Role of NS1 and TLR3 in Pathogenesis and Immunity of WNV
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Construction of Recombinant Baculoviruses for Expression of WNV Glycoprotein E- and NS1-Fragments
2.3. Virus Strains and Cell Lines
2.4. Animals and Protection Study
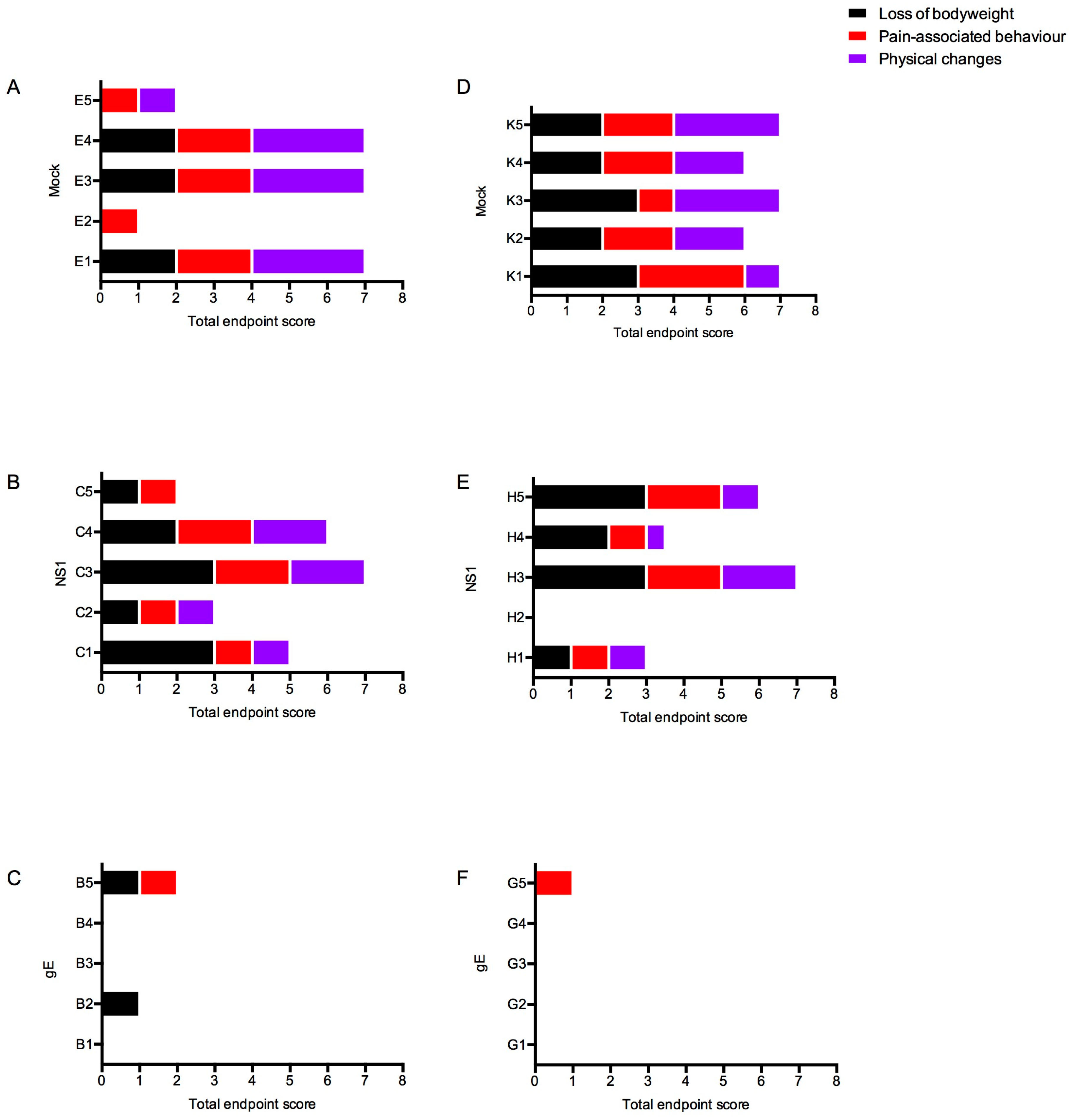
2.5. Clinical Scoring
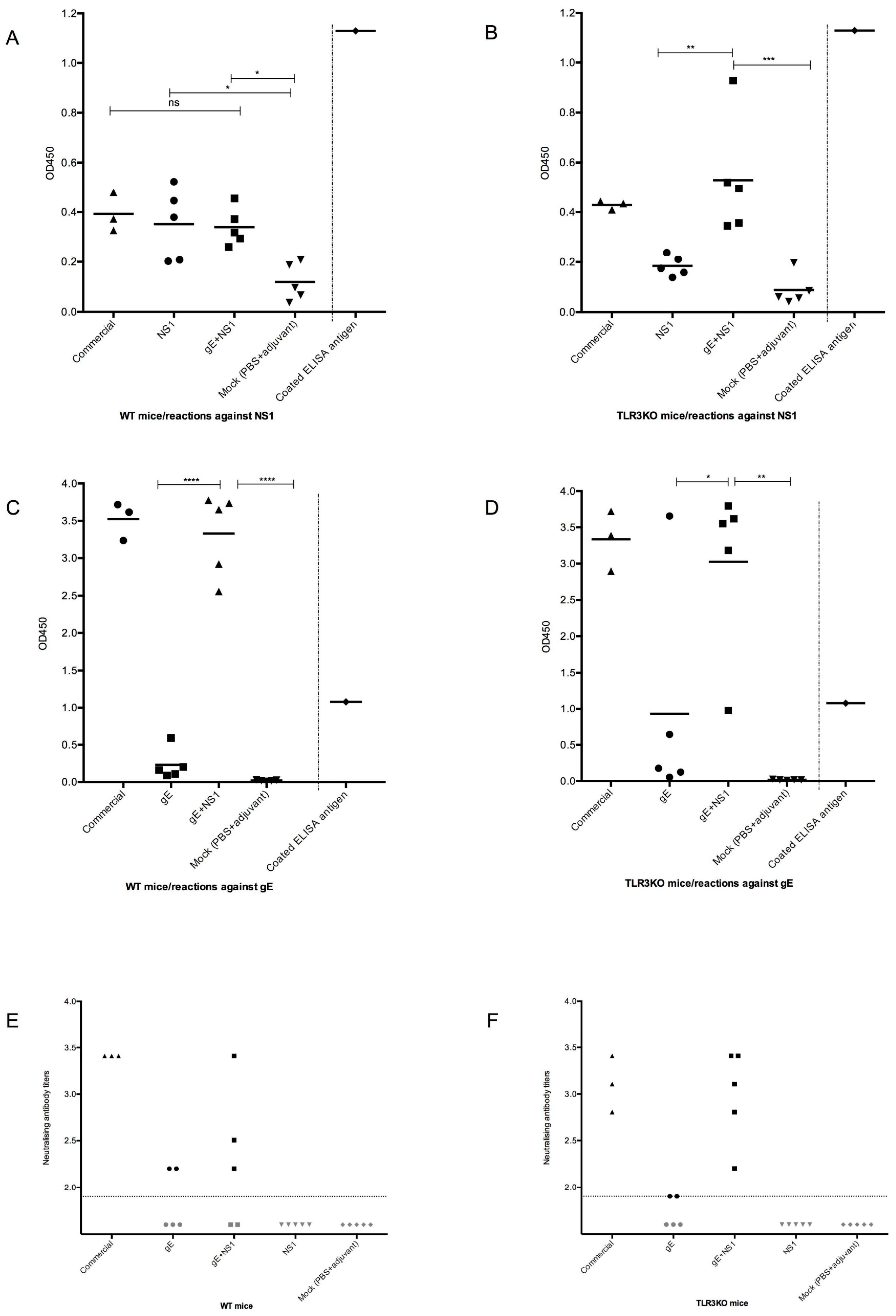
2.6. ELISA for gE and NS1 Specific Antibodies
2.7. WNV Neutralization Test
2.8. WNV RNA Detection in Plasma and Tissue Samples
2.9. Cytokine Quantification
2.10. Isolation of Leukocytes from Brain Tissues
2.11. Analysis of Leukocyte Infiltration into the CNS by Flow Cytometry
2.12. Histology of Brain Samples
2.13. Statistical Analysis
3. Results
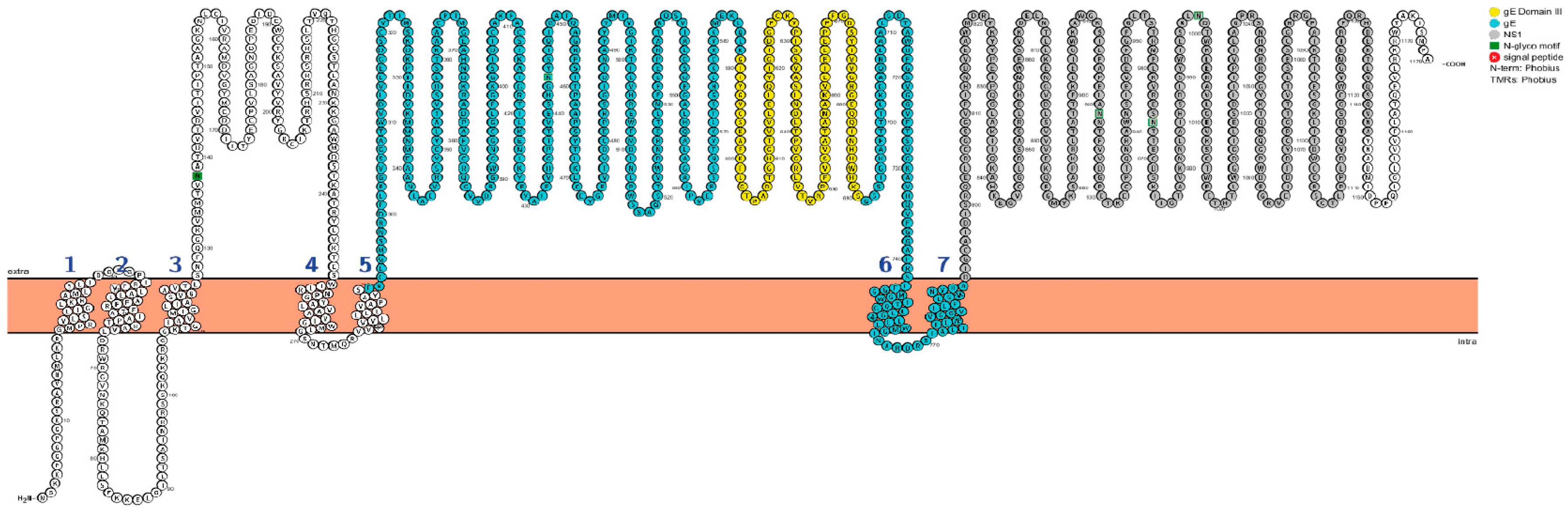
3.1. Construction of the Recombinant WNV gE and NS1 Proteins
3.2. Susceptibility of C57BL/6J Mice to WNV ITA09 Infection and Immunogenicity of WNV Antigens
3.3. Immune Protection of Wild Type and TLR3KO Mice Following NS1- and/or gE-Vaccination
3.3.1. Serologic Responses Prior to Challenge
3.3.2. Neutralizing Antibodies
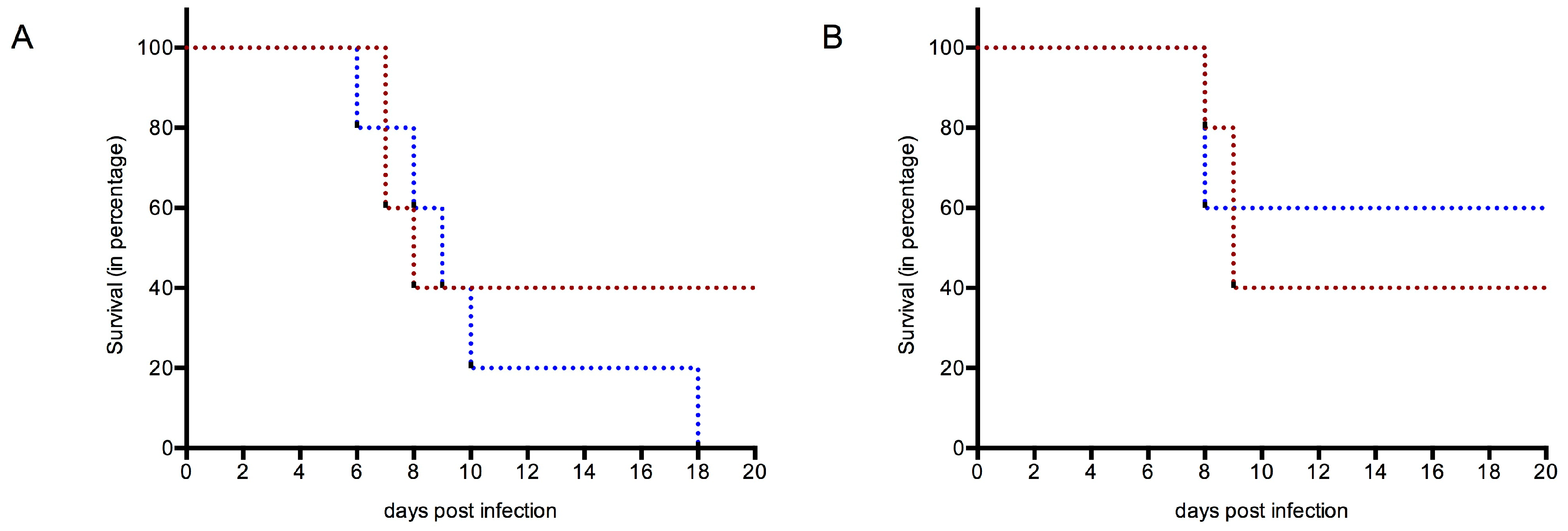
3.4. Protection against WNV Infection and Disease in WT and TLR3KO Mice Following Immunization
3.4.1. Survival
3.4.2. Clinical Scores
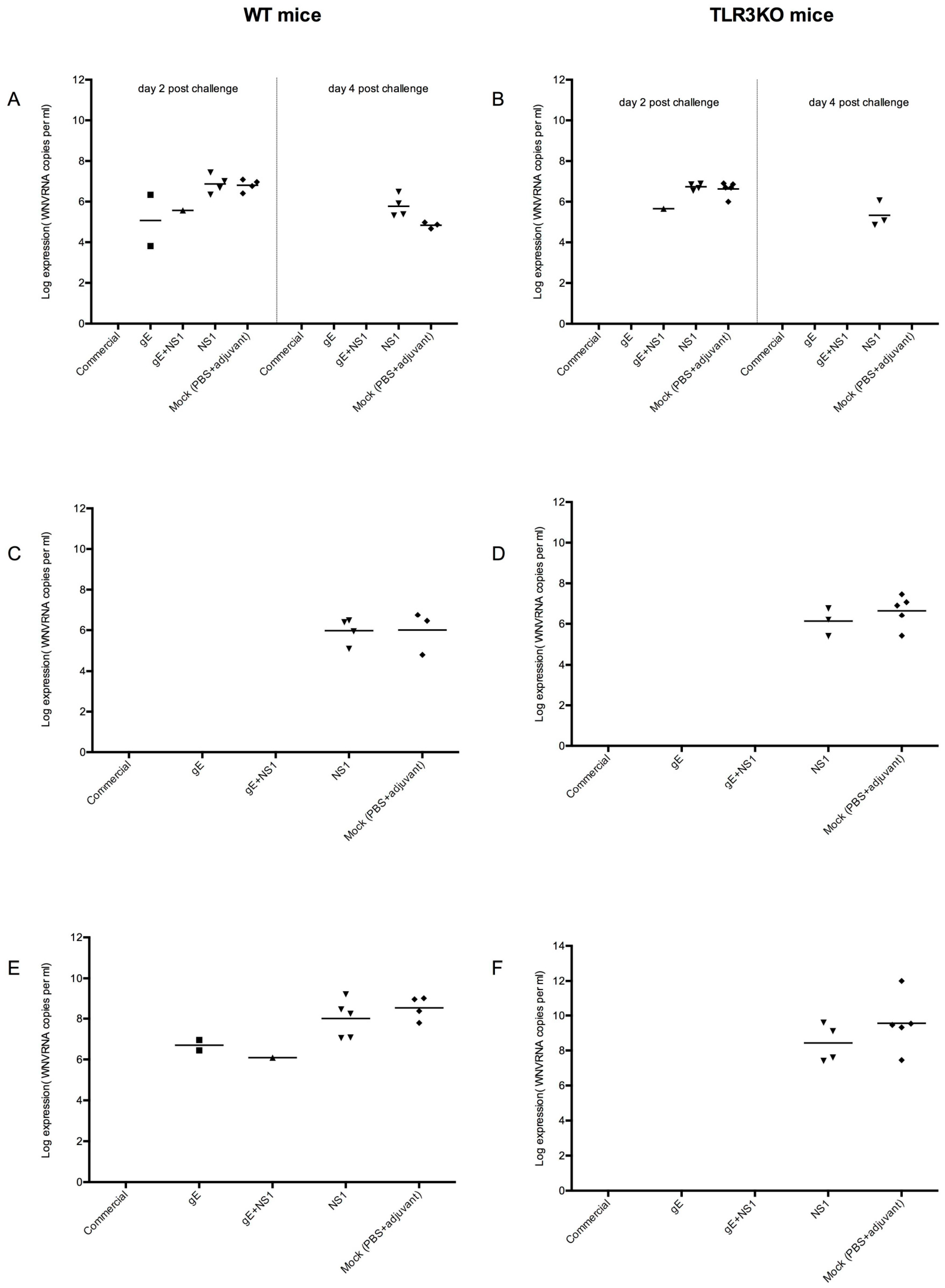
3.4.3. Viral Spread
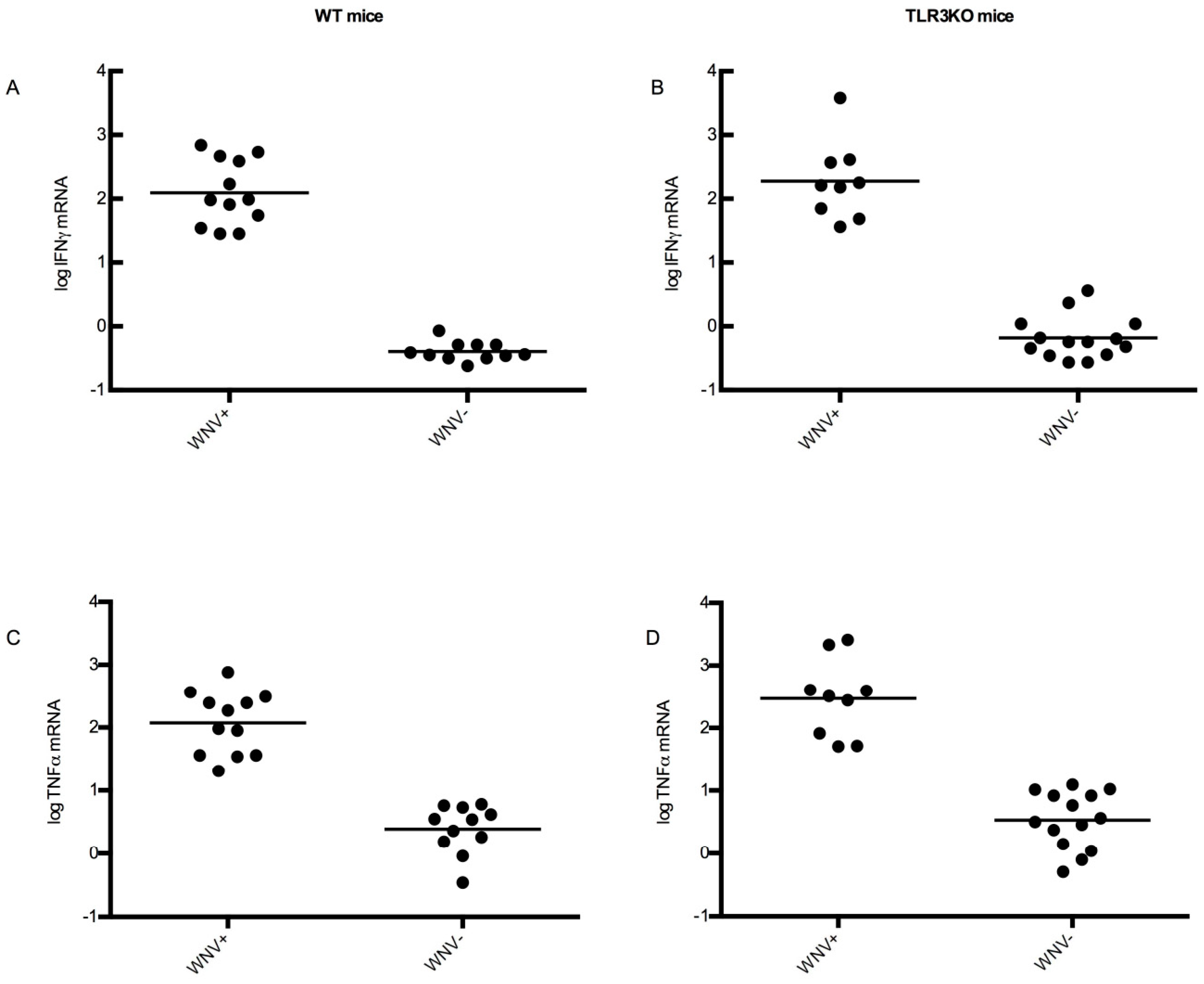
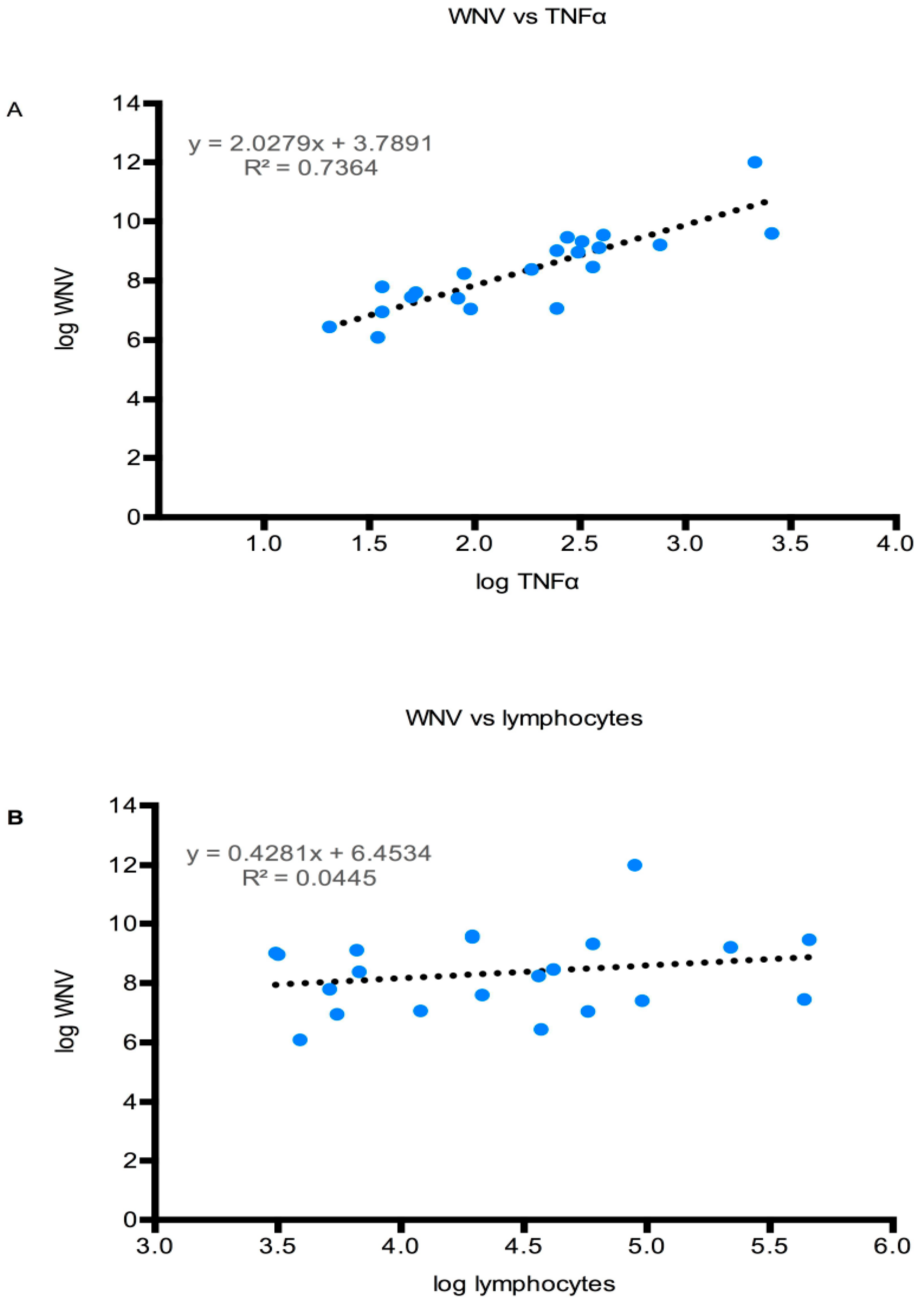
3.4.4. CNS Inflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kramer, L.D.; Styer, L.M.; Ebel, G.D. A Global Perspective on the Epidemiology of West Nile Virus. Annu. Rev. Entomol. 2008, 53, 61–81. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, E.; Gutierrez-Guzmán, A.; Del Amo, J.; Llorente, F.; El-Harrak, M.; Pérez-Ramírez, E.; Blanco, J.; Höfle, U.; Jiménez-Clavero, M. Pathogenicity of two recent Western Mediterranean West Nile virus isolates in a wild bird species indigenous to Southern Europe: The red-legged partridge. Vet. Res. 2011, 42, 11. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, J.; Llorente, F.; Figuerola, J.; Soriguer, R.C.; Moreno, A.M.; Cordioli, P.; Weissenböck, H.; Jiménez-Clavero, M.Á. Experimental infection of house sparrows (Passer domesticus) with West Nile virus isolates of Euro-Mediterranean and North American origins. Vet. Res. 2014, 45, 33. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramírez, E.; Llorente, F.; del Amo, J.; Nowotny, N.; Jiménez-Clavero, M.Á. Susceptibility and role as competent host of the red-legged partridge after infection with lineage 1 and 2 West Nile virus isolates of Mediterranean and Central European origin. Vet. Microbiol. 2018, 222, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Liu, J.; Kanesa-Thasan, N.; Myers, G.A.; Nichols, R.; Deary, A.; McCarthy, K.; Johnson, C.; Ermak, T.; Shin, S.; et al. A live, attenuated recombinant West Nile virus vaccine. Proc. Natl. Acad. Sci. USA 2006, 103, 6694–6699. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.E.; Pierson, T.C.; Hubka, S.; Rucker, S.; Gordon, I.J.; Enama, M.E.; Andrews, C.A.; Xu, Q.; Davis, B.S.; Nason, M.; et al. A West Nile Virus DNA Vaccine Induces Neutralizing Antibody in Healthy Adults during a Phase 1 Clinical Trial. J. Infect. Dis. 2007, 196, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Ledgerwood, J.E.; Pierson, T.C.; Hubka, S.A.; Desai, N.; Rucker, S.; Gordon, I.J.; Enama, M.E.; Nelson, S.; Nason, M.; Gu, W.; et al. A west nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial. J. Infect. Dis. 2011, 203, 1396–1404. [Google Scholar] [CrossRef]
- Biedenbender, R.; Bevilacqua, J.; Gregg, A.M.; Watson, M.; Dayan, G. Phase II, randomized, double-blind, placebo-controlled, multicenter study to investigate the immunogenicity and safety of a West Nile virus vaccine in healthy adults. J. Infect. Dis. 2011, 203, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Dayan, G.H.; Bevilacqua, J.; Coleman, D.; Buldo, A.; Risi, G. Phase II, dose ranging study of the safety and immunogenicity of single dose West Nile vaccine in healthy adults ≥50 years of age. Vaccine 2012, 30, 6656–6664. [Google Scholar] [CrossRef] [PubMed]
- Coller, B.-A.; Pai, V.; Weeks-Levy, C.L.; Ogata, S.A. Recombinant Subunit West Nile Virus Vaccine For Protections Of Human Subjects. U.S. Patent Application 13/322,784, 7 June 2012. [Google Scholar]
- Pierce, K.K.; Whitehead, S.S.; Kirkpatrick, B.D.; Grier, P.L.; Jarvis, A.; Kenney, H.; Carmolli, M.P.; Reynolds, C.; Tibery, C.M.; Lovchik, J.; et al. A Live Attenuated Chimeric West Nile Virus Vaccine, rWN/DEN4Δ30, Is Well Tolerated and Immunogenic in Flavivirus-Naive Older Adult Volunteers. J. Infect. Dis. 2017, 215, 52–55. [Google Scholar] [CrossRef]
- Durbin, A.P.; Wright, P.F.; Cox, A.; Kagucia, W.; Elwood, D.; Henderson, S.; Wanionek, K.; Speicher, J.; Whitehead, S.S.; Pletnev, A.G. The live attenuated chimeric vaccine rWN/DEN4Δ30 is well-tolerated and immunogenic in healthy flavivirus-naïve adult volunteers. Vaccine 2013, 31, 5772–5777. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, S.E.; Karlsson, K.H.; Reimer, J.M.; Corbach-Söhle, S.; Patel, S.; Richner, J.M.; Nowotny, N.; Barzon, L.; Bengtsson, K.L.; Ulbert, S.; et al. Matrix-MTM adjuvanted envelope protein vaccine protects against lethal lineage 1 and 2 West Nile virus infection in mice. Vaccine 2014, 32, 800–808. [Google Scholar] [CrossRef] [PubMed]
- De Filette, M.; Soehle, S.; Ulbert, S.; Richner, J.; Diamond, M.S.; Sinigaglia, A.; Barzon, L.; Roels, S.; Lisziewicz, J.; Lorincz, O.; et al. Vaccination of mice using the west nile virus E-protein in a DNA prime-protein boost strategy stimulates cell-mediated immunity and protects mice against a lethal challenge. PloS ONE 2014, 9, e87837. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.K.; Richner, J.M.; Poore, E.A.; Patil, P.P.; Amanna, I.J.; Slifka, M.K.; Diamond, M.S. A Hydrogen Peroxide-Inactivated Virus Vaccine Elicits Humoral and Cellular Immunity and Protects against Lethal West Nile Virus Infection in Aged Mice. J. Virol. 2013, 87, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.M.; Tesh, R.B.; Siirin, M.; da Rosa, A.T.; Newman, P.C.; Clements, D.E.; Ogata, S.; Coller, B.A.; Weeks-Levy, C.; Lieberman, M.M. Efficacy and durability of a recombinant subunit West Nile vaccine candidate in protecting hamsters from West Nile encephalitis. Vaccine 2007, 25, 2913–2918. [Google Scholar] [CrossRef] [PubMed]
- Siirin, M.T.; Travassos da Rosa, A.P.; Newman, P.; Weeks-Levy, C.; Coller, B.-A.; Xiao, S.-Y.; Lieberman, M.M.; Watts, D.M. Evaluation of the efficacy of a recombinant subunit West Nile vaccine in Syrian golden hamsters. Am. J. Trop. Med. Hyg. 2008, 79, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Anderson, J.F.; Magnarelli, L.A.; Wong, S.J.; Koski, R.A.; Fikrig, E. Immunization of Mice Against West Nile Virus with Recombinant Envelope Protein. J. Immunol. 2001, 167, 5273–5277. [Google Scholar] [CrossRef] [PubMed]
- Guy, B.; Guirakhoo, F.; Barban, V.; Higgs, S.; Monath, T.P.; Lang, J. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine 2010, 28, 632–649. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, J.; Miller, C.; Catalan, J.; Myers, G.A.; Ratterree, M.S.; Trent, D.W.; Monath, T.P. ChimeriVax-West Nile virus live-attenuated vaccine: preclinical evaluation of safety, immunogenicity, and efficacy. J. Virol. 2004, 78, 12497–12507. [Google Scholar] [CrossRef]
- Tesh, R.B.; Arroyo, J.; Travassos da Rosa, A.P.A.; Guzman, H.; Xiao, S.Y.; Monath, T.P. Efficacy of killed virus vaccine, live attenuated chimeric virus vaccine, and passive immunization for prevention of West Nile virus encephalitis in hamster model. Emerg. Infect. Dis. 2002, 8, 1392. [Google Scholar] [CrossRef]
- Iglesias, M.C.; Frenkiel, M.P.; Mollier, K.; Souque, P.; Despres, P.; Charneau, P. A single immunization with a minute dose of a lentiviral vector-based vaccine is highly effective at eliciting protective humoral immunity againts West Nile virus. J. Gene Med. 2006, 8, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.-H.J.; Chiang, C.-C.S.; Ng, M.-L. Immunization of flavivirus West Nile recombinant envelope domain III protein induced specific immune response and protection against West Nile virus infection. J. Immunol. 2007, 178, 2699–2705. [Google Scholar] [CrossRef] [PubMed]
- Spohn, G.; Jennings, G.T.; Martina, B.E.; Keller, I.; Beck, M.; Pumpens, P.; Osterhaus, A.D.; Bachmann, M.F. A VLP-based vaccine targeting domain III of the West Nile virus e protein protects from lethal infection in mice. Virol. J. 2010, 7, 146. [Google Scholar] [CrossRef] [PubMed]
- Amanna, I.J.; Raué, H.P.; Slifka, M.K. Development of a new hydrogen peroxide-based vaccine platform. Nat. Med. 2012, 18, 974. [Google Scholar] [CrossRef] [PubMed]
- Verstrepen, B.E.; Oostermeijer, H.; Fagrouch, Z.; van Heteren, M.; Niphuis, H.; Haaksma, T.; Kondova, I.; Bogers, W.M.; de Filette, M.; Sanders, N.; et al. Vaccine-Induced Protection of Rhesus Macaques against Plasma Viremia after Intradermal Infection with a European Lineage 1 Strain of West Nile Virus. PloS ONE 2014, 9, e112568. [Google Scholar] [CrossRef] [PubMed]
- Martina, B.E.; Koraka, P.; van den Doel, P.; van Amerongen, G.; Rimmelzwaan, G.F.; Osterhaus, A.D.M.E. Immunization with West Nile virus envelope domain III protects mice against lethal infection with homologous and heterologous virus. Vaccine 2008, 26, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S.; Pierson, T.C.; Fremont, D.H. The structural immunology of antibody protection against West Nile virus. Immunol. Rev. 2008, 225, 212–225. [Google Scholar] [CrossRef]
- McDonald, W.F.; Huleatt, J.W.; Foellmer, H.G.; Hewitt, D.; Tang, J.; Desai, P.; Price, A.; Jacobs, A.; Takahashi, V.N.; Huang, Y.; et al. A West Nile Virus Recombinant Protein Vaccine That Coactivates Innate and Adaptive Immunity. J. Infect. Dis. 2007, 195, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Kanai, R.; Kar, K.; Anthony, K.; Gould, L.H.; Ledizet, M.; Fikrig, E.; Marasco, W.A.; Koski, R.A.; Modis, Y. Crystal Structure of West Nile Virus Envelope Glycoprotein Reveals Viral Surface Epitopes. J. Virol. 2006, 80, 11000–11008. [Google Scholar] [CrossRef] [PubMed]
- Lindenbach, B.D.; Rice, C.M. trans-Complementation of Yellow Fever Virus NS1 Reveals a Role in Early RNA Replication. J. Virol. 1997, 71, 9608–9617. [Google Scholar]
- Whiteman, M.C.; Wicker, J.A.; Kinney, R.M.; Huang, C.Y.H.; Solomon, T.; Barrett, A.D.T. Multiple amino acid changes at the first glycosylation motif in NS1 protein of West Nile virus are necessary for complete attenuation for mouse neuroinvasiveness. Vaccine 2011, 29, 9702–9710. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.R.; de Sessions, P.F.; Leon, M.A.; Scholle, F. West Nile Virus Nonstructural Protein 1 Inhibits TLR3 Signal Transduction. J. Virol. 2008, 82, 8262–8271. [Google Scholar] [CrossRef] [PubMed]
- Baronti, C.; Sire, J.; de Lamballerie, X.; Quérat, G. Nonstructural NS1 proteins of several mosquito-borne Flavivirus do not inhibit TLR3 signaling. Virology 2010, 404, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Puerta-Guardo, H.; Glasner, D.R.; Espinosa, D.A.; Wang, C.; Beatty, P.R.; Correspondence, E.H. Flavivirus NS1 Triggers Tissue-Specific Vascular Endothelial Dysfunction Reflecting Disease Tropism. Cell Rep. 2019, 26, 1598–1613. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.M.; Liszewski, M.K.; Nybakken, G.; Davis, A.E.; Townsend, R.R.; Fremont, D.H.; Atkinson, J.P.; Diamond, M.S. West Nile virus nonstructural protein NS1 inhibits complement activation by binding the regulatory protein factor H. Proc. Natl. Acad. Sci. USA 2006, 103, 19111–19116. [Google Scholar] [CrossRef] [PubMed]
- Rebollo, B.; Llorente, F.; Pérez-Ramírez, E.; Sarraseca, J.; Gallardo, C.; Risalde, M.Á.; Höfle, U.; Figuerola, J.; Soriguer, R.C.; Venteo, Á.; et al. Absence of protection from West Nile virus disease and adverse effects in red legged partridges after non-structural NS1 protein administration. Comp. Immunol. Microbiol. Infect. Dis. 2018, 56, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Daffis, S.; Samuel, M.A.; Suthar, M.S.; Gale, M.; Diamond, M.S. Toll-Like Receptor 3 Has a Protective Role against West Nile Virus Infection. J. Virol. 2008, 82, 10349–10358. [Google Scholar] [CrossRef]
- Wang, T.; Town, T.; Alexopoulou, L.; Anderson, J.F.; Fikrig, E.; Flavell, R.A. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 2004, 10, 1366–1373. [Google Scholar] [CrossRef]
- Kong, K.-F.; Delroux, K.; Wang, X.; Qian, F.; Arjona, A.; Malawista, S.E.; Fikrig, E.; Montgomery, R.R. Dysregulation of TLR3 Impairs the Innate Immune Response to West Nile Virus in the Elderly. J. Virol. 2008, 82, 7613–7623. [Google Scholar] [CrossRef]
- Armstrong, P.M.; Vossbrinck, C.R.; Andreadis, T.G.; Anderson, J.F.; Pesko, K.N.; Newman, R.M.; Lennon, N.J.; Birren, B.W.; Ebel, G.D.; Henn, M.R. Molecular evolution of West Nile virus in a northern temperate region: Connecticut, USA 1999–2008. Virology 2011, 417, 203–210. [Google Scholar] [CrossRef]
- Chung, K.M.; Nybakken, G.E.; Thompson, B.S.; Engle, M.J.; Marri, A.; Fremont, D.H.; Diamond, M.S. Antibodies against West Nile Virus Nonstructural Protein NS1 Prevent Lethal Infection through Fc γ Receptor-Dependent and -Independent Mechanisms Antibodies against West Nile Virus Nonstructural Protein NS1 Prevent Lethal Infection through Fc ␥ Receptor-D. J. Virol. 2006, 80, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Koraka, P.; van Boheemen, S.; Roose, J.M.; Jaarsma, D.; van de Vijver, D.A.M.C.; Osterhaus, A.D.M.E.; Martina, B.E.E. Characterization of the Mouse Neuroinvasiveness of Selected European Strains of West Nile Virus. PLoS ONE 2013, 8, e74575. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barzon, L.; Franchin, E.; Squarzon, L.; Lavezzo, E.; Toppo, S.; Martello, T.; Bressan, S.; Pagni, S.; Cattai, M.; Piazza, A.; et al. Genome sequence analysis of the first human West Nile virus isolated in Italy in 2009. Euro Surveill. 2009, 14, 19384. [Google Scholar] [PubMed]
- Rizzoli, A.; Jimenez-Clavero, M.A.; Barzon, L.; Cordioli, P.; Figuerola, J.; Koraka, P.; Martina, B.; Moreno, A.; Nowotny, N.; Pardigon, N.; et al. The challenge of West Nile virus in Europe: knowledge gaps and research priorities. Euro Surveill. 2015, 20, 21135. [Google Scholar] [CrossRef] [PubMed]
- Barzon, L.; Pacenti, M.; Cusinato, R.; Cattai, M.; Franchin, E.; Pagni, S.; Martello, T.; Bressan, S.; Squarzon, L.; Cattelan, A.M.; et al. Human cases of West Nile virus infection in northeastern Italy, 15 June to 15 November 2010. Euro Surveill. 2011, 16, 19949. [Google Scholar] [PubMed]
- Barzon, L.; Pacenti, M.; Franchin, E.; Pagni, S.; Martello, T.; Cattai, M.; Cusinato, R.; Palù, G. Excretion of west nile virus in urine during acute infection. J. Infect. Dis. 2013, 208, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Cossarizza, A.; Chang, H.-D.; Radbruch, A.; Akdis, M.; Andrä, I.; Annunziato, F.; Bacher, P.; Barnaba, V.; Battistini, L.; Bauer, W.M.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur. J. Immunol. 2017, 47, 1584–1797. [Google Scholar] [CrossRef] [PubMed]
- Omasits, U.; Ahrens, C.H.; Müller, S.; Wollscheid, B. Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics 2014, 30, 884–886. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.A.; Diamond, M.S. Pathogenesis of West Nile Virus Infection: a Balance between Virulence, Innate and Adaptive Immunity, and Viral Evasion. J. Virol. 2006, 80, 9349–9360. [Google Scholar] [CrossRef]
- Kumar, M.; Verma, S.; Nerurkar, V.R. Pro-inflammatory cytokines derived from West Nile virus (WNV)-infected SK-N-SH cells mediate neuroinflammatory markers and neuronal death. J. Neuroinflammation 2010, 7, 73. [Google Scholar] [CrossRef]
- Volz, A.; Lim, S.; Kaserer, M.; Lülf, A.; Marr, L.; Jany, S.; Deeg, C.A.; Pijlman, G.P.; Koraka, P.; Osterhaus, A.D.M.E.; et al. Immunogenicity and protective efficacy of recombinant Modified Vaccinia virus Ankara candidate vaccines delivering West Nile virus envelope antigens. Vaccine 2016, 34, 1915–1926. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramírez, E.; Llorente, F.; Del Amo, J.; Fall, G.; Sall, A.A.; Lubisi, A.; Lecollinet, S.; Vázquez, A.; Jiménez-Clavero, M.Á. Pathogenicity evaluation of twelve west nile virus strains belonging to four lineages from five continents in a mouse model: Discrimination between three pathogenicity categories. J. Gen. Virol. 2017, 98, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Suthar, M.S.; Diamond, M.S.; Gale, M. West Nile virus infection and immunity. Nat. Rev. Microbiol. 2013, 11, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Sabouri, A.H.; Garibaldi Marcondes, M.C.; Flynn, C.; Berger, M.; Xiao, N.; Fox, H.S.; Sarvetnick, N.E. TLR signaling controls lethal encephalitis in WNV-infected brain. Brain Res. July 2014, 1574, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.W.; Choi, J.Y.; Uyangaa, E.; Kim, S.B.; Kim, J.H.; Kim, B.S.; Kim, K.; Eo, S.K. Distinct Dictation of Japanese Encephalitis Virus-Induced Neuroinflammation and Lethality via Triggering TLR3 and TLR4 Signal Pathways. PLoS Pathog. 2014, 10, e1004319. [Google Scholar] [CrossRef] [PubMed]


| Group | # | Genotype | Vaccination |
|---|---|---|---|
| A | 3 | aWT C57BL/6 | Commercial |
| B | 5 | WT C57BL/6 | cgE |
| C | 5 | WT C57BL/6 | dNS1 |
| D | 5 | WT C57BL/6 | gE + NS1 |
| E | 5 | WT C57BL/6 | Mock (ePBS + adjuvant) |
| F | 3 | bTLR3KO | Commercial |
| G | 5 | TLR3KO | gE |
| H | 5 | TLR3KO | NS1 |
| J | 5 | TLR3KO | gE + NS1 |
| K | 5 | TLR3KO | Mock (PBS + adjuvant) |
| Symptom | Observation | Score |
|---|---|---|
| Loss of body weight | Body weight within range of untreated group | 0 |
| 1–5% loss of body weight | 1 | |
| >5–15% loss | 2 | |
| >15% loss | 3 | |
| Pain-associated behavior | Normal activity | 0 |
| Reduced activity, moving after slight impulse, shivering | 1 | |
| Inactive, apathic, moving after moderate impulse | 2 | |
| Ataxia, paralysis, not moving even after moderate stimulation | 3 | |
| Normal posture | 0 | |
| Hunched posture | 1 | |
| Hunched posture, head lying on the cage floor | 2 | |
| Lying prone on the cage floor | 3 | |
| Physical changes | Normal hair coat | 0 |
| Ruffled | 1 | |
| Ruffled, loss of fur, ungroomed | 2 | |
| Normal eyes | 0 | |
| Eyes closed or squinted, no discharge | 1 | |
| Eyes closed or squinted, with discharge | 2 |
| Immunization | WT Mice | TLR3KO | ||
|---|---|---|---|---|
| Brain a | Spleen b | Brain | Spleen | |
| gE | 2/5 c | 0/5 | 0/5 | 0/5 |
| NS1 | 5/5 | 4/5 | 4/5 | 3/5 |
| gE + NS1 | 1/5 | 0/5 | 0/5 | 0/5 |
| Commercial vaccine | 0/3 | 0/3 | 0/3 | 0/3 |
| PBS + adjuvant | 4/5 | 3/5 | 5/5 | 5/5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, S.; Sinigaglia, A.; Barzon, L.; Fassan, M.; Sparber, F.; LeibundGut-Landmann, S.; Ackermann, M. Role of NS1 and TLR3 in Pathogenesis and Immunity of WNV. Viruses 2019, 11, 603. https://doi.org/10.3390/v11070603
Patel S, Sinigaglia A, Barzon L, Fassan M, Sparber F, LeibundGut-Landmann S, Ackermann M. Role of NS1 and TLR3 in Pathogenesis and Immunity of WNV. Viruses. 2019; 11(7):603. https://doi.org/10.3390/v11070603
Chicago/Turabian StylePatel, Sameera, Alessandro Sinigaglia, Luisa Barzon, Matteo Fassan, Florian Sparber, Salome LeibundGut-Landmann, and Mathias Ackermann. 2019. "Role of NS1 and TLR3 in Pathogenesis and Immunity of WNV" Viruses 11, no. 7: 603. https://doi.org/10.3390/v11070603
APA StylePatel, S., Sinigaglia, A., Barzon, L., Fassan, M., Sparber, F., LeibundGut-Landmann, S., & Ackermann, M. (2019). Role of NS1 and TLR3 in Pathogenesis and Immunity of WNV. Viruses, 11(7), 603. https://doi.org/10.3390/v11070603







