Usutu Virus: An Arbovirus on the Rise
Abstract
1. Introduction
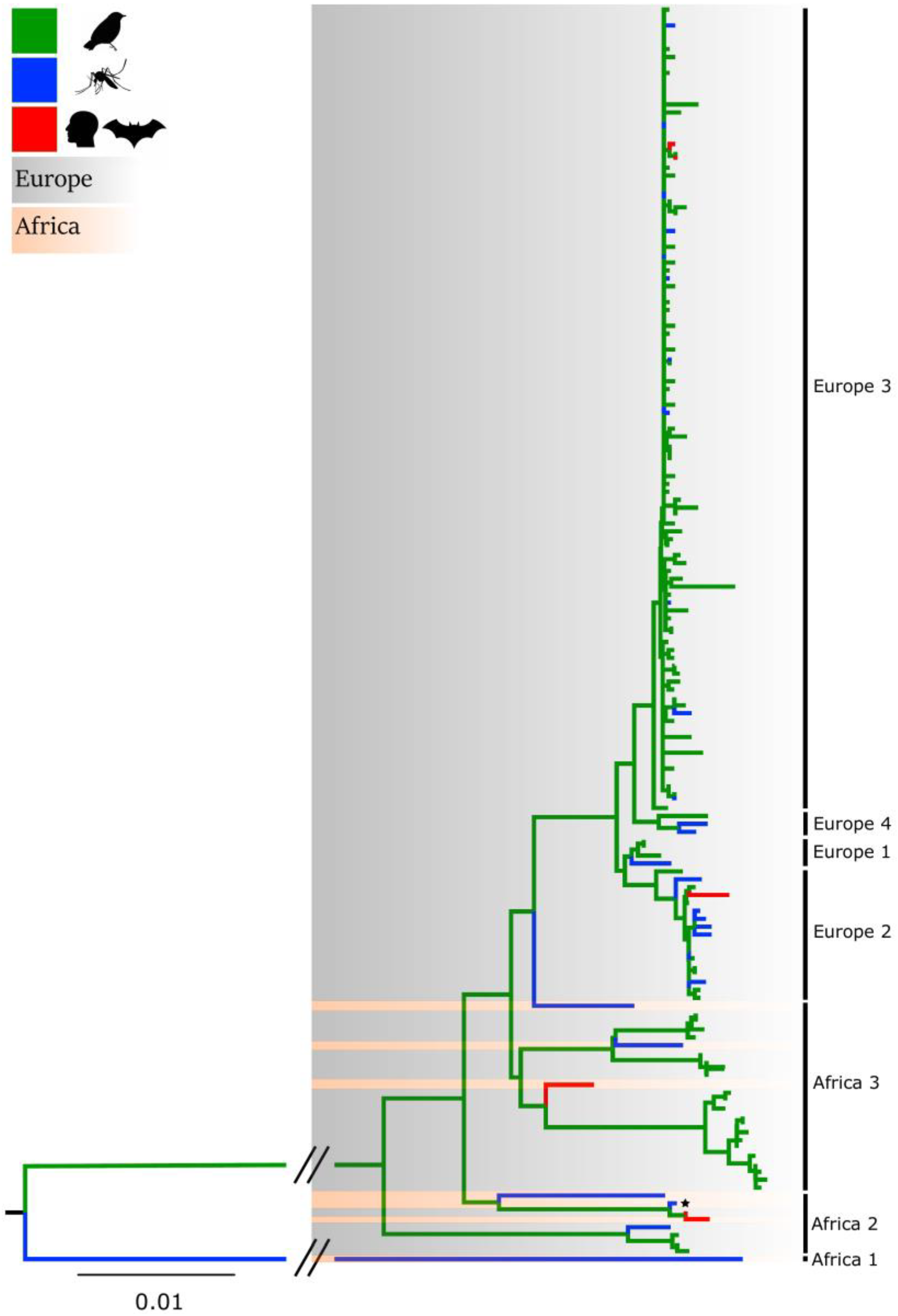
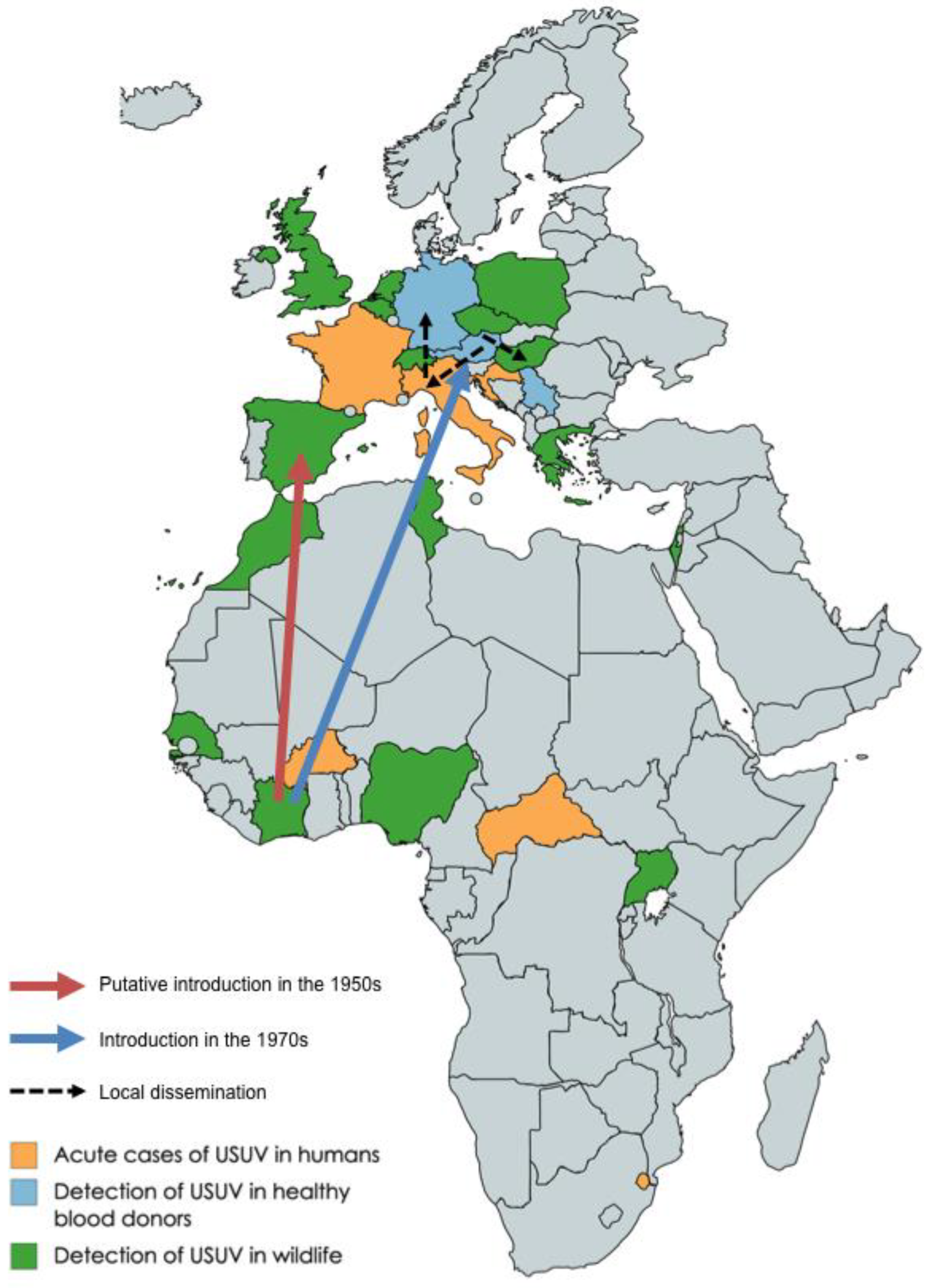
2. Introduction of USUV in Europe and Genetic Diversity
3. Transmission Dynamics
3.1. WNV and USUV Co-Circulation and Co-Infection Dynamics
3.2. Vector Species for USUV
3.3. Reservoir of USUV
3.4. Other Dead-End Hosts
4. Human Clinical Cases and Disease
5. Cellular Responses to USUV Infection
6. USUV Circulation in the Future: Surveillance and Mathematical Modeling
7. Concluding Remarks
Supplementary Materials
Funding
Conflicts of Interest
References
- Williams, M.C.; Simpson, D.I.; Haddow, A.J.; Knight, E.M. THE ISOLATION OF WEST NILE VIRUS FROM MAN AND OF USUTU VIRUS FROM THE BIRD-BITING MOSQUITO MANSONIA AURITES (THEOBALD) IN THE ENTEBBE AREA OF UGANDA. Ann. Trop. Med. Parasitol. 1964, 58, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Nikolay, B.; Diallo, M.; Boye, C.S.; Sall, A.A. Usutu virus in Africa. Vector Borne Zoonotic. Dis. 2011, 11, 1417–1423. [Google Scholar]
- Weissenbock, H.; Kolodziejek, J.; Url, A.; Lussy, H.; Rebel-Bauder, B.; Nowotny, N. Emergence of Usutu virus, an African mosquito-borne flavivirus of the Japanese encephalitis virus group, central Europe. Emerg. Infect. Dis. 2002, 8, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Weissenbock, H.; Bakonyi, T.; Rossi, G.; Mani, P.; Nowotny, N. Usutu virus, Italy, 1996. Emerg. Infect. Dis. 2013, 19, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Kemenesi, G.; Buzas, D.; Zana, B.; Kurucz, K.; Krtinic, B.; Kepner, A.; Foldes, F.; Jakab, F. First genetic characterization of Usutu virus from Culex pipiens mosquitoes Serbia, 2014. Transbound Emerg. Dis. 2018, 63, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Bakonyi, T.; Jungbauer, C.; Aberle, S.W.; Kolodziejek, J.; Dimmel, K.; Stiasny, K.; Allerberger, F.; Nowotny, N. Usutu virus infections among blood donors, Austria, July and August 2017-Raising awareness for diagnostic challenges. Euro Surveill. 2017, 22, 17-00644. [Google Scholar] [CrossRef] [PubMed]
- Engel, D.; Jost, H.; Wink, M.; Borstler, J.; Bosch, S.; Garigliany, M.M.; Jost, A.; Czajka, C.; Luhken, R.; Ziegler, U.; et al. Reconstruction of the Evolutionary History and Dispersal of Usutu Virus, a Neglected Emerging Arbovirus in Europe and Africa. MBio 2016, 7, e01938-15. [Google Scholar] [CrossRef]
- Cadar, D.; Luhken, R.; van der Jeugd, H.; Garigliany, M.; Ziegler, U.; Keller, M.; Lahoreau, J.; Lachmann, L.; Becker, N.; Kik, M.; et al. Widespread activity of multiple lineages of Usutu virus, western Europe, 2016. Euro Surveill. 2017, 22, 30452. [Google Scholar] [CrossRef]
- Calzolari, M.; Chiapponi, C.; Bonilauri, P.; Lelli, D.; Baioni, L.; Barbieri, I.; Lavazza, A.; Pongolini, S.; Dottori, M.; Moreno, A. Co-circulation of two Usutu virus strains in Northern Italy between 2009 and 2014. Infect. Genet. Evol. 2017, 51, 255–262. [Google Scholar] [CrossRef]
- Ben Hassine, T.; De Massis, F.; Calistri, P.; Savini, G.; BelHaj Mohamed, B.; Ranen, A.; Di Gennaro, A.; Sghaier, S.; Hammami, S. First detection of co-circulation of West Nile and Usutu viruses in equids in the south-west of Tunisia. Transbound Emerg. Dis. 2014, 61, 385–389. [Google Scholar] [CrossRef]
- Durand, B.; Haskouri, H.; Lowenski, S.; Vachiery, N.; Beck, C.; Lecollinet, S. Seroprevalence of West Nile and Usutu viruses in military working horses and dogs, Morocco, 2012: Dog as an alternative WNV sentinel species? Epidemiol. Infect. 2016, 144, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Mannasse, B.; Mendelson, E.; Orshan, L.; Mor, O.; Shalom, U.; Yeger, T.; Lustig, Y. Usutu Virus RNA in Mosquitoes, Israel, 2014–2015. Emerg. Infect. Dis. 2017, 23, 1699–1702. [Google Scholar] [CrossRef] [PubMed]
- Chaintoutis, S.C.; Dovas, C.I.; Papanastassopoulou, M.; Gewehr, S.; Danis, K.; Beck, C.; Lecollinet, S.; Antalis, V.; Kalaitzopoulou, S.; Panagiotopoulos, T.; et al. Evaluation of a West Nile virus surveillance and early warning system in Greece, based on domestic pigeons. Immunol. Microbiol. Infect. Dis. 2014, 37, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Eiden, M.; Gil, P.; Ziegler, U.; Rakotoarivony, I.; Marie, A.; Frances, B.; L’Ambert, G.; Simonin, Y.; Foulongne, V.; Groschup, M.H.; et al. Emergence of two Usutu virus lineages in Culex pipiens mosquitoes in the Camargue, France, 2015. Infect. Genet. Evol. 2018, 61, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Bakonyi, T.; Busquets, N.; Nowotny, N. Comparison of complete genome sequences of Usutu virus strains detected in Spain, Central Europe, and Africa. Vector Borne Zoonotic Dis. 2014, 14, 324–329. [Google Scholar] [CrossRef]
- Bazanow, B.; Jansen van Vuren, P. A Survey on West Nile and Usutu Viruses in Horses and Birds in Poland. Viruses 2018, 10, 87. [Google Scholar] [CrossRef]
- Bakonyi, T.; Erdelyi, K.; Ursu, K.; Ferenczi, E.; Csorgo, T.; Lussy, H.; Chvala, S.; Bukovsky, C.; Meister, T.; Weissenbock, H.; et al. Emergence of Usutu virus in Hungary. J. Clin. Microbiol. 2007, 45, 3870–3874. [Google Scholar] [CrossRef]
- Rudolf, I.; Bakonyi, T.; Sebesta, O.; Mendel, J.; Pesko, J.; Betasova, L.; Blazejova, H.; Venclikova, K.; Strakova, P.; Nowotny, N.; et al. Co-circulation of Usutu virus and West Nile virus in a reed bed ecosystem. Parasit Vectors 2015, 8, 520. [Google Scholar] [CrossRef]
- Buckley, A.; Dawson, A.; Moss, S.R.; Hinsley, S.A.; Bellamy, P.E.; Gould, E.A. Serological evidence of West Nile virus, Usutu virus and Sindbis virus infection of birds in the UK. J. Gen. Virol. 2003, 84, 2807–2817. [Google Scholar] [CrossRef]
- Barbic, L.; Vilibic-Cavlek, T.; Listes, E.; Stevanovic, V.; Gjenero-Margan, I.; Ljubin-Sternak, S.; Pem-Novosel, I.; Listes, I.; Mlinaric-Galinovic, G.; Di Gennaro, A.; et al. Demonstration of Usutu virus antibodies in horses, Croatia. Vector Borne Zoonotic Dis. 2013, 13, 772–774. [Google Scholar] [CrossRef]
- Rijks, J.M.; Kik, M.L.; Slaterus, R.; Foppen, R.; Stroo, A.; Jzer, J.I.; Stahl, J.; Grone, A.; Koopmans, M.; van der Jeugd, H.P.; et al. Widespread Usutu virus outbreak in birds in the Netherlands, 2016. Euro Surveill. 2016, 21, 30391. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, H.W.; Bakonyi, T.; Weissenbock, H.; Hatt, J.M.; Eulenberger, U.; Robert, N.; Hoop, R.; Nowotny, N. Emergence and establishment of Usutu virus infection in wild and captive avian species in and around Zurich, Switzerland--genomic and pathologic comparison to other central European outbreaks. Vet. Microbiol. 2011, 148, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Manarolla, G.; Bakonyi, T.; Gallazzi, D.; Crosta, L.; Weissenbock, H.; Dorrestein, G.M.; Nowotny, N. Usutu virus in wild birds in northern Italy. Vet. Microbiol. 2010, 141, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Jost, H.; Bialonski, A.; Maus, D.; Sambri, V.; Eiden, M.; Groschup, M.H.; Gunther, S.; Becker, N.; Schmidt-Chanasit, J. Isolation of usutu virus in Germany. Am. J. Trop. Med. Hyg. 2011, 85, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Sieg, M.; Schmidt, V.; Ziegler, U.; Keller, M.; Hoper, D.; Heenemann, K.; Ruckner, A.; Nieper, H.; Muluneh, A.; Groschup, M.H.; et al. Outbreak and Cocirculation of Three Different Usutu Virus Strains in Eastern Germany. PLoS Negl. Trop. Dis. 2017, 17, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, U.; Fast, C.; Eiden, M.; Bock, S.; Schulze, C.; Hoeper, D.; Ochs, A.; Schlieben, P.; Keller, M.; Zielke, D.E.; et al. Evidence for an independent third Usutu virus introduction into Germany. BMC Vet. Res. 2016, 192, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Gaibani, P.; Cavrini, F.; Gould, E.A.; Rossini, G.; Pierro, A.; Landini, M.P.; Sambri, V. Comparative genomic and phylogenetic analysis of the first Usutu virus isolate from a human patient presenting with neurological symptoms. PLoS ONE 2013, 8, e64761. [Google Scholar] [CrossRef] [PubMed]
- Cavrini, F.; Gaibani, P.; Longo, G.; Pierro, A.M.; Rossini, G.; Bonilauri, P.; Gerunda, G.E.; Di Benedetto, F.; Pasetto, A.; Girardis, M.; et al. Usutu virus infection in a patient who underwent orthotropic liver transplantation, Italy, August-September 2009. Euro Surveill. 2009, 14. [Google Scholar] [CrossRef]
- Nikolay, B. A review of West Nile and Usutu virus co-circulation in Europe: How much do transmission cycles overlap? Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 609–618. [Google Scholar] [CrossRef]
- Tamba, M.; Bonilauri, P.; Bellini, R.; Calzolari, M.; Albieri, A.; Sambri, V.; Dottori, M.; Angelini, P. Detection of Usutu virus within a West Nile virus surveillance program in Northern Italy. Vector Borne Zoonotic Dis. 2011, 11, 551–557. [Google Scholar] [CrossRef]
- Vilibic-Cavlek, T.; Kaic, B.; Barbic, L.; Pem-Novosel, I.; Slavic-Vrzic, V.; Lesnikar, V.; Kurecic-Filipovic, S.; Babic-Erceg, A.; Listes, E.; Stevanovic, V.; et al. First evidence of simultaneous occurrence of West Nile virus and Usutu virus neuroinvasive disease in humans in Croatia during the 2013 outbreak. Infection 2014, 42, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Aberle, S.W.; Kolodziejek, J.; Jungbauer, C.; Stiasny, K.; Aberle, J.H.; Zoufaly, A.; Hourfar, M.K.; Weidner, L.; Nowotny, N. Increase in human West Nile and Usutu virus infections, Austria, 2018. Euro Surveill. 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Nikolay, B.; Fall, G.; Boye, C.S.; Sall, A.A.; Skern, T. Validation of a structural comparison of the antigenic characteristics of Usutu virus and West Nile virus envelope proteins. Virus Res. 2014, 189, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ye, F.; Lin, S.; Yang, F.; Cheng, Y.; Cao, Y.; Chen, Z.; Lu, G. Crystal structure of Usutu virus envelope protein in the pre-fusion state. Virol. J. 2018, 15, 183. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.; Jimenez-Clavero, M.A.; Leblond, A.; Durand, B.; Nowotny, N.; Leparc-Goffart, I.; Zientara, S.; Jourdain, E.; Lecollinet, S. Flaviviruses in Europe: Complex circulation patterns and their consequences for the diagnosis and control of West Nile disease. Int. J. Environ. Res. Public Health 2013, 10, 6049–6083. [Google Scholar] [CrossRef] [PubMed]
- Llorente, F.; Garcia-Irazabal, A.; Perez-Ramirez, E.; Cano-Gomez, C.; Sarasa, M.; Vazquez, A.; Jimenez-Clavero, M.A. Influence of flavivirus co-circulation in serological diagnostics and surveillance: A model of study using West Nile, Usutu and Bagaza viruses. Transbound Emerg. Dis. 2019. [Google Scholar] [CrossRef] [PubMed]
- Cleton, N.B.; van Maanen, K.; Bergervoet, S.A.; Bon, N.; Beck, C.; Godeke, G.J.; Lecollinet, S.; Bowen, R.; Lelli, D.; Nowotny, N.; et al. A Serological Protein Microarray for Detection of Multiple Cross-Reactive Flavivirus Infections in Horses for Veterinary and Public Health Surveillance. Transbound Emerg. Dis. 2017, 64, 1801–1812. [Google Scholar] [CrossRef]
- Del Amo, J.; Sotelo, E.; Fernandez-Pinero, J.; Gallardo, C.; Llorente, F.; Aguero, M.; Jimenez-Clavero, M.A. A novel quantitative multiplex real-time RT-PCR for the simultaneous detection and differentiation of West Nile virus lineages 1 and 2, and of Usutu virus. J. Virol. Methods 2013, 189, 321–327. [Google Scholar] [CrossRef]
- Merino-Ramos, T.; Blazquez, A.B.; Escribano-Romero, E.; Canas-Arranz, R.; Sobrino, F.; Saiz, J.C.; Martin-Acebes, M.A. Protection of a single dose west nile virus recombinant subviral particle vaccine against lineage 1 or 2 strains and analysis of the cross-reactivity with Usutu virus. PLoS ONE 2014, 9, e108056. [Google Scholar] [CrossRef]
- Blazquez, A.B.; Escribano-Romero, E.; Martin-Acebes, M.A.; Petrovic, T.; Saiz, J.C. Limited susceptibility of mice to Usutu virus (USUV) infection and induction of flavivirus cross-protective immunity. Virology 2015, 482, 67–71. [Google Scholar] [CrossRef]
- Mancini, G.; Montarsi, F.; Calzolari, M.; Capelli, G.; Dottori, M.; Ravagnan, S.; Lelli, D.; Chiari, M.; Santilli, A.; Quaglia, M.; et al. Mosquito species involved in the circulation of West Nile and Usutu viruses in Italy. Vet. Ital. 2017, 53, 97–110. [Google Scholar] [PubMed]
- Lebl, K.; Zittra, C.; Silbermayr, K.; Obwaller, A.; Berer, D.; Brugger, K.; Walter, M.; Pinior, B.; Fuehrer, H.P.; Rubel, F. Mosquitoes (Diptera: Culicidae) and their relevance as disease vectors in the city of Vienna, Austria. Parasitol. Res. 2015, 114, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Fros, J.J.; Miesen, P.; Vogels, C.B.; Gaibani, P.; Sambri, V.; Martina, B.E.; Koenraadt, C.J.; van Rij, R.P.; Vlak, J.M.; Takken, W.; et al. Comparative Usutu and West Nile virus transmission potential by local Culex pipiens mosquitoes in north-western Europe. One Health 2015, 1, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Triana, L.M.; de Marco, M.F.; Mansfield, K.L.; Thorne, L.; Lumley, S.; Marston, D.; Fooks, A.A.; Johnson, N. Assessment of vector competence of UK mosquitoes for Usutu virus of African origin. PLoS Negl. Trop. Dis. 2018, 11, 381. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.L.; Huang, Y.S.; Lyons, A.C.; Alto, B.W.; Unlu, I.; Higgs, S.; Vanlandingham, D.L. North American Culex pipiens and Culex quinquefasciatus are competent vectors for Usutu virus. PLoS Negl. Trop. Dis. 2018, 12, e0006732. [Google Scholar] [CrossRef]
- Camp, J.V.; Kolodziejek, J.; Nowotny, N. Targeted surveillance reveals native and invasive mosquito species infected with Usutu virus. Parasit Vectors 2019, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Nikolay, B.; Diallo, M.; Faye, O.; Boye, C.S.; Sall, A.A. Vector competence of Culex neavei (Diptera: Culicidae) for Usutu virus. Am. J. Trop. Med. Hyg. 2012, 86, 993–996. [Google Scholar] [CrossRef]
- Calzolari, M.; Bonilauri, P.; Bellini, R.; Albieri, A.; Defilippo, F.; Maioli, G.; Galletti, G.; Gelati, A.; Barbieri, I.; Tamba, M.; et al. Evidence of simultaneous circulation of West Nile and Usutu viruses in mosquitoes sampled in Emilia-Romagna region (Italy) in 2009. PLoS ONE 2010, 5, e14324. [Google Scholar] [CrossRef]
- Calzolari, M.; Gaibani, P.; Bellini, R.; Defilippo, F.; Pierro, A.; Albieri, A.; Maioli, G.; Luppi, A.; Rossini, G.; Balzani, A.; et al. Mosquito, bird and human surveillance of West Nile and Usutu viruses in Emilia-Romagna Region (Italy) in 2010. PLoS ONE 2012, 7, e38058. [Google Scholar] [CrossRef]
- Puggioli, A.; Bonilauri, P.; Calzolari, M.; Lelli, D.; Carrieri, M.; Urbanelli, S.; Pudar, D.; Bellini, R. Does Aedes albopictus (Diptera: Culicidae) play any role in Usutu virus transmission in Northern Italy? Experimental oral infection and field evidences. Acta. Trop. 2017, 172, 192–196. [Google Scholar] [CrossRef]
- Llopis, I.V.; Tomassone, L.; Grego, E.; Silvano, F.; Rossi, L. Investigation into Usutu and West Nile viruses in ticks from wild birds in Northwestern Italy, 2012-2014. New Microbiol. 2017, 40, 56–57. [Google Scholar] [PubMed]
- Mancini, F.; Toma, L.; Ciervo, A.; Di Luca, M.; Faggioni, G.; Lista, F.; Rezza, G. Virus investigation in ticks from migratory birds in Italy. New Microbiol. 2013, 36, 433–434. [Google Scholar] [PubMed]
- Schuffenecker, I.; Iteman, I.; Michault, A.; Murri, S.; Frangeul, L.; Vaney, M.C.; Lavenir, R.; Pardigon, N.; Reynes, J.M.; Pettinelli, F.; et al. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006, 3, e263. [Google Scholar] [CrossRef] [PubMed]
- Benzarti, E.; Linden, A.; Desmecht, D.; Garigliany, M. Mosquito-borne epornitic flaviviruses: An update and review. J. Gen. Virol. 2019, 100, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Weissenbock, H.; Kolodziejek, J.; Fragner, K.; Kuhn, R.; Pfeffer, M.; Nowotny, N. Usutu virus activity in Austria, 2001–2002. Microbes. Infect. 2003, 5, 1132–1136. [Google Scholar] [CrossRef]
- Becker, N.; Jost, H.; Ziegler, U.; Eiden, M.; Hoper, D.; Emmerich, P.; Fichet-Calvet, E.; Ehichioya, D.U.; Czajka, C.; Gabriel, M.; et al. Epizootic emergence of Usutu virus in wild and captive birds in Germany. PLoS ONE 2012, 7, e32604. [Google Scholar] [CrossRef]
- Chvala, S.; Kolodziejek, J.; Nowotny, N.; Weissenbock, H. Pathology and viral distribution in fatal Usutu virus infections of birds from the 2001 and 2002 outbreaks in Austria. J. Comp. Pathol. 2004, 131, 176–185. [Google Scholar] [CrossRef]
- Bergsman, L.D.; Hyman, J.M.; Manore, C.A. A mathematical model for the spread of west nile virus in migratory and resident birds. Math. Biosci. Eng. 2016, 13, 401–424. [Google Scholar] [CrossRef]
- Lecollinet, S.; Blanchard, Y.; Manson, C.; Lowenski, S.; Laloy, E.; Quenault, H.; Touzain, F.; Lucas, P.; Eraud, C.; Bahuon, C.; et al. Dual Emergence of Usutu Virus in Common Blackbirds, Eastern France, 2015. Emerg. Infect. Dis. 2016, 22, 2225. [Google Scholar] [CrossRef]
- Meister, T.; Lussy, H.; Bakonyi, T.; Sikutova, S.; Rudolf, I.; Vogl, W.; Winkler, H.; Frey, H.; Hubalek, Z.; Nowotny, N.; et al. Serological evidence of continuing high Usutu virus (Flaviviridae) activity and establishment of herd immunity in wild birds in Austria. Vet. Microbiol. 2008, 127, 237–248. [Google Scholar] [CrossRef]
- Chvala, S.; Bakonyi, T.; Hackl, R.; Hess, M.; Nowotny, N.; Weissenbock, H. Limited pathogenicity of Usutu virus for the domestic chicken (Gallus domesticus). Avian Pathol. 2005, 34, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Chvala, S.; Bakonyi, T.; Hackl, R.; Hess, M.; Nowotny, N.; Weissenbock, H. Limited pathogenicity of usutu virus for the domestic goose (Anser anser f. domestica) following experimental inoculation. J. Vet. Med. B Infect. Dis. Vet. Public Health 2006, 53, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Rouffaer, L.O.; Steensels, M.; Verlinden, M.; Vervaeke, M.; Boonyarittichaikij, R.; Martel, A.; Lambrecht, B. Usutu Virus Epizootic and Plasmodium Coinfection in Eurasian Blackbirds (Turdus merula) in Flanders, Belgium. J. Wildl. Dis. 2018, 54, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Vanhomwegen, J.; Beck, C.; Despres, P.; Figuerola, A.; Garcia, R.; Lecollinet, S.; Lopez-Roig, M.; Manuguerra, J.C.; Serra-Cobo, J. Circulation of Zoonotic Arboviruses in Equine Populations of Mallorca Island (Spain). Vector Borne Zoonotic Dis. 2017, 17, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Lupulovic, D.; Martin-Acebes, M.A.; Lazic, S.; Alonso-Padilla, J.; Blazquez, A.B.; Escribano-Romero, E.; Petrovic, T.; Saiz, J.C. First serological evidence of West Nile virus activity in horses in Serbia. Vector Borne Zoonotic Dis. 2011, 11, 1303–1305. [Google Scholar] [CrossRef] [PubMed]
- Montagnaro, S.; Piantedosi, D.; Ciarcia, R.; Loponte, R.; Veneziano, V.; Fusco, G.; Amoroso, M.G.; Ferrara, G.; Damiano, S.; Iovane, G.; et al. Serological Evidence of Mosquito-Borne Flaviviruses Circulation in Hunting Dogs in Campania Region, Italy. Vector Borne Zoonotic Dis. 2019, 19, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Busani, L.; Capelli, G.; Cecchinato, M.; Lorenzetto, M.; Savini, G.; Terregino, C.; Vio, P.; Bonfanti, L.; Pozza, M.D.; Marangon, S. West Nile virus circulation in Veneto region in 2008-2009. Epidemiol. Infect. 2011, 139, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bocanegra, I.; Paniagua, J.; Gutierrez-Guzman, A.V.; Lecollinet, S.; Boadella, M.; Arenas-Montes, A.; Cano-Terriza, D.; Lowenski, S.; Gortazar, C.; Hofle, U. Spatio-temporal trends and risk factors affecting West Nile virus and related flavivirus exposure in Spanish wild ruminants. BMC Vet. Res. 2016, 12, 249. [Google Scholar] [CrossRef]
- Escribano-Romero, E.; Lupulovic, D.; Merino-Ramos, T.; Blazquez, A.B.; Lazic, G.; Lazic, S.; Saiz, J.C.; Petrovic, T. West Nile virus serosurveillance in pigs, wild boars, and roe deer in Serbia. Vet. Microbiol. 2015, 176, 365–369. [Google Scholar] [CrossRef]
- Cadar, D.; Becker, N.; Campos Rde, M.; Borstler, J.; Jost, H.; Schmidt-Chanasit, J. Usutu virus in bats, Germany, 2013. Emerg. Infect. Dis. 2014, 20, 1771–1773. [Google Scholar] [CrossRef]
- Romeo, C.; Lecollinet, S. Are tree squirrels involved in the circulation of flaviviruses in Italy? Transbound Emerg. Dis. 2018, 65, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Diagne, M.M.; Ndione, M.H.D.; Di Paola, N.; Fall, G.; Bedekelabou, A.P. Usutu Virus Isolated from Rodents in Senegal. Viruses 2019, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Cle, M.; Salinas, S.; Lecollinet, S.; Beck, C.; Gutierrez, S.; Baldet, T.; Vande Perre, P.; Foulongne, V.; Simonin, Y. Usutu virus: The phantom menace. Med. Sci. (Paris) 2018, 34, 709–716. [Google Scholar]
- Pecorari, M.; Longo, G.; Gennari, W.; Grottola, A.; Sabbatini, A.; Tagliazucchi, S.; Savini, G.; Monaco, F.; Simone, M.; Lelli, R.; et al. First human case of Usutu virus neuroinvasive infection, Italy, August-September 2009. Euro Surveill. 2009, 14. [Google Scholar] [CrossRef]
- Simonin, Y.; Sillam, O.; Carles, M.J.; Gutierrez, S.; Gil, P.; Constant, O.; Martin, M.F.; Girard, G.; Van de Perre, P.; Salinas, S.; et al. Human Usutu Virus Infection with Atypical Neurologic Presentation, Montpellier, France, 2016. Emerg. Infect. Dis. 2018, 24, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Allering, L.; Jost, H.; Emmerich, P.; Gunther, S.; Lattwein, E.; Schmidt, M.; Seifried, E.; Sambri, V.; Hourfar, K.; Schmidt-Chanasit, J. Detection of Usutu virus infection in a healthy blood donor from south-west Germany, 2012. Euro Surveill. 2012, 17. [Google Scholar] [CrossRef]
- Pierro, A.; Gaibani, P.; Spadafora, C.; Ruggeri, D.; Randi, V.; Parenti, S.; Finarelli, A.C.; Rossini, G.; Landini, M.P.; Sambri, V. Detection of specific antibodies against West Nile and Usutu viruses in healthy blood donors in northern Italy, 2010–2011. Clin. Microbiol. Infect. 2013, 19, E451–E453. [Google Scholar] [CrossRef]
- Cvjetković, I.H.; Petrović, T.; Petrić, D.; Cvjetković, D.; Kovačević, G.; Radovanov, J.; Galović, A.J.; Patić, A.; Nikolić, N.; Mikić, S.S. Seroprevalence of mosquito-born and tick-born microorganisms in human population of South Backa District. Arhiv. Veterinarske Med. 2016, 9, 23–30. [Google Scholar]
- Percivalle, E.; Sassera, D.; Rovida, F.; Isernia, P.; Fabbi, M.; Baldanti, F.; Marone, P. Usutu Virus Antibodies in Blood Donors and Healthy Forestry Workers in the Lombardy Region, Northern Italy. Vector Borne Zoonotic Dis. 2017, 17, 658–661. [Google Scholar] [CrossRef]
- Grottola, A.; Marcacci, M.; Tagliazucchi, S.; Gennari, W.; Di, A.G.; Orsini, M.; Monaco, F.; Marchegiano, P.; Marini, V.; Meacci, M.; et al. Usutu virus infections in humans: A retrospective analysis in the municipality of Modena, Italy. Clin. Microbiol. Infect. 2017, 23, 33–37. [Google Scholar] [CrossRef]
- Maggi, F.; Mazzetti, P.; Focosi, D.; Macera, L.; Scagnolari, C.; Manzin, A.; Antonelli, G.; Nelli, L.C. Lack of usutu virus RNA in cerebrospinal fluid of patients with encephalitis of unknown etiology, Tuscany, Italy. J. Med. Virol. 2015, 87, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Cordey, S.; Vieille, G.; Turin, L.; Kaiser, L. Usutu virus in cerebrospinal fluid: A 2-year survey in a Tertiary Care Hospital, Geneva, Switzerland. J. Med. Virol. 2018, 90, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Cavrini, F.; Della Pepa, M.E.; Gaibani, P.; Pierro, A.M.; Rossini, G.; Landini, M.P.; Sambri, V. A rapid and specific real-time RT-PCR assay to identify Usutu virus in human plasma, serum, and cerebrospinal fluid. J. Clin. Virol. 2011, 50, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Weissenbock, H.; Bakonyi, T.; Chvala, S.; Nowotny, N. Experimental Usutu virus infection of suckling mice causes neuronal and glial cell apoptosis and demyelination. Acta. Neuropathol. 2004, 108, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Salinas, S.; Constant, O.; Desmetz, C. Deleterious effect of Usutu virus on human neural cells. PLoS Negl. Trop. Dis. 2017, 11, e0005913. [Google Scholar] [CrossRef] [PubMed]
- Vielle, N.J.; Garcia-Nicolas, O.; Oliveira Esteves, B.I.; Brugger, M.; Summerfield, A.; Alves, M.P. The Human Upper Respiratory Tract Epithelium Is Susceptible to Flaviviruses. Front. Microbiol. 2019, 10, 811. [Google Scholar] [CrossRef] [PubMed]
- Sembene, P.M.; Faye, O.; Zanotto, P.M.A.; Sall, A.A.; Garcia-Nicolas, O.; Lewandowska, M.; Ricklin, M.E.; Summerfield, A. Monocyte-Derived Dendritic Cells as Model to Evaluate Species Tropism of Mosquito-Borne Flaviviruses. Viruses 2019, 9, 5. [Google Scholar]
- Cacciotti, G.; Caputo, B.; Selvaggi, C.; la Sala, A.; Vitiello, L.; Diallo, D.; Ceianu, C.; Antonelli, G.; Nowotny, N.; Scagnolari, C. Variation in interferon sensitivity and induction between Usutu and West Nile (lineages 1 and 2) viruses. Virology 2015, 485, 189–198. [Google Scholar] [CrossRef]
- Segura Guerrero, N.A.; Sharma, S.; Neyts, J.; Kaptein, S.J.F. Favipiravir inhibits in vitro Usutu virus replication and delays disease progression in an infection model in mice. Antiviral Res. 2018, 160, 137–142. [Google Scholar] [CrossRef]
- Blazquez, A.B.; Escribano-Romero, E.; Merino-Ramos, T.; Saiz, J.C.; Martin-Acebes, M.A. Infection with Usutu virus induces an autophagic response in mammalian cells. PLoS Negl. Trop. Dis. 2013, 7, e2509. [Google Scholar] [CrossRef]
- Echavarria-Consuegra, L.; Smit, J.M.; Reggiori, F. Role of autophagy during the replication and pathogenesis of common mosquito-borne flavi- and alphaviruses. Open Biol. 2019, 9, 190009. [Google Scholar] [CrossRef] [PubMed]
- Merino-Ramos, T.; Vazquez-Calvo, A.; Casas, J.; Sobrino, F.; Saiz, J.C.; Martin-Acebes, M.A. Modification of the Host Cell Lipid Metabolism Induced by Hypolipidemic Drugs Targeting the Acetyl Coenzyme A Carboxylase Impairs West Nile Virus Replication. Antimicrob. Agents. Chemother. 2016, 60, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Papa, A. Emerging arboviruses of medical importance in the Mediterranean region. J. Clin. Virol. 2019, 115, 5–10. [Google Scholar] [CrossRef]
- Walter, M.; Brugger, K.; Rubel, F. Usutu virus induced mass mortalities of songbirds in Central Europe: Are habitat models suitable to predict dead birds in unsampled regions? Prev. Vet. Med. 2018, 159, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Rubel, F.; Brugger, K.; Hantel, M.; Chvala-Mannsberger, S.; Bakonyi, T.; Weissenbock, H.; Nowotny, N. Explaining Usutu virus dynamics in Austria: Model development and calibration. Prev. Vet. Med. 2008, 85, 166–186. [Google Scholar] [CrossRef] [PubMed]
- Brugger, K.; Rubel, F. Simulation of climate-change scenarios to explain Usutu-virus dynamics in Austria. Prev. Vet. Med. 2009, 88, 24–31. [Google Scholar] [CrossRef]
- Cheng, Y.; Tjaden, N.B.; Jaeschke, A.; Luhken, R.; Ziegler, U.; Thomas, S.M.; Beierkuhnlein, C. Evaluating the risk for Usutu virus circulation in Europe: Comparison of environmental niche models and epidemiological models. Int. J. Health Geogr. 2018, 17, 35. [Google Scholar] [CrossRef]
- Tiawsirisup, S.; Kinley, J.R.; Tucker, B.J.; Evans, R.B.; Rowley, W.A.; Platt, K.B. Vector competence of Aedes vexans (Diptera: Culicidae) for West Nile virus and potential as an enzootic vector. J. Med. Entomol. 2008, 45, 452–457. [Google Scholar] [CrossRef]
- Veronesi, E.; Paslaru, A.; Silaghi, C.; Tobler, K.; Glavinic, U.; Torgerson, P.; Mathis, A. Experimental evaluation of infection, dissemination, and transmission rates for two West Nile virus strains in European Aedes japonicus under a fluctuating temperature regime. Parasitol. Res. 2018, 117, 1925–1932. [Google Scholar] [CrossRef]
- Fortuna, C.; Remoli, M.E.; Severini, F.; Di Luca, M.; Toma, L.; Fois, F.; Bucci, P.; Boccolini, D.; Romi, R.; Ciufolini, M.G. Evaluation of vector competence for West Nile virus in Italian Stegomyia albopicta (=Aedes albopictus) mosquitoes. Med. Vet. Entomol. 2015, 29, 430–433. [Google Scholar] [CrossRef]
- Kusne, S.; Smilack, J. Transmission of West Nile virus by organ transplantation. Liver Transpl. 2005, 11, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Hayes, E.B.; O’Leary, D.R. West Nile virus infection: A pediatric perspective. Pediatrics 2004, 113, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Zmurko, J.; Neyts, J.; Dallmeier, K. Flaviviral NS4b, chameleon and jack-in-the-box roles in viral replication and pathogenesis, and a molecular target for antiviral intervention. Rev. Med. Virol. 2015, 25, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Reisen, W.K.; Lothrop, H.D.; Wheeler, S.S.; Kennsington, M.; Gutierrez, A.; Fang, Y.; Garcia, S.; Lothrop, B. Persistent West Nile virus transmission and the apparent displacement St. Louis encephalitis virus in southeastern California, 2003–2006. J. Med. Entomol. 2008, 45, 494–508. [Google Scholar]
- Diaz, A.; Coffey, L.L.; Burkett-Cadena, N.; Day, J.F. Reemergence of St. Louis Encephalitis Virus in the Americas. Emerg. Infect. Dis. 2018, 24, 2150. [Google Scholar] [CrossRef] [PubMed]
| WNV | USUV | |
|---|---|---|
| Geographical Distribution | Africa, Europe, Middle East, North America and West Asia | Africa, Europe |
| Main Vector | Culex spp. | Culex spp. |
| Putative Seconday Vector | A. vexans [98], A. japonicus [99], A. albopictus [100] | A. albopictus [45,50] |
| Amplifying Host | Migratory birds | Migratory birds |
| Main Route of Transmission | Mosquito bite | Mosquito bite |
| Alternate Route of Transmission | Rare cases of contamination through organ transplant and transfusion [101] and of mother-to-child transmission [102] | Not described |
| Sensitivity to IFN | Strong [88] | Very strong [88] |
| Antagonism of IFN Response | Through NS4B [103] | Not described |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roesch, F.; Fajardo, A.; Moratorio, G.; Vignuzzi, M. Usutu Virus: An Arbovirus on the Rise. Viruses 2019, 11, 640. https://doi.org/10.3390/v11070640
Roesch F, Fajardo A, Moratorio G, Vignuzzi M. Usutu Virus: An Arbovirus on the Rise. Viruses. 2019; 11(7):640. https://doi.org/10.3390/v11070640
Chicago/Turabian StyleRoesch, Ferdinand, Alvaro Fajardo, Gonzalo Moratorio, and Marco Vignuzzi. 2019. "Usutu Virus: An Arbovirus on the Rise" Viruses 11, no. 7: 640. https://doi.org/10.3390/v11070640
APA StyleRoesch, F., Fajardo, A., Moratorio, G., & Vignuzzi, M. (2019). Usutu Virus: An Arbovirus on the Rise. Viruses, 11(7), 640. https://doi.org/10.3390/v11070640





