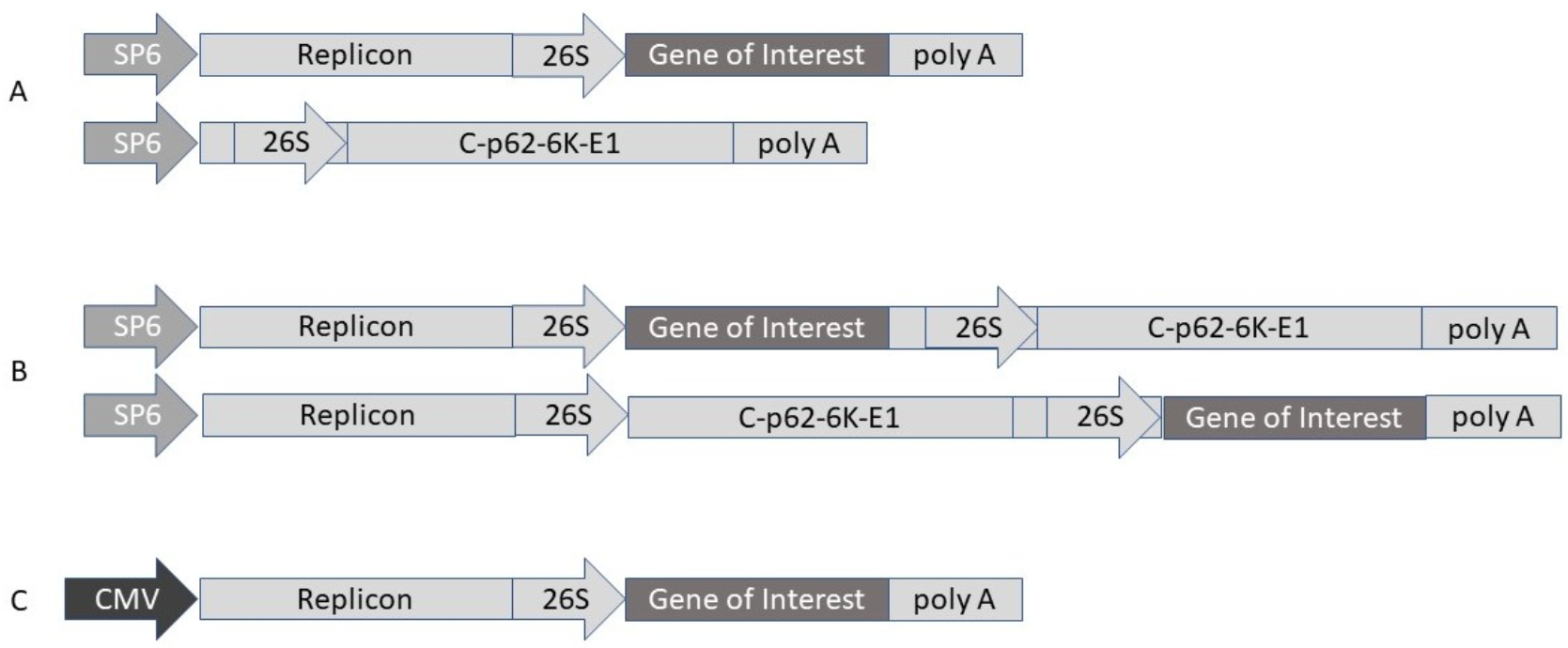
2.6. Alphavirus
3. Preclinical Studies
4. Clinical Trials
5. Conclusions
Funding
Conflicts of Interest
References
- Global Health Policy. Available online: https://www.google.com/covid19-map (accessed on 28 April 2020).
- Cherry, J.D. The chronology of the 2002–2003 SARS mini pandemic. Paediatr. Resp. Rev. 2004, 5, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Aleanizi, F.S.; Mohmed, N.; Alqahtani, F.Y.; El Hadi Mohamed, R.A. Outbreak of Middle east respiratory syndrome coronavirus in Saudi Arabia: A retrospective study. BMC Infect. Dis. 2017, 17, 23. [Google Scholar] [CrossRef]
- Lundstrom, K. Coronavirus Pandemic—Therapy and Vaccines. Biomedicines 2020, 8, 109. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Schiedner, G.; Morral, N.; Parks, R.S.; Wu, Y.; Koopmans, S.C.; Langston, C.; Graham, F.L.; Beaudet, A.L.; Kochanek, S. Genomic DNA transfer with a high-capacity adenovirus vector results in improved in vivo gene expression and decreased toxicity. Nat. Genet. 1998, 18, 180–183. [Google Scholar] [CrossRef]
- Feng, L.; Wang, Q.; Shan, C.; Yang, C.; Feng, Y.; Wu, J.; Liu, X.; Zhou, Y.; Jian, R.; Hu, P.; et al. An adenovirus vectored COVID-19 vaccine confers protection from SARS-CoV-2 challenge in rhesus macaques. Nat. Commun. 2020, 11, 4207. [Google Scholar] [CrossRef]
- Chen, K.-D.; Wu, X.-X.; Yu, D.-S.; Ou, H.-L.; Li, Y.-H.; Zhou, Y.-Q.; Li, L.-J. Process optimization for the rapid production of adenoviral vectors for clinical trials in a disposable bioreactor system. Appl. Microbiol. Biotechnol. 2018, 102, 6469–6477. [Google Scholar] [CrossRef]
- Kwak, H.; Honig, H.; Kaufmann, H.L. Poxviruses as vectors for cancer immunotherapy. Curr. Opin. Drug Discov. Devel. 2003, 6, 161–168. [Google Scholar] [PubMed]
- Gomez, C.E.; Perdiguero, B.; Jimenez, V.; Filali, A.; Haddad, E.K.; Quakkelaar, E.D.; Delaloye, J.; Harari, A.; Roger, T.; Dunhen, T.; et al. Ssytems Analysis of MVA-C Induced Immune Response Reveals Its Significance as a Vaccine Candidate against HIV/AIDS of Clade C. PLoS ONE 2012, 7, e35485. [Google Scholar] [CrossRef]
- Kreijtz, J.H.; Suezer, Y.; van Amerongen, G.; de Mutsert, G.; Schnierle, B.S.; Wood, J.M.; Kuiken, T.; Fouchier, R.A.; Lower, J.; Osterhaus, A.D.; et al. Recombinant modified vaccinia virus Ankara-based vaccine induces protective immunity in mice against infection with influenza virus H5N1. J. Infect. Dis. 2007, 195, 1598–1606. [Google Scholar] [CrossRef]
- Volz, A.; Sutter, G. Modified Vaccinia Virus Ankara: History, Value in Basic Research, and Current Perspectives for Vaccine Development. Adv. Virus Res. 2017, 197, 187–243. [Google Scholar]
- Vigna, E.; Naldini, L. Lentiviral vectors: Excellent tools for experimental gene transfer and promising candidates for gene therapy. J. Gen. Med. 2000, 2, 308–316. [Google Scholar] [CrossRef]
- Kay, M.A.; Glorioso, J.C.; Naldini, L. Viral vectors for gene therapy: The art of turning infectious agents into vehicles of therapeutics. Nat. Med. 2001, 7, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Manceur, A.P.; Kim, H.; Misic, V.; Andreev, N.; Dorion-Thibaudeau, J.; Lanthier, S.; Bernier, A.; Tremblay, S.; Gélinas, A.M.; Broussau, S.; et al. Scalable Lentiviral Vector Production Using Stable HEK293SF Producer Cell Lines. Hum. Gene Ther. Methods 2017, 28, 330–339. [Google Scholar] [CrossRef]
- Schambach, A.; Galla, M.; Modlich, U.; Will, E.; Chandra, S.; Reeves, L.; Colbert, M.; Williams, D.A.; von Kalle, C.; Baum, C.; et al. Lentiviral vectors pseudotyped with murine ecotropic envelope: Increased biosafety and convenience in preclinical research. Exp. Hematol. 2006, 34, 588–592. [Google Scholar] [CrossRef]
- Lévy, C.; Verhoeyen, E.; Cosset, F.L. Surface engineering of lentiviral vectors for gene transfer into gene therapy target cells. Curr. Opin. Pharmacol. 2015, 24, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Suzuki, Y. Gene Regulatable Lentiviral Vector System. In Viral Gene Therapy; Xu, K., Ed.; Intech Open: London, UK, 2011. [Google Scholar] [CrossRef]
- Tokgun, U.; Fiorentino, F.P.; Tokgun, P.E.; Yokota, J.; Akca, H. Design of a Lentiviral Vector for the Inducible Expression of MYC: A New Strategy for Construction Approach. Mol. Biotechnol. 2017, 59, 200–206. [Google Scholar] [CrossRef]
- Lundstrom, K. Self-replicating RNA viral vectors in vaccine development and gene therapy. Future Virol. 2016, 11, 345–356. [Google Scholar] [CrossRef]
- Radecke, F.; Spielhofer, P.; Schneider, H.; Kaelin, K.; Huber, M.; Dötsch, C.; Christiansen, G.; Billeter, M.A. Rescue of measles viruses from cloned DNA. EMBO J. 1995, 14, 5773–5784. [Google Scholar] [CrossRef]
- Singh, M.; Cattaneo, R.; Billeter, M.A. A recombinant measles virus expressing hepatitis B surface antigen induces humoral responses in genetically modified mice. J. Virol. 1999, 73, 4823–4828. [Google Scholar] [CrossRef]
- Lyles, D.S.; Rupprecht, C.E. Rhabdoviridiae. In Fields’ Virology, 5th ed.; Knipe, D.M., Howley, P.M., Eds.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 1364–1408. [Google Scholar]
- Osakada, F.; Callaway, E.M. Design and generation of recombinant rabies virus vectors. Nat. Protoc. 2013, 8, 1583–1601. [Google Scholar] [CrossRef]
- An, H.; Kim, G.N.; Kang, C.Y. Genetically modified VSV(NJ) vector is capable of accommodating a large foreign gene insert and allows high level gene expression. Virus Res. 2013, 171, 168–177. [Google Scholar] [CrossRef]
- Ohara, S.; Inoue, K.; Yamada, M.; Yamawaki, T.; Koganezawa, N.; Tsutsui, K.; Witter, M.P.; Iijima, T. Dual transneural tracing in the rat entorhoinal-hippocampal circuit by intracerebral injection of recombinant rabies virus vectors. Front. Neuroanat. 2009, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Racine, T.; Kobinger, G.P.; Arts, E.J. Development of an HIV vaccine using vesicular stomatitis virus vector expressing designer HIV-1 envelope glycoproteins to enhance humoral responses. AIDS Res. Ther. 2017, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Takayama-Ito, M.; Yamada, K.; Hosokawa, J.; Sugiyama, M.; Minamoto, N. Improved recovery of rabies virus from cloned cDNA using a vaccinia virus-free reverse genetics system. Microbiol. Immunol. 2003, 47, 613–617. [Google Scholar] [CrossRef]
- Harty, R.N.; Brown, M.E.; Hayes, F.P.; Wright, N.T.; Schnell, M.J. Vaccinia virus-free recovery of vesicular stomatitis virus. J. Mol. Microbiol. Biotechnol. 2001, 3, 513–517. [Google Scholar] [PubMed]
- Liljestrom, P.; Garoff, H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology 1991, 9, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Levis, R.; Shen, P.; Schlesinger, S.; Rice, C.M.; Huang, H.V. Sindbis virus: An efficient, broad host range vector for gene expression in animal cells. Science 1989, 243, 1188–1191. [Google Scholar] [CrossRef]
- Davis, N.L.; Willis, L.V.; Smith, J.F.; Johnston, R.E. In vitro synthesis of infectious Venezuelan equine encephalitis virus RNA from a cDNA clone: Analysis of a viable deletion mutant. Virology 1989, 171, 189–204. [Google Scholar] [CrossRef]
- Lundstrom, K. Application of Viral Vectors for Vaccine Development with a Special Emphasis on COVID-19. Viruses 2020, 12, 1324. [Google Scholar] [CrossRef]
- Safronetz, D.; Mire, C.; Rosenke, K.; Feldmann, F.; Haddock, E.; Geissbert, T.; Feldmann, H. A recombinant vesicular stomatitis virus-based Lassa fever vaccine protects guinea pigs and macaques against challenge with geographically and genetically distinct Lassa viruses. PLoS Negl. Trop. Dis. 2015, 9, e0003736. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A.; Aquilar, P.V.; Bopp, N.E.; Yarovinsky, T.O.; Weaver, S.C.; Rose, J.K. A recombinant virus vaccine that protects both against Chikungunya and Zika virus infections. Vaccine 2018, 36, 3894–3900. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, N.J.; Geisbert, T.W.; Geisbert, J.B.; Shedlock, D.J.; Xu, L.; Lamoreaux, L.; Custers, J.H.H.V.; Popernack, P.M.; Yang, Z.-Y.; Pau, M.G.; et al. Immune protection of nonhuman primates against Ebola virus with single low-dose adenovirus vectors encoding modified GPs. PLoS Med. 2006, 3, e177. [Google Scholar] [CrossRef] [PubMed]
- Henao-Restrepo, A.M.; Longini, I.M.; Egger, M.; Dean, N.E.; Edmunds, W.J.; Camacho, A.; Carroll, M.W.; Doumbia, M.; Draguez, B.; Duraffour, S. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: Interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 2015, 386, 857–866. [Google Scholar] [CrossRef]
- Ollmann Saphire, E. A vaccine against Ebola virus. Cell 2020, 181, 6. [Google Scholar] [CrossRef]
- King, R.G.; Silva-Sanchez, A.; Peel, J.N.; Botta, D.; Meza Perez, S.; Allie, S.R.; Schultz, M.D.; Liu, M.; Bradley, J.E.; Qiu, S.; et al. Single-dose intranasal administration of AdCOVID elicits systemic and mucosal immunity against SARS-CoV-2 in mice. bioRxiv 2020. [Google Scholar] [CrossRef]
- Tostanoski, L.H.; Wegmann, F.; Martinot, A.J.; Loos, C.; McMahan, K.; Mercado, N.B.; Yu, J.; Chan, C.N.; Bondoc, S.; Starke, C.E.; et al. Ad26 vaccine protects against SARS-CoV-2 severe clinical disease in hamsters. Nat. Med. 2020, 26, 1694–1700. [Google Scholar] [CrossRef]
- Mercado, N.N.B.; Zahn, R.; Wegmann, F.; Loos, C.; Chandrashekar, A.; Yu, J.; Liu, J.; Peter, L.; McMahan, K.; Tostanoski, H.; et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 2020, 586, 583–588. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatullin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Lambe, T.; Spencer, A.; Belij-Rammerstorfer, S.; Purushotham, J.N.; Port, J.R.; Avanzato, V.A.; Bushmaker, T.; Flaxman, A.; Ulaszewska, M.; et al. ChAdOx1 nCov-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 2020, 586, 578–582. [Google Scholar] [CrossRef]
- Hassan, A.O.; Kafai, N.M.; Dmitriev, I.P.; Fox, J.M.; Smith, B.K.; Harvey, I.B.; Chen, R.E.; Winkler, E.S.; Wessel, A.W.; Case, J.B.; et al. A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2. Cell 2020, 183, 169–184. [Google Scholar] [CrossRef]
- Ku, M.-W.; Bourgine, M.; Authie, P.; Lopez, J.; Nemirov, K.; Moncoq, F.; Noirat, A.; Vesin, B.; Nevo, F.; Blanc, C.; et al. Intranasal vaccination with a lentiviral vector protects against SARS-CoV-2 in preclinical animal models. Cell Host Microbe 2021, 29, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chiuppesi, F.; Salazar, M.D.; Contreras, H.; Nguyen, V.H.; Martinez, J.; Park, Y.; Nguyen, J.; Kha, M.; Iniguez, A.; Zhou, Q.; et al. Development of a multi-antigenic SARS-CoV-2 vaccine candidate using a synthetic poxvirus platform. Nat. Commun. 2020, 11, 6021. [Google Scholar] [CrossRef] [PubMed]
- García-Arriaza, J.; Garaigorta, U.; Pérez, P.; Lázaro-Frías, A.; Zamora, C.; Gastaminza, P.; Del Fresno, C.; Casasnovas, J.M.; Sorzano, C.Ó.S.; Sancho, D.; et al. COVID-19 vaccine candidates based on modified vaccinia Ankara expressing the SARS-CoV-2 spike induce robust T- and B-cell immune responses and fully efficacy in mice. J. Virol. 2021. [Google Scholar] [CrossRef]
- Sun, W.; McCroskery, S.; Liu, W.C.; Leist, S.R.; Liu, Y.; Albrecht, R.A.; Slamanig, S.; Oliva, J.; Amanat, F.; Schäfer, A.; et al. A Newcastle Disease Virus (NDV) Expressing a Membrane-Anchored Spike as a Cost-Effective Inactivated SARS-CoV-2 Vaccine. Vaccines 2020, 8, 771. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Leist, S.R.; McCroskery, S.; Liu, Y.; Slamanig, S.; Oliva, J.; Amanat, F.; Schäfer, A.; Dinnon, K.H., 3rd; García-Sastre, A.; et al. Newcastle disease virus (NDV) expressing the spike protein of SARS-CoV-2 as a live virus vaccine candidate. EBioMedicine 2020, 62, 103132. [Google Scholar] [CrossRef]
- Hörner, C.; Schürmann, C.; Auste, A.; Ebenig, A.; Muraleedharan, S.; Dinnon, K.H., 3rd; Scholz, T.; Herrmann, M.; Schnierle, B.S.; Baric, R.S.; et al. A highly immunogenic and effective measles virus-based Th1-biased COVID-19 Vaccine. Proc. Natl. Acad. Sci. USA 2020, 117, 32657–32666. [Google Scholar] [CrossRef] [PubMed]
- Brett, J.B.; Rothlauf, P.W.; Chen, R.E.; Kafai, N.M.; Fox, J.M.; Smith, B.K.; Shrihari, S.; McCune, B.T.; Harvey, I.B.; Keeler, S.P.; et al. Replication-Competent Vesicular Stomatitis Virus Vaccine Vector Protects against SARS-CoV-2-Mediated Pathogenesis in Mice. Cell Host Microbe 2020, 28, 465–474. [Google Scholar]
- Yahalom-Ronen, Y.; Tamir, H.; Melamed, S.; Politi, B.; Shifman, O.; Achdout, H.; Vitner, E.B.; Israeli, O.; Milrot, E.; Stein, D.; et al. A single dose of recombinant VSV-∆G-spike provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020, 11, 6402. [Google Scholar] [CrossRef]
- McKay, P.F.; Hu, K.; Blakney, A.K.; Samnuan, K.; Brown, J.C.; Penn, R.; Zhou, J.; Bouton, C.R.; Rogers, P.; Polra, K.; et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibodies in mice. Nat. Commun. 2020, 11, 3523. [Google Scholar] [CrossRef]
- VBI Announces Progress of Coronavirus Vaccine Program. Available online: www.vbivaccines.com/wire/coronavirus-vaccine-program-update/ (accessed on 9 February 2021).
- Loes, A.N.; Gentles, L.E.; Greaney, A.J.; Crawford, K.H.D.; Bloom, J.D. Attenuated Influenza Virions Expressing the SARS-CoV-2 Receptor-Binding Domain Induce Neutralizing Antibodies in Mice. Viruses 2020, 12, 987. [Google Scholar] [CrossRef] [PubMed]
- Draft Landscape of COVID-19 Vaccines. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 14 January 2021).
- Förster, R.; Fleige, H.; Sutter, G. Combating COVID-19: MVA vector vaccines applied to the respiratory tract as promising toward protective immunity in the lung. Front. Immunol. 2020, 11, 1959. [Google Scholar] [CrossRef] [PubMed]
- Kirchmeier, M.; Fluckiger, A.-C.; Soare, C.; Bozic, J.; Ontsouka, B.; Ahmed, T.; Diress, A.; Pereira, L.; Schödel, F.; Plotkin, S.; et al. Enveloped virus-like particle expression of human cytomegalovirus glycoprotein B antigen induces antibodies with potent and broad neutralizing activity. Clin. Vaccine Immunol. 2014, 21, 174–180. [Google Scholar] [CrossRef]
- Zhu, F.C.; Li, Y.-H.; Guan, X.-H.; Hou, L.H.; Wang, W.J.; Li, J.X.; Wu, S.P.; Wang, B.S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Zhu, F.C.; Guan, X.H.; Li, Y.H.; Huang, J.Y.; Jiang, T.; Hou, L.H.; Li, J.X.; Yang, B.F.; Wang, L.; Wang, W.J. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years and older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Phase III Trial of A COVID-19 Vaccine of Adenovirus Vector in Adults 18 Years Old. Available online: https://clinicaltrials.gov/ct2/show/NCT04526990 (accessed on 22 January 2021).
- Clinical Trial of Recombinant Novel Coronavirus Vaccine (Adenovirus Type 5 Vector) Against COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04540419 (accessed on 22 January 2021).
- A Study of Ad26.COV2.S in Adults (COVID-19). Available online: https://clinicaltrials.gov/ct2/show/NCT04436276 (accessed on 22 January 2021).
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1-2a Trial of Ad26.COV.S Covid-19 Vaccine. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- A Study of Ad26.COV2.S for the Prevention of SARS-CoV-2-Mediated COVID-19 in Adult Participants (ENSEMBLE). Available online: https://clinicaltrials.gov/ct2/show/NCT04505722 (accessed on 22 January 2021).
- A study of the Ad26.COV2.S Vaccine Candidate for the Prevention of SARS-CoV-2-Mediated COVID-19 in Adults. Available online: https://doi.org/10.1186/ISRCTN14722499 (accessed on 22 January 2021).
- Clinical Trial of Efficacy, Safety, and Immunogenicity of Gam-COVID-Vac Vaccine against COVID-19 (RESIST). Available online: https://clinicaltrials.gov/ct2/show/NCT04530396 (accessed on 22 January 2021).
- Clinical Trial of Efficacy, Safety, and Immunogenicity of Gam-COVID-Vac Vaccine against COVID-19 in Belarus. Available online: https://clinicaltrials.gov/ct2/show/NCT04564716 (accessed on 22 January 2021).
- Clinical Trial of the Immunogenicity, Safety, and Efficacy of the Gam-COVID-Vac Vaccine against COVID-19 in Venezuela. Available online: https://clinicaltrials.gov/ct2//show/NCT04642339 (accessed on 22 January 2021).
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhavatulin, A.Y.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19: An interim analysis of a randomised controlled phase 3 in Russia. Lancet 2020. [Google Scholar] [CrossRef]
- Callaway, E. Russia’s fast-track coronavirus vaccine draws outrage over safety. Nature 2020, 584, 334–335. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAsOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2 single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- A Phase 2/3 Study to Determine the Efficacy, Safety and Immunogenicity of the Candidate Coronavirus Disease (COVID-19) Vaccine ChAdOx1 nCoV-19 in Healthy UK Volunteers. Available online: https://clinicaltrials.gov/ct2/show/NCT04400838 (accessed on 22 January 2021).
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Phase III Double-Blind, Placebo-Controlled Study of AZD1222 for the Prevention of COVID-19 in Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT04516746 (accessed on 22 January 2021).
- AZD1222 Vaccine for the Prevention of COVID-19. Available online: https://clinicaltriasl.gov/ct/show/NCT04540393 (accessed on 22 January 2021).
- A Phase III Study to Investigate a Vaccine against COVID-19. Available online: https://doi.org/10.11186/ISRCTN89951424 (accessed on 22 January 2021).
- A Phase 2/3 Observer-Blind, Randomized, Controlled Study to Determine the Safety and Immunogenicity of Covshield (COVID-19) in Healthy Indian Adults. Available online: http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=46186&EncHid=&userName=covid-19%20vaccine (accessed on 22 January 2021).
- Regulatory Approval of COVID-19 Vaccine AstraZeneca—GOV.UK. Available online: www.gov.uk (accessed on 25 January 2021).
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Qasim, E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Safety, Tolerability and Immunogenicity of the Candidate Vaccine MVA-SARS-2-S against COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04569383 (accessed on 22 January 2021).
- A Synthetic MVA-based SARS-CoV-2 Vaccine, COH04S1, for the Prevention of COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04639466 (accessed on 25 January 2021).
- Immunity and Safety of Covid-19 Synthetic Minigene Vaccine. Available online: https://clinicaltrials.gov/ct2/show/NCT04276896 (accessed on 22 January 2021).
- Merck Discontinues Development of SARS-CoV-2/COVID-19 Vaccine Candidates; Continues Development of Two Investigational Therapeutic Candidates. Available online: www.merck.com/news/merck-discontinues-development-of-sars-cov-2-covid-19-vaccine-candidates-continues-development-of-two-investigational-therapeutic-candidates/ (accessed on 9 February 2021).
- Merck and IAVI Discontinue Development of COVID-19 Vaccine Candidate V590. Available online: www.iavi.org/news-resources/press-releases/2021/merck-and-iavi-discontinue-development-of-covid-19-vaccine-candidate-v590 (accessed on 9 February 2021).
- Evaluate the Safety, Immunogenicity and Potential Efficacy of an rVSV-SARS-CoV-2-S Vaccine. Available online: https://clinicaltrials.gov/ct2/show/NCT04608305 (accessed on 25 January 2021).
- A Phase I Clinical Trial of Influenza virus Vector COVID-19 Vaccine for intranasal Spray (DelNS1-2019-nCoV-RBD-OPT1). ChiCTR2000037782. Available online: www.chictr.org.cn/showprojen.aspx?proj=55421 (accessed on 25 January 2021).
- A Phase II Clinical Trial of Influenza virus Vector COVID-19 Vaccine for intranasal Spray (DelNS1-2019-nCoV-RBD-OPT1). ChiCTR2000039715. Available online: www.chictr.org.cn/showprojen.aspx?proj=63754 (accessed on 25 January 2021).
- Clinical Trial to Evaluate the Safety and Immunogenicity of the COVID-19 Vaccine (COVID-19-101). Available online: https://clinicaltrials.gov/ct2/show/NCT04497298 (accessed on 22 January 2021).
- Dose Ranging Trial to Assess Safety and Immunogenicity of V590 (COVID-19 Vaccine) in Healthy Adults (V590-001). Available online: https://clinicaltrials.gov/ct2/show/NCT04569786 (accessed on 22 January 2021).
- Higgins, T.S.; Wu, A.W.; Illing, E.A.; Sokoloski, K.J.; Weaver, B.A.; Anthony, B.P.; Hughes, N.; Ting, J.Y. Intranasal Antiviral Drug Delivery and Coronavirus Disease 2019 (COVID-19): A State of the Art Review. Otolaryngol. Head Neck Surg. 2020, 163, 682–694. [Google Scholar] [CrossRef]
- Phillips, N.; Cyranoski, D.; Mallapaty, S. A leading coronavirus vaccine trial is on hold: Scientists react. Nature 2020. [Google Scholar] [CrossRef]
- Global Clinical Trials of COVID-19 Vaccine Resume. Available online: https://www.ox.ac.uk/news/2020-11-23-global-clinical-trials-covid-19-vaccine-resume (accessed on 25 January 2021).
- Al Idrus, A. Russia Posts 91.4 % Efficacy for COVID-19 Vaccine after Jumping the Gun to Approval. Available online: https://www.fiercebiotech.com/biotech/russia-posts-91-efficacy-for-covid-19-vaccine-after-jumping-gun-to-approval (accessed on 25 January 2021).
- Balfour, H. AstraZeneca to test combination of AZD1222 and Sputnik V vaccines. Eur. Pharmaceutical Rev. 2020. [Google Scholar]
- Spencer, A.J.; McKay, P.F.; Belij-Rammerstorfer, S.; Ulaszewska, M.; Bissett, C.D.; Hu, K.; Samnuan, K.; Wright, D.; Sharpe, H.R.; Gilbride, C.; et al. Heterologous vaccination regimens with self-amplifying RNA and Adenoviral 2 COVID vaccines induce superior immune responses than single dose vaccine 3 regimens in mice. bioRxiv 2021. [Google Scholar] [CrossRef]
- Ledford, H. Could mixing COVID vaccines boost immune response? Nature 2021. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trial to Assess the Safety of a Coronavirus Vaccine in Healthy Men and Women. Available online: https://doi.org/10.1186/ISRCTN17072692 (accessed on 25 January 2021).
- Leung, K.; Shum, M.H.; Leung, G.M.; Lam, T.T.; Wu, J.T. Early transmissibility assessment of the N501Y mutant strains of SARS-CoV-2 in the United Kingdom, October to November 2020. Euro Surveill. 2021, 26, 2002106. [Google Scholar] [CrossRef]
- Galloway, S.E.; Paul, P.; MacCannell, D.R.; Johansson, M.A.; Brooks, J.T.; MacNeil, A.; Slayton, R.B.; Tong, S.; Silk, B.J.; Armstrong, G.L.; et al. Emergence of SARS-CoV-2 B.1.1.7 Lineage—United States, 29 December 2020–12 January 2021. MMWR Morb. Mortal Wkly Rep. 2021, 70, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Schmidt, F.; Weisblum, Y.; Muecksch, F.; Barnes, C.O.; Finkin, S.; Schaefer-Babajew, D.; Cipolla, M.; Gaebler, C.; Lieberman, J.A.; et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. bioRxiv 2021. [Google Scholar] [CrossRef]
- Emary, K.R.W.; Golubchik, T.; Lay, P.K.; Ariani, C.V.; Angus, B.; Bibi, S.; Blane, B.; Bonsall, D.; Cicconi, P.; Charlton, S.; et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 VOC 202012/01 (B.1.1.7). Soc. Sci. Electron. Publ. 2021. [Google Scholar] [CrossRef]
- Mahase, E. Covid-19: Novavax vaccine efficacy is 86% against UK variant and 60% against South African variant. Br. Med. J. 2021, 372. [Google Scholar] [CrossRef]
- Xie, X.; Zou, J.; Fontes-Garfias, C.R.; Xia, H.; Swanson, K.A.; Cutler, M.; Cooper, D.; Menachery, V.D.; Scott Weaver, S.; Dormitzer, P.R.; et al. Neutralization of N501Y mutant SARS-CoV-2 by BNT162b vaccine-elicited sera. bioRxiv 2021. [Google Scholar] [CrossRef]
| Vector | Construct | Response | Ref |
|---|---|---|---|
| Adenovirus | |||
| Ad5 | Ad5-S-nb2 | Protection against SARS-CoV-2 in macaques | [7] |
| Ad5 | Ad5-S-RBD | Systemic & mucosal response in mice (i.n. administration) | [39] |
| Ad26 | Ad26.COV.S | Protection against SARS-CoV-2 pneumonia in hamsters | [40] |
| Ad26 | Ad26.COV.S | Protection against SARS-CoV-2 in macaques | [41] |
| Ad26/Ad5 | rAd26-S/Ad5-S | Good preclinical safety and immunogenicity profiles | [42] |
| ChAdOx1 | ChAdOx1 nCOV-19 | Protection against SARS-CoV-2 in macaques | [43] |
| ChAdOx1 | ChAd-SARS-CoV-2-S | Protection of mice after i.n. administration | [44] |
| Lentivirus | |||
| LV | LV-SARS-CoV-2-S | Protection in hamsters after i.n. administration | [45] |
| Influenza | |||
| IFV | ΔNA(RBD)-Flu | Robust antibody responses after i.n. administration | [47] |
| Poxvirus | |||
| MVA | MVA SARS-CoV-2-S/N | Humoral & cellular immune responses in mice | [46] |
| MVA | MVA-COV2-S | Protection against SARS-CoV-2 in mice | [47] |
| NDV | |||
| NDV | NDV SARS-CoV-2-S | Protection against SARS-CoV-2 in mice and hamsters | [48] |
| NDV | NDV-SARS-CoV-2-S | Protection against SARS-CoV-2 in mice | [49] |
| Measles virus | |||
| MV | MV-SARS-CoV-2-S | Neutralizing & T cell antibody responses in mice | [50] |
| Rhabdovirus | |||
| VSV | VSV-SARS-CoV-2-S | Protection against SARS-CoV-2 in mice | [51] |
| VSV | VSV-Δ | Protection against SARS-CoV-2 in hamsters | [52] |
| Alphavirus | |||
| VEE | VEE-SARS-CoV-2-S | High-level neutralizing antibodies in mice | [53] |
| VLPs | |||
| eVLPs | MLV Gag SARS-CoV-2 S | Immunogenicity and efficacy in hamsters | [54] |
| Vector | Phase | Response | Ref |
|---|---|---|---|
| Ad5-SARS-CoV-2-S | I | SARS-CoV-2-specific cellular and humoral responses | [59] |
| Ad5-SARS-CoV-2-S | II | Strong neutralizing SARS-CoV-2-specific antibodies | [60] |
| Ad5-SARS-CoV-2-S | III | Recruitment in progress | [61] |
| Ad5-SARS-CoV-2-S | III | Study in progress | [62] |
| Ad26.COV2 S | I/II | Good safety, strong immunogenicity | [63,64] |
| Ad26.COV2 S | III | Recruitment in progress | [65] |
| Ad26.COV2 S | III | Recruitment in progress | [66] |
| rAd26-S/rAd5-S | I/II | Good safety, strong immune responses | [42] |
| rAd26-S/rAd5-S | III | Recruitment in progress | [67] |
| rAd26-S/rAd5-S | III | Study in progress | [68] |
| rAd26-S/rAd5-S | III | Study planned | [69] |
| rAd26-S/rAd5-S | III | 91.6% vaccine efficacy from interim results | [70] |
| ChAdOx1 nCOV-19 | I/II | Humoral and cellular immune responses | [72] |
| ChAdOx1 nCOV-19 | II/III | Similar nAb responses in all age groups | [74] |
| ChAdOx1 nCoV-19 | III | Recruitment in progress | [75] |
| ChAdOx1 nCoV-19 | III | Recruitment in progress | [76] |
| ChAdOx1 nCoV-19 | III | Study in progress | [77] |
| ChAdOx1 nCoV-19 | III | Study in progress | [78] |
| ChAdOx1 nCoV-19 | III | Interim results: 62.1–90.0% efficacy in 4 trials | [80] |
| MVA-SARS-COV-2 | I | Recruitment in progress | [81] |
| MVA-SARS-COV-2 | I | Recruitment in progress | [82] |
| LV-DC + CTL Ag | I | Recruitment in progress | [83] |
| MV-SASR-CoV-2-S | I | Trial discontinued | [84] |
| VSV | I | Trial discontinued | [85] |
| VSV | I/II | Recruitment in progress | [86] |
| IFV-CoV-2 S RBD | I | Registered trial | [87] |
| IFV-CoV-2 S RBD | II | Registered trial | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lundstrom, K. Viral Vectors for COVID-19 Vaccine Development. Viruses 2021, 13, 317. https://doi.org/10.3390/v13020317
Lundstrom K. Viral Vectors for COVID-19 Vaccine Development. Viruses. 2021; 13(2):317. https://doi.org/10.3390/v13020317
Chicago/Turabian StyleLundstrom, Kenneth. 2021. "Viral Vectors for COVID-19 Vaccine Development" Viruses 13, no. 2: 317. https://doi.org/10.3390/v13020317
APA StyleLundstrom, K. (2021). Viral Vectors for COVID-19 Vaccine Development. Viruses, 13(2), 317. https://doi.org/10.3390/v13020317





