Culex torrentium: A Potent Vector for the Transmission of West Nile Virus in Central Europe
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Rearing of Mosquitoes
2.2. Experimental Infection and Analysis
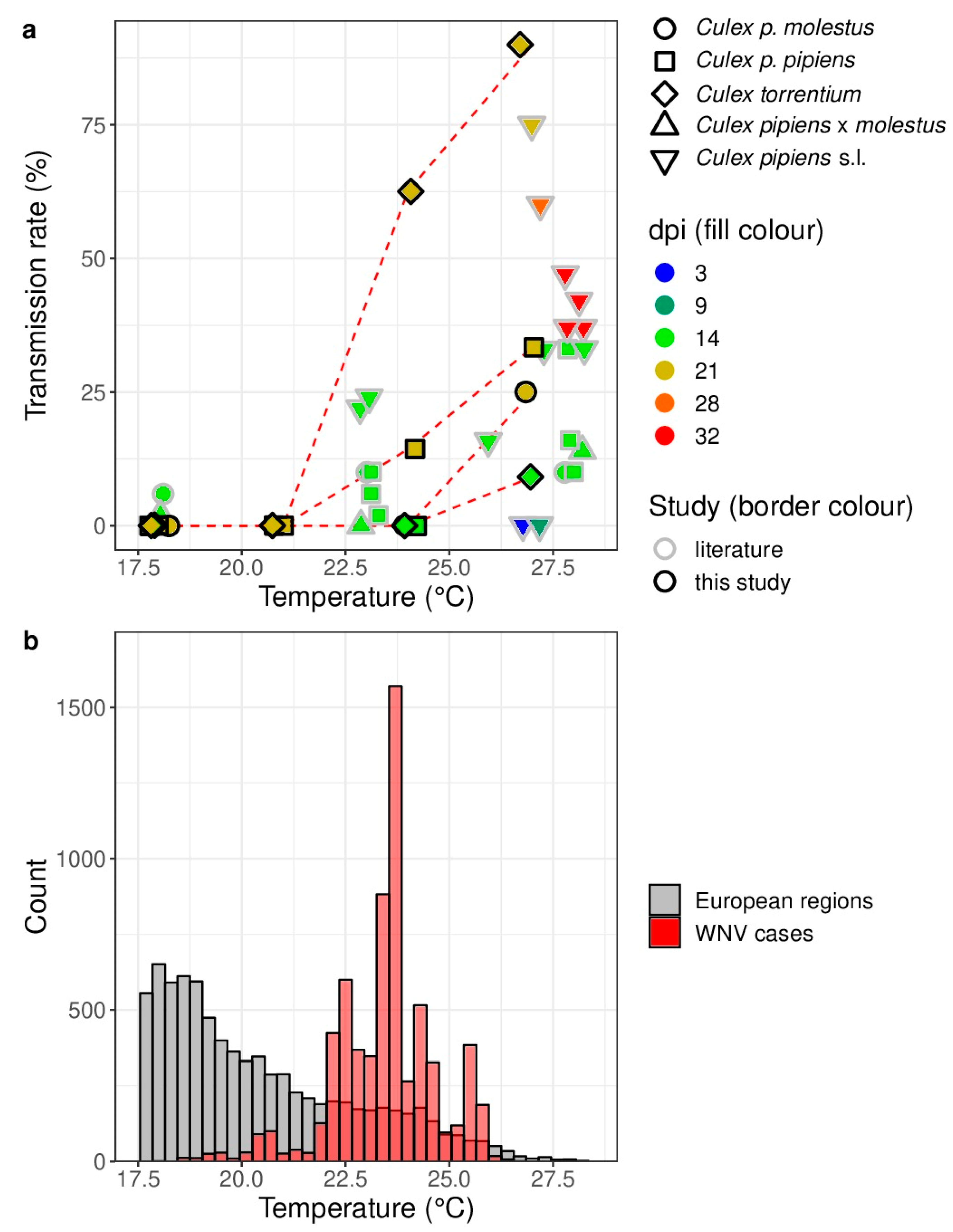
2.3. Comparison of the Study Results with Previous Vector Competence Studies and the Actual Circulation of WNV in Europe
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Calisher, C.H.; Karabatsos, N.; Dalrymple, J.M.; Shope, R.E.; Porterfield, J.S.; Westaway, E.G.; Brandt, W.E. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J. Gen. Virol. 1989, 70, 37–43. [Google Scholar] [CrossRef]
- Campbell, G.L.; Marfin, A.A.; Lanciotti, R.S.; Gubler, D.J. West Nile virus. Lancet. Infect. Dis. 2002, 2, 519–529. [Google Scholar] [CrossRef]
- Gyure, K.A. West Nile virus infections. J. Neuropathol. Exp. Neurol. 2009, 68, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.R.; Brault, A.C.; Nasci, R.S. West Nile virus: review of the literature. JAMA 2013, 310, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention (CDC). West Nile Virus: Final Cumulative Maps & Data for 1999–2017. Available online: https://www.cdc.gov/westnile/statsmaps/cumMapsData.html (accessed on 28 September 2018).
- European Centre for Disease Prevention and Control (ECDC). No Surveillance and Disease Data for West Nile. Available online: https://ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data (accessed on 28 September 2018).
- Harbach, R.E. Culex pipiens: species versus species complex—Taxonomic history and perspective. J. Am. Mosq. Control. Assoc. 2012, 28, 10–23. [Google Scholar] [CrossRef]
- Scheuch, D.E.; Schäfer, M.; Eiden, M.; Heym, E.C.; Ziegler, U.; Walther, D.; Schmidt-Chanasit, J.; Keller, M.; Groschup, M.H.; Kampen, H. Detection of Usutu, Sindbis, and Batai viruses in moquitoes (Diptera: Culicidae) collected in Germany 2011–2016. Viruses 2018, 10, 389. [Google Scholar] [CrossRef]
- Hesson, J.C.; Rettich, F.; Merdić, E.; Vignjević, G.; Ostman, O.; Schäfer, M.; Schaffner, F.; Foussadier, R.; Besnard, G.; Medlock, J.; et al. The arbovirus vector Culex torrentium is more prevalent than Culex pipiens in northern and central Europe. Med. Vet. Entomol. 2014, 28, 179–186. [Google Scholar] [CrossRef]
- Zittra, C.; Flechl, E.; Kothmayer, M.; Vitecek, S.; Rossiter, H.; Zechmeister, T.; Fuehrer, H.P. Ecological characterization and molecular differentiation of Culex pipiens complex taxa and Culex torrentium in eastern Austria. Parasit. Vector 2016, 9, 197. [Google Scholar] [CrossRef]
- Rudolf, M.; Czajika, C.; Bürstler, J.; Melaun, C.; Jöst, H.; von Thien, H.; Badusche, M.; Becker, N.; Schmidt-Chanasit, J.; Krüger, A.; et al. First nationwide surveillance of Culex pipiens complex and Culex torrentium mosquitoes demonstrated the presence of Culex pipiens biotype pipiens/molestus hybrids in Germany. PLoS ONE 2013, 8, e71832. [Google Scholar] [CrossRef] [PubMed]
- Lühken, R.; Steinke, S.; Leggewie, M.; Tannich, E.; Krüger, A.; Becker, S.; Kiel, E. Physio-chemical characteristics of Culex pipiens sensu lato and Culex torrentium (Diptera: Culicidae) breeding sites in Germany. J. Med. Entomol. 2015, 52, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.; Petrić, D.; Zgomba, M.; Boase, C.; Madon, M.; Dahk, C.; Kaiser, A. Mosquitoes and Their Control, 2nd ed.; Springer: Heidelberg, Germany, 2010; p. 196. [Google Scholar]
- Börstler, J.; Lühken, R.; Rudolf, M.; Steinke, S.; Melaun, C.; Becker, S.; Garms, R.; Krüger, A. The use of morphometric wing characters to discriminate female Culex pipiens and Culex torrentium. J. Vector Ecol. 2014, 39, 204–212. [Google Scholar]
- Vogels, C.B.; van de Peppe, L.J.; van Vliet, A.J.; Westenberh, M.; Ibañez_Justica, A.; Stroo, A.; Viesser, T.M.; Koenrasdt, C.J. Winter activity and aboveground hybridization between the two biotypes of the West Nile virus vector Culex pipiens. Vector Borne Zoonotic Dis. 2015, 15, 619–626. [Google Scholar] [CrossRef]
- Calzolari, M.; Bonilauri, P.; Bellini, R.; Becker, S.; Dottori, M. Wide recognition of Culex pipiens and lack of detection of Culex torrentium through biomolecular differentiation of mosquitoes in the Emilia-Romagna region, Northern Italy. Med. Vet. Entomol. 2016, 30, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Hesson, J.C.; Schäfer, M.; Lundström, J.O. First report on human-biting Culex pipiens in Sweden. Parasites Vectors 2016, 9, 632. [Google Scholar] [CrossRef]
- Lundström, J.O.; Niklasson, B.; Francy, D.B. Swedish Culex torrentium and Cx. pipiens (Diptera: Culicidae) as experimental vectors of Ockelbo virus. J. Med. Entomol. 1990, 27, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Börstler, J.; Jöst, H.; Garms, R.; Krüger, A.; Tannich, E.; Becker, N.; Schmidt-Chanasit, J.; Lühken, R. Host-feeding patterns of mosquito species in Germany. Parasites Vectors 2016, 9, 318. [Google Scholar] [CrossRef]
- Osorio, H.C.; Ze-Ze, L.; Amaro, F.; Nunes, A.; Alves, M.J. Sympatric occurrence of Culex pipiens (Diptera, Culicidae) biotypes pipiens, molestus and their hybrids in Portugal, Western Europe: feeding patterns and habitat determinants. Med. Vet. Entomol. 2014, 28, 103–109. [Google Scholar] [CrossRef]
- Vogels, C.B.F.; Göertz, G.P.; Pijlman, G.P.; Koenraadt, C.J.M. Vector competence of European mosquitoes for West Nile virus. Emerg. Microbes. Infect. 2017, 6, e96. [Google Scholar] [CrossRef] [PubMed]
- Romo, H.; Papa, A.; Kading, R.; Clark, R.; Delorey, M.; Brault, A.C. Comparative vector competence of North American Culex pipiens and Culex quinquefasciatus for African and European Lineage 2 West Nile viruses. Am. J. Trop. Med. Hyg. 2018, 98, 1863–1869. [Google Scholar] [CrossRef]
- Camp, J.V.; Kolodziejek, J.; Nowotny, N. Targeted surveillance reveals native and invasive mosquito species infected with Usutu virus. Parasites Vectors 2019, 12, 46. [Google Scholar] [CrossRef]
- Boccolini, D.; Toma, L.; Di Luca, M.; Severini, F.; Romi, R.; Remoli, M.E.; Sabatucci, M.; Venturi, G.; Rezza, G.; Fortuna, C. Experimental investigation of the susceptibility of Italian Culex pipiens mosquitoes to Zika virus infection. Eurosurveillance 2016, 21, pii30328. [Google Scholar] [CrossRef]
- Heitmann, A.; Jansen, S.; Lühken, R.; Helms, M.; Pluskota, B.; Becker, N.; Kuhn, C.; Schmidt-Chanasit, J.; Tannich, E. Experimental transmission of Zika virus by mosquitoes from central Europe. Eurosurveillance 2017, 22, pii30437. [Google Scholar] [CrossRef]
- Talbalaghi, A.; Moutailler, S.; Vazeille, M.; Failloux, A.B. Are Aedes albopictus or other mosquito species from Northern Italy competent to sustain new arboviral outbreaks? Med. Vet. Entomol. 2010, 24, 83–87. [Google Scholar] [CrossRef]
- Leggewie, M.; Badusche, M.; Rudolf, M.; Jansen, S.; Börstler, J.; Hiber, K.; Krüger, A.; Schmidt-Chansit, J.; Tannich, E.; Becker, S. Culex pipiens and Culex torrentium populations from Central Europe are susceptible to West Nile virus infection. One Health 2016, 2, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Hesson, J.C.; Verner_Carlsson, J.; Larsson, A.; Ahmed, R.; Lundquist, A.; Lundströhm, J.L. Culex torrentium mosquito role as major enzootic vector defined by rate of Sindbis virus infection, Sweden, 2009. Emerg. Infect. Dis. 2015, 21, 875–878. [Google Scholar] [CrossRef]
- Gabriel, M.; Emmerich, P.; Frank, C.; Fiedler, M.; Rashidi-Alvaijeh, J.; Jochhum, C.; Günther, S.; Auerhammer, K.; Rupprecht, H.J.; Blank, R.T.; et al. Increase in West Nile virus infections imported to Germany in 2012. J. Clin. Virol. 2013, 58, 587–589. [Google Scholar] [CrossRef]
- Calistri, P.; Savini, L.; Candeloro, L.; Di Sabatini, D.; Cito, F.; Bruno, R.; Danzetta, M.L. A transitional model for the evaluation of West Nile Virus transmission in Italy. Transbound. Emerg. Dis. 2016, 63, 485–496. [Google Scholar] [CrossRef]
- Ziegler, U.; Lühken, R.; Keller, M.; Cafar, D.; van der Grinten, E.; Michel, F.; Albrecht, K.; Eiden, M.; Rinder, M.; Lachmann, L.; et al. West Nile virus epizootic in Germany, 2018. Antivir. Res. 2019, 162, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Rossini, G.; Carletti, F.; Bordi, L.; Cavrini, F.; Gaibani, P.; Landini, M.P.; Pierro, A.; Capobianchi, M.R.; Di Caro, A.; Sambi, V. Phylogenetic analysis of West Nile Virus isolates, Italy, 2008–2009. Emerg. Infect. Dis. 2011, 17, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.-Y.; Davis., B.S.; Chang, G.J.J. Development of multiplex real-time reverse transcriptase PCR assays for detecting eight medically important flaviviruses in mosquitoes. J. Clin. Microbiol. 2007, 45, 584–589. [Google Scholar] [CrossRef]
- Eshoo, M.W.; Whitehouse, C.A.; Zoll, S.T.; Massire, C.; Pennella, T.T.; Blyn, L.B.; Sampath, R.; Ecker, J.A.; Desai, W.; Wasieloski, L.P. Direct broad-range detection of alphaviruses in mosquito extracts. Virology 2007, 368, 286–295. [Google Scholar] [CrossRef]
- Lambert, A.J.; Lanciotti, R.S. Consensus amplification and novel multiplex sequencing method for S segment species identification of 47 viruses of the Orthobunyavirus, Phlebovirus, and Nairovirus genera of the family Bunyaviridae. J. Clin. Microbiol. 2009, 47, 2398–2404. [Google Scholar] [CrossRef]
- Jansen, S.; Heitmann, A.; Lühken, R.; Jöst, H.; Helms, M.; Vapalahti, O.; Schmidt-Chanasit, J.; Tannich, E. Experimental transmission of Zika virus by Aedes japonicus japonicus from Southwest Germany. Emerg. Microbes Infect. 2018, 7, 192. [Google Scholar] [CrossRef]
- Heitmann, A.; Jansen, S.; Lühken, R.; Leggewie, M.; Schmidt-Chanasit, J.; Tannich, E. Forced salivation as a method to analyze vector competence of mosquitoes. J. Vis. Exp. 2018, 138, e57980. [Google Scholar] [CrossRef]
- Heitmann, A.; Jansen, S.; Lühken, R.; Helms, M.; Pluskota, B.; Becker, N.; Kuhn, C.; Schmidt-Chanasit, J.; Tannich, E. Experimental risk assessment for chikungunya virus transmission based on vector competence, distribution and temperature suitability in Europe, 2018. Eurosurveillance 2018, 23, pii1800033. [Google Scholar] [CrossRef]
- Veronesi, E.; Paslaru, A.; Silagi, C.; Tobler, K.; Glavinic, U.; Torgerson, P.; Mathis, A. Experimental evaluation of infection, dissemination, and transmission rates for two West Nile virus strains in European Aedes japonicus under a fluctuating temperature regime. Parasitol. Res. 2018, 117, 1925–1932. [Google Scholar] [CrossRef]
- Fros, J.J.; Geertsema, C.; Vogels, C.B.; Roosjen, P.P.; Failloux, A.B.; Vlak, J.M.; Koenradt, C.J.; Takken, W.; Pijlmann, G.P. West Nile virus: high transmission rate in North-Western European mosquitoes indicates its epidemic potential and warrants increased surveillance. PLoS Negl. Trop. Dis. 2015, 9, e0003956. [Google Scholar] [CrossRef] [PubMed]
- Haylock, M.R.; Hofstra, N.; Klein Tank, A.M.G.; Klok, E.J.; Jones, P.D.; New, M. A European daily high-resolution gridded data set of surface temperature and precipitation for 1950–2006. J. Geophys. Res. Atmos. 2008, 113, D20119. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 23 May 2019).
- Wilke, C.O. Cowplot: Streamlined Plot theme and Plot Annotations for ‘ggplot2’. R Package Version 0.9.2. Available online: https://CRAN.R-project.org/package=cowplot (accessed on 23 May 2019).
- Wickham, H.; Francois, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation. R Package Version 0.7.4. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 23 May 2019).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 1st ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Bache, S.M.; Wickham, H. Magrittr: A Forward-Pipe Operator for R. R Package Version 1.5. Available online: https://CRAN.R-project.org/package=magrittr (accessed on 23 May 2019).
- Rivand, R.; Lewin-Koh, N. Maptools: Tools for Reading and Handling Spatial Objects. R package version 0.9–2. Available online: https://CRAN.R-project.org/package=maptools (accessed on 23 May 2019).
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling. R package version 2.6–7. Available online: https://CRAN.R-project.org/package=raster (accessed on 23 May 2019).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultanous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Caraguel, C.G.B.; Stryhn, H.; Gagné, N.; Dohoo, I.R.; Hammell, K.L. Selection of a cutoff for real-time polymerase chain reaction results to fit a diagnostic purpose: analytical and epidemiologic approaches. J. Vet. Diagn. Investig. 2001, 23, 2–15. [Google Scholar] [CrossRef]
- Leggewie, M.; Krumkamp, R.; Badusche, M.; Heitmann, A.; Jansen, S.; Schmidt-Chanasit, J.; Tannich, E.; Becker, S.C. Culex torrentium mosquitoes from Germany are negative for Wolbachia. Med. Vet. Entomol. 2018, 32, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, U.; Seidowski, D.; Angenvoort, J.; Eiden, M.; Nowotny, N.; Groshup, M.H. Monitoring of West Nile virus infections in Germany. Zoonoses Public Health 2012, 59 (Suppl. 2), 95–101. [Google Scholar] [CrossRef]
- Michel, F.; Fischer, D.; Eiden, M.; Fast, C.; Reuschel, M.; Müller, R.; Rinder, M.; Urbaniak, S.; Brandes, F.; Schwehn, R.; et al. West Nile Virus and Usutu virus monitoring of wild birds in Germany. Int. J. Environ. Res. Public Health 2018, 15, 171. [Google Scholar] [CrossRef]
- Börstler, J.; Engel, D.; Petersen, M.; Poggensee, C.; Jansen, S.; Schmidt-Chanasit, J.; Lühken, R. Surveillance of maternal antibodies against West Nile virus in chicken eggs in South-West Germany. Trop. Med. Int. Health 2016, 21, 687–690. [Google Scholar] [CrossRef]
- Kenney, J.L.; Brault, A.C. The role of environmental, virological and vector interactions in dictating biological transmission of arthtopod-borne viruses by mosquitoes. Adv. Virus Res. 2014, 89, 39–83. [Google Scholar]
- Vogels, C.B.F.; Göertz, G.P.; Pijlman, G.P.; Koenradt, C.J.M. Vector competence of northern and southern European Culex pipiens pipiens mosquitoes for West Nile virus across a gradient of temperatures. Med. Vet. Entomol. 2017, 31, 358–364. [Google Scholar] [CrossRef]
- Cadar, D.; Lühken, R.; van der Jeugd, H.; Gariglyani, M.; Ziegler, U.; Keller, M.; Lahoreau, J.; Lachmann, L.; Becker, N.; Kik, M. Widespread activity of multiple lineages of Usutu virus, Western Europe, 2016. Eurosurveillance 2017, 22, pii30452. [Google Scholar] [CrossRef]
- Lühken, R.; Jöst, H.; Cadar, D.; Thomas, S.M.; Bosch, S.; Tannich, E.; Becker, N.; Ziegler, U.; Lachmann, L.; Schmidt-Chanasit, J. Distribution of Usutu Virus in Germany and its effect on breeding bird populations. Emerg. Infect. Dis. 2017, 23, 1994–2001. [Google Scholar] [CrossRef]
| 14 Days Post Infection | 21 Days Post Infection | ||||||
|---|---|---|---|---|---|---|---|
| Mosquito Taxa | T in °C | IR (%) | TR (%) | TE (%) | IR (%) | TR (%) | TE (%) |
| Culex p. molestus | 18 | 0/29 (0.0) | NA | NA | 1/29 (3.4) | 0/1 (0.0) | NA |
| 24 | 0/31 (0.0) | NA | NA | 1/31 (3.2) | 0/1 (0.0) | NA | |
| 27 | 0/31 (0.0) | NA | NA | 4/62 (6.4) | 1/4 (25.0) | 1/62 (1.6) | |
| Culex p. pipiens | 18 | 1/32 (3.1) | 0/1 (0.0) | NA | 2/33 (6.1) | 0/2 (0.0) | NA |
| 21 | 1/30 (3.3) | 0/1 (0.0) | NA | 3/31 (9.7) | 0/3 (0.0) | NA | |
| 24 | 1/30 (3.3) | 0/1 (0.0) | NA | 7/31 (22.6) | 1/7 (14.3%) | 1/31 (3.2) | |
| 27 | 0/35 (0.0) | NA | NA | 3/33 (9.1) | 1/3 (33.3) | 1/33 (3.0) | |
| Culex torrentium | 18 | 2/32 (6.2) | 0/2 (0.0) | NA | 5/33 (15.2) | 0/5 (0.0) | NA |
| 21 | 0/31 (0.0) | NA | NA | 4/32 (12.5) | 0/4 (0.0) | NA | |
| 24 | 2/31 (6.4) | 0/2 (0.0) | NA | 8/29 (27.6) | 5/8 (62.5) | 5/29 (17.2) | |
| 27 | 11/34 (32.4) | 1/11 (9.1) | 1/34 (2.9) | 10/38 (26.3) | 9/10 (90.0) | 9/38 (23.7) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansen, S.; Heitmann, A.; Lühken, R.; Leggewie, M.; Helms, M.; Badusche, M.; Rossini, G.; Schmidt-Chanasit, J.; Tannich, E. Culex torrentium: A Potent Vector for the Transmission of West Nile Virus in Central Europe. Viruses 2019, 11, 492. https://doi.org/10.3390/v11060492
Jansen S, Heitmann A, Lühken R, Leggewie M, Helms M, Badusche M, Rossini G, Schmidt-Chanasit J, Tannich E. Culex torrentium: A Potent Vector for the Transmission of West Nile Virus in Central Europe. Viruses. 2019; 11(6):492. https://doi.org/10.3390/v11060492
Chicago/Turabian StyleJansen, Stephanie, Anna Heitmann, Renke Lühken, Mayke Leggewie, Michelle Helms, Marlis Badusche, Giada Rossini, Jonas Schmidt-Chanasit, and Egbert Tannich. 2019. "Culex torrentium: A Potent Vector for the Transmission of West Nile Virus in Central Europe" Viruses 11, no. 6: 492. https://doi.org/10.3390/v11060492
APA StyleJansen, S., Heitmann, A., Lühken, R., Leggewie, M., Helms, M., Badusche, M., Rossini, G., Schmidt-Chanasit, J., & Tannich, E. (2019). Culex torrentium: A Potent Vector for the Transmission of West Nile Virus in Central Europe. Viruses, 11(6), 492. https://doi.org/10.3390/v11060492






