Establishment and Comparison of Pathogenicity and Related Neurotropism in Two Age Groups of Immune Competent Mice, C57BL/6J Using an Indian Isolate of Chikungunya Virus (CHIKV)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics Statement
2.2. CHIKV Amplification and Quantification and Characterization
2.3. Determination of Optimum Dose to Establish CHIKV Infection in C57BL/6J Mice
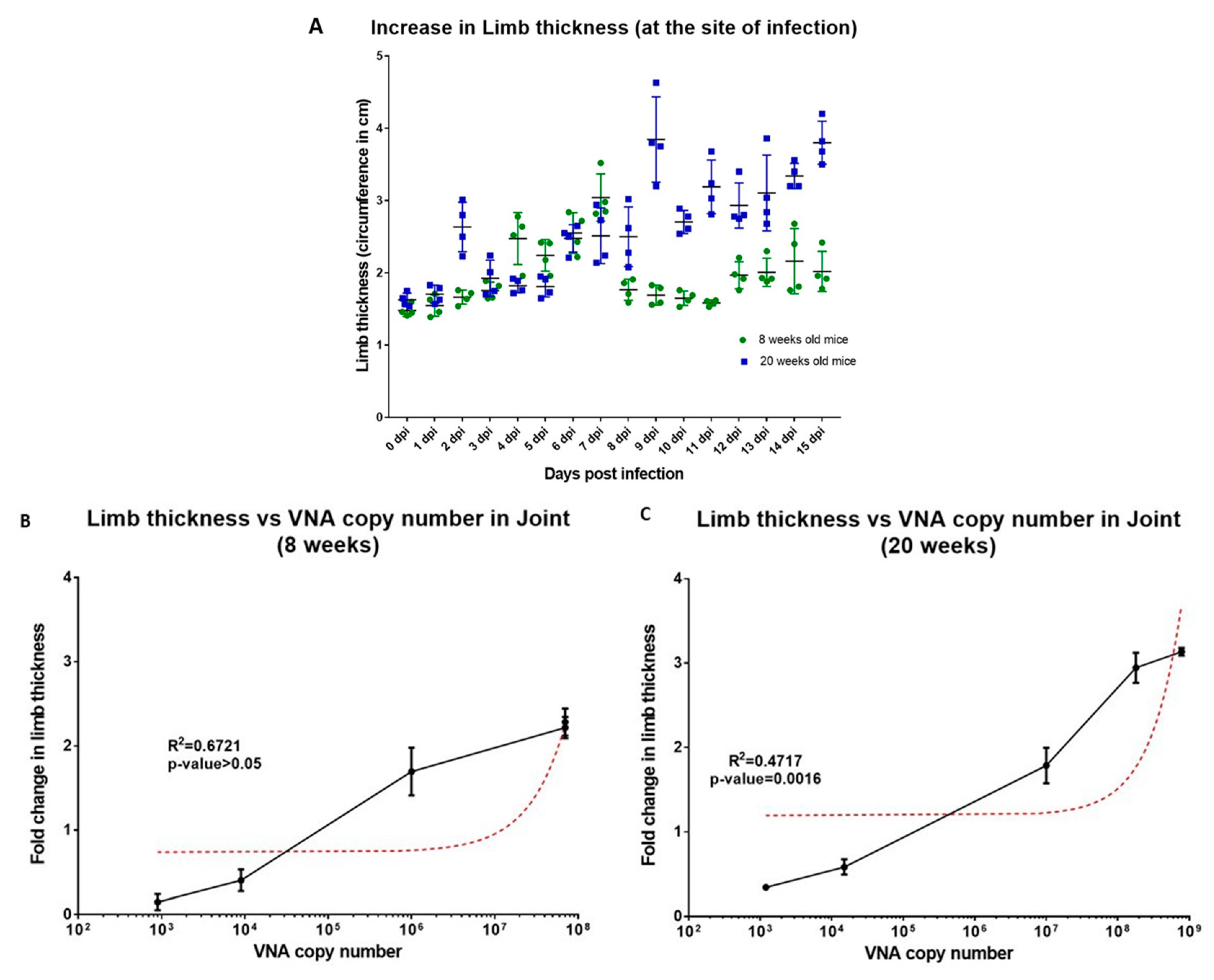
2.4. Evaluation of Disease Progression in C57BL/6J Mice Using CHIK/DEL/2010/01 CHIKV Isolate
2.4.1. Day-Wise Viral Load Estimation
2.4.2. Blood Sampling Method and Sample Handling
2.4.3. Clinical Chemistry Parameters
2.4.4. Estimation of the Plaque Forming Units
2.4.5. Estimation of Presence of Binding Abs
2.4.6. Neutralization Status of the Binding Abs
2.4.7. Cytokine and Chemokine Estimation
2.4.8. Immuno-Histopathology and Confocal Microscopy
2.5. Statistical Analysis
3. Results and Discussion
3.1. CHIKV Strains Differ in Their Pathogenic Potential in C57BL/6J Mice
3.2. Pathogenicity of CHIKV#01 Strain Is Dose-Dependent
3.3. Hematological Markers Demonstrate Severity of Acute Infection in Older Mice and Development of RA Factors in the Post–Acute Phase of Infection
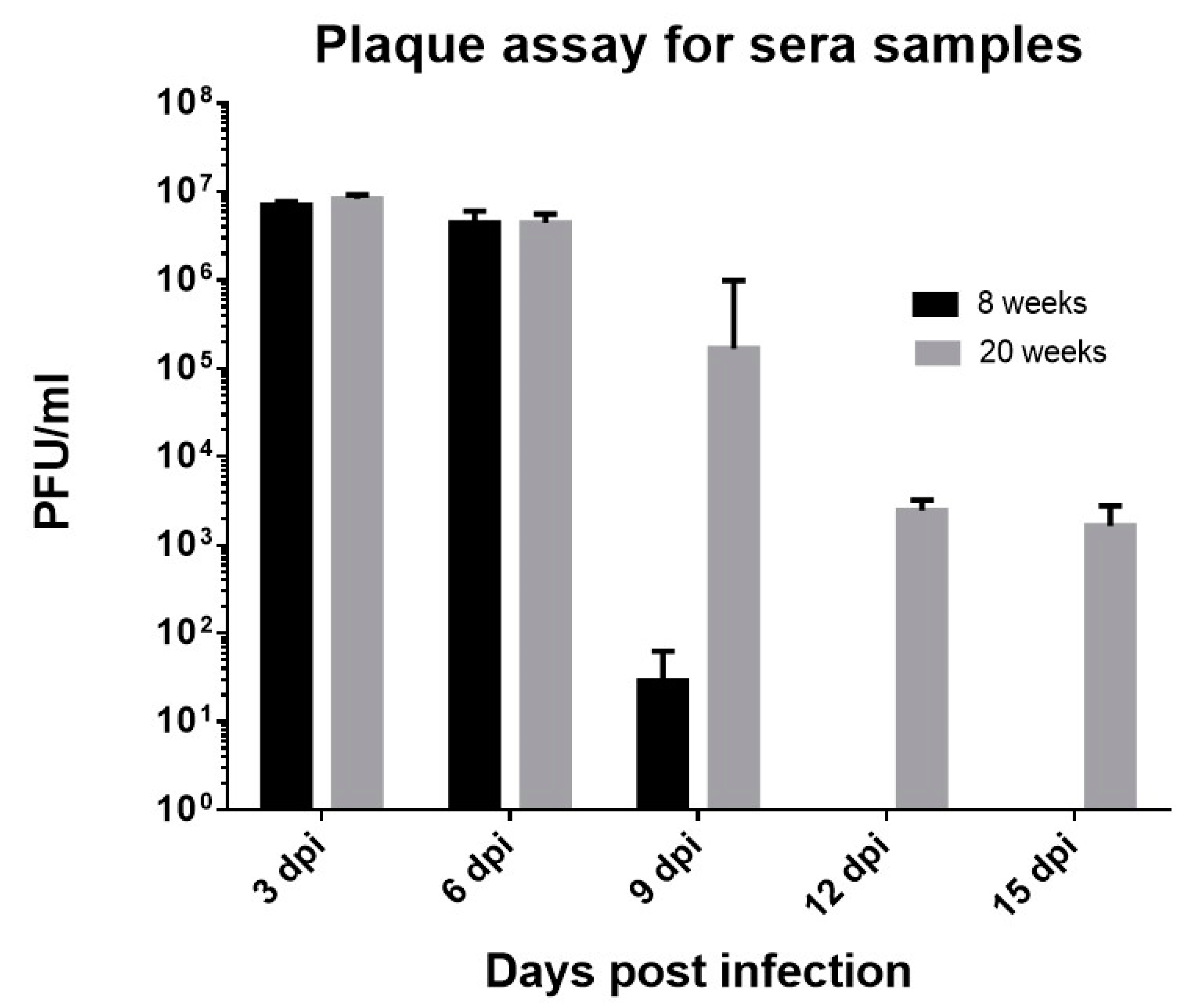
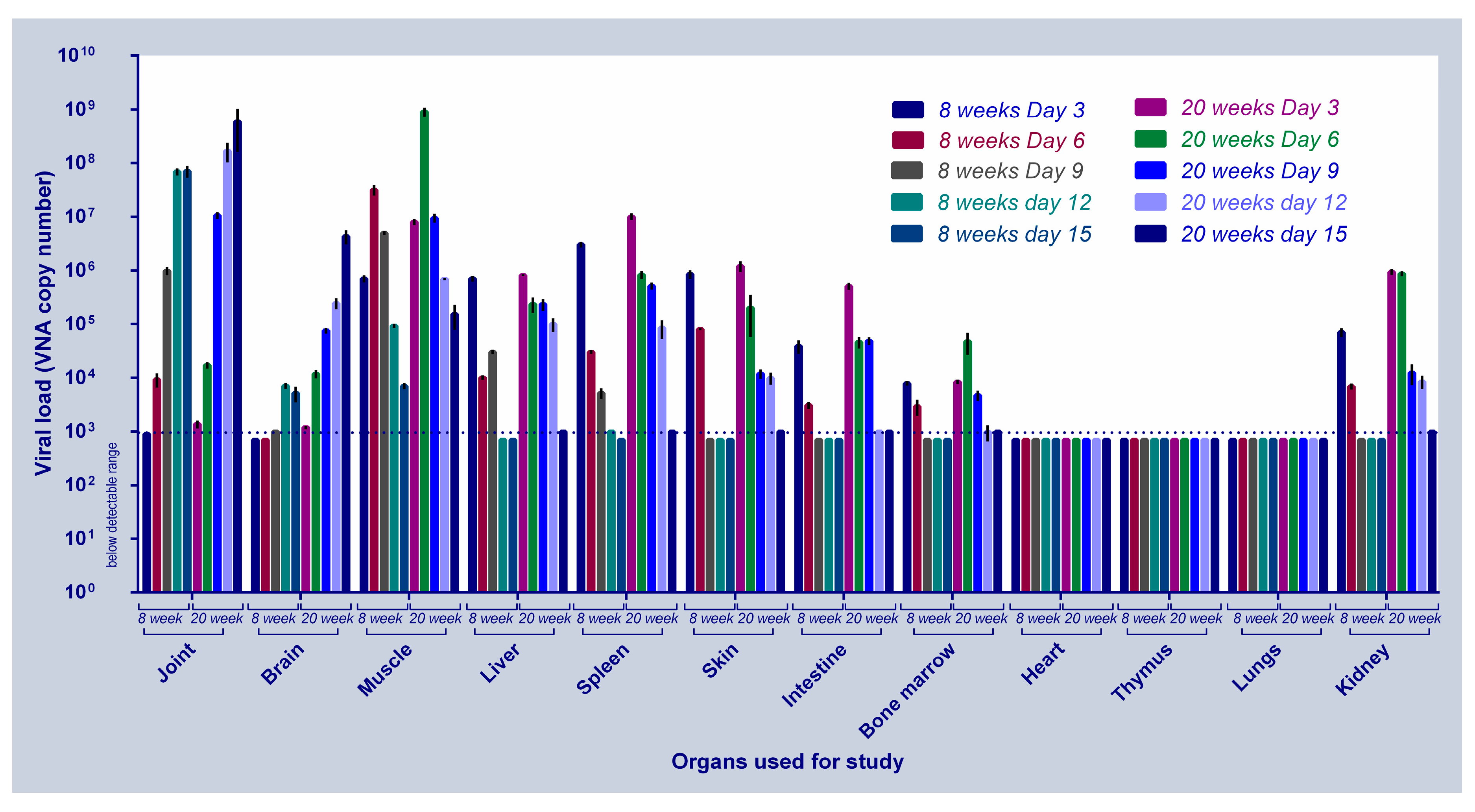
3.4. CHIKV#01 Persists Longer and Have Severe Disease Progression in Older Mice as Compared to Younger Mice
3.5. Disease Severity Is Correlated with Changes in Cell–Mediated Immunity in Older Mice
3.6. Neutralizing Role of Binding Abs (Anti-CHIKV IgM and Anti-CHIKV IgG)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Strauss, E.G.; Strauss, J.H. Structure and replication of the alphavirus genome. In The Togaviridae and Flaviviridae; Springer: Berlin, Germany, 1986; pp. 35–90. [Google Scholar]
- Porterfield, J. Antigenic characteristics and classification of Togaviridae. In Togaviruses; Academic Press: Cambridge, MA, USA, 1980; pp. 13–46. [Google Scholar]
- Nakkhara, P.; Chongsuvivatwong, V.; Thammapalo, S. Risk factors for symptomatic and asymptomatic chikungunya infection. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, R.V.; Trinta, K.S. Chikungunya virus: Clinical aspects and treatment—A Review. Memórias Inst. Oswaldo Cruz 2017, 112, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Rai, S.; Chakravarti, A. Chikungunya: A review. Trop. Dr. 2008, 38, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Staples, J.E.; Breiman, R.F.; Powers, A.M. Chikungunya fever: An epidemiological review of a re-emerging infectious disease. Clin. Infect. Dis. 2009, 49, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Thiboutot, M.M.; Kannan, S.; Kawalekar, O.U.; Shedlock, D.J.; Khan, A.S.; Sarangan, G.; Srikanth, P.; Weiner, D.B.; Muthumani, K. Chikungunya: A potentially emerging epidemic? PLoS Negl. Trop. Dis. 2010, 4, e623. [Google Scholar] [CrossRef] [PubMed]
- Schilte, C.; Staikovsky, F.; Couderc, T.; Madec, Y.; Carpentier, F.; Kassab, S.; Albert, M.L.; Lecuit, M.; Michault, A. Chikungunya virus-associated long-term arthralgia: A 36-month prospective longitudinal study. PLoS Negl. Trop. Dis. 2013, 7, e2137. [Google Scholar] [CrossRef]
- Soumahoro, M.-K.; Gérardin, P.; Boëlle, P.-Y.; Perrau, J.; Fianu, A.; Pouchot, J.; Malvy, D.; Flahault, A.; Favier, F.; Hanslik, T. Impact of Chikungunya virus infection on health status and quality of life: A retrospective cohort study. PLoS ONE 2009, 4, e7800. [Google Scholar] [CrossRef]
- Rezza, G.; Nicoletti, L.; Angelini, R.; Romi, R.; Finarelli, A.C.; Panning, M.; Cordioli, P.; Fortuna, C.; Boros, S.; Magurano, F.; et al. Infection with chikungunya virus in Italy: An outbreak in a temperate region. Lancet 2007, 370, 1840–1846. [Google Scholar] [CrossRef]
- Economopoulou, A.; Dominguez, M.; Helynck, B.; Sissoko, D.; Wichmann, O.; Quenel, P.; Germonneau, P.; Quatresous, I. Atypical Chikungunya virus infections: Clinical manifestations, mortality and risk factors for severe disease during the 2005–2006 outbreak on Reunion. Epidemiol. Infect. 2009, 137, 534–541. [Google Scholar] [CrossRef]
- Tandale, B.V.; Sathe, P.S.; Arankalle, V.A.; Wadia, R.; Kulkarni, R.; Shah, S.V.; Shah, S.K.; Sheth, J.K.; Sudeep, A.; Tripathy, A.S. Systemic involvements and fatalities during Chikungunya epidemic in India, 2006. J. Clin. Virol. 2009, 46, 145–149. [Google Scholar] [CrossRef]
- Queyriaux, B.; Simon, F.; Grandadam, M.; Michel, R.; Tolou, H.; Boutin, J.-P. Clinical burden of chikungunya virus infection. Lancet Infect. Dis. 2008, 8, 2–3. [Google Scholar] [CrossRef]
- Sissoko, D.; Malvy, D.; Ezzedine, K.; Renault, P.; Moscetti, F.; Ledrans, M.; Pierre, V. Post-epidemic Chikungunya disease on Reunion Island: Course of rheumatic manifestations and associated factors over a 15-month period. PLoS Negl. Trop. Dis. 2009, 3, e389. [Google Scholar] [CrossRef] [PubMed]
- Jain, J.; Nayak, K.; Tanwar, N.; Gaind, R.; Gupta, B.; Shastri, J.S.; Bhatnagar, R.K.; Kaja, M.K.; Chandele, A.; Sunil, S. Clinical, Serological and Virological analysis of 572 chikungunya patients during the years 2010-2013 from India. Clin. Infect. Dis. 2017, 65, 133–140. [Google Scholar] [CrossRef]
- Langsjoen, R.M.; Haller, S.L.; Roy, C.J.; Vinet-Oliphant, H.; Bergren, N.A.; Erasmus, J.H.; Livengood, J.A.; Powell, T.D.; Weaver, S.C.; Rossi, S.L. Chikungunya Virus Strains Show Lineage-Specific Variations in Virulence and Cross-Protective Ability in Murine and Nonhuman Primate Models. MBio 2018, 9, e02449-17. [Google Scholar] [CrossRef]
- Beasley, D.W.; Li, L.; Suderman, M.T.; Barrett, A.D. Mouse neuroinvasive phenotype of West Nile virus strains varies depending upon virus genotype. Virology 2002, 296, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-H.; Lum, F.-M.; Ng, L. Limitations of current in vivo mouse models for the study of chikungunya virus pathogenesis. Med Sci. 2015, 3, 64–77. [Google Scholar] [CrossRef]
- Haese, N.N.; Broeckel, R.M.; Hawman, D.W.; Heise, M.T.; Morrison, T.E.; Streblow, D.N. Animal models of chikungunya virus infection and disease. J. Infect. Dis. 2016, 214, S482–S487. [Google Scholar] [CrossRef]
- Couderc, T.; Chrétien, F.; Schilte, C.; Disson, O.; Brigitte, M.; Guivel-Benhassine, F.; Touret, Y.; Barau, G.; Cayet, N.; Schuffenecker, I. A mouse model for Chikungunya: Young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 2008, 4, e29. [Google Scholar] [CrossRef]
- Werneke, S.W.; Schilte, C.; Rohatgi, A.; Monte, K.J.; Michault, A.; Arenzana-Seisdedos, F.; Vanlandingham, D.L.; Higgs, S.; Fontanet, A.; Albert, M.L. ISG15 is critical in the control of Chikungunya virus infection independent of UbE1L mediated conjugation. PLoS Pathog. 2011, 7, e1002322. [Google Scholar] [CrossRef]
- Rudd, P.A.; Wilson, J.; Gardner, J.; Larcher, T.; Babarit, C.; Le, T.T.; Anraku, I.; Kumagai, Y.; Loo, Y.-M.; Gale, M. Interferon response factors 3 and 7 protect against Chikungunya virus hemorrhagic fever and shock. J. Virol. 2012, 86, 9888–9898. [Google Scholar] [CrossRef]
- Schilte, C.; Buckwalter, M.R.; Laird, M.E.; Diamond, M.S.; Schwartz, O.; Albert, M.L. Cutting edge: Independent roles for IRF-3 and IRF-7 in hematopoietic and nonhematopoietic cells during host response to Chikungunya infection. J. Immunol. 2012, 188, 2967–2971. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.L.; Burke, C.W.; Higgs, S.T.; Klimstra, W.B.; Ryman, K.D. Interferon-alpha/beta deficiency greatly exacerbates arthritogenic disease in mice infected with wild-type chikungunya virus but not with the cell culture-adapted live-attenuated 181/25 vaccine candidate. Virology 2012, 425, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Plante, K.; Wang, E.; Partidos, C.D.; Weger, J.; Gorchakov, R.; Tsetsarkin, K.; Borland, E.M.; Powers, A.M.; Seymour, R.; Stinchcomb, D.T. Novel chikungunya vaccine candidate with an IRES-based attenuation and host range alteration mechanism. PLoS Pathog. 2011, 7, e1002142. [Google Scholar] [CrossRef] [PubMed]
- Hawman, D.W.; Stoermer, K.A.; Montgomery, S.A.; Pal, P.; Oko, L.; Diamond, M.S.; Morrison, T.E. Chronic joint disease caused by persistent Chikungunya virus infection is controlled by the adaptive immune response. J. Virol. 2013, 87, 13878–13888. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.E.; Oko, L.; Montgomery, S.A.; Whitmore, A.C.; Lotstein, A.R.; Gunn, B.M.; Elmore, S.A.; Heise, M.T. A Mouse Model of Chikungunya Virus–Induced Musculoskeletal Inflammatory Disease: Evidence of Arthritis, Tenosynovitis, Myositis, and Persistence. Am. J. Pathol. 2011, 178, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Yeo, L.S.; Chu, J.J.H. Recent developments and challenges in mouse models of Chikungunya virus infection. Future Virol. 2013, 8, 423–426. [Google Scholar] [CrossRef]
- Wang, E.; Volkova, E.; Adams, A.P.; Forrester, N.; Xiao, S.-Y.; Frolov, I.; Weaver, S.C. Chimeric alphavirus vaccine candidates for chikungunya. Vaccine 2008, 26, 5030–5039. [Google Scholar] [CrossRef]
- Kuo, S.-C.; Wang, Y.-M.; Ho, Y.-J.; Chang, T.-Y.; Lai, Z.-Z.; Tsui, P.-Y.; Wu, T.-Y.; Lin, C.-C. Suramin treatment reduces chikungunya pathogenesis in mice. Antivir. Res. 2016, 134, 89–96. [Google Scholar] [CrossRef]
- Poo, Y.S.; Rudd, P.A.; Gardner, J.; Wilson, J.A.; Larcher, T.; Colle, M.-A.; Le, T.T.; Nakaya, H.I.; Warrilow, D.; Allcock, R. Multiple immune factors are involved in controlling acute and chronic chikungunya virus infection. PLoS Negl. Trop. Dis. 2014, 8, e3354. [Google Scholar] [CrossRef]
- Muthumani, K.; Lankaraman, K.M.; Laddy, D.J.; Sundaram, S.G.; Chung, C.W.; Sako, E.; Wu, L.; Khan, A.; Sardesai, N.; Kim, J.J. Immunogenicity of novel consensus-based DNA vaccines against Chikungunya virus. Vaccine 2008, 26, 5128–5134. [Google Scholar] [CrossRef]
- Hallengärd, D.; Kakoulidou, M.; Lulla, A.; Kümmerer, B.M.; Johansson, D.X.; Mutso, M.; Lulla, V.; Fazakerley, J.K.; Roques, P.; Le Grand, R. Novel attenuated Chikungunya vaccine candidates elicit protective immunity in C57BL/6 mice. J. Virol. 2014, 88, 2858–2866. [Google Scholar] [CrossRef]
- Gérardin, P.; Fianu, A.; Michault, A.; Mussard, C.; Boussaïd, K.; Rollot, O.; Grivard, P.; Kassab, S.; Bouquillard, E.; Borgherini, G. Predictors of Chikungunya rheumatism: A prognostic survey ancillary to the TELECHIK cohort study. Arthritis Res. Ther. 2013, 15, R9. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Jain, J.; Kumar, A.; Narang, M.; Zakaria, M.K.; Marcello, A.; Kumar, D.; Gaind, R.; Sunil, S. Chikungunya outbreak in Delhi, India, 2016: Report on coinfection status and comorbid conditions in patients. New Microbes New Infect. 2017, 20, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, V.; Neeraja, M.; Subbalaxmi, M.; Parida, M.; Dash, P.; Santhosh, S.; Rao, P. Clinical features and molecular diagnosis of Chikungunya fever from South India. Clin. Infect. Dis. 2008, 46, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.-O.; Park, S.; Kwak, J.-S.; Won, Y.; Choi, W.-S.; Rhee, J.; Chun, C.-H.; Ryu, J.-H.; Kim, D.-K.; Choi, H.-S. Estrogen-related receptor γ causes osteoarthritis by upregulating extracellular matrix-degrading enzymes. Nat. Commun. 2017, 8, 2133. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.J.; Andrews, N.; Ball, D.; Bellantuono, I.; Gray, J.; Hachoumi, L.; Holmes, A.; Latcham, J.; Petrie, A.; Potter, P. Does age matter? The impact of rodent age on study outcomes. Lab. Anim. 2017, 51, 160–169. [Google Scholar] [CrossRef]
- Chandak, N.H.; Kashyap, R.S.; Kabra, D.; Karandikar, P.; Saha, S.S.; Morey, S.H.; Purohit, H.J.; Taori, G.M.; Daginawala, H.F. Neurological complications of Chikungunya virus infection. Neurol. India 2009, 57, 177. [Google Scholar]
- Jain, J.; Pai, S.; Sunil, S. Standardization of in vitro assays to evaluate the activity of polyherbal siddha formulations against Chikungunya virus infection. Virusdisease 2018, 29, 32–39. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Morton, D.; Griffiths, P. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet. Rec. 1985, 116, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Santhosh, S.; Tiwari, M.; Lakshmana Rao, P.; Parida, M. Assessment of in vitro prophylactic and therapeutic efficacy of chloroquine against Chikungunya virus in vero cells. J. Med. Virol. 2010, 82, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.A.; Lu, L.; da Rosa, A.P.T.; Xiao, S.-Y.; Tesh, R.B. An animal model for studying the pathogenesis of chikungunya virus infection. Am. J. Trop. Med. Hyg. 2008, 79, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Phanthanawiboon, S.; Limkittikul, K.; Sakai, Y.; Takakura, N.; Saijo, M.; Kurosu, T. Acute systemic infection with dengue virus leads to vascular leakage and death through tumor necrosis factor-α and Tie2/angiopoietin signaling in mice lacking type I and II interferon receptors. PLoS ONE 2016, 11, e0148564. [Google Scholar] [CrossRef] [PubMed]
- Goupil, B.A.; Mores, C.N. A review of chikungunya virus-induced arthralgia: Clinical manifestations, therapeutics, and pathogenesis. Open Rheumatol. J. 2016, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, O.; Albert, M.L. Biology and pathogenesis of chikungunya virus. Nat. Rev. Microbiol. 2010, 8, 491. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.F.; Chow, A.; Sun, Y.-J.; Kwek, D.J.; Lim, P.-L.; Dimatatac, F.; Ng, L.-C.; Ooi, E.-E.; Choo, K.-H.; Her, Z. IL-1β, IL-6, and RANTES as biomarkers of Chikungunya severity. PLoS ONE 2009, 4, e4261. [Google Scholar] [CrossRef]
- Higgs, S.; Ziegler, S.A. A nonhuman primate model of chikungunya disease. J. Clin. Investig. 2010, 120, 657–660. [Google Scholar] [CrossRef]
- Labadie, K.; Larcher, T.; Joubert, C.; Mannioui, A.; Delache, B.; Brochard, P.; Guigand, L.; Dubreil, L.; Lebon, P.; Verrier, B. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J. Clin. Investig. 2010, 120, 894–906. [Google Scholar] [CrossRef]
- Gasque, P.; Bandjee, M.C.J.; Reyes, M.M.; Viasus, D. Chikungunya pathogenesis: From the clinics to the bench. J. Infect. Dis. 2016, 214, S446–S448. [Google Scholar] [CrossRef]
- Bouquillard, É.; Combe, B. A report of 21 cases of rheumatoid arthritis following Chikungunya fever. A mean follow-up of two years. Jt. Bone Spine 2009, 76, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.; Anuradha, V.; Lagoo-Joshi, V.; Kunjir, V.; Salvi, S.; Saluja, M. Chikungunya virus aches and pains: An emerging challenge. Arthritis Rheum. 2008, 58, 2921–2922. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, A.; Angheben, A.; Bisoffi, Z.; Monteiro, G.; Marocco, S.; Calleri, G.; Lipani, F.; Gobbi, F.; Canta, F.; Castelli, F. Imported chikungunya infection, Italy. Emerg. Infect. Dis. 2007, 13, 1264. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Solomon, T. Pathogenic flaviviruses. Lancet 2008, 371, 500–509. [Google Scholar] [CrossRef]
- Weissenböck, H.; Hubálek, Z.; Bakonyi, T.; Nowotny, N. Zoonotic mosquito-borne flaviviruses: Worldwide presence of agents with proven pathogenicity and potential candidates of future emerging diseases. Vet. Microbiol. 2010, 140, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.E. Arboviruses and the central nervous system. In Springer Seminars in Immunopathology; Springer: Berlin, Germany, 1995; pp. 121–132. [Google Scholar]
- Goto, A.; Hayasaka, D.; Yoshii, K.; Mizutani, T.; Kariwa, H.; Takashima, I. A BHK-21 cell culture-adapted tick-borne encephalitis virus mutant is attenuated for neuroinvasiveness. Vaccine 2003, 21, 4043–4051. [Google Scholar] [CrossRef]
- Jones, P.H.; Maric, M.; Madison, M.N.; Maury, W.; Roller, R.J.; Okeoma, C.M. BST-2/tetherin-mediated restriction of chikungunya (CHIKV) VLP budding is counteracted by CHIKV non-structural protein 1 (nsP1). Virology 2013, 438, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Poo, Y.S.; Nakaya, H.; Gardner, J.; Larcher, T.; Schroder, W.A.; Le, T.T.; Major, L.D.; Suhrbier, A. CCR2 deficiency promotes exacerbated chronic erosive neutrophil-dominated chikungunya virus arthritis. J. Virol. 2014, 88, 6862–6872. [Google Scholar] [CrossRef] [PubMed]
- Suhrbier, A.; La Linn, M. Clinical and pathologic aspects of arthritis due to Ross River virus and other alphaviruses. Curr. Opin. Rheumatol. 2004, 16, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, C.; Kavanaugh, A. Tumor necrosis factor as a therapeutic target of rheumatologic disease. Expert Opin. Ther. Targets 2007, 11, 1369–1384. [Google Scholar] [CrossRef] [PubMed]
- Dupuis-Maguiraga, L.; Noret, M.; Brun, S.; Le Grand, R.; Gras, G.; Roques, P. Chikungunya disease: Infection-associated markers from the acute to the chronic phase of arbovirus-induced arthralgia. PLoS Negl. Trop. Dis. 2012, 6, e1446. [Google Scholar] [CrossRef] [PubMed]
- Suhrbier, A.; Mahalingam, S. The immunobiology of viral arthritides. Pharmacol. Ther. 2009, 124, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Santiago, F.W.; Halsey, E.S.; Siles, C.; Vilcarromero, S.; Guevara, C.; Silvas, J.A.; Ramal, C.; Ampuero, J.S.; Aguilar, P.V. Long-term arthralgia after Mayaro virus infection correlates with sustained pro-inflammatory cytokine response. PLoS Negl. Trop. Dis. 2015, 9, e0004104. [Google Scholar] [CrossRef] [PubMed]
- Derdeyn, C.A.; Moore, P.L.; Morris, L. Development of broadly neutralizing antibodies from autologous neutralizing antibody responses. Curr. Opin. HIV Aids 2014, 9, 210. [Google Scholar] [CrossRef]

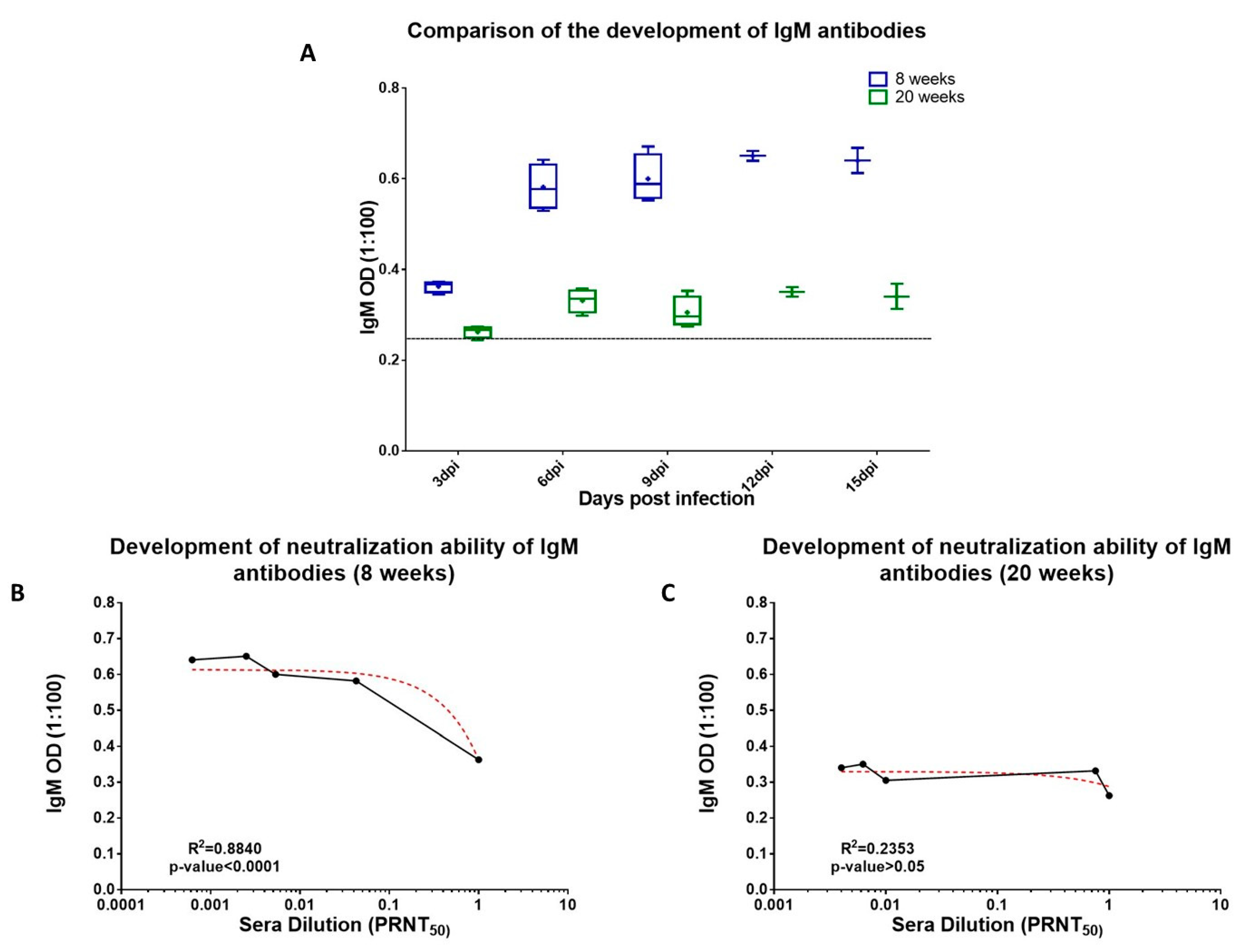
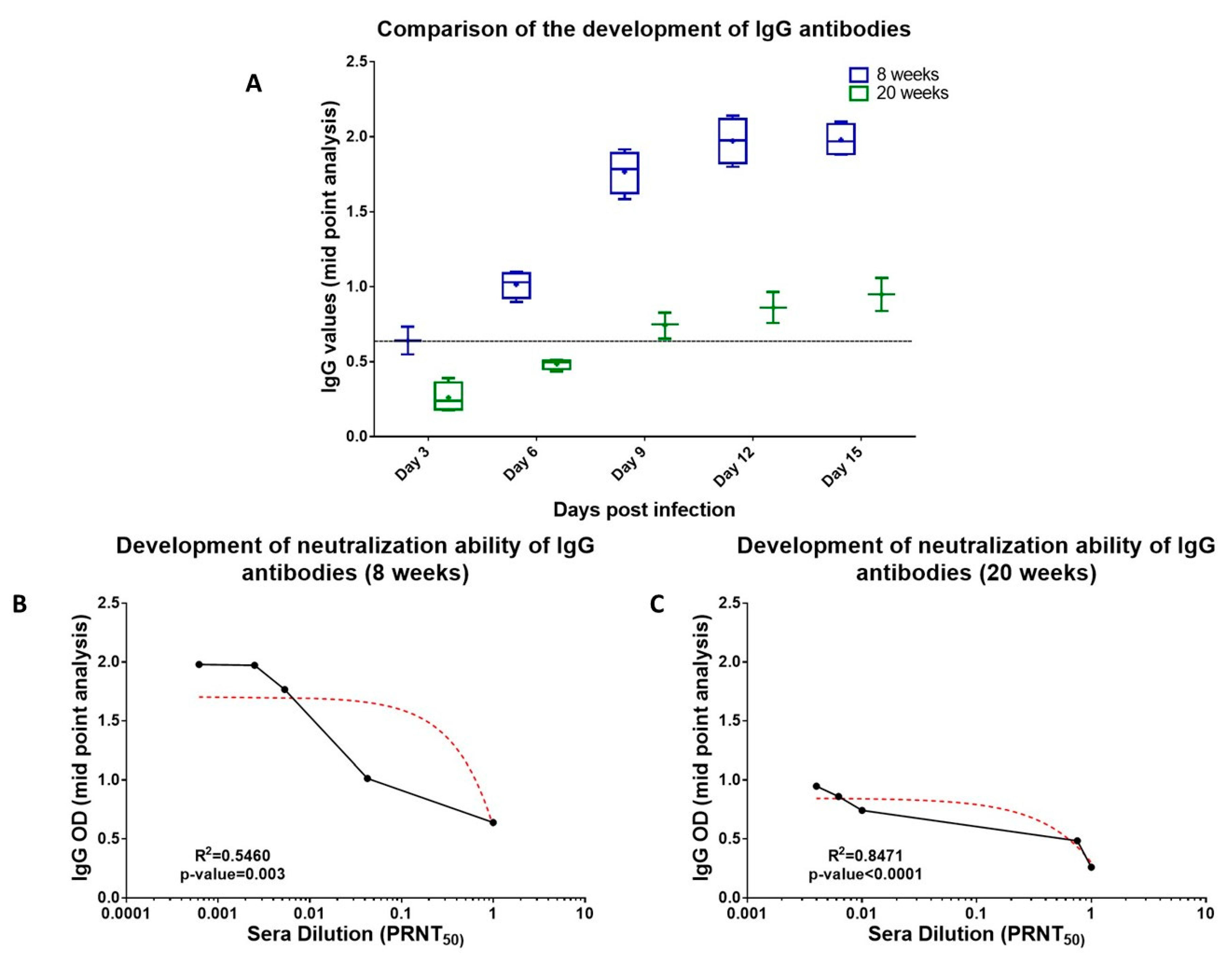
| (a) | |||||||||||
| Days of Infection | Day 3 | Day 6 | Day 9 | Day 12 | Day 15 | Normal Range | |||||
| Parameters | Mean | Stdev | Mean | Stdev | Mean | Stdev | Mean | Stdev | Mean | Stdev | |
| Hb (g/dL) | 15.6 | 0.62 | 15.28 | 0.68 | 13.88 | 1.23 | 15.18 | 0.28 | 15.25 | 0.58 | 11.2–16.4 |
| TLC (×103/mm3) | 3.05 | 0.56 | 5.25 | 0.90 | 6.30 | 0.54 | 5.00 | 1.01 | 5.00 | 1.45 | 1.8–5.2 |
| DLC-neutrophils (%) | 18.75 | 0.96 | 20.75 | 2.50 | 22.25 | 4.50 | 19.00 | 1.41 | 19.75 | 3.10 | 8–20 |
| DLC lymphocytes (%) | 75.25 | 0.96 | 78.00 | 2.16 | 80.25 | 4.99 | 75.25 | 0.96 | 74.75 | 3.86 | 76–91 |
| RBC (×106/mm3) | 10.78 | 0.46 | 10.18 | 0.36 | 9.49 | 0.98 | 10.08 | 0.24 | 10.24 | 0.65 | 6.1–10.7 |
| MCV (fL) | 47.15 | 0.67 | 47.65 | 1.27 | 47.35 | 1.33 | 47.65 | 0.62 | 46.83 | 2.00 | 43.4–47.8 |
| MCH (pg) | 14.5 | 0.22 | 15.00 | 0.45 | 14.63 | 0.35 | 15.10 | 0.18 | 14.90 | 0.54 | 14.8–17.6 |
| MCHC (%) | 30.75 | 0.45 | 26.98 | 9.35 | 30.90 | 1.15 | 31.63 | 0.30 | 31.85 | 0.35 | 29.3–35.9 |
| Platelet count (mm3) | 1097 | 257.27 | 1193.00 | 243.68 | 770.00 | 478.09 | 997.25 | 299.24 | 1009.00 | 407.56 | 285–890 |
| (b) | |||||||||||
| Days of Infection | Day 3 | Day 6 | Day 9 | Day 12 | Day 15 | Normal Range | |||||
| Parameters | Mean | Stdev | Mean | Stdev | Mean | Stdev | Mean | Stdev | Mean | Stdev | |
| Hb (g/dl) | 14.575 | 0.359398 | 14.38 | 1.06 | 13.43 | 0.96 | 14.05 | 0.75 | 15.17 | 0.81 | 11.2–16.4 |
| TLC (×103/mm3) | 4.28025 | 1.211589 | 4.76 | 1.28 | 5.75 | 1.45 | 5.80 | 1.68 | 6.95 | 0.83 | 1.8–5.2 |
| DLC-neutrophils (%) | 12.75 | 0.732006 | 45.00 | 0.17 | 42.50 | 0.10 | 61.25 | 0.30 | 53.33 | 0.49 | 8–20 |
| DLC lymphocytes (%) | 90.275 | 4.453744 | 96.38 | 1.39 | 91.75 | 10.50 | 96.70 | 1.70 | 97.47 | 0.64 | 76–91 |
| RBC (×106/mm3) | 9.8675 | 0.314788 | 9.68 | 0.51 | 8.97 | 0.55 | 9.53 | 0.52 | 9.66 | 0.68 | 6.1–10.7 |
| MCV (fL) | 51.075 | 1.05317 | 51.60 | 0.93 | 50.83 | 1.32 | 51.95 | 1.81 | 52.70 | 1.21 | 43.4–47.8 |
| MCH (pg) | 18.8 | 0.182574 | 19.83 | 0.46 | 20.95 | 0.31 | 19.73 | 0.13 | 20.20 | 0.85 | 14.8–17.6 |
| MCHC (%) | 38.925 | 0.713559 | 38.73 | 1.28 | 39.40 | 0.50 | 38.38 | 1.18 | 38.90 | 1.73 | 29.3–35.9 |
| Platelet count (×103/mm3) | 897.25 | 181.9146 | 907.75 | 94.89 | 1021.75 | 384.15 | 1102.25 | 216.78 | 935.00 | 137.18 | 285–890 |
| DOI | 3 dpi (Mean ± Standard Deviation) | 6 dpi (Mean ± Standard Deviation) | 9 dpi (Mean ± Standard Deviation) | 12 dpi (Mean ± Standard Deviation) | 15 dpi (Mean ± Standard Deviation) | Average Values in C57BL/6ICGEB Mice | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 8 Weeks | 20 Weeks | 8 Weeks | 20 Weeks | 8 Weeks | 20 Weeks | 8 Weeks | 20 Weeks | 8 Weeks | 20 Weeks | ||
| AST/SGOT (U/L) | 90.4 ± 5.23 | 135.95 ± 1.34 | 116.4 ± 7.58 | 135.55 ± 4.52 | 135.8 ± 4.36 | 257.42 ± 2.36 | 131.6 ± 5.33 | 145.51 ± 5.23 | 134 ± 6.3 | 140.65 ± 5.89 | 62.21–87.7 | >0.05 |
| ALT/SGPT (U/L) | 37.2 ± 4.54 | 94.00 ± 8.48 | 47 ± 9.3 | 71.1 ± 11.15 | 38.6 ± 10.28 | 76.46 ± 5.68 | 64.2 ± 9.18 | 78.85 ± 8.56 | 50.2 ± 10.19 | 80.24 ± 9.65 | 23.18–30.82 | >0.05 |
| ALP (U/L) | 297 ± 5.25 | 230.2 ± 2.58 | 295 ± 8.28 | 280 ± 6.28 | 294 ± 11.19 | 300 ± 2.19 | 248 ± 5.34 | 305 ± 6.34 | 304 ± 5.23 | 318 ± 4.23 | 35–96 | >0.05 |
| RF | Absent | Absent | Absent | Absent | Absent | Absent | Absent | Present (50%) | Absent | Present (50%) | Not applicable | |
| Cytokines and Chemokines (Semi Quantitative Expression with Respect to Controls) | ||||
|---|---|---|---|---|
| Age Group | 8 Weeks | 8 Weeks | 20 Weeks | 20 Weeks |
| Days post infections (dpi) | Day 1–Day 6 | Day 7–Day 15 | Day 1–Day 6 | Day 7–Day 15 |
| Clinical Manifestations | Asymptomatic Acute | Post-Acute | Severe Acute | Post-Acute |
| IFN-α | + | ++ | ++ | +++ |
| CCR1 | ++ | - | ||
| CCR2 | ++ | + | ||
| BST-2 | ++ | + | + | |
| TNF-α | ++ | + | +++ | + |
| VEGF | + | ++ | ||
| IL-4 | ++ | ++ | ||
| GM-CSF | + | + | ||
| CCL2(MCP-1) | + | ++ | ||
| RANTES | + | |||
| RANKL | + | ++ | ||
| IL-1β | + | + | ++ | +++ |
| IL-6 | + | ++ | +++ | +++ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, J.; Narayanan, V.; Kumar, A.; Shrinet, J.; Srivastava, P.; Chaturvedi, S.; Sunil, S. Establishment and Comparison of Pathogenicity and Related Neurotropism in Two Age Groups of Immune Competent Mice, C57BL/6J Using an Indian Isolate of Chikungunya Virus (CHIKV). Viruses 2019, 11, 578. https://doi.org/10.3390/v11060578
Jain J, Narayanan V, Kumar A, Shrinet J, Srivastava P, Chaturvedi S, Sunil S. Establishment and Comparison of Pathogenicity and Related Neurotropism in Two Age Groups of Immune Competent Mice, C57BL/6J Using an Indian Isolate of Chikungunya Virus (CHIKV). Viruses. 2019; 11(6):578. https://doi.org/10.3390/v11060578
Chicago/Turabian StyleJain, Jaspreet, Vimal Narayanan, Ankit Kumar, Jatin Shrinet, Priyanshu Srivastava, Shivam Chaturvedi, and Sujatha Sunil. 2019. "Establishment and Comparison of Pathogenicity and Related Neurotropism in Two Age Groups of Immune Competent Mice, C57BL/6J Using an Indian Isolate of Chikungunya Virus (CHIKV)" Viruses 11, no. 6: 578. https://doi.org/10.3390/v11060578
APA StyleJain, J., Narayanan, V., Kumar, A., Shrinet, J., Srivastava, P., Chaturvedi, S., & Sunil, S. (2019). Establishment and Comparison of Pathogenicity and Related Neurotropism in Two Age Groups of Immune Competent Mice, C57BL/6J Using an Indian Isolate of Chikungunya Virus (CHIKV). Viruses, 11(6), 578. https://doi.org/10.3390/v11060578





