Survivin Overexpression Has a Negative Effect on Feline Calicivirus Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Virus Infection
2.2. Plasmids
2.3. Transient Transfections and Plaque Assays
2.4. Western Blotting Analysis
2.5. Immunofluorescence Assays
2.6. Virus-Binding Assay by Flow Cytometry
3. Results
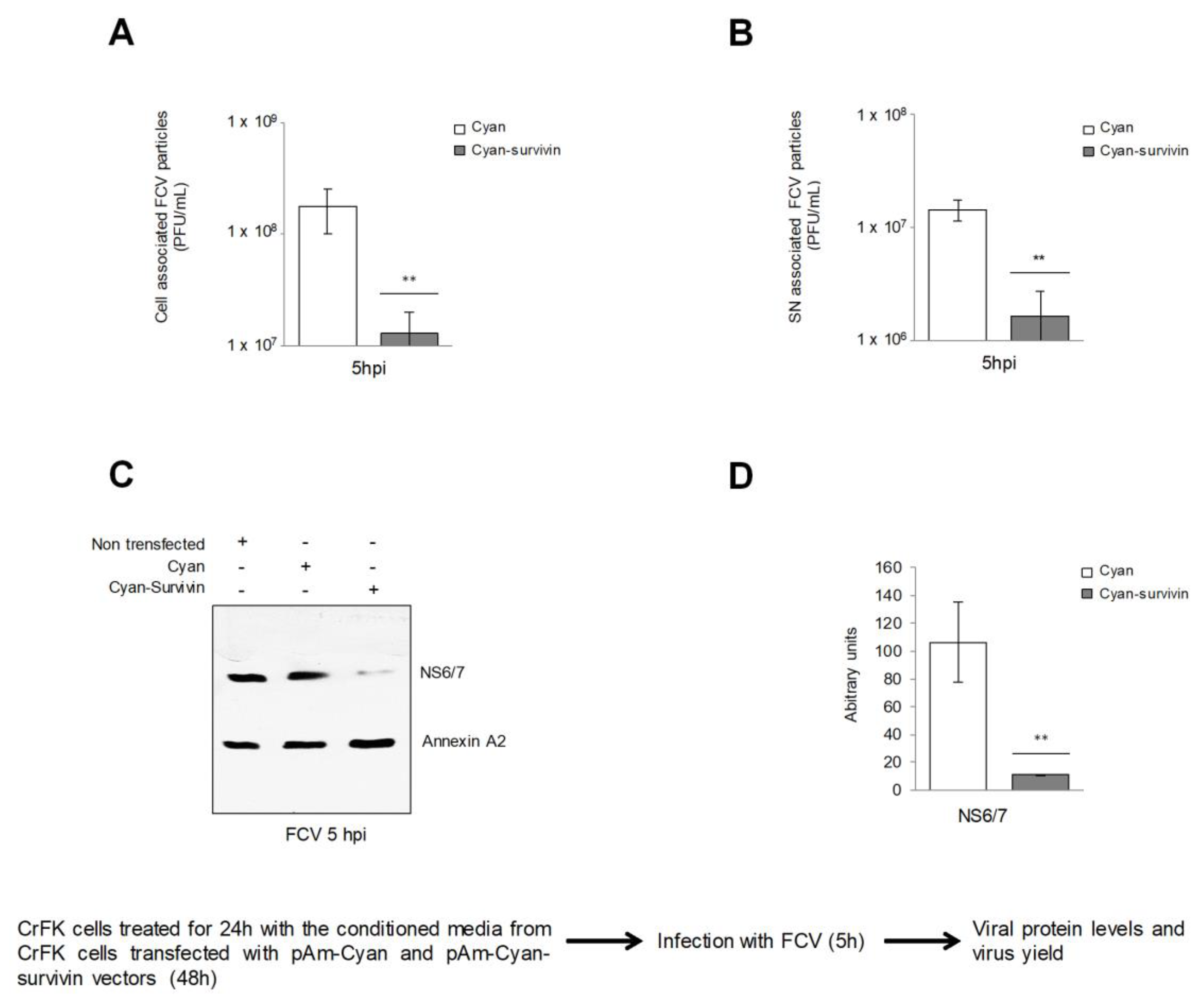
3.1. Overexpression of Survivin Has an Autocrine and a Paracrine Effect against FCV Infection
3.2. Supernatants Collected from CrFK Cells Overexpressing Survivin Had Reduced FCV Infection
3.3. The Protective Effect of the Conditioned Medium from CrFK Cells that Overexpress Survivin on FCV Infection is Specific
3.4. Overexpression of Survivin in RAW 264.7 Cells Reduces MNV-1 Release
3.5. The Overexpression of Survivin Interferes with FCV Binding to the Target Cells
3.6. The Amount of fJAM-1 Protein is Reduced in CrFK Cells Overexpressing Survivin and in Cells Treated with Conditioned Medium
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Radford, A.D.; Coyne, K.P.; Dawson, S.; Porter, C.J.; Gaskell, R.M. Feline calicivirus. Vet. Res. 2007, 38, 319–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oka, T.; Takagi, H.; Tohya, Y. Development of a novel single step reverse genetics system for feline calicivirus. J. Virol. Methods 2014, 207, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Sosnovtsev, S.; Green, K.Y. Rna transcripts derived from a cloned full-length copy of the feline calicivirus genome do not require vpg for infectivity. Virology 1995, 210, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Makino, A.; Shimojima, M.; Miyazawa, T.; Kato, K.; Tohya, Y.; Akashi, H. Junctional adhesion molecule 1 is a functional receptor for feline calicivirus. J. Virol. 2006, 80, 4482–4490. [Google Scholar] [CrossRef]
- Ossiboff, R.J.; Parker, J.S. Identification of regions and residues in feline junctional adhesion molecule required for feline calicivirus binding and infection. J. Virol. 2007, 81, 13608–13621. [Google Scholar] [CrossRef]
- Stuart, A.D.; Brown, T.D. Entry of feline calicivirus is dependent on clathrin-mediated endocytosis and acidification in endosomes. J. Virol. 2006, 80, 7500–7509. [Google Scholar] [CrossRef]
- Gutiérrez-Escolano, A.L. Host-cell factors involved in the calicivirus replicative cycle. Future Virol. 2014, 9, 147–160. [Google Scholar] [CrossRef]
- Natoni, A.; Kass, G.E.; Carter, M.J.; Roberts, L.O. The mitochondrial pathway of apoptosis is triggered during feline calicivirus infection. J. Gen. Virol. 2006, 87, 357–361. [Google Scholar] [CrossRef]
- Sosnovtsev, S.V.; Prikhod’ko, E.A.; Belliot, G.; Cohen, J.I.; Green, K.Y. Feline calicivirus replication induces apoptosis in cultured cells. Virus Res. 2003, 94, 1–10. [Google Scholar] [CrossRef]
- Al-Molawi, N.; Beardmore, V.A.; Carter, M.J.; Kass, G.E.N.; Roberts, L.O. Caspase-mediated cleavage of the feline calicivirus capsid protein. J. Gen. Virol. 2003, 84, 1237–1244. [Google Scholar] [CrossRef]
- Bok, K.; Prikhodko, V.G.; Green, K.Y.; Sosnovtsev, S.V. Apoptosis in murine norovirus-infected raw264.7 cells is associated with downregulation of survivin. J. Virol. 2009, 83, 3647–3656. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Vazquez, O.S.; Cancio-Lonches, C.; Hernandez-Gonzalez, O.; Chavez-Munguia, B.; Villegas-Sepulveda, N.; Gutierrez-Escolano, A.L. The feline calicivirus leader of the capsid protein causes survivin and xiap downregulation and apoptosis. Virology 2019, 527, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Sanchez, C.; Cancio-Lonches, C.; Mora-Heredia, J.E.; Santos-Valencia, J.C.; Barrera-Vazquez, O.S.; Yocupicio-Monroy, M.; Gutierrez-Escolano, A.L. Negative effect of heat shock on feline calicivirus release from infected cells is associated with the control of apoptosis. Virus Res. 2015, 198, 44–52. [Google Scholar] [CrossRef] [PubMed]
- López-Manriquez, E.; Vashist, S.; Ueña, L.; Goodfellow, I.; Chavez, P.; Mora-Heredia, J.E.; Cancio-Lonches, C.; Garrido, E.; Gutiérrez-Escolano, A.L. Norovirus genome circularisation and efficient replication is facilitated by binding of pcbps and hnrnp a1. J. Virol. 2013, 87, 11371–11387. [Google Scholar]
- Cancio-Lonches, C.; Yocupicio-Monroy, M.; Sandoval-Jaime, C.; Galvan-Mendoza, I.; Urena, L.; Vashist, S.; Goodfellow, I.; Salas-Benito, J.; Lorena Gutierrez-Escolano, A. Nucleolin interacts with the feline calicivirus 3′ untranslated region and the protease-polymerase ns6 and ns7 proteins, playing a role in virus replication. J. Virol. 2011, 85, 8056–8068. [Google Scholar] [CrossRef]
- Wobus, C.E.; Karst, S.M.; Thackray, L.B.; Chang, K.O.; Sosnovtsev, S.V.; Belliot, G.; Krug, A.; Mackenzie, J.M.; Green, K.Y.; Virgin, H.W. Replication of norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2004, 2, e432. [Google Scholar] [CrossRef]
- Garcia, A.D.; Meseda, C.A.; Mayer, A.E.; Kumar, A.; Merchlinsky, M.; Weir, J.P. Characterization and use of mammalian-expressed vaccinia virus extracellular membrane proteins for quantification of the humoral immune response to smallpox vaccines. Clin. Vaccine Immunol. 2007, 14, 1032–1044. [Google Scholar] [CrossRef]
- Escobar-Herrera, J.; Medina-Ramírez, F.J.; Gutiérrez-Escolano, A.L. A carboxymethyl-cellulose plaque assay for feline calicivirus. J. Virol. Methods 2007, 146, 393–396. [Google Scholar] [CrossRef]
- Hwang, S.; Alhatlani, B.; Arias, A.; Caddy, S.L.; Christodoulou, C.; Cunha, J.B.; Emmott, E.; Gonzalez-Hernandez, M.; Kolawole, A.; Lu, J. Murine norovirus: Propagation, quantification, and genetic manipulation. Curr. Protoc. Microbiol. 2014, 33, 15K.2.1–15K.2.61. [Google Scholar]
- Garg, H.; Suri, P.; Gupta, J.C.; Talwar, G.P.; Dubey, S. Survivin: A unique target for tumor therapy. Cancer Cell Int. 2016, 16, 49. [Google Scholar] [CrossRef]
- Haga, K.; Fujimoto, A.; Takai-Todaka, R.; Miki, M.; Doan, Y.H.; Murakami, K.; Yokoyama, M.; Murata, K.; Nakanishi, A.; Katayama, K. Functional receptor molecules cd300lf and cd300ld within the cd300 family enable murine noroviruses to infect cells. Proc. Natl. Acad. Sci. USA 2016, 113, E6248–E6255. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, D.T.; Nakatsuji, T.; Wang, Z.; di Nardo, A.; Gallo, R.L. Vaccinia virus binds to the scavenger receptor marco on the surface of keratinocytes. J. Invest. Derm. 2015, 135, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, S.P.; Altieri, D.C. Survivin at a glance. J. Cell Sci. 2019, 132, jcs223826. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Jutzy, J.M.; Aspe, J.R.; McGregor, D.W.; Neidigh, J.W.; Wall, N.R. Survivin is released from cancer cells via exosomes. Apoptosis 2011, 16, 1–12. [Google Scholar] [CrossRef]
- Khan, S.; Bennit, H.F.; Wall, N.R. The emerging role of exosomes in survivin secretion. Histol. Histopathol. 2015, 30, 43–50. [Google Scholar]
- Mera, S.; Magnusson, M.; Tarkowski, A.; Bokarewa, M. Extracellular survivin up-regulates adhesion molecules on the surface of leukocytes changing their reactivity pattern. J. Leukoc. Biol. 2008, 83, 149–155. [Google Scholar] [CrossRef]
- Tsuneki, M.; Madri, J.A. Cd44 regulation of endothelial cell proliferation and apoptosis via modulation of cd31 and ve-cadherin expression. J. Biol. Chem. 2014, 289, 5357–5370. [Google Scholar] [CrossRef]
- Tsuneki, M.; Madri, J.A. Adhesion molecule-mediated hippo pathway modulates hemangioendothelioma cell behavior. Mol. Cell. Biol. 2014, 34, 4485–4499. [Google Scholar] [CrossRef]
- Fernandez, J.G.; Rodriguez, D.A.; Valenzuela, M.; Calderon, C.; Urzua, U.; Munroe, D.; Rosas, C.; Lemus, D.; Diaz, N.; Wright, M.C.; et al. Survivin expression promotes vegf-induced tumor angiogenesis via pi3k/akt enhanced beta-catenin/tcf-lef dependent transcription. Mol. Cancer 2014, 13, 209. [Google Scholar] [CrossRef]
- Mestdagt, M.; Polette, M.; Buttice, G.; Noel, A.; Ueda, A.; Foidart, J.M.; Gilles, C. Transactivation of mcp-1/ccl2 by beta-catenin/tcf-4 in human breast cancer cells. Int. J. Cancer 2006, 118, 35–42. [Google Scholar] [CrossRef]
- Stamatovic, S.M.; Sladojevic, N.; Keep, R.F.; Andjelkovic, A.V. Relocalization of junctional adhesion molecule a during inflammatory stimulation of brain endothelial cells. Mol. Cell. Biol. 2012, 32, 3414–3427. [Google Scholar] [CrossRef] [PubMed]
- Emmott, E.; Sorgeloos, F.; Caddy, S.L.; Vashist, S.; Sosnovtsev, S.; Lloyd, R.; Heesom, K.; Locker, N.; Goodfellow, I. Norovirus-mediated modification of the translational landscape via virus and host-induced cleavage of translation initiation factors. Mol. Cell Proteom. 2017, 16, S215–S229. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Oviedo, J.M.; Martin-Alonso, J.M.; Diaz, E.; Boga, J.A.; Parra, F. Programmed cell death in the pathogenesis of rabbit hemorrhagic disease. Arch. Virol. 1998, 143, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Troeger, H.; Loddenkemper, C.; Schneider, T.; Schreier, E.; Epple, H.J.; Zeitz, M.; Fromm, M.; Schulzke, J.D. Structural and functional changes of the duodenum in human norovirus infection. Gut 2009, 58, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Herod, M.R.; Salim, O.; Skilton, R.J.; Prince, C.A.; Ward, V.K.; Lambden, P.R.; Clarke, I.N. Expression of the murine norovirus (mnv) orf1 polyprotein is sufficient to induce apoptosis in a virus-free cell model. PLoS ONE 2014, 9, e90679. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrera-Vázquez, O.S.; Cancio-Lonches, C.; Miguel-Rodríguez, C.E.; Valdes Pérez, M.M.; Gutiérrez-Escolano, A.L. Survivin Overexpression Has a Negative Effect on Feline Calicivirus Infection. Viruses 2019, 11, 996. https://doi.org/10.3390/v11110996
Barrera-Vázquez OS, Cancio-Lonches C, Miguel-Rodríguez CE, Valdes Pérez MM, Gutiérrez-Escolano AL. Survivin Overexpression Has a Negative Effect on Feline Calicivirus Infection. Viruses. 2019; 11(11):996. https://doi.org/10.3390/v11110996
Chicago/Turabian StyleBarrera-Vázquez, Oscar Salvador, Clotilde Cancio-Lonches, Carlos Emilio Miguel-Rodríguez, Monica Margarita Valdes Pérez, and Ana Lorena Gutiérrez-Escolano. 2019. "Survivin Overexpression Has a Negative Effect on Feline Calicivirus Infection" Viruses 11, no. 11: 996. https://doi.org/10.3390/v11110996
APA StyleBarrera-Vázquez, O. S., Cancio-Lonches, C., Miguel-Rodríguez, C. E., Valdes Pérez, M. M., & Gutiérrez-Escolano, A. L. (2019). Survivin Overexpression Has a Negative Effect on Feline Calicivirus Infection. Viruses, 11(11), 996. https://doi.org/10.3390/v11110996





