Control of Acute Arboviral Infection by Natural Killer Cells
Abstract
1. Introduction
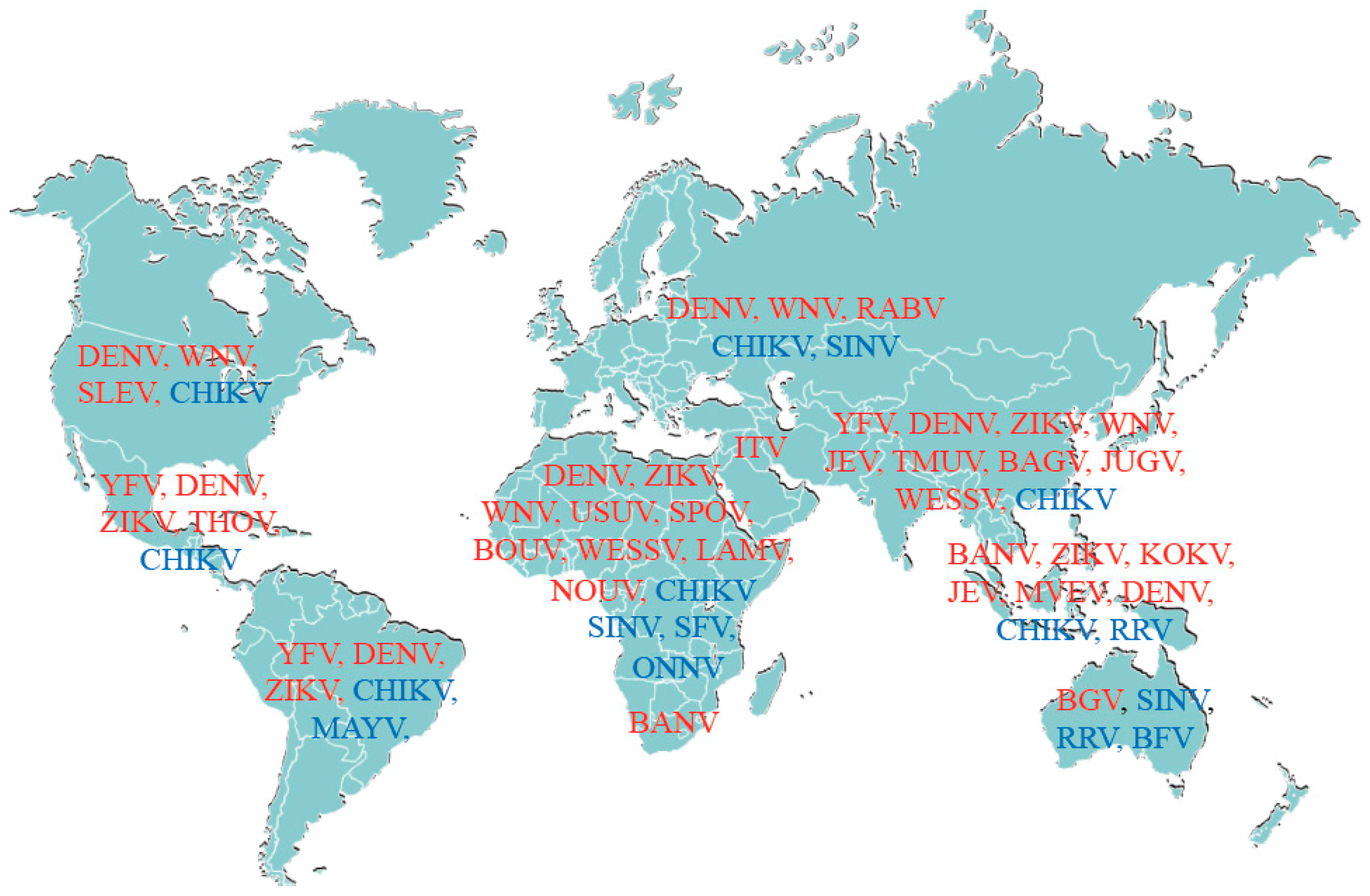
1.1. Emerging Arboviruses: A Global Public Health Threat
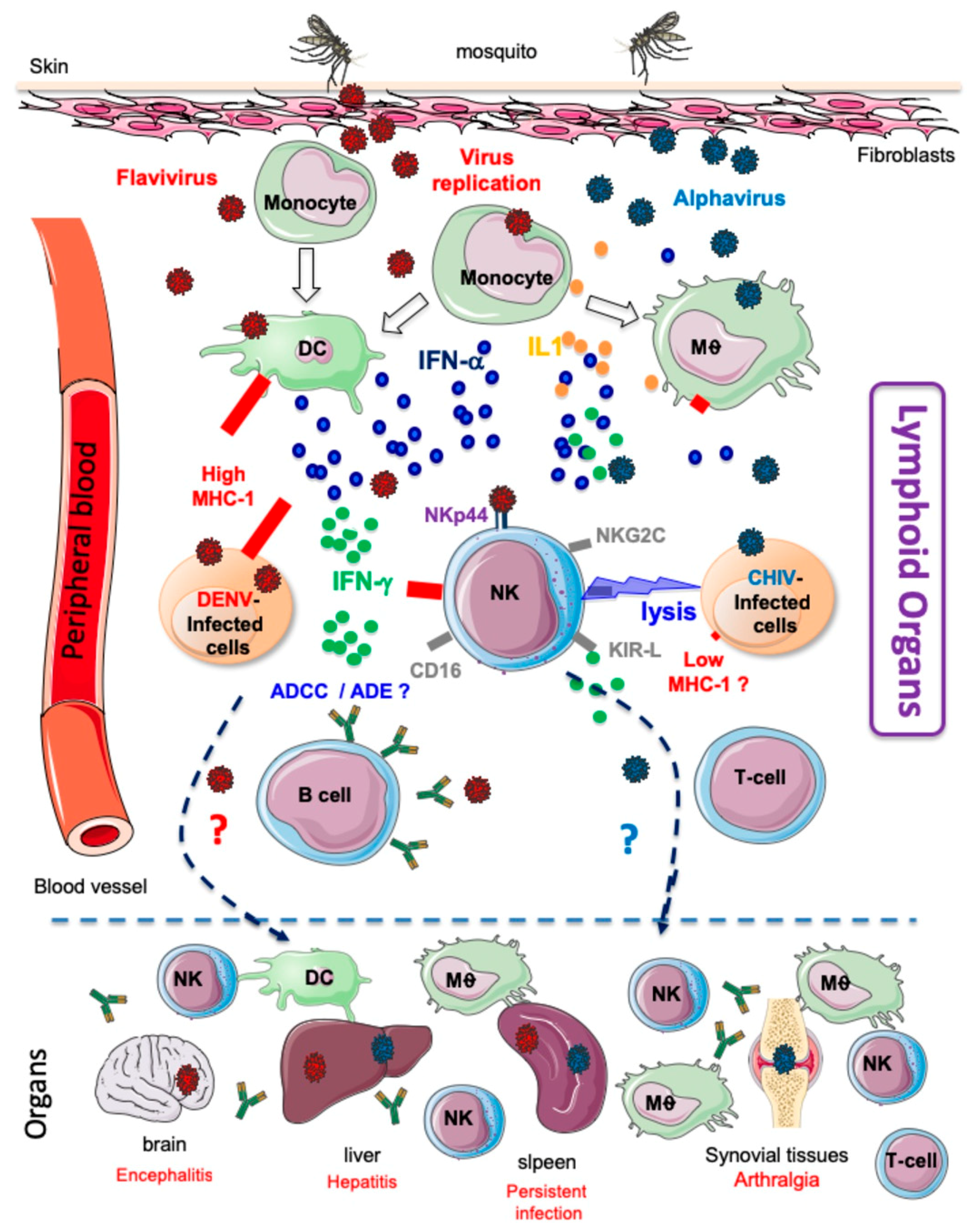
1.2. Host Immune Responses to Mosquito Bites and Arbovirus Infection
1.3. NK Cells: A Critical First-Line Defence of the Immune System against Viral Infections
- (1)
- (2)
- (3)
- NK cells can control a viral infection in the absence of a T cell response. This was shown in a 3-month-old girl with a T−B+NK+ severe combined immunodeficiency phenotype who recovered from cytomegalovirus (CMV) infection without antiviral therapy. In this patient, the high number of NK cells (>2 × 1010/L) present at the peak of viremia, as well as the correlation between viral load and the number of NK cells during follow-up, were suggestive of a causal relation [35].
- (4)
- Adaptive NKG2C+ NK cells can be expanded in response to different viral infections in the context of human CMV seropositivity [36,37,38]. These hyper-reactive and long-lived NK cells have been described after certain viral infections and are able to mount stronger protective responses upon re-encounter of the same pathogen, at least in mice [39,40,41,42], which could redefine the notion of immunological memory for NK cells. These adaptive NK cells will be discussed in further detail later, in the context of arbovirus infection.
2. NK Cell Responses following Arbovirus Infection
2.1. Alphaviviruses
2.2. Flaviviruses
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gould, E.A.; Solomon, T. Pathogenic flaviviruses. Lancet 2008, 371, 500–509. [Google Scholar] [CrossRef]
- Strauss, J.H.; Strauss, E.G. The alphaviruses: Gene expression, replication and evolution. Microbiol. Rev. 1994, 58, 491–562. [Google Scholar]
- Musso, D.; Rodriguez-Morales, A.J.; Levi, J.E.; Cao-Lormeau, V.M.; Gubler, D.J. Unexpected outbreaks of arbovirus infections: Lessons learned from the Pacific and tropical America. Lancet Infect. Dis. 2018. [Google Scholar] [CrossRef]
- Harrington, L.C.; Buonaccorsi, J.P.; Edman, J.D.; Costero, A.; Kittayapong, P.; Clark, G.G.; Scott, T.W. Analysis of survival of young and old Aedes aegypti (Diptera: Culicidac) from Puerto Rico and Thailand. J. Med. Entomol. 2001, 38, 537–547. [Google Scholar] [CrossRef]
- Lambrechts, L.; Scott, T.W.; Gubler, D.J. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl. Trop. Dis. 2010, 4, e646. [Google Scholar] [CrossRef]
- Petitdemange, C.; Wauquier, N.; Vieillard, V. Control of immunopathology during chikungunya virus infection. J. Allergy Clin. Immunol. 2015, 135, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Stanaway, J.D. The global economic burden of dengue: A systematic analysis. Lancet Infect. Dis. 2016, 16, 935–941. [Google Scholar] [CrossRef]
- Laureti, M.; Narayanan, D.; Rodriguez-Andres, J.; Fazakerley, J.K.; Kedzierski, L. Flavivirus Receptors: Diversity, Identity and Cell Entry. Front. Immunol. 2018, 9, 2180. [Google Scholar] [CrossRef]
- Van Duijl-Richter, M.K.; Hoornweg, T.E.; Rodenhuis-Zybert, I.A.; Smit, J.M. Early Events in Chikungunya Virus Infection-From Virus Cell Binding to Membrane Fusion. Viruses 2015, 7, 3647–3674. [Google Scholar] [CrossRef]
- Palha, N.; Guivel-Benhassine, F.; Briolat, V.; Lutfalla, G.; Sourisseau, M.; Ellett, F.; Wang, C.H.; Lieschke, G.J.; Herbomel, P.; Schwartz, O.; et al. Real-time whole-body visualization of Chikungunya Virus infection and host interferon response in zebrafish. PLoS Pathog. 2013, 9, e1003619. [Google Scholar] [CrossRef]
- Schwartz, O.; Albert, M.L. Biology and pathogenesis of chikungunya virus. Nat. Rev. Microbiol. 2010, 8, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Plante, K.; Wang, E.; Partidos, C.D.; Weger, J.; Gorchakov, R.; Tsetsarkin, K.; Borland, E.M.; Powers, A.M.; Seymour, R.; Stinchcomb, D.T.; et al. Novel chikungunya vaccine candidate with an IRES-based attenuation and host range alteration mechanism. PLoS Pathog. 2011, 7, e1002142. [Google Scholar] [CrossRef]
- Pingen, M.; Bryden, S.R.; Pondeville, E.; Schnettler, E.; Kohl, A.; Merits, A.; Fazakerley, J.K.; Graham, G.J.; McKimmie, C.S. Host Inflammatory Response to Mosquito Bites Enhances the Severity of Arbovirus Infection. Immunity 2016, 44, 1455–1469. [Google Scholar] [CrossRef] [PubMed]
- Pingen, M.; Schmid, M.A.; Harris, E.; McKimmie, C.S. Mosquito Biting Modulates Skin Response to Virus Infection. Trends Parasitol. 2017, 33, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Petitdemange, C.; Wauquier, N.; Rey, J.; Hervier, B.; Leroy, E.; Vieillard, V. Control of acute dengue virus infection by natural killer cells. Front. Immunol. 2014, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Wikan, N.; Smith, D.R. Zika virus: History of a newly emerging arbovirus. Lancet Infect. Dis. 2016, 16, e119–e126. [Google Scholar] [CrossRef]
- Auerswald, H.; Boussioux, C.; In, S.; Mao, S.; Ong, S.; Huy, R.; Leang, R.; Chan, M.; Duong, V.; Ly, S.; et al. Broad and long-lasting immune protection against various Chikungunya genotypes demonstrated by participants in a cross-sectional study in a Cambodian rural community. Emerg. Microbes Infect. 2018, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Gresh, L.; Halloran, M.E.; Mercado, J.C.; Kuan, G.; Gordon, A.; Balmaseda, A.; Harris, E. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017, 358, 929–932. [Google Scholar] [CrossRef]
- Hammer, Q.; Rückert, T.; Romagnani, C. Natural killer cell specificity for viral infections. Nat. Immunol. 2018, 19, 800–808. [Google Scholar] [CrossRef]
- Farber, D.L.; Yudanin, N.A.; Restifo, N.P. Human memory T cells: Generation, compartmentalization and homeostasis. Nat. Rev. Immunol. 2014, 14, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wallace, D.L.; de Lara, C.M.; Ghattas, H.; Asquith, B.; Worth, A.; Griffin, G.E.; Taylor, G.P.; Tough, D.F.; Beverley, P.C.; et al. In vivo kinetics of human natural killer cells: The effects of ageing and acute and chronic viral infection. Immunology 2007, 121, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Orange, J.S. Natural killer cell deficiency. J. Allergy Clin. Immunol. 2013, 132, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Mace, E.M.; Orange, J.S. Emerging insights into human health and NK cell biology from the study of NK cell deficiencies. Immunol. Rev. 2019, 287, 202–225. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Jost, S.; Altfeld, M. Control of human viral infections by natural killer cells. Annu. Rev. Immunol. 2013, 31, 163–194. [Google Scholar] [CrossRef] [PubMed]
- Björkström, N.K.; Ljunggren, H.G.; Michaëlsson, J. Emerging insights into natural killer cells in human peripheral tissues. Nat. Rev. Immunol. 2016, 16, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Della Chiesa, M.; Sivori, S.; Carlomagno, S.; Moretta, L.; Moretta, A. Activating KIRs and NKG2C in Viral Infections: Toward NK Cell Memory? Front. Immunol. 2015, 6, 573. [Google Scholar] [CrossRef]
- Naiyer, M.M.; Cassidy, S.A.; Magri, A.; Cowton, V.; Chen, K.; Mansour, S.; Kranidioti, H.; Mbirbindi, B.; Rettman, P.; Harris, S.; et al. KIR2DS2 recognizes conserved peptides derived from viral helicases in the context of HLA-C. Sci. Immunol. 2017, 2, eaal5296. [Google Scholar] [CrossRef] [PubMed]
- Long, E.O.; Kim, H.S.; Liu, D.; Peterson, M.E.; Rajagopalan, S. Controlling natural killer cell responses: Integration of signals for activation and inhibition. Annu. Rev. Immunol. 2013, 31, 227–258. [Google Scholar] [CrossRef]
- Strauss-Albee, D.M.; Fukuyama, J.; Liang, E.C.; Yao, Y.; Jarrell, J.A.; Drake, A.L.; Kinuthia, J.; Montgomery, R.R.; John-Stewart, G.; Holmes, S.; et al. Human NK cell repertoire diversity reflects immune experience and correlates with viral susceptibility. Sci. Transl. Med. 2015, 7, 297ra115. [Google Scholar] [CrossRef]
- Kulkarni, S.; Martin, M.P.; Carrington, M. The Yin and Yang of HLA and KIR in human disease. Semin. Immunol. 2008, 20, 343–352. [Google Scholar] [CrossRef]
- Petitdemange, C.; Wauquier, N.; Jacquet, J.M.; Theodorou, I.; Leroy, E.; Vieillard, V. Association of HLA class-I and inhibitory KIR genotypes in Gabonese patients infected by Chikungunya or Dengue type-2 viruses. PLoS ONE 2014, 9, e108798. [Google Scholar] [CrossRef]
- Boudreau, J.E.; Hsu, K.C. Natural killer cell education in human health and disease. Curr. Opin. Immunol. 2018, 50, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, T.W.; Baars, P.A.; Dantin, C.; van den Burg, M.; van Lier, R.A.; Roosnek, E. Human NK cells can control CMV infection in the absence of T cells. Blood 2008, 112, 914–915. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.A.; Fehniger, T.A. Human Adaptive Natural Killer Cells: Beyond NKG2C. Trends Immunol. 2016, 37, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, K.J.; Beziat, V.; Ljunggren, H.G. Spotlight on NKG2C and the human NK-cell response to CMV infection. Eur. J. Immunol. 2012, 42, 3141–3145. [Google Scholar] [CrossRef]
- López-Botet, M.; Muntasell, A.; Vilches, C. The CD94/NKG2C+ NK-cell subset on the edge of innate and adaptive immunity to human cytomegalovirus infection. Semin. Immunol. 2014, 26, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.C.; Beilke, J.N.; Lanier, L.L. Adaptive immune features of natural killer cells. Nature 2009, 457, 557–561. [Google Scholar] [CrossRef]
- Paust, S.; Gill, H.S.; Wang, B.Z.; Flynn, M.P.; Moseman, E.A.; Senman, B.; Szczepanik, M.; Telenti, A.; Askenase, P.W.; Compans, R.W.; et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat. Immunol. 2010, 11, 1127–1135. [Google Scholar] [CrossRef]
- Adams, N.M.; O’Sullivan, T.E.; Geary, C.D.; Karo, J.M.; Amezquita, R.A.; Joshi, N.S.; Kaech, S.M.; Sun, J.C. NK Cell Responses Redefine Immunological Memory. J. Immunol. 2016, 197, 2963–2970. [Google Scholar] [CrossRef] [PubMed]
- Cerwenka, A.; Lanier, L.L. Natural killer cell memory in infection, inflammation and cancer. Nat. Rev. Immunol. 2016, 16, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Murray, K.O. Dengue, West Nile virus, chikungunya, Zika-and now Mayaro? PLoS Negl. Trop. Dis. 2017, 11, e0005462. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Ampudia, Y.; Monsalve, D.M.; Rodríguez, Y.; Pacheco, Y.; Anaya, J.M.; Ramírez-Santana, C. Mayaro: An emerging viral threat? Emerg. Microbes Infect. 2018, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Petitdemange, C.; Becquart, P.; Wauquier, N.; Béziat, V.; Debré, P.; Leroy, E.M.; Vieillard, V. Unconventional repertoire profile is imprinted during acute chikungunya infection for natural killer cells polarization toward cytotoxicity. PLoS Pathog. 2011, 7, e1002268. [Google Scholar] [CrossRef] [PubMed]
- Petitdemange, C.; Wauquier, N.; Devilliers, H.; Yssel, H.; Mombo, I.; Caron, M.; Nkoghé, D.; Debré, P.; Leroy, E.; Vieillard, V. Longitudinal Analysis of Natural Killer Cells in Dengue Virus-Infected Patients in Comparison to Chikungunya and Chikungunya/Dengue Virus-Infected Patients. PLoS Negl. Trop. Dis. 2016, 10, e0004499. [Google Scholar] [CrossRef] [PubMed]
- Thanapati, S.; Das, R.; Tripathy, A.S. Phenotypic and functional analyses of NK and NKT-like populations during the early stages of chikungunya infection. Front. Microbiol. 2015, 6, 895. [Google Scholar] [CrossRef]
- Fox, J.M.; Diamond, M.S. Immune-Mediated Protection and Pathogenesis of Chikungunya Virus. J. Immunol. 2016, 197, 4210–4218. [Google Scholar] [CrossRef]
- Hoarau, J.J.; Jaffar Bandjee, M.C.; Krejbich Trotot, P.; Das, T.; Li-Pat-Yuen, G.; Dassa, B.; Denizot, M.; Guichard, E.; Ribera, A.; Henni, T.; et al. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J. Immunol. 2010, 184, 5914–5927. [Google Scholar] [CrossRef]
- Della Chiesa, M.; Marcenaro, E.; Sivori, S.; Carlomagno, S.; Pesce, S.; Moretta, A. Human NK cell response to pathogens. Semin. Immunol. 2014, 26, 152–160. [Google Scholar] [CrossRef]
- Gumá, M.; Angulo, A.; Vilches, C.; Gómez-Lozano, N.; Malats, N.; López-Botet, M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood 2004, 104, 3664–3671. [Google Scholar] [CrossRef] [PubMed]
- Björkström, N.K.; Lindgren, T.; Stoltz, M.; Fauriat, C.; Braun, M.; Evander, M.; Michaëlsson, J.; Malmberg, K.J.; Klingström, J.; Ahlm, C.; et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J. Exp. Med. 2011, 208, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, B.; Varchetta, S.; Paudice, E.; Michelone, G.; Zaramella, M.; Mavilio, D.; De Filippi, F.; Bruno, S.; Mondelli, M.U. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology 2009, 137, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Béziat, V.; Dalgard, O.; Asselah, T.; Halfon, P.; Bedossa, P.; Boudifa, A.; Hervier, B.; Theodorou, I.; Martinot, M.; Debré, P.; et al. CMV drives clonal expansion of NKG2C+ NK cells expressing self-specific KIRs in chronic hepatitis patients. Eur. J. Immunol. 2012, 42, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Rölle, A.; Brodin, P. Immune Adaptation to Environmental Influence: The Case of NK Cells and HCMV. Trends Immunol. 2016, 37, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Rölle, A.; Pollmann, J.; Ewen, E.M.; Le, V.T.; Halenius, A.; Hengel, H.; Cerwenka, A. IL-12-producing monocytes and HLA-E control HCMV-driven NKG2C+ NK cell expansion. J. Clin. Investig. 2014, 124, 5305–5316. [Google Scholar] [CrossRef]
- Braun, M.; Björkström, N.K.; Gupta, S.; Sundström, K.; Ahlm, C.; Klingström, J.; Ljunggren, H.G. NK cell activation in human hantavirus infection explained by virus-induced IL-15/IL15Rα expression. PLoS Pathog. 2014, 10, e1004521. [Google Scholar] [CrossRef]
- Rölle, A.; Meyer, M.; Calderazzo, S.; Jäger, D.; Momburg, F. Distinct HLA-E Peptide Complexes Modify Antibody-Driven Effector Functions of Adaptive NK Cells. Cell Rep. 2018, 24, 1967–1976. [Google Scholar] [CrossRef]
- Lam, V.C.; Lanier, L.L. NK cells in host responses to viral infections. Curr. Opin. Immunol. 2017, 44, 43–51. [Google Scholar] [CrossRef]
- Sun, J.C.; Lanier, L.L. Is There Natural Killer Cell Memory and Can It Be Harnessed by Vaccination? NK Cell Memory and Immunization Strategies against Infectious Diseases and Cancer. Cold Spring Harb. Perspect. Biol. 2018, 1010, a029538. [Google Scholar] [CrossRef]
- Teo, T.H.; Her, Z.; Tan, J.J.; Lum, F.M.; Lee, W.W.; Chan, Y.H.; Ong, R.Y.; Kam, Y.W.; Leparc-Goffart, I.; Gallian, P.; et al. Caribbean and La Réunion Chikungunya Virus Isolates Differ in Their Capacity To Induce Proinflammatory Th1 and NK Cell Responses and Acute Joint Pathology. J. Virol. 2015, 89, 7955–7969. [Google Scholar] [CrossRef]
- Huss, R.S.; Huddleston, J.I.; Goodman, S.B.; Butcher, E.C.; Zabel, B.A. Synovial tissue-infiltrating natural killer cells in osteoarthritis and periprosthetic inflammation. Arthritis Rheum. 2010, 62, 3799–3805. [Google Scholar] [CrossRef] [PubMed]
- Jaime, P.; García-Guerrero, N.; Estella, R.; Pardo, J.; García-Álvarez, F.; Martinez-Lostao, L. CD56+/CD16− Natural Killer cells expressing the inflammatory protease granzyme A are enriched in synovial fluid from patients with osteoarthritis. Osteoarthr. Cartil. 2017, 25, 1708–1718. [Google Scholar] [CrossRef] [PubMed]
- Strode, G.K. Yellow Fever; McGraw-Hill: New York, NY, USA, 1951. [Google Scholar]
- Jia, X.Y.; Briese, T.; Jordan, I.; Rambaut, A.; Chi, H.C.; Mackenzie, J.S.; Hall, R.A.; Scherret, J.; Lipkin, W.I. Genetic analysis of West Nile New York 1999 encephalitis virus. Lancet 1999, 354, 1971–1972. [Google Scholar] [CrossRef]
- Ngono, A.E.; Shresta, S. Immune Response to Dengue and Zika. Annu. Rev. Immunol. 2018, 36, 279–308. [Google Scholar] [CrossRef]
- Becquart, P.; Wauquier, N.; Nkoghe, D.; Ndjoyi-Mbiguino, A.; Padilla, C.; Souris, M.; Leroy, E.M. Acute dengue virus 2 infection in Gabonese patients is associated with an early innate immune response, including strong interferon alpha production. BMC Infect. Dis. 2010, 10, 356. [Google Scholar] [CrossRef]
- Sun, P.; García, J.; Comach, G.; Vahey, M.T.; Wang, Z.; Forshey, B.M.; Morrison, A.C.; Sierra, G.; Bazan, I.; Rocha, C.; et al. Sequential waves of gene expression in patients with clinically defined dengue illnesses reveal subtle disease phases and predict disease severity. PLoS Negl. Trop. Dis. 2013, 7, e2298. [Google Scholar] [CrossRef]
- Biron, C.A.; Nguyen, K.B.; Pien, G.C.; Cousens, L.P.; Salazar-Mather, T.P. Natural killer cells in antiviral defense: Function and regulation by innate cytokines. Annu. Rev. Immunol. 1999, 17, 189–220. [Google Scholar] [CrossRef]
- Costa, V.V.; Ye, W.; Chen, Q.; Teixeira, M.M.; Preiser, P.; Ooi, E.E.; Chen, J. Dengue Virus-Infected Dendritic Cells but Not Monocytes, Activate Natural Killer Cells through a Contact-Dependent Mechanism Involving Adhesion Molecules. mBio 2017, 8, e00741-17. [Google Scholar] [CrossRef]
- Keawvichit, R.; Khowawisetsut, L.; Lertjuthaporn, S.; Tangnararatchakit, K.; Apiwattanakul, N.; Yoksan, S.; Chuansumrit, A.; Chokephaibulkit, K.; Ansari, A.A.; Onlamoon, N.; et al. Differences in activation and tissue homing markers of natural killer cell subsets during acute dengue infection. Immunology 2018, 153, 455–465. [Google Scholar] [CrossRef]
- Hsieh, M.F.; Lai, S.L.; Chen, J.P.; Sung, J.M.; Lin, Y.L.; Wu-Hsieh, B.A.; Gerard, C.; Luster, A.; Liao, F. Both CXCR3 and CXCL10/IFN-inducible protein 10 are required for resistance to primary infection by dengue virus. J. Immunol. 2006, 177, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, E.L.; De Oliveira-Pinto, L.M.; Zagne, S.M.; Cerqueira, D.I.; Nogueira, R.M.; Kubelka, C.F. NK cells, displaying early activation, cytotoxicity and adhesion molecules, are associated with mild dengue disease. Clin. Exp. Immunol. 2006, 143, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Green, S.; Pichyangkul, S.; Vaughn, D.W.; Kalayanarooj, S.; Nimmannitya, S.; Nisalak, A.; Kurane, I.; Rothman, A.L.; Ennis, F.A. Early CD69 expression on peripheral blood lymphocytes from children with dengue hemorrhagic fever. J. Infect. Dis. 1999, 180, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Strauss-Albeen, D.M.; Zhou, J.Q.; Malawista, A.; Garcia, M.N.; Murray, K.O.; Blish, C.A.; Montgomery, R.R. The natural killer cell response to West Nile virus in young and old individuals with or without a prior history of infection. PLoS ONE 2017, 12, e0172625. [Google Scholar] [CrossRef] [PubMed]
- Béziat, V.; Sleiman, M.; Goodridge, J.P.; Kaarbø, M.; Liu, L.L.; Rollag, H.; Ljunggren, H.G.; Zimmer, J.; Malmberg, K.J. Polyclonal Expansion of NKG2C(+) NK Cells in TAP-Deficient Patients. Front. Immunol. 2015, 6, 507. [Google Scholar] [CrossRef] [PubMed]
- Neves, P.C.; Matos, D.C.; Marcovistz, R.; Galler, R. TLR expression and NK cell activation after human yellow fever vaccination. Vaccine 2009, 27, 5543–5549. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, N.; Ivarsson, M.A.; Blom, K.; Gonzalez, V.D.; Braun, M.; Falconer, K.; Gustafsson, R.; Fogdell-Hahn, A.; Sandberg, J.K.; Michaëlsson, J. The Human NK Cell Response to Yellow Fever Virus 17D Is Primarily Governed by NK Cell Differentiation Independently of NK Cell Education. J. Immunol. 2015, 195, 3262–3272. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, L.M.; Sell, A.M.; Moliterno, R.A.; Clementino, S.L.; Cardozo, D.M.; Dalalio, M.M.; Fonzar, U.J.; Visentainer, J.E. Influence of KIR genes and their HLA ligands in susceptibility to dengue in a population from southern Brazil. Tissue Antigens 2013, 82, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Alagarasu, K.; Bachal, R.V.; Shah, P.S.; Cecilia, D. Profile of killer cell immunoglobulin-like receptor and its human leucocyte antigen ligands in dengue-infected patients from Western India. Int. J. Immunogenet. 2015, 42, 432–438. [Google Scholar] [CrossRef]
- Alter, G.; Heckerman, D.; Schneidewind, A.; Fadda, L.; Kadie, C.M.; Carlson, J.M.; Oniangue-Ndza, C.; Martin, M.; Li, B.; Khakoo, S.I.; et al. HIV-1 adaptation to NK-cell-mediated immune pressure. Nature 2011, 476, 96–100. [Google Scholar] [CrossRef]
- Townsley, E.; O’Connor, G.; Cosgrove, C.; Woda, M.; Co, M.; Thomas, S.J.; Kalayanarooj, S.; Yoon, I.K.; Nisalak, A.; Srikiatkhachorn, A.; et al. Interaction of a dengue virus NS1-derived peptide with the inhibitory receptor KIR3DL1 on natural killer cells. Clin. Exp. Immunol. 2016, 183, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Blom, K.; Braun, M.; Pakalniene, J.; Lunemann, S.; Enqvist, M.; Dailidyte, L.; Schaffer, M.; Lindquist, L.; Mickiene, A.; Michaëlsson, J.; et al. NK Cell Responses to Human Tick-Borne Encephalitis Virus Infection. J. Immunol. 2016, 197, 2762–2771. [Google Scholar] [CrossRef] [PubMed]
- Meresse, B.; Curran, S.A.; Ciszewski, C.; Orbelyan, G.; Setty, M.; Bhagat, G.; Lee, L.; Tretiakova, M.; Semrad, C.; Kistner, E.; et al. Reprogramming of CTLs into natural killer-like cells in celiac disease. J. Exp. Med. 2006, 203, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.; Bottino, C.; Sivori, S.; Sanseverino, L.; Castriconi, R.; Marcenaro, E.; Augugliaro, R.; Moretta, L.; Moretta, A. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J. Exp. Med. 1998, 187, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Baychelier, F.; Sennepin, A.; Ermonval, M.; Dorgham, K.; Debré, P.; Vieillard, V. Identification of a cellular ligand for the natural cytotoxicity receptor NKp44. Blood 2013, 122, 2935–2942. [Google Scholar] [CrossRef] [PubMed]
- Hershkovitz, O.; Rosental, B.; Rosenberg, L.A.; Navarro-Sanchez, M.E.; Jivov, S.; Zilka, A.; Gershoni-Yahalom, O.; Brient-Litzler, E.; Bedouelle, H.; Ho, J.W.; et al. NKp44 receptor mediates interaction of the envelope glycoproteins from the West Nile and dengue viruses with NK cells. J. Immunol. 2009, 183, 2610–2621. [Google Scholar] [CrossRef] [PubMed]
- Lobigs, M.; Müllbacher, A.; Regner, M. MHC class I up-regulation by flaviviruses: Immune interaction with unknown advantage to host or pathogen. Immunol. Cell. Biol. 2003, 81, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Glasner, A.; Oiknine-Djian, E.; Weisblum, Y.; Diab, M.; Panet, A.; Wolf, D.G.; Mandelboim, O. Zika Virus Escapes NK Cell Detection by Upregulating Major Histocompatibility Complex Class I Molecules. J. Virol. 2017, 91, e00785-17. [Google Scholar] [CrossRef]
- Cheng, Y.; King, N.J.; Kesson, A.M. Major histocompatibility complex class I (MHC-I) induction by West Nile virus: Involvement of 2 signaling pathways in MHC-I up-regulation. J. Infect. Dis. 2004, 189, 658–668. [Google Scholar] [CrossRef]
- Drews, E.; Adam, A.; Htoo, P.; Townsley, E.; Mathew, A. Upregulation of HLA-E by dengue and not Zika viruses. Clin. Transl. Immunol. 2018, 7, e1039. [Google Scholar] [CrossRef]
- Zhang, M.; Daniel, S.; Huang, Y.; Chancey, C.; Huang, Q.; Lei, Y.F.; Grinev, A.; Mostowski, H.; Rios, M.; Dayton, A. Anti-West Nile virus activity of in vitro expanded human primary natural killer cells. BMC Immunol. 2010, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, J.A.; Barros, V.L.; Pagliari, C.; Fernandes, E.R.; Guedes, F.; Takakura, C.F.; Andrade, H.F., Jr.; Vasconcelos, P.F.; Duarte, M.I. Revisiting the liver in human yellow fever: Virus-induced apoptosis in hepatocytes associated with TGF-beta, TNF-alpha and NK cells activity. Virology 2006, 345, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Kurane, I.; Hebblewaite, D.; Brandt, W.E.; Ennis, F.A. Lysis of dengue virus-infected cells by natural cell-mediated cytotoxicity and antibody-dependent cell-mediated cytotoxicity. J. Virol. 1984, 52, 223–230. [Google Scholar]
- Laoprasopwattana, K.; Libraty, D.H.; Endy, T.P.; Nisalak, A.; Chunsuttiwat, S.; Ennis, F.A.; Rothman, A.L.; Green, S. Antibody-dependent cellular cytotoxicity mediated by plasma obtained before secondary dengue virus infections: Potential involvement in early control of viral replication. J. Infect. Dis. 2007, 195, 1108–1116. [Google Scholar] [CrossRef]
- Sun, P.; Morrison, B.J.; Beckett, C.G.; Liang, Z.; Nagabhushana, N.; Li, A.; Porter, K.R.; Williams, M. NK cell degranulation as a marker for measuring antibody-dependent cytotoxicity in neutralizing and non-neutralizing human sera from dengue patients. J. Immunol. Methods 2017, 441, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.M.; Thompson, B.S.; Fremont, D.H.; Diamond, M.S. Antibody recognition of cell surface-associated NS1 triggers Fc-gamma receptor-mediated phagocytosis and clearance of West Nile Virus-infected cells. J. Virol. 2007, 81, 9551–9555. [Google Scholar] [CrossRef] [PubMed]
- Khandia, R.; Munjal, A.; Dhama, K.; Karthik, K.; Tiwari, R.; Malik, Y.S.; Singh, R.K.; Chaicumpa, W. Modulation of Dengue/Zika Virus Pathogenicity by Antibody-Dependent Enhancement and Strategies to Protect Against Enhancement in Zika Virus Infection. Front. Immunol. 2018, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Castanha, P.M.S.; Nascimento, E.J.M.; Braga, C.; Cordeiro, M.T.; de Carvalho, O.V.; de Mendonça, L.R.; Azevedo, E.A.N.; França, R.F.O.; Dhalia, R.; Marques, E.T.A. Enhancement of Zika Infection by Dengue-Specific Antibodies Does Not Alter the Production of Interleukin 6 in FcγRII-Expressing K562 Cells. J. Infect. Dis. 2017, 216, 614–615. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Neutralization and antibody-dependent enhancement of dengue viruses. Adv. Virus Res. 2003, 60, 421–467. [Google Scholar]
- Sun, P.; Williams, M.; Nagabhushana, N.; Morrison, B.J. Combating Antibody-dependent Enhancement of Dengue Viral infection in Peripheral Blood Monocytes by NK Cell-mediated Antibody Dependent Cell Cytotoxicity. J. Immunol. 2017, 198, 122.11. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maucourant, C.; Petitdemange, C.; Yssel, H.; Vieillard, V. Control of Acute Arboviral Infection by Natural Killer Cells. Viruses 2019, 11, 131. https://doi.org/10.3390/v11020131
Maucourant C, Petitdemange C, Yssel H, Vieillard V. Control of Acute Arboviral Infection by Natural Killer Cells. Viruses. 2019; 11(2):131. https://doi.org/10.3390/v11020131
Chicago/Turabian StyleMaucourant, Christopher, Caroline Petitdemange, Hans Yssel, and Vincent Vieillard. 2019. "Control of Acute Arboviral Infection by Natural Killer Cells" Viruses 11, no. 2: 131. https://doi.org/10.3390/v11020131
APA StyleMaucourant, C., Petitdemange, C., Yssel, H., & Vieillard, V. (2019). Control of Acute Arboviral Infection by Natural Killer Cells. Viruses, 11(2), 131. https://doi.org/10.3390/v11020131



