Rapid Resolution of Non-Effusive Feline Infectious Peritonitis Uveitis with an Oral Adenosine Nucleoside Analogue and Feline Interferon Omega
Abstract
1. Introduction
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Addie, D.D.; Toth, S.; Murray, G.D.; Jarrett, O. The risk of feline infectious peritonitis in cats naturally infected with feline coronavirus. Am. J. Vet. Res. 1995, 56, 429–434. [Google Scholar] [PubMed]
- Kipar, A.; May, H.; Menger, S.; Weber, M.; Leukert, W.; Reinacher, M. Morphologic features and development of granulomatous vasculitis in feline infectious peritonitis. Vet. Pathol. 2005, 42, 321–330. [Google Scholar] [CrossRef] [PubMed]
- De Groot, R.J.; Baker, S.C.; Baric, R.; Enjuanes, L.; Gorbalenya, A.E.; Holmes, K.V.; Perlman, S.; Poon, L.; Rottier, P.J.M.; Talbot, P.J.; et al. Family Coronaviridae. In Ninth Report International Committee on Taxonomy of Viruses; King AMQ; Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 806–828. [Google Scholar]
- Pedersen, N.C.; Perron, M.; Bannasch, M.; Montgomery, E.; Murakami, E.; Liepnieks, M.; Liu, H. Efficacy and safety of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis. J. Feline Med. Surg. 2019, 21, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Addie, D.D.; Curran, S.; Bellini, F.; Crowe, B.; Sheehan, E.; Ukrainchuk, L.; Decaro, N. Oral Mutian® X stopped faecal feline coronavirus shedding by naturally infected cats. Res. Vet. Sci. 2020, 130, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.W.; Egberink, H.F.; Halpin, R.; Spiro, D.J.; Rottier, P.J. Spike protein fusion peptide and feline coronavirus virulence. Emerg. Infect. Dis. 2012, 18, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Duthie, S.; Eckersall, P.D.; Addie, D.D.; Lawrence, C.E.; Jarrett, O. Value of alpha1-acid glycoprotein in the diagnosis of feline infectious peritonitis. Vet. Rec. 1997, 141, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Shelly, S.M.; Scarlett-Kranz, J.; Blue, J.T. Protein electrophoresis on effusions from cats as a diagnostic test for feline infectious peritonitis. J. Am. Anim. Hosp. Assoc. 1988, 24, 495–500. [Google Scholar]
- Pedersen, N.C.; Kim, Y.; Liu, H.; Galasiti, K.A.C.; Eckstrand, C.; Groutas, W.C.; Bannasch, M.; Meadows, J.M.; Chang, K.O. Efficacy of a 3C-like protease inhibitor in treating various forms of acquired feline infectious peritonitis. J. Feline Med. Surg. 2018, 20, 378–392. [Google Scholar] [CrossRef]
- Dunbar, D.; Kwok, W.; Graham, E.; Armitage, A.; Irvine, R.; Johnston, P.; McDonald, M.; Montgomery, D.; Nicolson, L.; Robertson, E.; et al. Diagnosis of non-effusive feline infectious peritonitis by reverse transcriptase quantitative polymerase chain reaction from mesenteric lymph node fine needle aspirates. J. Feline Med. Surg. 2019, 21, 910–921. [Google Scholar] [CrossRef]
- Felten, S.; Leutenegger, C.M.; Balzer, H.J.; Pantchev, N.; Matiasek, K.; Wess, G.; Egberink, H.; Hartmann, K. Sensitivity and specificity of a real-time reverse transcriptase polymerase chain reaction detecting feline coronavirus mutations in effusion and serum/plasma of cats to diagnose feline infectious peritonitis. BMC Vet. Res. 2017, 13, 228. [Google Scholar] [CrossRef]
- Fish, E.J.; Diniz, P.P.V.; Juan, Y.C.; Bossong, F.; Collisson, E.W.; Drechsler, Y.; Kaltenboeck, B. Cross-sectional quantitative RT-PCR study of feline coronavirus viremia and replication in peripheral blood of healthy shelter cats in Southern California. J. Feline Med. Surg. 2018, 20, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Simons, F.A.; Vennema, H.; Rofina, J.E.; Pol, J.M.; Horzinek, M.C.; Rottier, P.J.; Egberink, H.F. A mRNA PCR for the diagnosis of feline infectious peritonitis. J. Virol. Methods 2005, 124, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Herrewegh, A.A.P.M.; de Groot, R.J.; Cepica, A.; Egberink, H.F.; Horzinek, M.C.; Rottier, P.J.M. Detection of feline coronavirus RNA in feces, tissue, and body fluids of naturally infected cats by reverse transcriptase PCR. J. Clin. Microbiol. 1995, 33, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Tasker, S. Diagnosis of feline infectious peritonitis: Update on evidence supporting available tests. J. Feline Med. Surg. 2018, 20, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Porter, E.; Tasker, S.; Day, M.J.; Harley, R.; Kipar, A.; Siddell, S.G.; Helps, C.R. Amino acid changes in the spike protein of feline coronavirus correlate with systemic spread of virus from the intestine and not with feline infectious peritonitis. Vet. Res. 2014, 45, 49. [Google Scholar] [CrossRef]
- Giori, L.; Giordano, A.; Giudice, C.; Grieco, V.; Paltrinieri, S. Performances of different diagnostic tests for feline infectious peritonitis in challenging clinical cases. J. Small Anim. Pract. 2011, 52, 152–157. [Google Scholar] [CrossRef]
- Tsai, H.Y.; Chueh, L.L.; Lin, C.N.; Su, B.L. Clinicopathological findings and disease staging of feline infectious peritonitis: 51 cases from 2003 to 2009 in Taiwan. J. Feline Med. Surg. 2011, 13, 74–80. [Google Scholar] [CrossRef]
- Rohrer, C.; Suter, P.F.; Lutz, H. The diagnosis of feline infectious peritonitis (FIP): A retrospective and prospective study. Kleinterpraxis 1993, 38, 379–389. [Google Scholar]
- Riemer, F.; Kuehner, K.A.; Ritz, S.; Sauter-Louis, C.; Hartmann, K. Clinical and laboratory features of cats with feline infectious peritonitis–A retrospective study of 231 confirmed cases (2000–2010). J. Feline Med. Surg. 2016, 18, 348–356. [Google Scholar] [CrossRef]
- Addie, D.D.; Jarrett, J.O. Use of a reverse-transcriptase polymerase chain reaction for monitoring feline coronavirus shedding by healthy cats. Vet. Rec. 2001, 148, 649–653. [Google Scholar] [CrossRef]
- Jinks, M.R.; English, R.V.; Gilger, B.C. Causes of endogenous uveitis in cats presented to referral clinics in North Carolina. Vet. Ophthalmol. 2016, 19 (Suppl. S1), 30–37. [Google Scholar] [CrossRef] [PubMed]
- Legendre, A.M.; Kuritz, T.; Galyon, G.; Baylor, V.M.; Heidel, R.E. Polyprenyl Immunostimulant Treatment of Cats with Presumptive Non-Effusive Feline Infectious Peritonitis in a Field Study. Front. Vet. Sci. 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Fischer, Y.; Ritz, S.; Weber, K.; Sauter-Louis, C.; Hartmann, K. Randomized, placebo controlled study of the effect of propentofylline on survival time and quality of life of cats with feline infectious peritonitis. J. Vet. Intern. Med. 2011, 25, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Ritz, S.; Egberink, H.; Hartmann, K. Effect of feline interferon-omega on the survival time and quality of life of cats with feline infectious peritonitis. J. Vet. Intern. Med. 2007, 21, 1193–1197. [Google Scholar] [CrossRef]
- Jepson, R.E.; Syme, H.M.; Vallance, C.; Elliott, J. Plasma asymmetric dimethylarginine, symmetric dimethylarginine, l-arginine, and nitrite/nitrate concentrations in cats with chronic kidney disease and hypertension. J. Vet. Intern. Med. 2008, 22, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Relford, R.; Robertson, J.; Clements, C. Symmetric Dimethylarginine: Improving the Diagnosis and Staging of Chronic Kidney Disease in Small Animals. Vet. Clin. N. Am. Small Anim. Pract. 2016, 46, 941–960. [Google Scholar] [CrossRef] [PubMed]
- Paltrinieri, S.; Giraldi, M.; Prolo, A.; Scarpa, P.; Piseddu, E.; Beccati, M.; Graziani, B.; Bo, S. Serum symmetric dimethylarginine and creatinine in Birman cats compared with cats of other breeds. J. Feline Med. Surg. 2018, 20, 905–912. [Google Scholar] [CrossRef]
- Gil, S.; Leal, R.O.; McGahie, D.; Sepúlveda, N.; Duarte, A.; Niza, M.M.; Tavares, L. Oral Recombinant Feline Interferon-Omega as an alternative immune modulation therapy in FIV positive cats, clinical and laboratory evaluation. Res. Vet. Sci. 2014, 96, 79–85. [Google Scholar] [CrossRef]
- Addie, D. Feline coronavirus infections. In Infectious Diseases of the Dog and Cat; Green, C., Ed.; Elsevier: Maryland Heights, MO, USA, 2012; pp. 92–108. [Google Scholar]
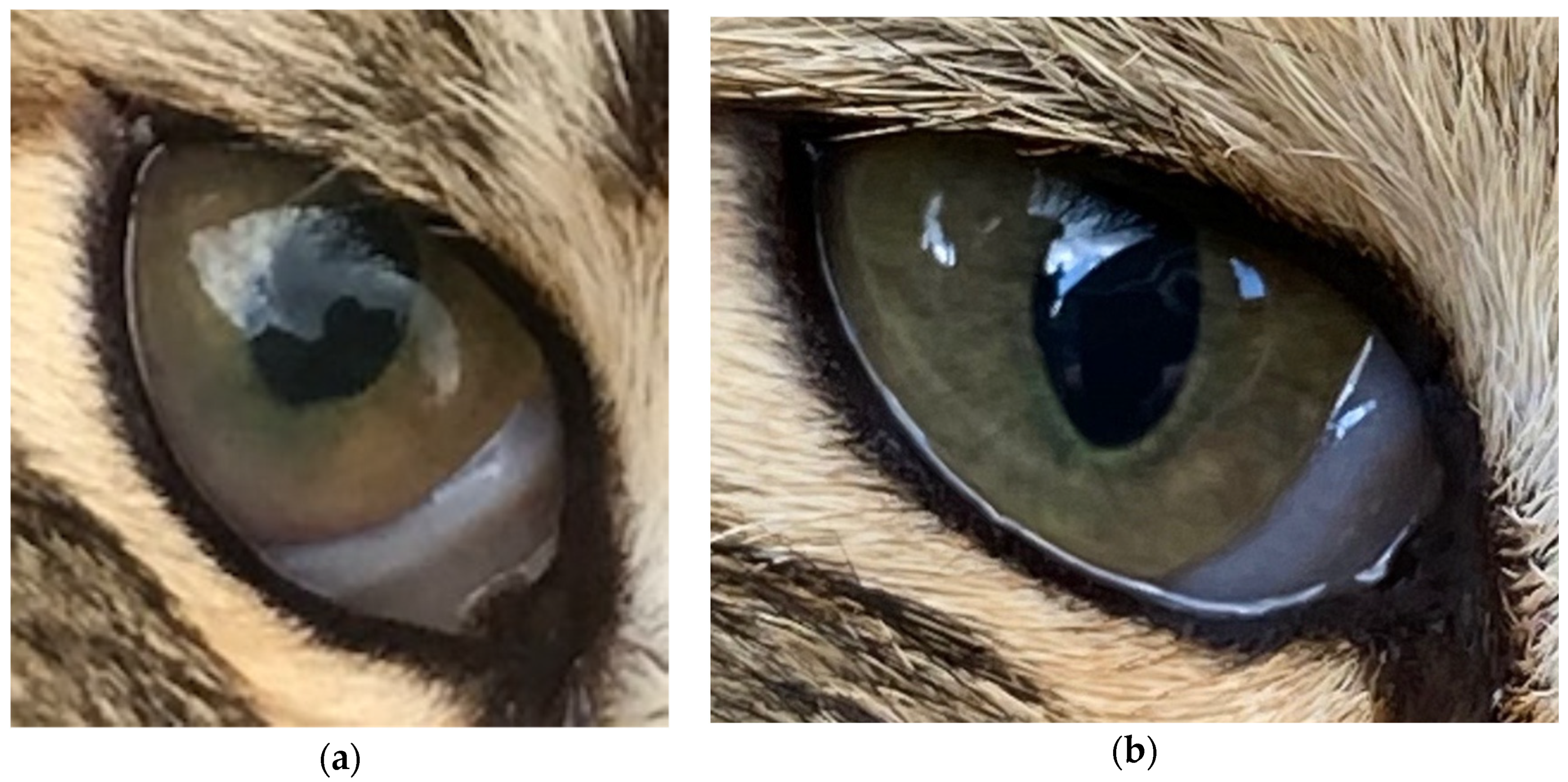
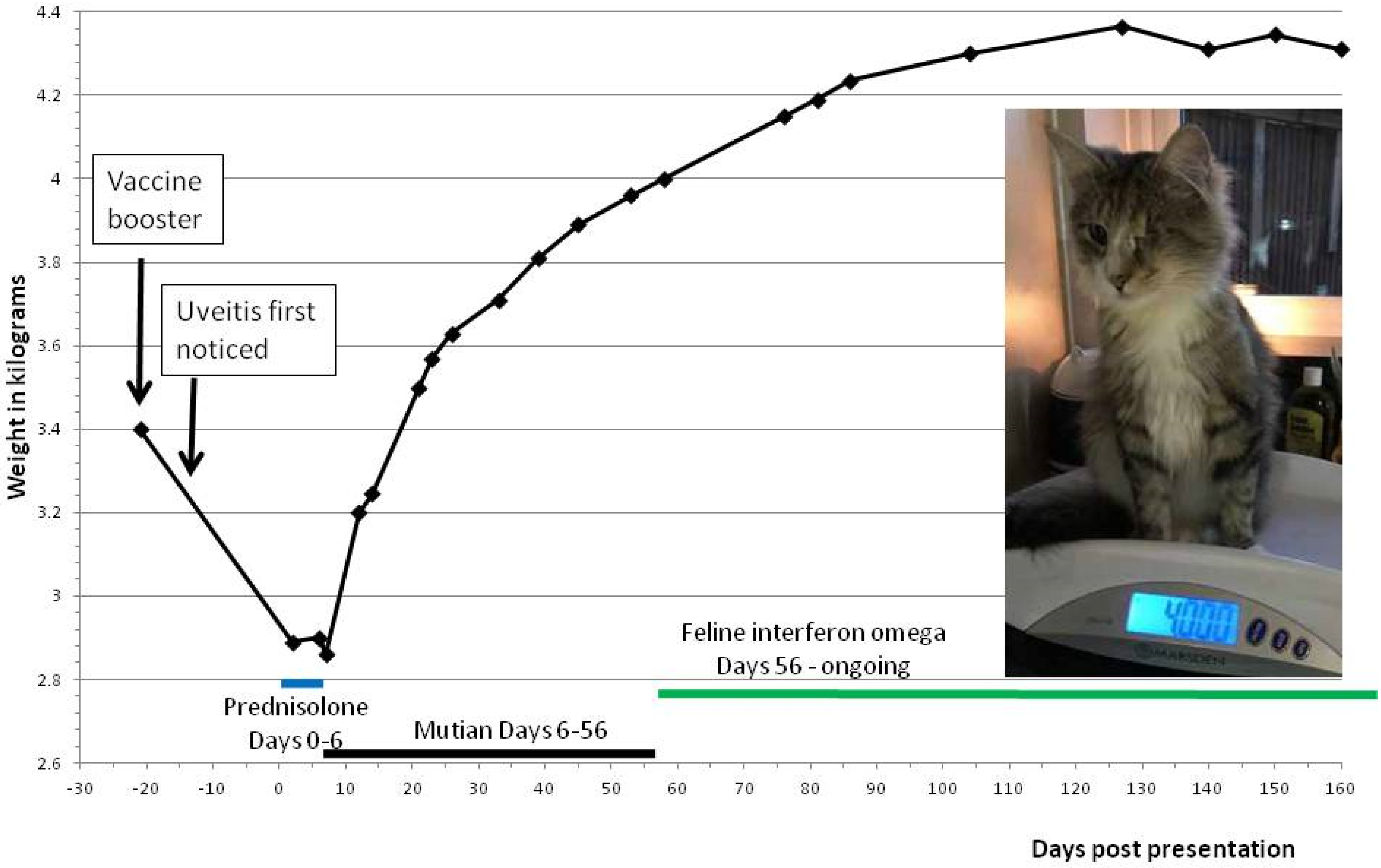
| Day −29 | Day −19 | Day 7 | Day 41 | ||
|---|---|---|---|---|---|
| Skywise | 2yo Norwegian forest cat (NFC) FIP | >10,240 | CT 30 | Neg | |
| Paddy | 2yo NFC persistent pyrexia, lethargy | >10,240 | CT 18 | Neg | |
| Oliver | 8 yo Domestic shorthair cat | >10,240 | CT 20 | Neg | |
| Link | 1 yo NFC | >10,240 | CT 20 | Neg | |
| Zelda | 1 yo NFC | 640 | Neg | Neg |
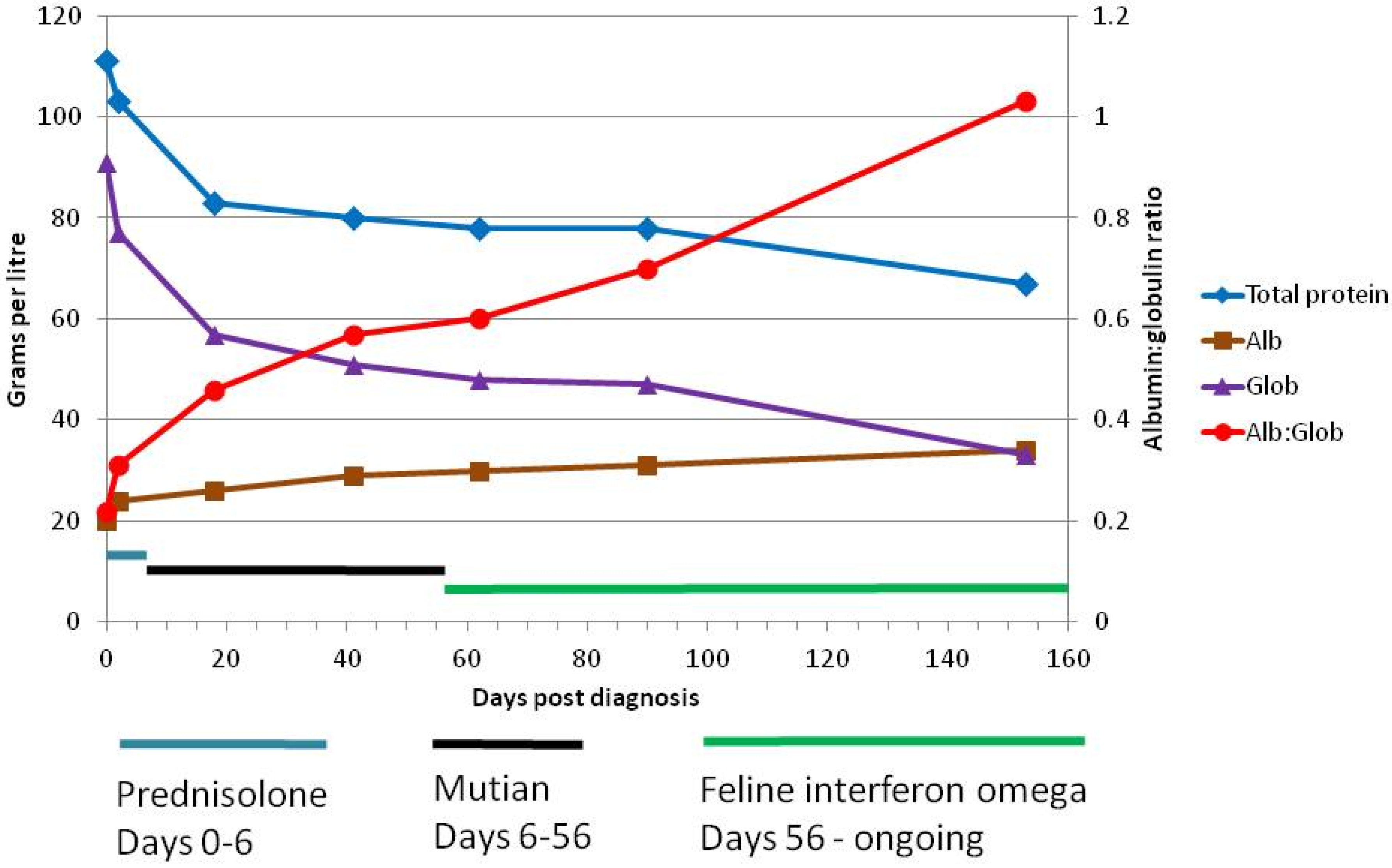
| AGP | TP | Alb | Glob | A:G | Bilirubin | Hct | Lymph. Count | ALT | AP | GGT | SDMA | Creat | Urea | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref. Range | <500 μg/mL | 57–89 g/L | 22–40 g/L | 28–51 g/L | >0.8 * | 0–15 μmol/L | 30–52% | 0.92–6.88 × 109 | 12–130 U/L | 14–111 U/L | 0–4 U/L | 0–14 μg/dL | 71–212 μmol/L | 5.7–12.9 mmol/L |
| Day 0 | ND | 111 | 20 | 91 | 0.22 | 11 | 25.7 | 0.95 | 64 | ND | ND | ND | ND | ND |
| Day 2 | 3100 | 103 | 24 | 77 | 0.31 | ND | 22.0 | 2.07 | ND | ND | ND | ND | ND | ND |
| Day 18 | 700 | 83 | 26 | 57 | 0.46 | 6 | 34.4 | 3.61 | 27 | 32 | 0 | ND | 88 | 9.6 |
| Day 41 | 400 | 80 | 29 | 51 | 0.57 | 5 | 35.1 | 3.85 | 67 | 40 | 0 | 28 | 93 | 8.3 |
| Day 62 | 400 | 78 | 30 | 48 | 0.60 | 5 | 35.2 | 2.64 | 61 | 40 | 0 | 19 | 106 | 6.5 |
| Day 90 | 300 | 78 | 31 | 47 | 0.70 | 6 | 40.0 | 4.15 | 81 | 40 | 0 | 14 | 113 | 10.0 |
| Day 153 | 400 | 67 | 34 | 33 | 1.03 | 1 | 35.2 | 4.49 | 81 | 46 | 2 | ND ** | 98 | 7.8 |
| Treatment | Day | Ophthalmoscopic Findings | ||
|---|---|---|---|---|
| Systemic prednisolone | 0 | Day of presentation to primary veterinary surgeon. | ||
| 1 | Eye examined using a standard ophthalmoscope. Cornea unremarkable, anterior chamber slightly cloudy, but sedation would have been required for detailed examination. Cat not visual and was distressed. | |||
| 2 | Blood samples and mesenteric lymph node fine-needle aspirates taken under sedation. | |||
| Topical prednisolone acetate 1% tid | 4 | Keratic (protein) precipitates on lens present, partially obscuring fundus. Pupillary light reflex present but reduced. | ||
| Mutian at 8 mg/k for 19 days, then 6 mg/kg | 6 | Cat’s guardian reported that she thought the cat could see. | ||
| 17 | Cat’s guardian reported that the cat chased a bird. | |||
| 18 | Uveitis resolving, anterior chamber clear. Keratic precipitates as before. | |||
| 41 | Uveitis appeared largely resolved, keratic precipitates as before. Fundus seen this time, possible bulla seen, resolving chorioretinitis, but cat’s temperament precluded detailed examination. Excellent visual acuity. | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Addie, D.D.; Covell-Ritchie, J.; Jarrett, O.; Fosbery, M. Rapid Resolution of Non-Effusive Feline Infectious Peritonitis Uveitis with an Oral Adenosine Nucleoside Analogue and Feline Interferon Omega. Viruses 2020, 12, 1216. https://doi.org/10.3390/v12111216
Addie DD, Covell-Ritchie J, Jarrett O, Fosbery M. Rapid Resolution of Non-Effusive Feline Infectious Peritonitis Uveitis with an Oral Adenosine Nucleoside Analogue and Feline Interferon Omega. Viruses. 2020; 12(11):1216. https://doi.org/10.3390/v12111216
Chicago/Turabian StyleAddie, Diane D., Johanna Covell-Ritchie, Oswald Jarrett, and Mark Fosbery. 2020. "Rapid Resolution of Non-Effusive Feline Infectious Peritonitis Uveitis with an Oral Adenosine Nucleoside Analogue and Feline Interferon Omega" Viruses 12, no. 11: 1216. https://doi.org/10.3390/v12111216
APA StyleAddie, D. D., Covell-Ritchie, J., Jarrett, O., & Fosbery, M. (2020). Rapid Resolution of Non-Effusive Feline Infectious Peritonitis Uveitis with an Oral Adenosine Nucleoside Analogue and Feline Interferon Omega. Viruses, 12(11), 1216. https://doi.org/10.3390/v12111216





