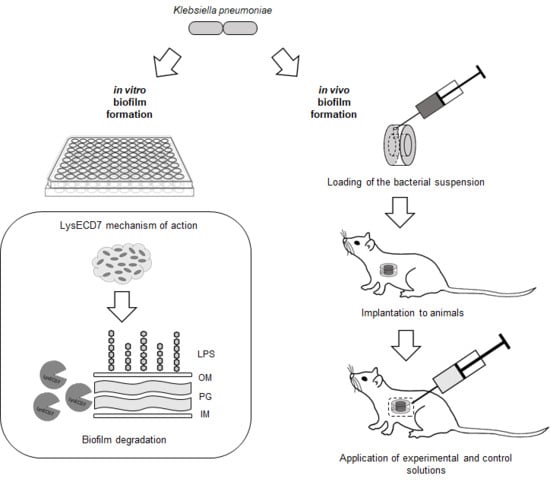
Antibiofilm Activity of a Broad-Range Recombinant Endolysin LysECD7: In Vitro and In Vivo Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain
2.2. Construct Cloning
2.3. Recombinant Expression and Purification of LysECD7-8his
2.4. Evaluation of the K. pneumoniae Antibiotic Susceptibility
2.5. In Vitro Antibiofilm Activity
2.6. In Vivo Model
2.6.1. Diffusion Chambers
2.6.2. Experimental Animals
2.6.3. Implantation and Explantation of Diffusion Chambers
2.6.4. Estimation of In Vivo Biofilm Formation
2.6.5. In Vivo Assessment of the LysECD7 on Preformed Biofilms
2.6.6. Biofilm Biomass Measurement
2.6.7. Assessment of Amount of Culturable Bacteria
2.6.8. Sterility Assessment
2.7. Statistical Analysis
3. Results and Discussion
3.1. LysECD7 Is Active against K. pneumoniae Biofilm In Vitro
3.2. In Vivo Biofilm Formation
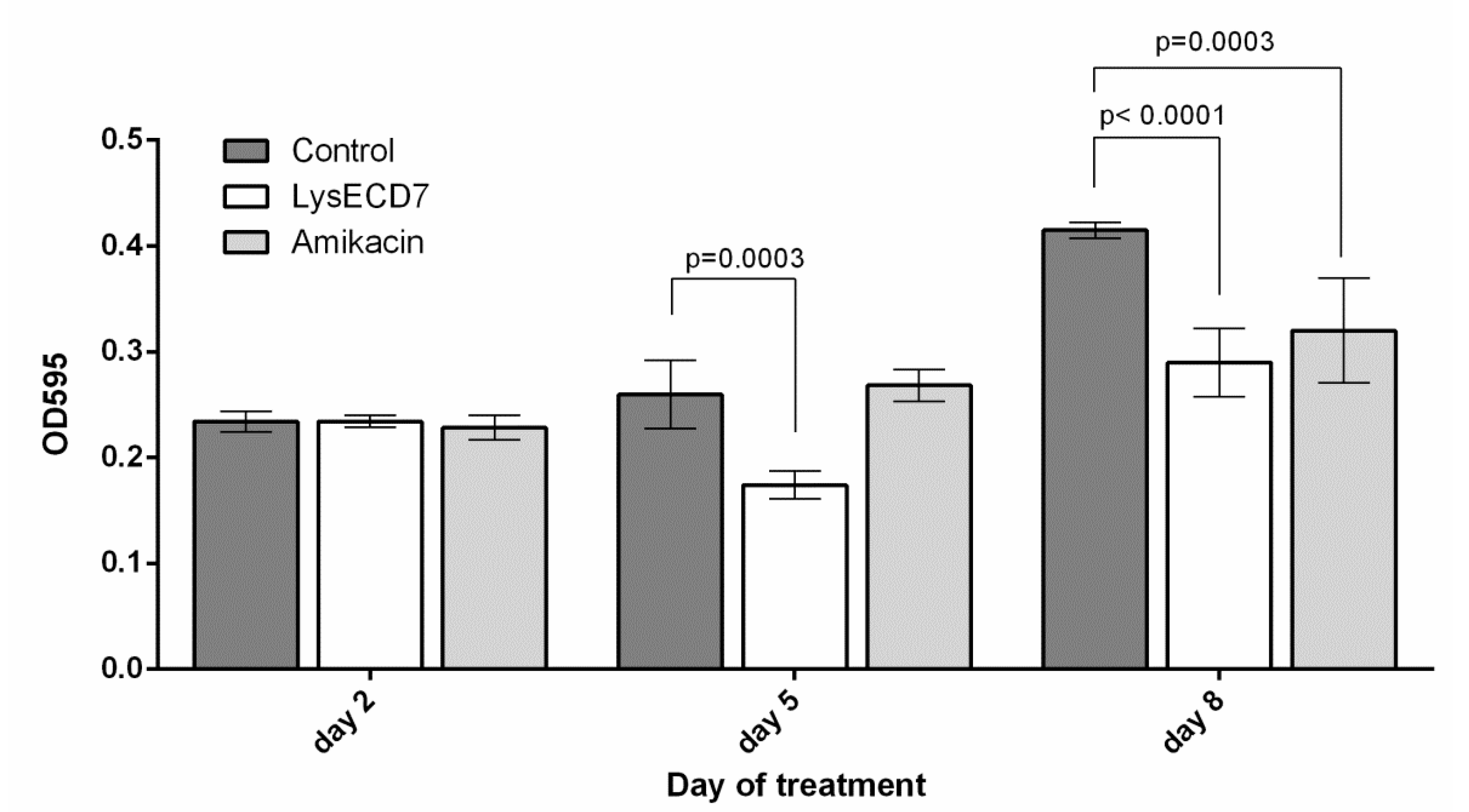
3.3. In Vivo Efficacy of the LysECD7
3.3.1. Intraoperative Clinical Picture
3.3.2. Biofilms Development Dynamics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stewart, P.S. Antimicrobial tolerance in biofilms. Microbiol. Spectr. 2015, 3, 269–285. [Google Scholar] [CrossRef]
- Lebeaux, D.; Ghigo, J.M.; Beloin, C. Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef]
- Malone, M.; Barjnsholt, T.; McBain, A.J.; James, G.A.; Stoodley, P.; Leaper, D.; Tachi, M.; Shultz, G.; Swanson, T.; Wolcott, R.D. The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. J. Wound Care 2017, 26, 202–205. [Google Scholar] [CrossRef]
- Darouiche, R.O. Treatment of infections associated with surgical implants. N Engl. J. Med. 2004, 350, 14221–14429. [Google Scholar] [CrossRef]
- Sohail, M.R.; Uslan, D.Z.; Khan, A.H.; Friedman, P.A.; Hayes, D.L.; Wilson, W.R.; Steckelberg, J.M.; Stoner, S.; Baddour, L.M. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J. Am. Coll. Cardiol. 2007, 49, 1851–1859. [Google Scholar] [CrossRef]
- National Institutes of Health. Research on Microbial Biofilms. Report No. PA-03-047 (National Institutes of Health, Bethesda, 2002). Available online: https://grants.nih.gov/grants/guide/pa-files/pa-03-047.html (accessed on 1 February 2020).
- Labrou, N. Therapeutic Enzymes: Function and Clinical Implication; Springer: Singapore, 2019. [Google Scholar]
- Briers, Y.; Walmagh, M.; Grymonprez, B.; Biebl, M.; Pirnay, J.P.; Defraine, V.; Michiels, J.; Cenens, W.; Aertsen, A.; Miller, S.; et al. Art-175 is a highly efficient antibacterial against multidrug-resistant strains and persisters of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 3774–3784. [Google Scholar] [CrossRef]
- Lai, M.J.; Lin, N.T.; Hu, A.; Soo, P.C.; Chen, L.K.; Chen, L.H.; Chang, K.C. Antibacterial activity of Acinetobacter baumannii phage ΦaB2 endolysin (LysAB2) against both Gram-positive and Gram-negative bacteria. Appl. Microbiol. Biotechnol. 2011, 90, 529–539. [Google Scholar] [CrossRef]
- Lai, M.J.; Soo, P.C.; Lin, N.T.; Hu, A.; Chen, Y.J.; Chen, L.K.; Chang, K.C. Identification and characterisation of the putative phage-related endolysins through full genome sequence analysis in Acinetobacter baumannii ATCC 17978. Int. J. Antimicrob. Agents. 2013, 42, 141–148. [Google Scholar] [CrossRef]
- Huang, G.; Shen, X.; Gong, Y.; Dong, Z.; Zhao, X.; Shen, W.; Wang, J.; Hu, F.; Peng, Y. Antibacterial properties of Acinetobacter baumannii phage Abp1 endolysin (PlyAB1). BMC Infect. Dis. 2014, 14, 681. [Google Scholar]
- Briers, Y.; Volckaert, G.; Cornelissen, A.; Lagaert, S.; Michiels, C.W.; Hertveldt, K.; Lavigne, R. Muralytic activity and modular structure of the endolysins of Pseudomonas aeruginosa bacteriophages φKZ and EL. Mol. Microbiol. 2007, 65, 1334–1344. [Google Scholar] [CrossRef]
- Lood, R.; Winer, B.Y.; Pelzek, A.J.; Diez-Martinez, R.; Thandar, M.; Euler, C.W.; Schuch, R.; Fischetti, V.A. Novel phage Lysin capable of killing the multidrug-resistant gram-negative bacterium Acinetobacter baumannii in a mouse bacteremia model. Antimicrob. Agents Chemother. 2015, 59, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Boas, D.V.; Mesnage, S.; Kluskens, L.D.; Lavigne, R.; Sillankorva, S.; Secundo, F.; Azeredo, J. Structural and enzymatic characterization of ABgp46, a novel phage endolysin with broad anti-gram-negative bacterial activity. Front. Microbiol. 2016, 7, 208. [Google Scholar] [CrossRef] [PubMed]
- Thummeepak, R.; Kitti, T.; Kunthalert, D.; Sitthisak, S. Enhanced antibacterial activity of Acinetobacter baumannii bacteriophage ØABP-01 endolysin (LysABP-01) in combination with colistin. Front. Microbiol. 2016, 7, 1402. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, R.; García, E.; García, P. Phage lysins for fighting bacterial respiratory infections: A new generation of antimicrobials. Front. Immunol. 2018, 9, 2252. [Google Scholar] [CrossRef] [PubMed]
- Raz, A.; Serrano, A.; Hernandez, A.; Euler, C.W.; Fischetti, V.A. Isolation of phage lysins that effectively kill Pseudomonas aeruginosa in mouse models of lung and skin infection. Antimicrob. Agents Chemother. 2019, 63, e000241–e000249. [Google Scholar] [CrossRef]
- Dams, D.; Briers, Y. Enzybiotics: Enzyme-based antibacterials as therapeutics. Adv. Exp. Med. Biol. 2019, 1148, 233–253. [Google Scholar]
- Antonova, N.P.; Vasina, D.V.; Lendel, A.M.; Usachev, E.V.; Makarov, V.V.; Gintsburg, A.L.; Tkachuk, A.P.; Gushchin, V.A. Broad bactericidal activity of the myoviridae bacteriophage lysins LysAm24, LysECD7, LysSi3 against Gram-negative ESKAPE pathogens. Viruses 2019, 11, 284. [Google Scholar] [CrossRef]
- Antonova, N.P.; Vasina, D.V.; Rubalsky, E.O.; Fursov, M.V.; Savinova, A.S.; Grigoriev, I.V.; Usachev, E.V.; Shevlyagina, N.V.; Zhukhovitsky, V.G.; Balabanyan, V.U.; et al. Modulation of endolysin LysECD7 bactericidal activity by different peptide tag fusion. Biomolecules 2020, 10, 440. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Cirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. Apmis. 2007, 115, 8918–8999. [Google Scholar] [CrossRef]
- Arbuthnott, J.P.; Arbuthnott, E.R.; Arbuthnott, A.D.; Pike, W.J.; Cockayne, A. Investigation of microbial growth in vivo: Evaluation of a novel in vivo chamber implant system. Fems. Microbiol. Lett. 1992, 100, 75–79. [Google Scholar] [CrossRef]
- Pike, W.J.; Cockayne, A.; Webster, C.A.; Slack, R.C.; Shelton, A.P.; Arbuthnott, J.P. Development and design of a novel in vivo chamber implant for the analysis of microbial virulence and assessment of antimicrobial therapy. Microb. Pathog. 1991, 10, 443–450. [Google Scholar] [CrossRef]
- Ruemke, S.; Rubalskii, E.; Mashaqi, B.; Burgwitz, K.; Haverich, A.; Salmoukas, C.; Kuehn, C. Evaluation of Gram-positive and Gram-negative bacterial adherence on four different vascular prosthetic grafts in vitro. Austin J. Surg. 2019, 6, 1200. [Google Scholar]
- Vuotto, C.; Longo, F.; Pascolini, C.; Donelli, G. Balice.; M.P. Libori.; M.F. Tiracchia.; V. Salvia.; A. Varaldo.; P.E. Biofilm formation and antibiotic resistance in Klebsiella pneumoniae urinary strains. J. Appl. Microbiol. 2017, 123, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.; Thomson, A.H.; Brown, N.M.; Semple, Y.; Sluman, C.; MacGowan, A.; Lovering, A.M.; Wiffen, P.J. Amikacin use and therapeutic drug monitoring in adults: Do dose regimens and drug exposures affect either outcome or adverse events? A systematic review. J. Antimicrob. Chemother. 2016, 71, 2754–2759. [Google Scholar] [CrossRef]
- Larpin, Y.; Oechslin, F.; Moreillon, P.; Resch, G.; Entenza, J.M.; Mancini, S. In vitro characterization of PlyE146, a novel phage lysin that targets Gram-negative bacteria. PLoS ONE 2018, 13, e0192507. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Schmelcher, M.; Shen, Y.; Nelson, D.C.; Eugster, M.R.; Eichenseher, F.; Hanke, D.C.; Loessner, M.J.; Dong, S.; Pritchard, D.G.; Lee, J.C.; et al. Evolutionarily distinct bacteriophage endolysins featuring conserved peptidoglycan cleavage sites protect mice from MRSA infection. J. Antimicrob. Chemother. 2015, 70, 1453–1465. [Google Scholar] [CrossRef]
- Kiedrowski, M.R.; Horswill, A.R. New approaches for treating staphylococcal biofilm infections. Ann. N. Y. Acad. Sci. 2011, 1241, 104–121. [Google Scholar] [CrossRef]
- Łusiak-Szelachowska, M.; Weber-Dąbrowska, B.; Górski, A. Bacteriophages and lysins in biofilm control. Virol. Sin. 2020, 35, 125–133. [Google Scholar] [CrossRef]
- Guo, M.; Feng, C.; Ren, J.; Zhuang, X.; Zhang, Y.; Zhu, Y.; Dong, K.; He, P.; Guo, X.; Qin, J. A Novel antimicrobial endolysin, LysPA26, against Pseudomonas aeruginosa. Front. Microbiol. 2017, 8, 293. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Ruas-Madiedo, P.; Martínez, B.; Rodríguez, A.; García, P. Effective removal of staphylococcal biofilms by the endolysin LysH5. PLoS ONE 2014, 9, e107307. [Google Scholar]


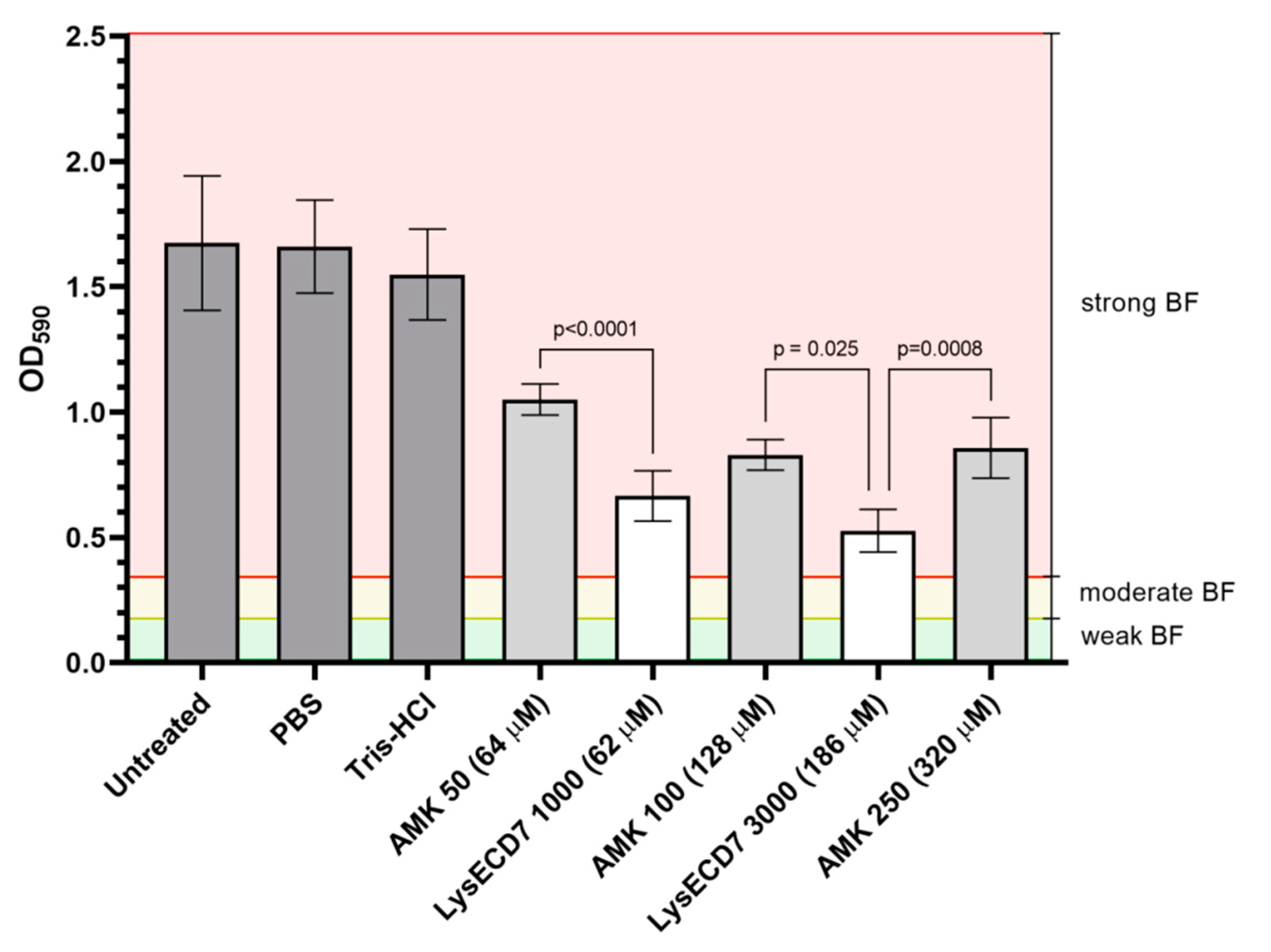
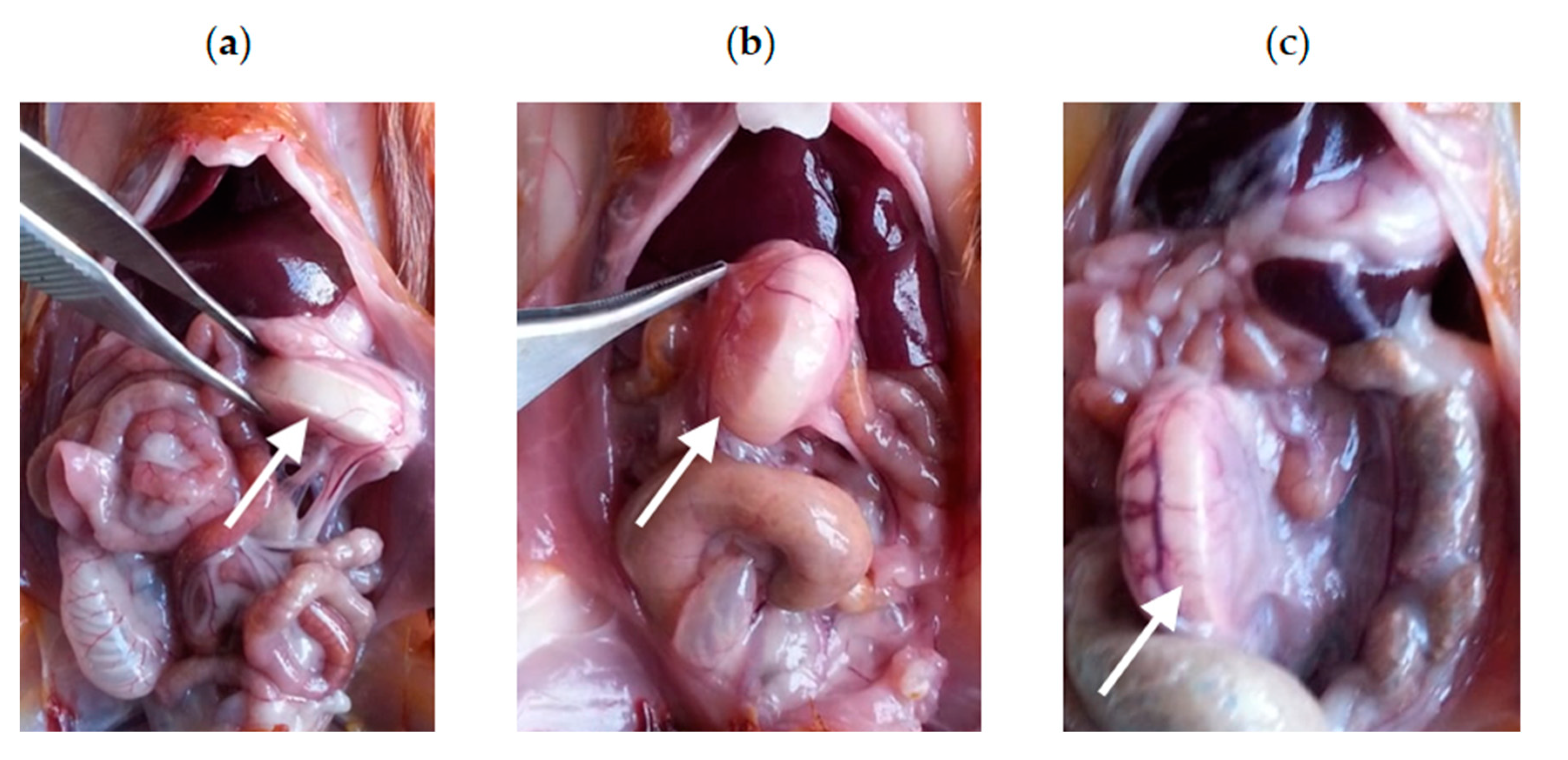
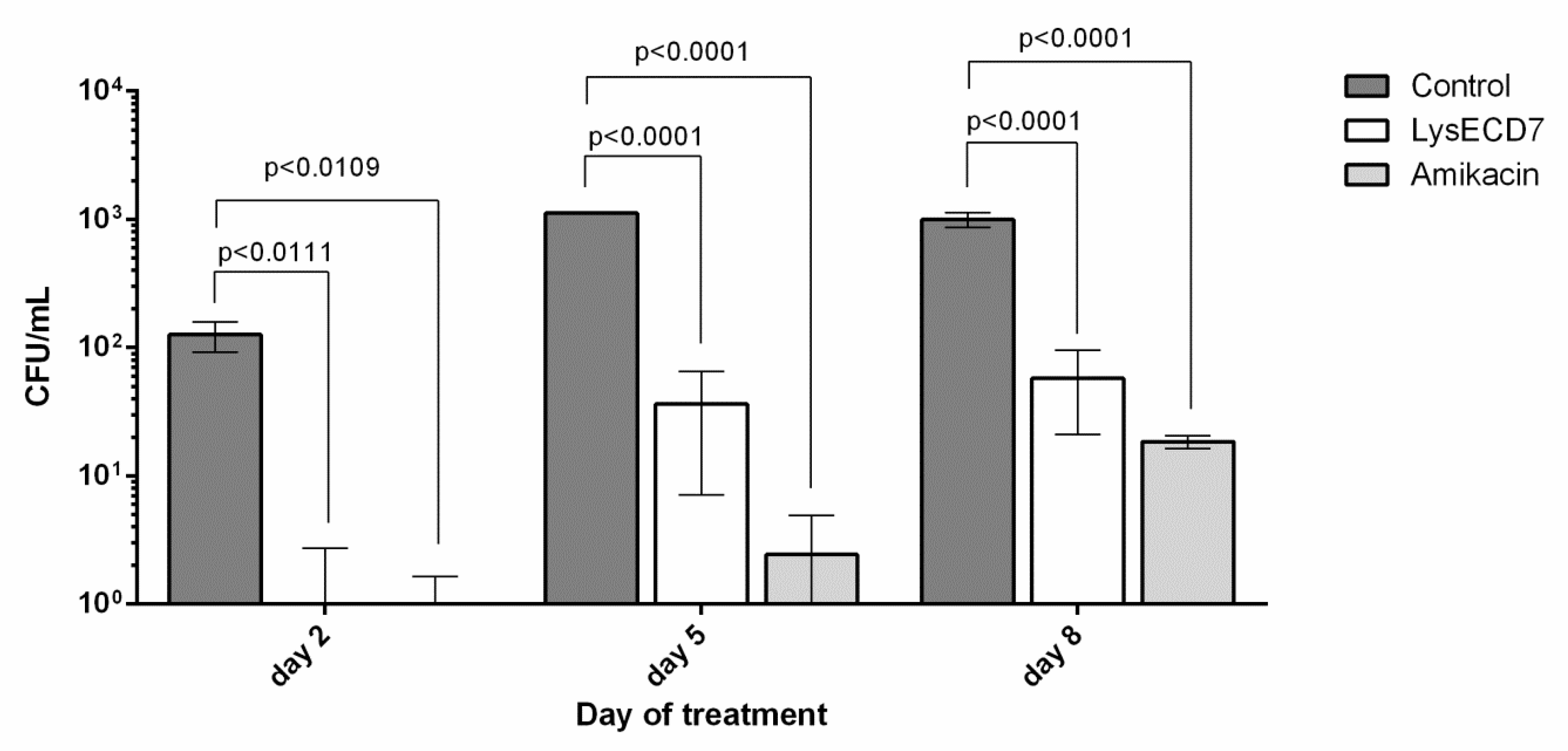
| Indicator | Before Implantation | Two Days After Implantation | Four Days After Implantation | Six Days After Implantation |
|---|---|---|---|---|
| General clinical condition | Not changed | Not changed | Not changed | The animal is inactive, with a lack of appetite |
| Postoperative wound condition | – | Primary intention healing | Primary intention healing | Primary healing, slight inflammation of the postoperative wound |
| Visual inflammation of surrounding tissues | – | Slight inflammatory infiltration of tissue surrounding the chamber | Absent | Inflammatory infiltration of tissues surrounding the chamber, peri implant zone exudate |
| Sterility of the surrounding tissues | – | Sterile | Sterile | Sterile |
| Sterility of the implant external surface | Sterile | Sterile | Sterile | Sterile |
| Visual characteristics of the frame inner surface | No visible BF | No visible BF | The presence of mucous BF | The presence of a dense, non-stretching BF |
| Visual characteristics of the membrane inner surface | No visible biofilm | No visible biofilm | No visible biofilm | The presence of a dense, non-stretching biofilm |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fursov, M.V.; Abdrakhmanova, R.O.; Antonova, N.P.; Vasina, D.V.; Kolchanova, A.D.; Bashkina, O.A.; Rubalsky, O.V.; Samotrueva, M.A.; Potapov, V.D.; Makarov, V.V.; et al. Antibiofilm Activity of a Broad-Range Recombinant Endolysin LysECD7: In Vitro and In Vivo Study. Viruses 2020, 12, 545. https://doi.org/10.3390/v12050545
Fursov MV, Abdrakhmanova RO, Antonova NP, Vasina DV, Kolchanova AD, Bashkina OA, Rubalsky OV, Samotrueva MA, Potapov VD, Makarov VV, et al. Antibiofilm Activity of a Broad-Range Recombinant Endolysin LysECD7: In Vitro and In Vivo Study. Viruses. 2020; 12(5):545. https://doi.org/10.3390/v12050545
Chicago/Turabian StyleFursov, Mikhail V., Radmila O. Abdrakhmanova, Nataliia P. Antonova, Daria V. Vasina, Anastasia D. Kolchanova, Olga A. Bashkina, Oleg V. Rubalsky, Marina A. Samotrueva, Vasiliy D. Potapov, Valentine V. Makarov, and et al. 2020. "Antibiofilm Activity of a Broad-Range Recombinant Endolysin LysECD7: In Vitro and In Vivo Study" Viruses 12, no. 5: 545. https://doi.org/10.3390/v12050545
APA StyleFursov, M. V., Abdrakhmanova, R. O., Antonova, N. P., Vasina, D. V., Kolchanova, A. D., Bashkina, O. A., Rubalsky, O. V., Samotrueva, M. A., Potapov, V. D., Makarov, V. V., Yudin, S. M., Gintsburg, A. L., Tkachuk, A. P., Gushchin, V. A., & Rubalskii, E. O. (2020). Antibiofilm Activity of a Broad-Range Recombinant Endolysin LysECD7: In Vitro and In Vivo Study. Viruses, 12(5), 545. https://doi.org/10.3390/v12050545