Novel Victorivirus from a Pakistani Isolate of Alternaria alternata Lacking a Typical Translational Stop/Restart Sequence Signature
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Culture and Isolation Condition
2.2. RNA Preparation
2.3. Viral Genome Sequencing using RNA-Seq
2.4. Database Search and Sequence Analysis
2.5. Phylogenetic Analysis
2.6. RT-PCR for Confirmation of Contigs and RLM-RACE for Terminal Sequence Determination
2.7. Purification of Virus Particles
2.8. Transfection of C. parasitica Spheroplasts
2.9. Protoplast Fusion
3. Results
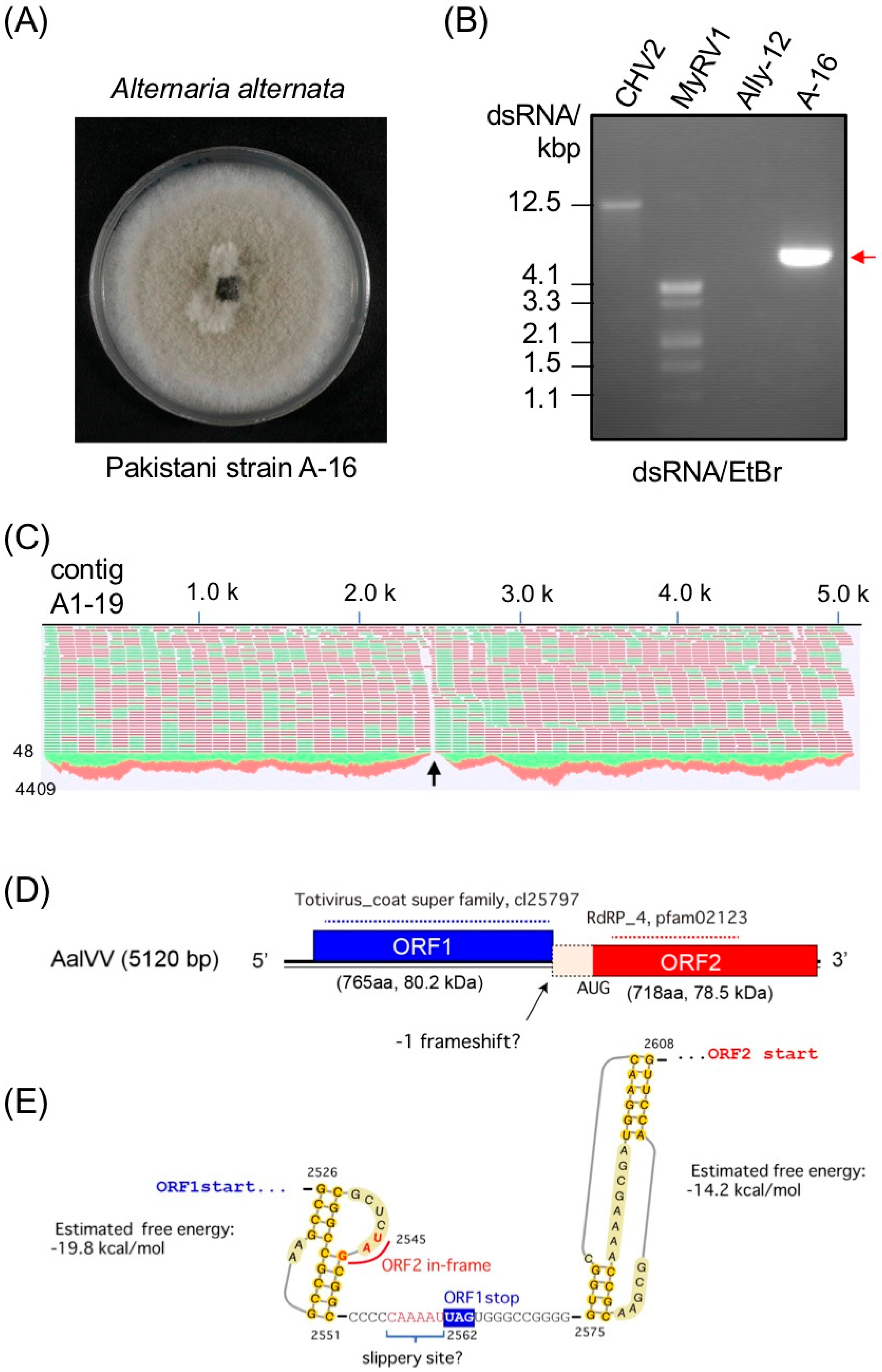
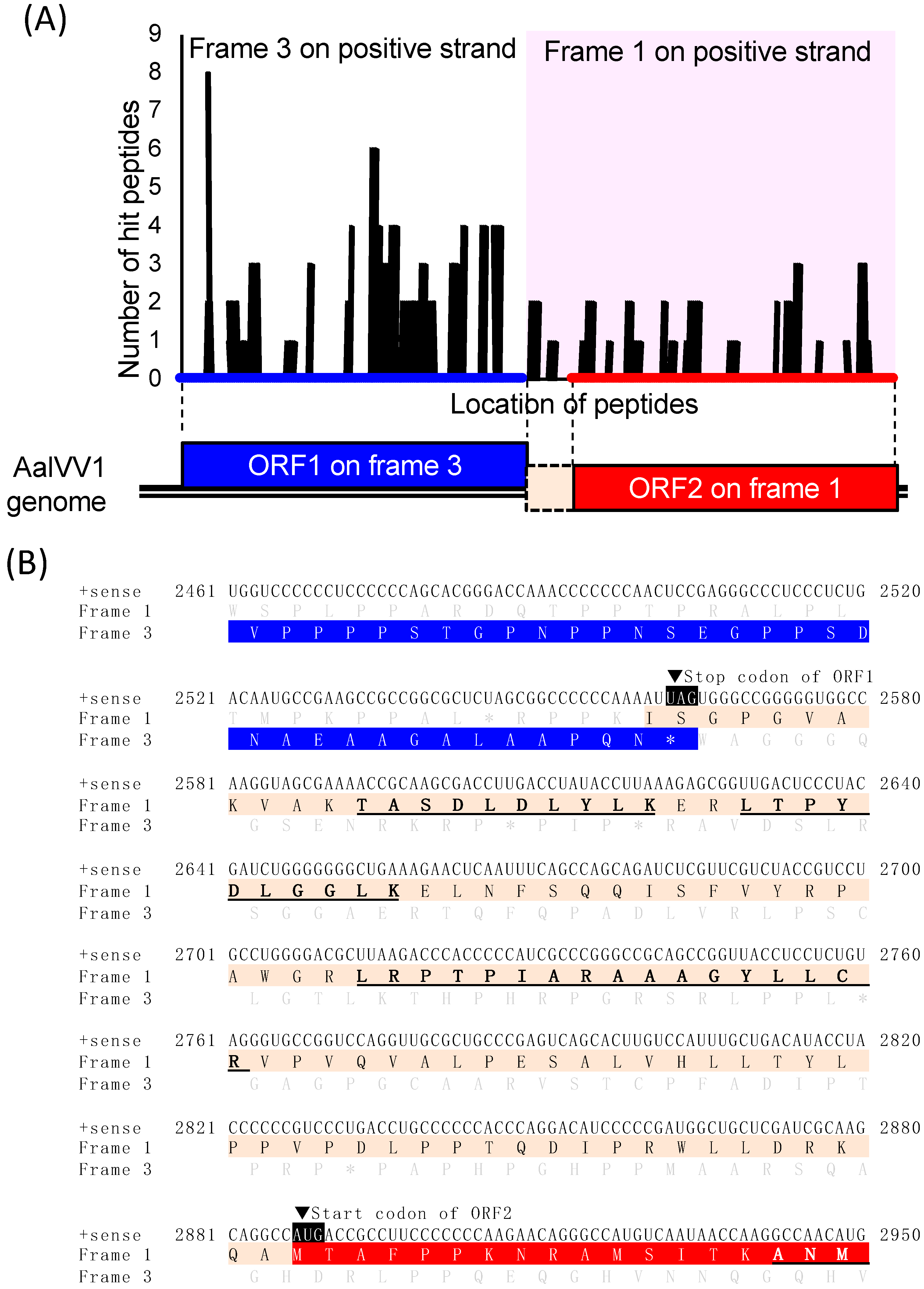
3.1. Genome Organization of a Victorivirus Alternaria alternata victorivirus 1
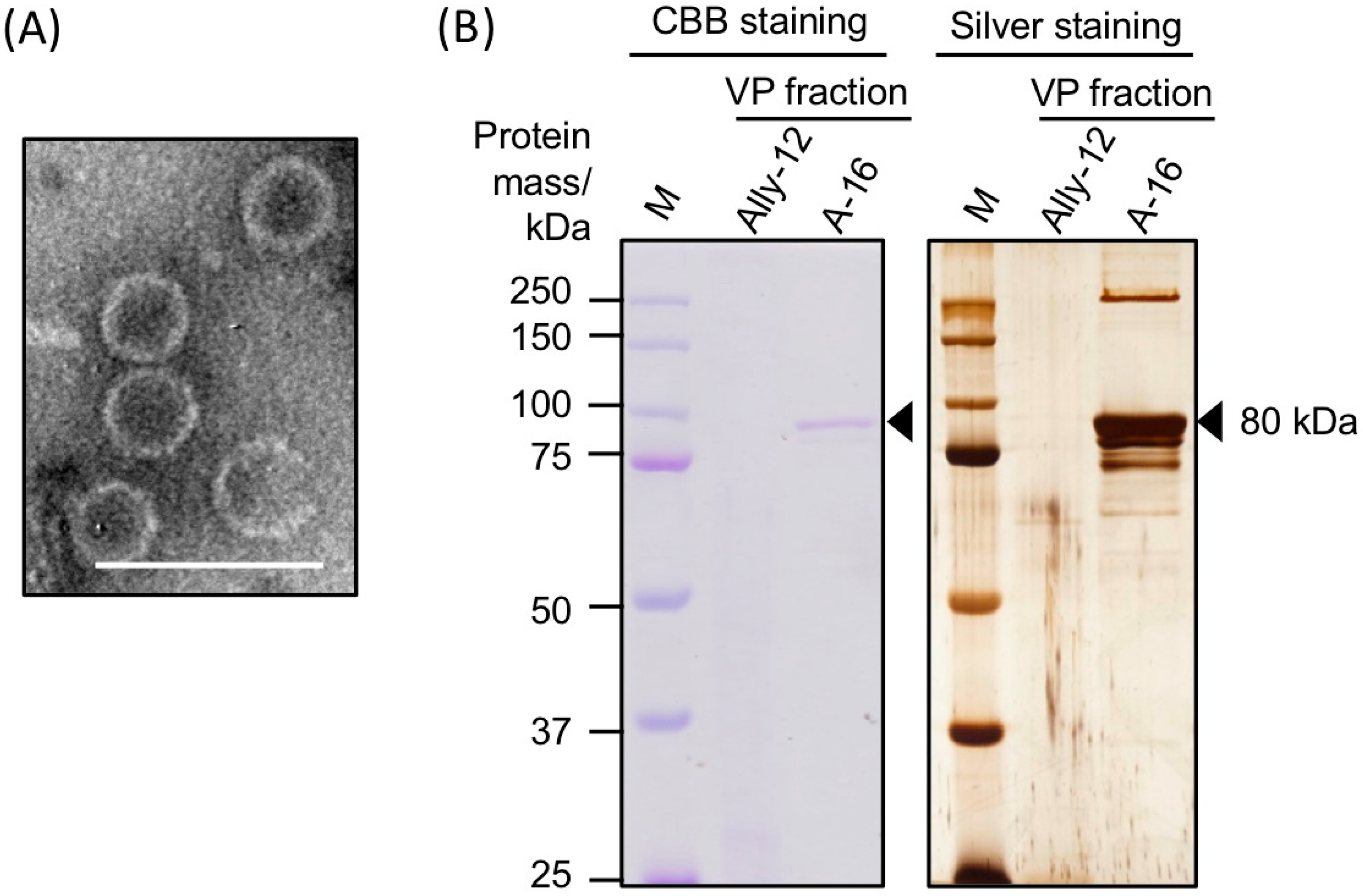
3.2. Morphology and Protein Components of Purified AalVV1 Particles
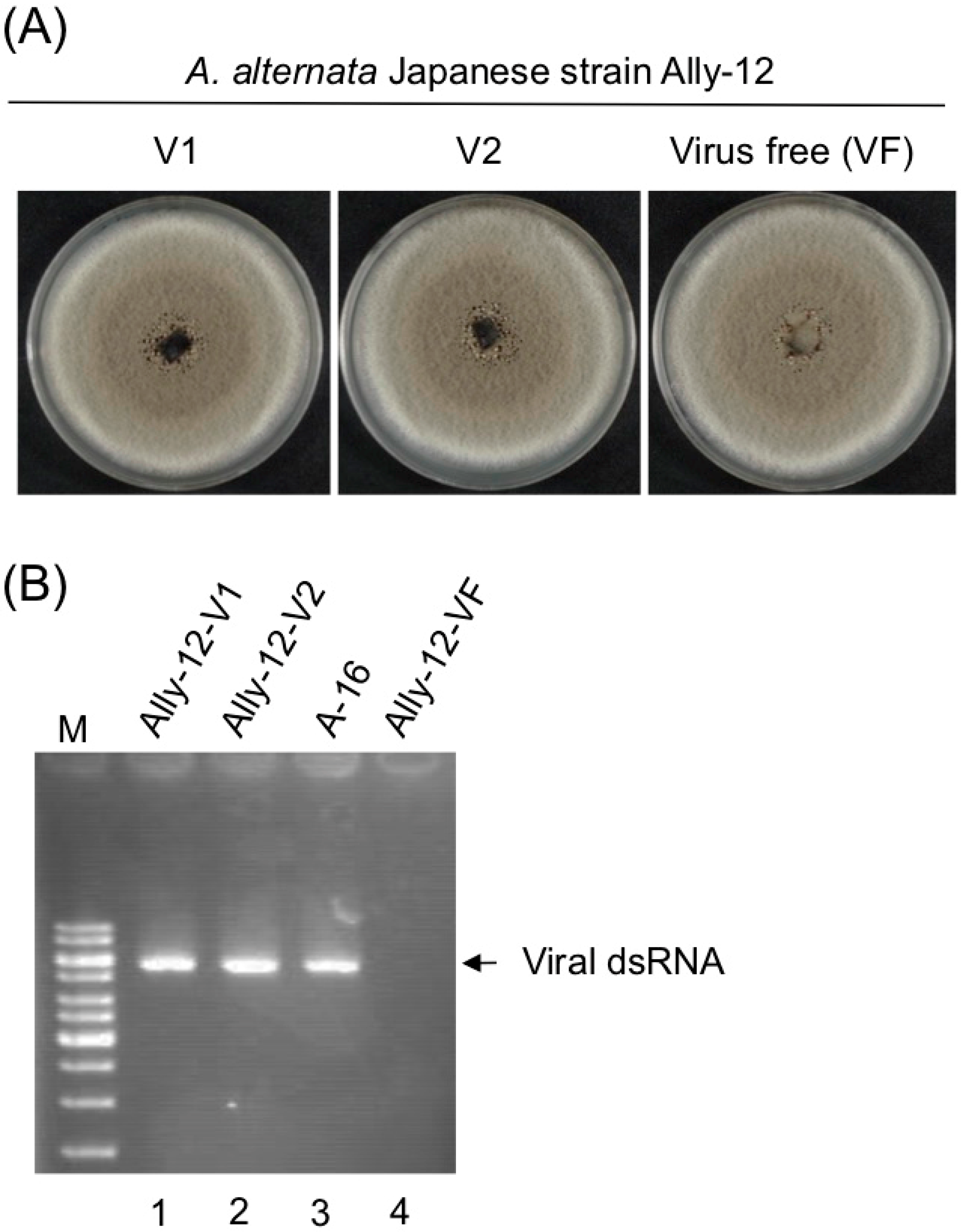
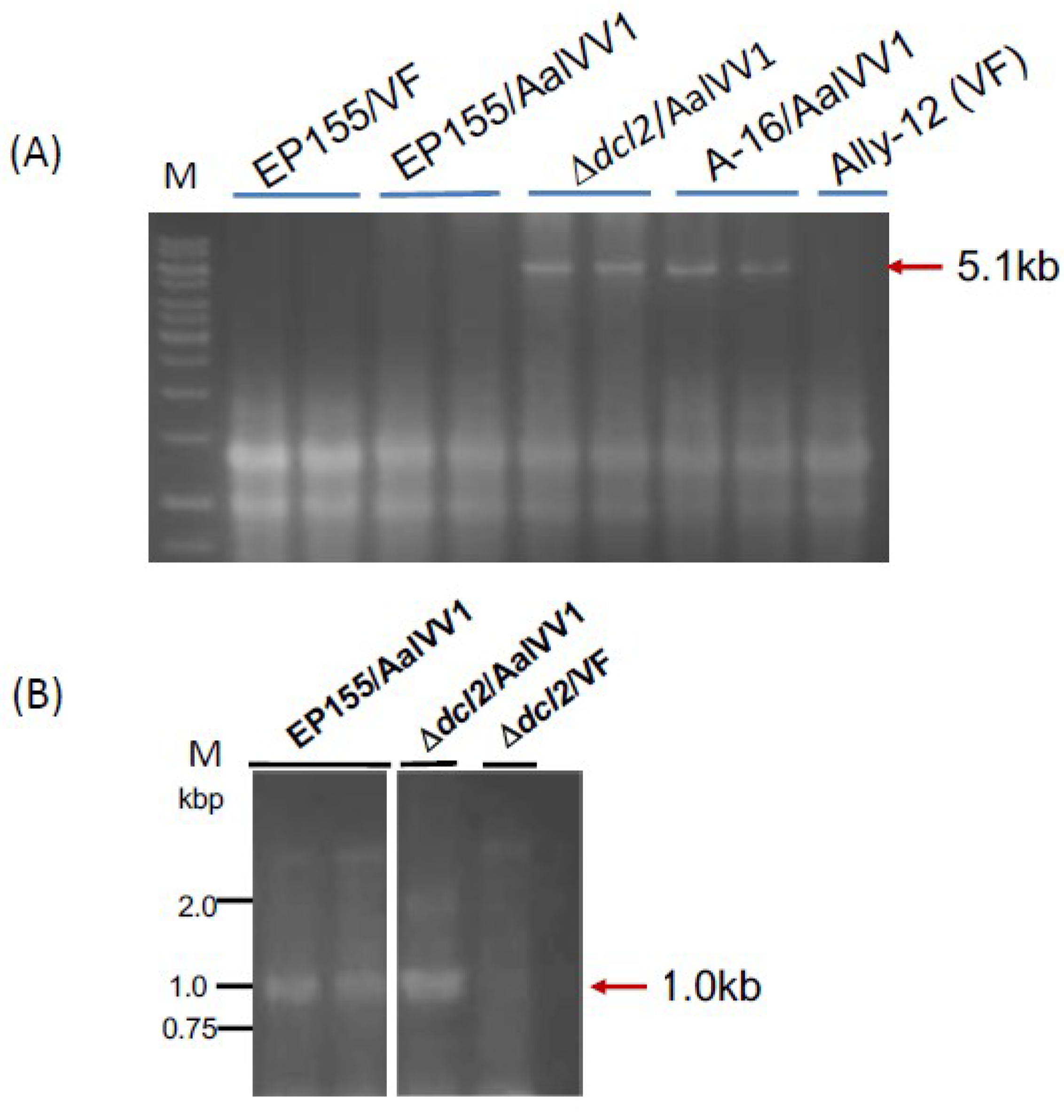
3.3. Asymptomatic Infection of A. alternata by AalVV1
3.4. AalVV1 is Infectious to the Fungus C. parasitica as Particles and Shows Symptomless Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wickner, R.B.; Ghabrial, S.A.; Nibert, M.L.; Patterson, J.L.; Wang, C.C. Family Totiviridae. In Virus Taxonomy: Ninth Report of the International Committee for the Taxonomy of Viruses; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowits, E.J., Eds.; Elsevier, Academic Press: New York, NY, USA, 2011; pp. 639–650. [Google Scholar]
- Sabanadzovic, S.; Valverde, R.A.; Brown, J.K.; Martin, R.R.; Tzanetakis, I.E. Southern tomato virus: The link between the families Totiviridae and Partitiviridae. Virus Res. 2009, 140, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.R.; Zhou, J.; Tzanetakis, I.E. Blueberry latent virus: An amalgam of the Partitiviridae and Totiviridae. Virus Res. 2011, 155, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Kozlakidis, Z.; Hacker, C.V.; Bradley, D.; Jamal, A.; Phoon, X.; Webber, J.; Brasier, C.M.; Buck, K.W.; Coutts, R.H. Molecular characterisation of two novel double-stranded RNA elements from Phlebiopsis gigantea. Virus Genes 2009, 39, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fu, Y.; Xie, J.; Cheng, J.; Ghabrial, S.A.; Li, G.; Peng, Y.; Yi, X.; Jiang, D. Evolutionary genomics of mycovirus-related dsRNA viruses reveals cross-family horizontal gene transfer and evolution of diverse viral lineages. BMC Evol. Biol. 2012, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Arjona-Lopez, J.M.; Telengech, P.; Jamal, A.; Hisano, S.; Kondo, H.; Yelin, M.D.; Arjona-Girona, M.I.; Kanematsu, S.; Lopez-Herrera, C.; Suzuki, N. Novel, diverse RNA viruses from Mediterranean isolates of the phytopathogenic fungus, Rosellinia necatrix: Insights into evolutionary biology of fungal viruses. Env. Microbiol 2018, 20, 1464–1483. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Havens, W.M.; Nibert, M.L.; Ghabrial, S.A. RNA sequence determinants of a coupled termination-reinitiation strategy for downstream open reading frame translation in Helminthosporium victoriae virus 190S and other victoriviruses (family Totiviridae). J. Virol. 2011, 85, 7343–7352. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Havens, W.M.; Nibert, M.L.; Ghabrial, S.A. An RNA cassette from Helminthosporium victoriae virus 190S necessary and sufficient for stop/restart translation. Virology 2015, 474, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Nibert, M.L.; Pyle, J.D.; Firth, A.E. A+1 ribosomal frameshifting motif prevalent among plant amalgaviruses. Virology 2016, 498, 201–208. [Google Scholar] [CrossRef]
- Atkins, J.F.; Loughran, G.; Bhatt, P.R.; Firth, A.E.; Baranov, P.V. Ribosomal frameshifting and transcriptional slippage: From genetic steganography and cryptography to adventitious use. Nucleic Acids Res. 2016, 44, 7007–7078. [Google Scholar] [CrossRef]
- Dinman, J.D.; Icho, T.; Wickner, R.B. A-1 ribosomal frameshift in a double-stranded RNA virus of yeast forms a gag-pol fusion protein. Proc. Natl. Acad. Sci. USA 1991, 88, 174–178. [Google Scholar] [CrossRef]
- Caston, J.R.; Trus, B.L.; Booy, F.P.; Wickner, R.B.; Wall, J.S.; Steven, A.C. Structure of L-A virus: A specialized compartment for the transcription and replication of double-stranded RNA. J. Cell Biol. 1997, 138, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Luque, D.; Mata, C.P.; Suzuki, N.; Ghabrial, S.A.; Caston, J.R. Capsid structure of dsRNA fungal viruses. Viruses 2018, 10, 481. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Hisano, S.; Chiba, S.; Maruyama, K.; Andika, I.B.; Toyoda, K.; Fujimori, F.; Suzuki, N. Sequence and phylogenetic analyses of novel totivirus-like double-stranded RNAs from field-collected powdery mildew fungi. Virus Res. 2016, 213, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cao, L.; Huang, Q.; Qian, Y.; Zhou, X. The complete genome sequence of a novel maize-associated totivirus. Arch. Virol. 2016, 161, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Roossinck, M.J. Evolutionary and ecological links between plant and fungal viruses. New Phytol. 2019, 221, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Esteban, R. Cap-snatching mechanism in yeast L-A double-stranded RNA virus. Proc. Natl. Acad. Sci. USA 2011, 108, 17667–17671. [Google Scholar] [CrossRef]
- Wickner, R.B.; Fujimura, T.; Esteban, R. Viruses and prions of Saccharomyces cerevisiae. Adv. Virus Res. 2013, 86, 1–36. [Google Scholar]
- Cheng, R.H.; Caston, J.R.; Wang, G.J.; Gu, F.; Smith, T.J.; Baker, T.S.; Bozarth, R.F.; Trus, B.L.; Cheng, N.; Wickner, R.B.; et al. Fungal virus capsids, cytoplasmic compartments for the replication of double-stranded RNA, formed as icosahedral shells of asymmetric Gag dimers. J. Mol. Biol. 1994, 244, 255–258. [Google Scholar] [CrossRef]
- Naitow, H.; Tang, J.; Canady, M.; Wickner, R.B.; Johnson, J.E. L-A virus at 3.4 A resolution reveals particle architecture and mRNA decapping mechanism. Nat. Struct. Biol. 2002, 9, 725–728. [Google Scholar] [CrossRef]
- Caston, J.R.; Luque, D.; Trus, B.L.; Rivas, G.; Alfonso, C.; Gonzalez, J.M.; Carrascosa, J.L.; Annamalai, P.; Ghabrial, S.A. Three-dimensional structure and stoichiometry of Helmintosporium victoriae190S totivirus. Virology 2006, 347, 323–332. [Google Scholar] [CrossRef]
- Chiba, S.; Jamal, A.; Suzuki, N. First evidence for internal ribosomal entry sites in diverse fungal virus genomes. MBio 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.H.; Sun, L.; Chiba, S.; Araki, H.; Suzuki, N. Coupled termination/reinitiation for translation of the downstream open reading frame B of the prototypic hypovirus CHV1-EP713. Nucleic Acids Res. 2009, 37, 3645–3659. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Havens, W.M.; Lin, Y.H.; Suzuki, N.; Ghabrial, S.A. The victorivirus Helminthosporium victoriae virus 190S is the primary cause of disease/hypovirulence in its natural host and a heterologous host. Virus Res. 2016, 213, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Eusebio-Cope, A.; Sun, L.; Tanaka, T.; Chiba, S.; Kasahara, S.; Suzuki, N. The chestnut blight fungus for studies on virus/host and virus/virus interactions: From a natural to a model host. Virology 2015, 477, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Lin, Y.H.; Kondo, H.; Kanematsu, S.; Suzuki, N. A novel victorivirus from a phytopathogenic fungus, Rosellinia necatrix is infectious as particles and targeted by RNA silencing. J. Virol. 2013, 87, 6727–6738. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Suzuki, N. Highly activated RNA silencing via strong induction of dicer by one virus can interfere with the replication of an unrelated virus. Proc. Natl. Acad. Sci. USA 2015, 112, E4911–E4918. [Google Scholar] [CrossRef] [PubMed]
- International Rules for Seed Testing 2018. Available online: https://www.seedtest.org/upload/cms/user/ISTARules2018SHmethod7-014_updated20171109.pdf (accessed on 22 June 2019).
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Eusebio-Cope, A.; Suzuki, N. Mycoreovirus genome rearrangements associated with RNA silencing deficiency. Nucleic Acids Res. 2015, 43, 3802–3813. [Google Scholar] [CrossRef] [PubMed]
- Taga, M.; Murata, M. Visualization of mitotic chromosomes in filamentous fungi by fluorescence staining and fluorescence in situ hybridization. Chromosoma 1994, 103, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Segers, G.C.; Zhang, X.; Deng, F.; Sun, Q.; Nuss, D.L. Evidence that RNA silencing functions as an antiviral defense mechanism in fungi. Proc. Natl. Acad. Sci. USA 2007, 104, 12902–12906. [Google Scholar] [CrossRef]
- EnzymeX version 3.3.3. Available online: http://nucleobytes.com/enzymex/index.html (accessed on 22 June 2019).
- Sperschneider, J.; Datta, A. DotKnot: Pseudoknot prediction using the probability dot plot under a refined energy model. Nucleic Acids Res. 2010, 38, e103. [Google Scholar] [CrossRef]
- Byun, Y.; Han, K. PseudoViewer3: Generating planar drawings of large-scale RNA structures with pseudoknots. Bioinformatics 2009, 25, 1435–1437. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Chiba, S.; Suzuki, N. Detection and analysis of non-retroviral RNA virus-like elements in plant, fungal, and insect genomes. Methods Mol. Biol. 2015, 1236, 73–88. [Google Scholar] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Lefort, V.; Longueville, J.E.; Gascuel, O. SMS: Smart Model Selection in PhyML. Mol. Biol. Evol. 2017, 34, 2422–2424. [Google Scholar] [CrossRef]
- FigTree version 1.3.1. Available online: http://tree.bio.ed.ac.uk/software/ (accessed on 22 June 2019).
- Suzuki, N.; Supyani, S.; Maruyama, K.; Hillman, B.I. Complete genome sequence of Mycoreovirus-1/Cp9B21, a member of a novel genus within the family Reoviridae, isolated from the chestnut blight fungus Cryphonectria parasitica. J. Gen. Virol. 2004, 85, 3437–3448. [Google Scholar] [CrossRef]
- Shamsi, W.; Sato, Y.; Jamal, A.; Shahi, S.; Kondo, H.; Suzuki, N.; Bhatti, M.F. Molecular and biological characterization of a novel botybirnavirus identified from a Pakistani isolate of Alternaria alternata. Virus Res. 2019, 263, 119–128. [Google Scholar] [CrossRef]
- Hillman, B.I.; Supyani, S.; Kondo, H.; Suzuki, N. A reovirus of the fungus Cryphonectria parasitica that is infectious as particles and related to the Coltivirus genus of animal pathogens. J. Virol. 2004, 78, 892–898. [Google Scholar] [CrossRef]
- Chiba, S.; Lin, Y.H.; Kondo, H.; Kanematsu, S.; Suzuki, N. A novel betapartitivirus RnPV6 from Rosellinia necatrix tolerates host RNA silencing but is interfered by its defective RNAs. Virus Res. 2016, 219, 62–72. [Google Scholar] [CrossRef]
- Shahi, S.; Eusebio-Cope, A.; Kondo, H.; Hillman, B.I.; Suzuki, N. Investigation of host range of and host defense against a mitochondrially replicating mitovirus. J. Virol. 2019, 93, e01503-18. [Google Scholar] [CrossRef] [PubMed]
- CBS-KNAW Collections in short. Available online: http://www.westerdijkinstitute.nl/collections/ (accessed on 3 April 2018).
- Ghabrial, S.A.; Nibert, M.L. Victorivirus, a new genus of fungal viruses in the family Totiviridae. Arch. Virol. 2009, 154, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Staple, D.W.; Butcher, S.E. Pseudoknots: RNA structures with diverse functions. PLoS Biol. 2005, 3, e213. [Google Scholar] [CrossRef] [PubMed]
- Fulbright, D.W. Effect of eliminating dsRNA in hypovirulent Endothia parasitica. Phytopathology 1984, 74, 722–724. [Google Scholar] [CrossRef]
- Aoki, N.; Moriyama, H.; Kodama, M.; Arie, T.; Teraoka, T.; Fukuhara, T. A novel mycovirus associated with four double-stranded RNAs affects host fungal growth in Alternaria alternata. Virus Res. 2009, 140, 179–187. [Google Scholar] [CrossRef]
- Xavier, A.D.S.; Barros, A.P.O.; Godinho, M.T.; Zerbini, F.M.; Souza, F.O.; Bruckner, F.P.; Alfenas-Zerbini, P. A novel mycovirus associated to Alternaria alternata comprises a distinct lineage in Partitiviridae. Virus Res. 2018, 244, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Fu, M.; Hong, N.; Zhai, L.; Xiao, F.; Wang, G. Characterization of a novel botybirnavirus isolated from a phytopathogenic Alternaria fungus. Arch. Virol. 2017, 162, 3907–3911. [Google Scholar] [CrossRef]
- Ma, G.; Liang, Z.; Hua, H.; Zhou, T.; Wu, X. Complete genome sequence of a new botybirnavirus isolated from a phytopathogenic Alternaria alternata in China. Arch. Virol. 2019, 164, 1225–1228. [Google Scholar] [CrossRef]
- Okada, R.; Ichinose, S.; Takeshita, K.; Urayama, S.I.; Fukuhara, T.; Komatsu, K.; Arie, T.; Ishihara, A.; Egusa, M.; Kodama, M.; et al. Molecular characterization of a novel mycovirus in Alternaria alternata manifesting two-sided effects: Down-regulation of host growth and up-regulation of host plant pathogenicity. Virology 2018, 519, 23–32. [Google Scholar] [CrossRef]
- Komatsu, K.; Katayama, Y.; Omatsu, T.; Mizutani, T.; Fukuhara, T.; Kodama, M.; Arie, T.; Teraoka, T.; Moriyama, H. Genome sequence of a novel victorivirus identified in the phytopathogenic fungus Alternaria arborescens. Arch. Virol. 2016, 161, 1701–1704. [Google Scholar] [CrossRef]
- Komatsu, K.; Katayama, Y.; Omatsu, T.; Mizutani, T.; Fukuhara, T.; Kodama, M.; Arie, T.; Teraoka, T.; Moriyama, H. Genome sequence of a novel mitovirus identified in the phytopathogenic fungus Alternaria arborescens. Arch. Virol. 2016, 161, 2627–2631. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shang, H.H.; Yang, H.Q.; Da Gao, B.; Zhong, J. A mitovirus isolated from the phytopathogenic fungus Alternaria brassicicola. Arch. Virol. 2017, 162, 2869–2874. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Shang, H.H.; Zhu, C.X.; Zhu, J.Z.; Zhu, H.J.; Hu, Y.; Gao, B.D. Characterization of a novel single-stranded RNA virus, closely related to fusariviruses, infecting the plant pathogenic fungus Alternaria brassicicola. Virus Res. 2016, 217, 1–7. [Google Scholar] [CrossRef]
- Shang, H.H.; Zhong, J.; Zhang, R.J.; Chen, C.Y.; Gao, B.D.; Zhu, H.J. Genome sequence of a novel endornavirus from the phytopathogenic fungus Alternaria brassicicola. Arch. Virol. 2015, 160, 1827–1830. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Tsuge, T.; Kobayashi, H.; Nishimura, S. The presence of double-stranded RNAs in Alternaria alternata Japanese pear pathotype and their participation in AK-toxin productivity. Ann. Phytopath. Soc. Jpn. 1988, 54, 250–252. [Google Scholar] [CrossRef]
- Ghabrial, S. Totiviruses. In Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., Van Regenmortel, M.H.V., Eds.; Elsevier: Oxford, UK, 2008; Volume 5, pp. 163–174. [Google Scholar]
- Meyers, G. Translation of the minor capsid protein of a calicivirus is initiated by a novel termination-dependent reinitiation mechanism. J. Biol. Chem. 2003, 278, 34051–34060. [Google Scholar] [CrossRef] [PubMed]
- Meyers, G. Characterization of the sequence element directing translation reinitiation in RNA of the calicivirus rabbit hemorrhagic disease virus. J. Virol. 2007, 81, 9623–9632. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Linder-Basso, D.; Hillman, B.I.; Kaneko, S.; Milgroom, M.G. Evidence for interspecies transmission of viruses in natural populations of filamentous fungi in the genus Cryphonectria. Mol. Ecol. 2003, 12, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Xu, R.; Boland, G.J. Hypovirulence-associated double-stranded RNA from Sclerotinia homoeocarpa is conspecific with Ophiostoma novo-ulmi mitovirus 3a-Ld. Phytopathology 2003, 93, 1407–1414. [Google Scholar] [CrossRef]
- Yaegashi, H.; Nakamura, H.; Sawahata, T.; Sasaki, A.; Iwanami, Y.; Ito, T.; Kanematsu, S. Appearance of mycovirus-like double-stranded RNAs in the white root rot fungus, Rosellinia necatrix, in an apple orchard. Fems. Microbiol. Ecol. 2013, 83, 49–62. [Google Scholar] [CrossRef]
- Kashif, M.; Hyder, R.; de Vega Perez, D.; Hantula, J.; Vainio, E.J. Heterobasidion wood decay fungi host diverse and globally distributed viruses related to Helicobasidium mompa partitivirus V70. Virus Res. 2015, 195, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ghabrial, S.A. Organization and expression of the double-stranded RNA genome of Helminthosporium victoriae 190S virus, a totivirus infecting a plant pathogenic filamentous fungus. Proc. Natl. Acad. Sci. USA 1996, 93, 12541–12546. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Choi, G.H.; Nuss, D.L. A single Argonaute gene is required for induction of RNA silencing antiviral defense and promotes viral RNA recombination. Proc. Natl. Acad. Sci. USA 2009, 106, 17927–17932. [Google Scholar] [CrossRef] [PubMed]
- Andika, I.B.; Jamal, A.; Kondo, H.; Suzuki, N. SAGA complex mediates the transcriptional up-regulation of antiviral RNA silencing. Proc. Natl. Acad. Sci. USA 2017, 114, E3499–E3506. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Lin, Y.H.; Kondo, H.; Kanematsu, S.; Suzuki, N. Effects of defective-interfering RNA on symptom induction by, and replication of a novel partitivirus from a phytopathogenic fungus Rosellinia. necatrix. J. Virol. 2013, 87, 2330–2341. [Google Scholar] [CrossRef] [PubMed]
- Aulia, A.; Andika, I.B.; Kondo, H.; Hillman, B.I.; Suzuki, N. A symptomless hypovirus, CHV4, facilitates stable infection of the chestnut blight fungus by a coinfecting reovirus likely through suppression of antiviral RNA silencing. Virology 2019, 533, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.; Chiba, C.; Kondo, H.; Suzuki, N. Cryphonectria nitschkei chrysovirus 1 with unique molecular features and a very narrow host range. 2019. (in preparation) [Google Scholar]

| Query/Virus name | QC * | E-Value | Identity | Accession |
|---|---|---|---|---|
| Query: AalVV1 ORF1 (putative capsid protein, CP) ** | ||||
| Phomopsis vexans RNA virus1 | 92% | 0.0 | 69.82% | YP_009115491.1 |
| Nigrospora oryzae victorivirus 1 | 92% | 0.0 | 68.86% | YP_009254735.1 |
| Fusarium poae victorivirus 1 | 93% | 0.0 | 66.34% | YP_009272904.1 |
| Ustilaginoidea virens RNA virus 3 | 99% | 0.0 | 64.01% | YP_009004155.1 |
| Colletotrichum caudatum totivirus 1 | 98% | 0.0 | 62.97% | AZT88630.1 |
| Alternaria arborescens victorivirus 1 | 92% | 0.0 | 67.37% | YP_009553477.1 |
| Coniothyrium minitans RNA virus | 92% | 0.0 | 66.95% | ALM62230.1 |
| Ustilaginoidea virens RNA virus 6 | 94% | 0.0 | 60.00% | ART91348.1 |
| Magnaporthe oryzae virus 3 | 94% | 0.0 | 60.22% | YP_009143306.1 |
| Gremmeniella abietina RNA virus L2 | 93% | 0.0 | 61.53% | YP_044806.1 |
| Query: AalVV1 ORF2 (putative RNA-dependent RNA-polymerase, RdRp) ** | ||||
| Nigrospora oryzae victorivirus 1 | 99% | 0.0 | 61.36% | YP_009254736.1 |
| Phomopsis vexans RNA virus | 99% | 0.0 | 59.53% | YP_009115492.1 |
| Ustilaginoidea virens RNA virus 3 | 99% | 0.0 | 56.39% | YP_009004156.1 |
| Ustilaginoidea virens RNA virus 6 | 99% | 0.0 | 54.86% | ART91349.1 |
| Fusarium poae victorivirus 1 | 99% | 0.0 | 56.39% | YP_009272905.1 |
| Colletotrichum caudatum totivirus 1 | 99% | 0.0 | 53.69% | AZT88631.1 |
| Coniothyrium minitans RNA virus | 99% | 0.0 | 50.21% | YP_392467.1 |
| Phomopsis longicolla totivirus 1 | 99% | 0.0 | 50.14% | ALD89108.1 |
| Aspergillus mycovirus 178 | 99% | 0.0 | 48.68% | ABX79995.1 |
| Magnaporthe oryzae virus 3 | 99% | 0.0 | 49.58% | YP_009143307.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamal, A.; Sato, Y.; Shahi, S.; Shamsi, W.; Kondo, H.; Suzuki, N. Novel Victorivirus from a Pakistani Isolate of Alternaria alternata Lacking a Typical Translational Stop/Restart Sequence Signature. Viruses 2019, 11, 577. https://doi.org/10.3390/v11060577
Jamal A, Sato Y, Shahi S, Shamsi W, Kondo H, Suzuki N. Novel Victorivirus from a Pakistani Isolate of Alternaria alternata Lacking a Typical Translational Stop/Restart Sequence Signature. Viruses. 2019; 11(6):577. https://doi.org/10.3390/v11060577
Chicago/Turabian StyleJamal, Atif, Yukiyo Sato, Sabitree Shahi, Wajeeha Shamsi, Hideki Kondo, and Nobuhiro Suzuki. 2019. "Novel Victorivirus from a Pakistani Isolate of Alternaria alternata Lacking a Typical Translational Stop/Restart Sequence Signature" Viruses 11, no. 6: 577. https://doi.org/10.3390/v11060577
APA StyleJamal, A., Sato, Y., Shahi, S., Shamsi, W., Kondo, H., & Suzuki, N. (2019). Novel Victorivirus from a Pakistani Isolate of Alternaria alternata Lacking a Typical Translational Stop/Restart Sequence Signature. Viruses, 11(6), 577. https://doi.org/10.3390/v11060577






