Effects of Regulatory T Cell Depletion on NK Cell Responses against Listeria monocytogenes in Feline Immunodeficiency Virus Infected Cats
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals, Viral Inoculum, and Monoclonal Antibody Administration
2.3. Listeria monocytogenes Inoculum and Bromodeoxyuridine (BrdU) Administration
2.4. Sample Collection and Processing, and Lm Quantification
2.5. Immunophenotyping
2.6. Perforin and Granzyme A Production by NK and NKT Cells
2.7. Viral Parameters
2.8. Statistical Analysis
3. Results
3.1. The Effect of Anti-CD25 mAb Treatment on Treg, NK and NKT Cells and on Viral Burden
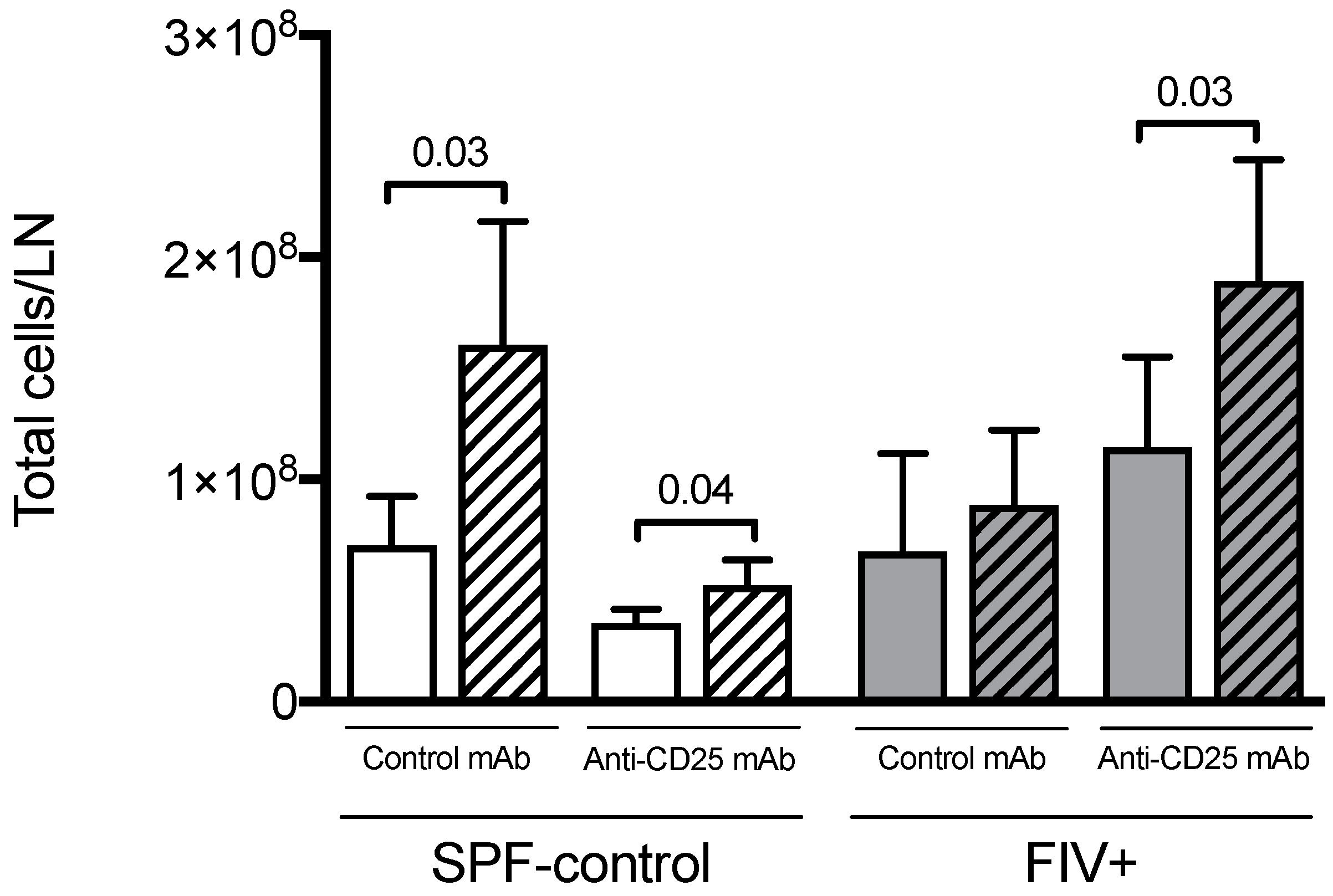
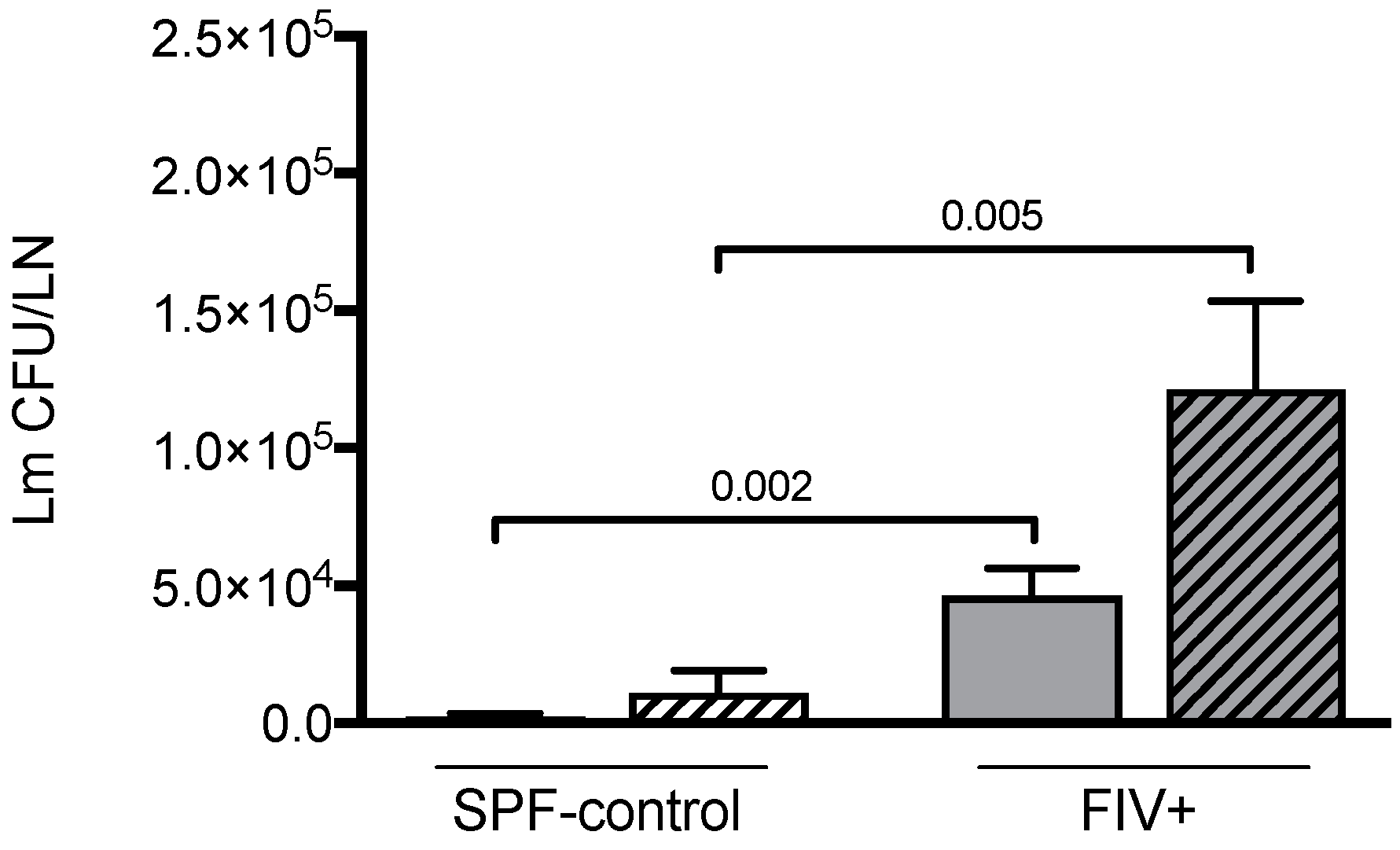
3.2. The Effect of Treg Depletion on the Quantitative Cellular Response to Lm
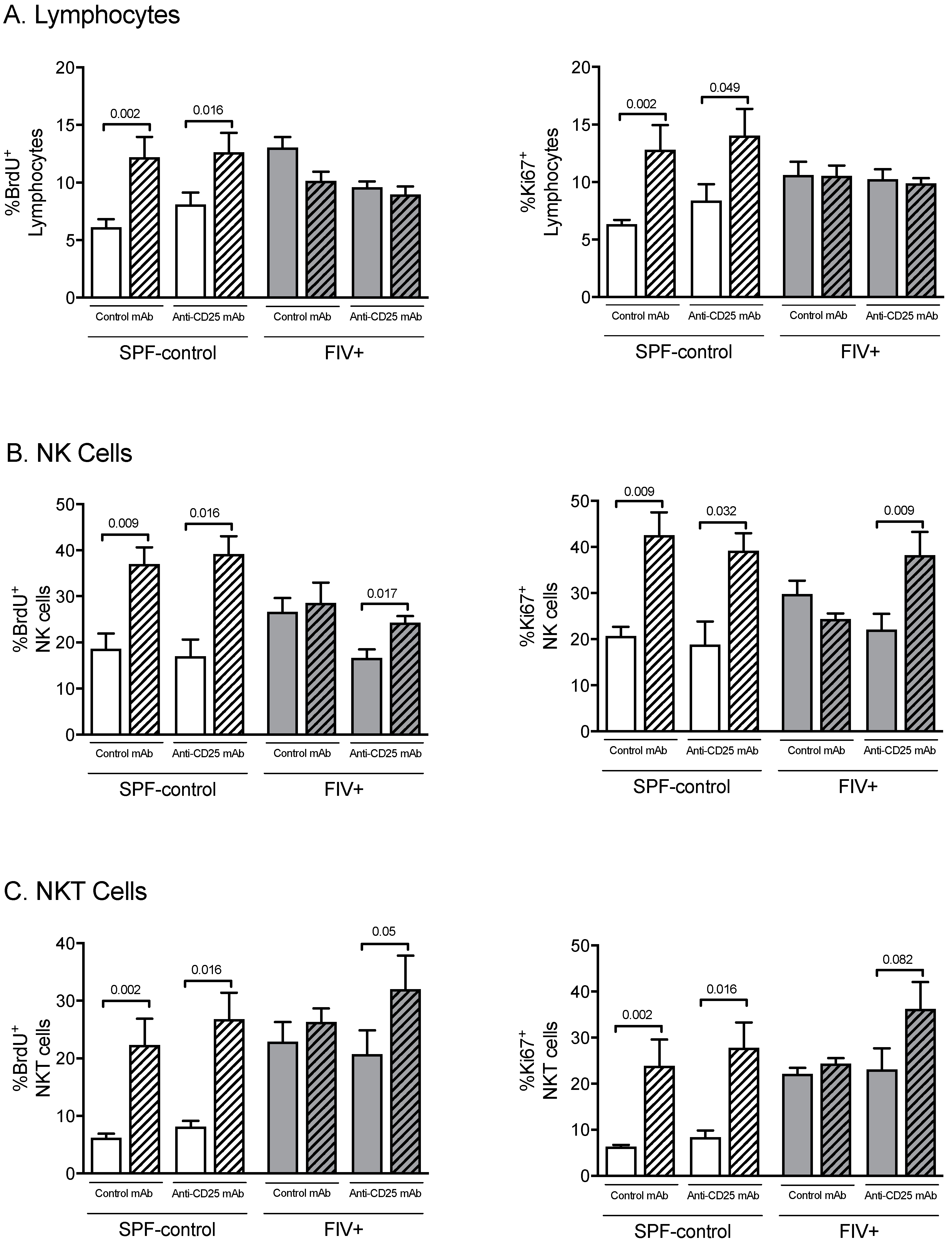
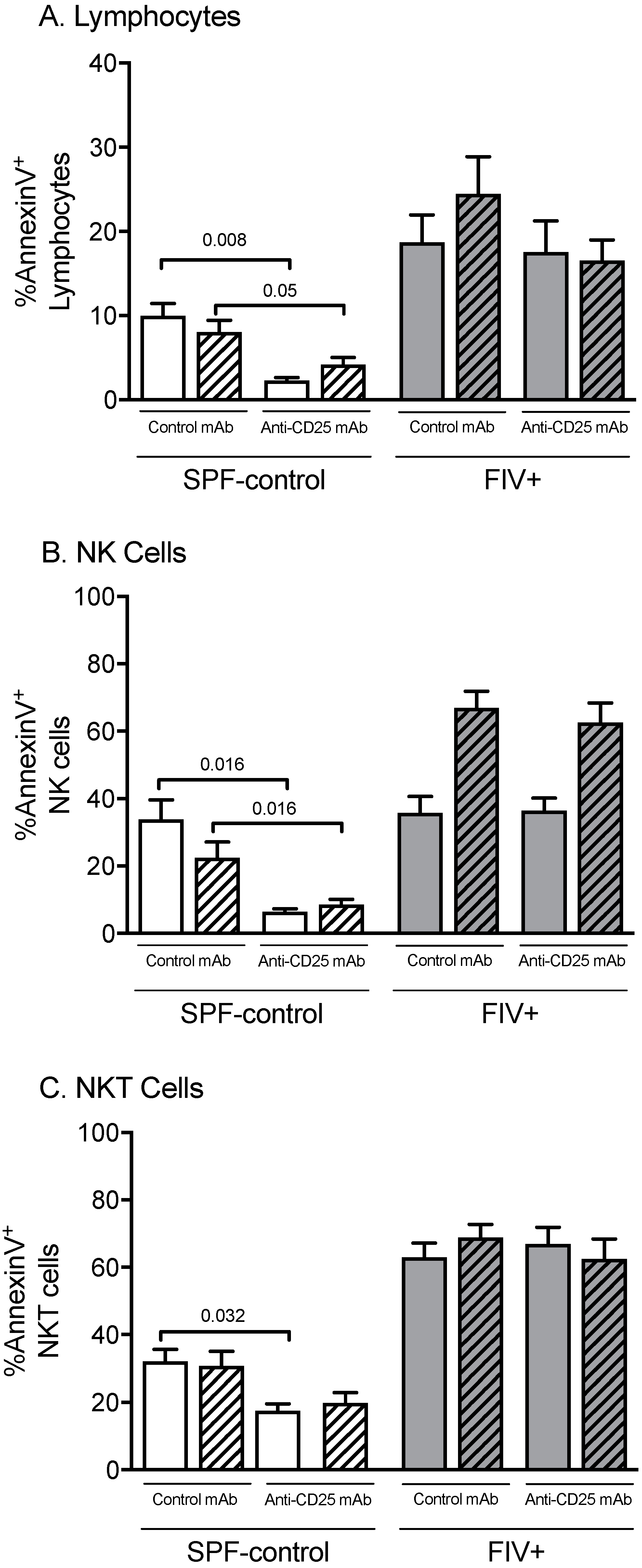
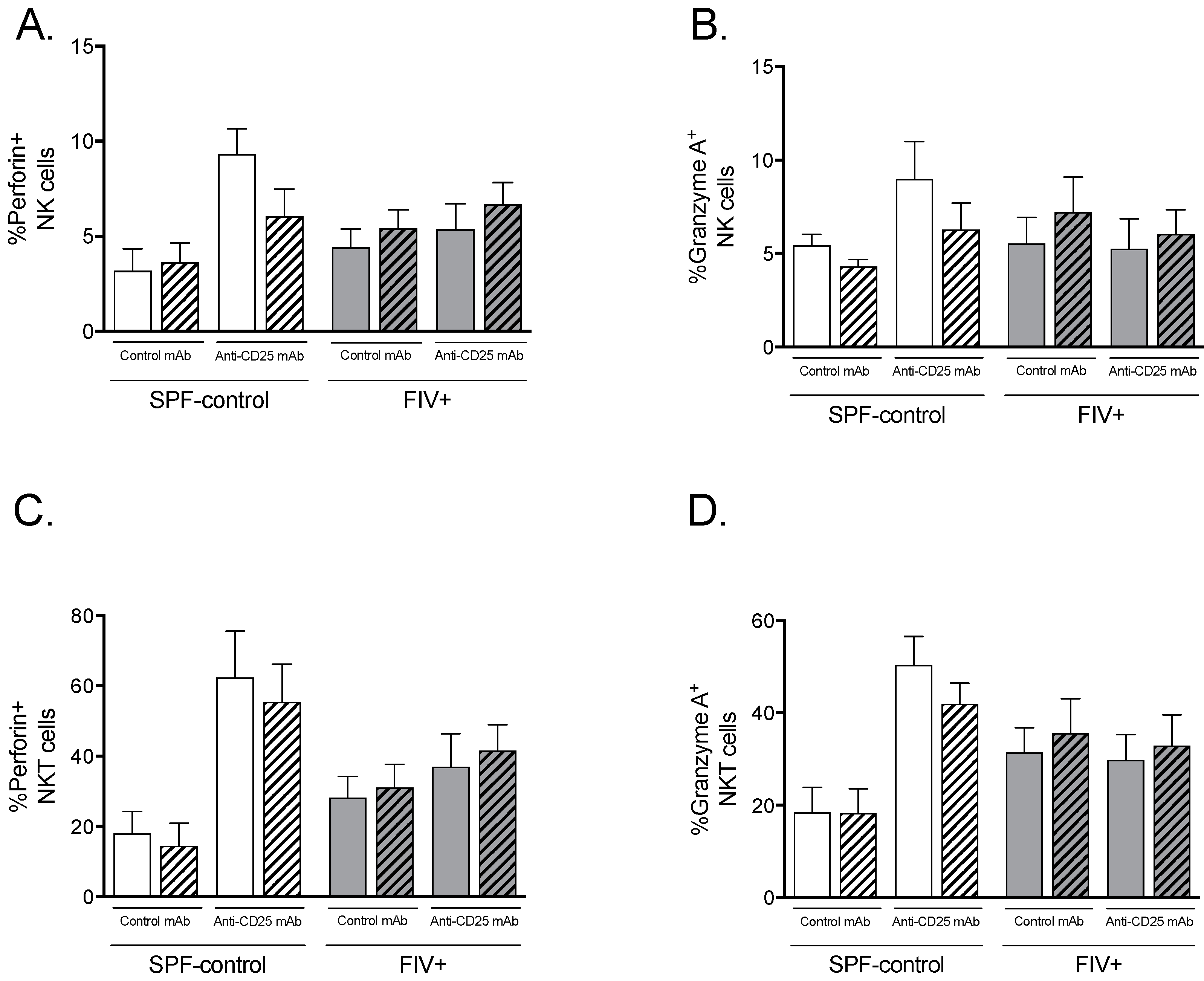
3.3. The Effect of Treg Depletion on the Functional Response to Lm
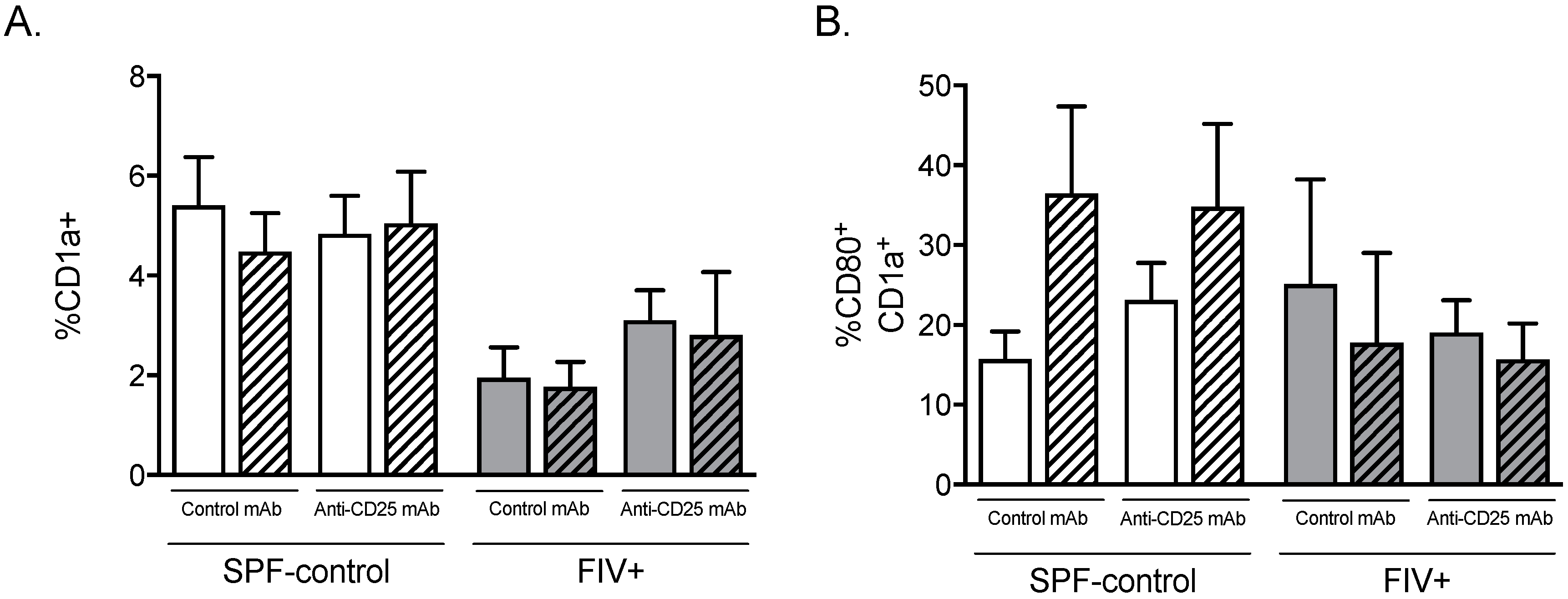
3.4. Treg Depletion Does Not Affect Dendritic Cell Percentage or Activation Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hasenkrug, K.J.; Chougnet, C.A.; Dittmer, U. Regulatory T cells in retroviral infections. PLoS Pathog. 2018, 14, e1006776. [Google Scholar] [CrossRef] [PubMed]
- Rouse, B.T.; Sarangi, P.P.; Suvas, S. Regulatory t cells in virus infections. Immunol. Rev. 2006, 212, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Kinter, A.; McNally, J.; Riggin, L.; Jackson, R.; Roby, G.; Fauci, A.S. Suppression of HIV-specific T cell activity by lymph node cd25+ regulatory T cells from HIV-infected individuals. Proc. Natl. Acad. Sci. USA 2007, 104, 3390–3395. [Google Scholar] [CrossRef] [PubMed]
- Kinter, A.L.; Horak, R.; Sion, M.; Riggin, L.; McNally, J.; Lin, Y.; Jackson, R.; O’Shea, A.; Roby, G.; Kovacs, C.; et al. Cd25+ regulatory T cells isolated from HIV-infected individuals suppress the cytolytic and nonlytic antiviral activity of HIV-specific cd8+ T cells in vitro. AIDS Res. Hum. Retrovir. 2007, 23, 438–450. [Google Scholar] [CrossRef]
- Pereira, L.E.; Villinger, F.; Onlamoon, N.; Bryan, P.; Cardona, A.; Pattanapanysat, K.; Mori, K.; Hagen, S.; Picker, L.; Ansari, A.A. Simian immunodeficiency virus (SIV) infection influences the level and function of regulatory T cells in SIV-infected rhesus macaques but not SIV-infected sooty mangabeys. J. Virol. 2007, 81, 4445–4456. [Google Scholar] [CrossRef]
- Vahlenkamp, T.W.; Tompkins, M.B.; Tompkins, W.A. Feline immunodeficiency virus infection phenotypically and functionally activates immunosuppressive CD4 + CD 25+ T regulatory cells. J. Immunol. 2004, 172, 4752–4761. [Google Scholar] [CrossRef]
- Mikkelsen, S.R.; Reckling, S.K.; Egan, E.A.; Dean, G.A. In vivo depletion of cd4 (+) cd25 (hi) regulatory t cells is associated with improved antiviral responses in cats chronically infected with feline immunodeficiency virus. Virology 2010, 403, 163–172. [Google Scholar] [CrossRef]
- Dean, G.; Bernales, J.; Pedersen, N. Effect of feline immunodeficiency virus on cytokine response to Listeria monocytogenes in vivo. Vet. Immunol. Immunopathol. 1998, 65, 125–138. [Google Scholar] [CrossRef]
- Dean, G.; Lavoy, A.; Yearley, J.; Stanton, C. Cytokine modulation of the innate immune response in feline immunodeficiency virus-infected cats. J. Infect. Dis. 2006, 193, 1520–1527. [Google Scholar] [CrossRef][Green Version]
- Simoes, R.D.; Howard, K.E.; Dean, G.A. In vivo assessment of natural killer cell responses during chronic feline immunodeficiency virus infection. PLoS ONE 2012, 7, e37606. [Google Scholar] [CrossRef][Green Version]
- Bancroft, G.J. The role of natural killer cells in innate resistance to infection. Curr. Opin. Immunol. 1993, 5, 503–510. [Google Scholar] [CrossRef]
- Bancroft, G.J.; Schreiber, R.D.; Bosma, G.C.; Bosma, M.J.; Unanue, E.R. A T cell-independent mechanism of macrophage activation by interferon-γ. J. Immunol. 1987, 139, 1104–1107. [Google Scholar] [PubMed]
- Shegarfi, H.; Sydnes, K.; Lovik, M.; Inngjerdingen, M.; Rolstad, B.; Naper, C. The role of natural killer cells in resistance to the intracellular bacterium Listeria monocytogenes in rats. Scand. J. Immunol. 2009, 70, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Dunn, P.L.; North, R.J. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect. Immun. 1991, 59, 2892–2900. [Google Scholar]
- Yang, J.S.; English, R.V.; Ritchey, J.W.; Davidson, M.G.; Wasmoen, T.; Levy, J.K.; Gebhard, D.H.; Tompkins, M.B.; Tompkins, W.A. Molecularly cloned feline immunodeficiency virus NCSU1 JSY3 induces immunodeficiency in specific-pathogen-free cats. J. Virol. 1996, 70, 3011–3017. [Google Scholar]
- Ohno, K.; Goitsuka, R.; Kitamura, K.; Hasegawa, A.; Tokunaga, T.; Honda, M. Production of a monoclonal antibody that defines the α-subunit of the feline IL-2 receptor. Hybridoma 1992, 11, 595–605. [Google Scholar] [CrossRef]
- Stevens, R.; Howard, K.; Nordone, S.; Burkhard, M.; Dean, G. Oral immunization with recombinant Listeria monocytogenes controls virus load after vaginal challenge with feline immunodeficiency virus. J. Virol. 2004, 78, 8210–8218. [Google Scholar] [CrossRef]
- Dean, G.; Pedersen, N. Cytokine response in multiple lymphoid tissues during the primary phase of feline immunodeficiency virus infection. J. Virol. 1998, 72, 9436–9440. [Google Scholar]
- Nishimura, Y.; Shimojima, M.; Sato, E.; Izumiya, Y.; Tohya, Y.; Mikami, T.; Miyazawa, T. Downmodulation of Cd3y expression in cd8α+β-T cells of feline immunodeficiency virus-infected cats. J. Gen. Virol. 2004, 85, 2585–2589. [Google Scholar] [CrossRef]
- Tompkins, M.B.; Gebhard, D.H.; Bingham, H.R.; Hamilton, M.J.; Davis, W.C.; Tompkins, W.A. Characterization of monoclonal antibodies to feline T lymphocytes and their use in the analysis of lymphocyte tissue distribution in the cat. Vet. Immunol. Immunopathol. 1990, 26, 305–317. [Google Scholar] [CrossRef]
- Tompkins, M.B.; Bull, M.E.; Dow, J.L.; Ball, J.M.; Collisson, E.W.; Winslow, B.J.; Phadke, A.P.; Vahlenkamp, T.W.; Tompkins, W.A. Feline immunodeficiency virus infection is characterized by B7 + CTLA4 + T cell apoptosis. J. Infect. Dis. 2002, 185, 1077–1093. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.C.; Moore, P.F. A feline homologue of CD1 is defined using a feline-specific monoclonal antibody. Tissue Antigens 1997, 49, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Smithberg, S.R.; Fogle, J.E.; Mexas, A.M.; Reckling, S.K.; Lankford, S.M.; Tompkins, M.B.; Dean, G.A. In vivo depletion of CD4 + CD25 + regulatory T cells in cats. J. Immunol. Methods 2008, 329, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Bettelli, E.; Dastrange, M.; Oukka, M. FOXP3 interacts with nuclear factor of activated t cells and NF-κ b to repress cytokine gene expression and effector functions of T helper cells. Proc. Natl. Acad. Sci. USA 2005, 102, 5138–5143. [Google Scholar] [CrossRef] [PubMed]
- Lankford, S.; Petty, C.; LaVoy, A.; Reckling, S.; Tompkins, W.; Dean, G.A. Cloning of feline FOXP3 and detection of expression in CD4 + CD25 + regulatory T cells. Vet. Immunol. Immunopathol. 2008, 122, 159–166. [Google Scholar] [CrossRef][Green Version]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. FOXP3 programs the development and function of CD4 + CD25 + regulatory t cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef]
- Humann, J.; Lenz, L.L. Activation of naive NK cells in response to Listeria monocytogenes requires IL-18 and contact with infected dendritic cells. J. Immunol. 2010, 184, 5172–5178. [Google Scholar] [CrossRef]
- Jones, L.E.; Perelson, A.S. Opportunistic infection as a cause of transient viremia in chronically infected HIV patients under treatment with haart. Bull. Math. Biol. 2005, 67, 1227–1251. [Google Scholar] [CrossRef] [PubMed]
- Hazenberg, M.D.; Stuart, J.W.; Otto, S.A.; Borleffs, J.C.; Boucher, C.A.; de Boer, R.J.; Miedema, F.; Hamann, D. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: A longitudinal analysis in patients before and during highly active antiretroviral therapy (Haart). Blood 2000, 95, 249–255. [Google Scholar] [CrossRef]
- Giorgi, J.V.; Hultin, L.E.; McKeating, J.A.; Johnson, T.D.; Owens, B.; Jacobson, L.P.; Shih, R.; Lewis, J.; Wiley, D.J.; Phair, J.P.; et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 1999, 179, 859–870. [Google Scholar] [CrossRef]
- De Boer, R.J.; Mohri, H.; Ho, D.D.; Perelson, A.S. Turnover rates of B cells, T cells, and NK cells in simian immunodeficiency virus-infected and uninfected rhesus macaques. J. Immunol. 2003, 170, 2479–2487. [Google Scholar] [CrossRef] [PubMed]
- Mohri, H.; Bonhoeffer, S.; Monard, S.; Perelson, A.S.; Ho, D.D. Rapid turnover of t lymphocytes in SIV-infected rhesus macaques. Science 1998, 279, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Mohri, H.; Perelson, A.S.; Tung, K.; Ribeiro, R.M.; Ramratnam, B.; Markowitz, M.; Kost, R.; Hurley, A.; Weinberger, L.; Cesar, D.; et al. Increased turnover of T lymphocytes in HIV-1 infection and its reduction by antiretroviral therapy. J. Exp. Med. 2001, 194, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, J.A.; Lempicki, R.A.; Sidorov, I.A.; Adelsberger, J.W.; Herpin, B.; Metcalf, J.A.; Sereti, I.; Polis, M.A.; Davey, R.T.; Tavel, J.; et al. Identification of dynamically distinct subpopulations of t lymphocytes that are differentially affected by HIV. J. Exp. Med. 2001, 194, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Teng, M.W.; Swann, J.; Kyparissoudis, K.; Godfrey, D.I.; Hayakawa, Y. CD4 + CD25 + T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J. Immunol. 2006, 176, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, C.A.; Shevach, E.M. Naturally-occurring CD4 + CD25 + immunoregulatory T cells: Central players in the arena of peripheral tolerance. Semin. Immunol. 2004, 16, 81–88. [Google Scholar] [CrossRef]
- Wing, K.; Larsson, P.; Sandstrom, K.; Lundin, S.B.; Suri-Payer, E.; Rudin, A. CD4+ CD25+ FOXP3+ regulatory T cells from human thymus and cord blood suppress antigen-specific T cell responses. Immunology 2005, 115, 516–525. [Google Scholar] [CrossRef]
- Lim, H.W.; Hillsamer, P.; Banham, A.H.; Kim, C.H. Cutting edge: Direct suppression of B cells by CD4+ CD25+ regulatory T cells. J. Immunol. 2005, 175, 4180–4183. [Google Scholar] [CrossRef]
- Fallarino, F.; Grohmann, U.; Hwang, K.W.; Orabona, C.; Vacca, C.; Bianchi, R.; Belladonna, M.L.; Fioretti, M.C.; Alegre, M.L.; Puccetti, P. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 2003, 4, 1206–1212. [Google Scholar] [CrossRef]
- Taams, L.S.; van Amelsfort, J.M.; Tiemessen, M.M.; Jacobs, K.M.; de Jong, E.C.; Akbar, A.N.; Bijlsma, J.W.; Lafeber, F.P. Modulation of monocyte/macrophage function by human CD4 + CD25 + regulatory t cells. Hum. Immunol. 2005, 66, 222–230. [Google Scholar] [CrossRef]
- Azuma, T.; Takahashi, T.; Kunisato, A.; Kitamura, T.; Hirai, H. Human CD4+ CD25+ regulatory T cells suppress NKT cell functions. Cancer Res. 2003, 63, 4516–4520. [Google Scholar] [PubMed]
- Ghiringhelli, F.; Menard, C.; Martin, F.; Zitvogel, L. The role of regulatory T cells in the control of natural killer cells: Relevance during tumor progression. Immunol. Rev. 2006, 214, 229–238. [Google Scholar] [CrossRef]
- Smyth, M.J.; Hayakawa, Y.; Takeda, K.; Yagita, H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat. Rev. Cancer 2002, 2, 850–861. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, G.; Tsang, M.L.; Moretta, L.; Melioli, G.; Steinman, R.M.; Munz, C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKP30 receptor by activated NK cells. J. Exp. Med. 2002, 195, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, N.C.; Lozier, A.; Flament, C.; Ricciardi-Castagnoli, P.; Bellet, D.; Suter, M.; Perricaudet, M.; Tursz, T.; Maraskovsky, E.; Zitvogel, L. Dendritic cells directly trigger NK cell functions: Cross-talk relevant in innate anti-tumor immune responses in vivo. Nat. Med. 1999, 5, 405–411. [Google Scholar] [CrossRef]
- Zhang, L.; Reckling, S.; Dean, G.A. Phenotypic and functional analysis of CD1A + dendritic cells from cats chronically infected with feline immunodeficiency virus. Comp. Immunol. Microbiol. Infect. Dis. 2015, 42, 53–59. [Google Scholar] [CrossRef]
- Littwitz-Salomon, E.; Malyshkina, A.; Schimmer, S.; Dittmer, U. The cytotoxic activity of natural killer cells is suppressed by IL-10(+) regulatory T cells during acute retroviral infection. Front. Immunol. 2018, 9, 1947. [Google Scholar] [CrossRef]
- Littwitz-Salomon, E.; Schimmer, S.; Dittmer, U. Natural killer T cells contribute to the control of acute retroviral infection. Retrovirology 2017, 14, 5. [Google Scholar] [CrossRef]
- Garrido, C.; Abad-Fernandez, M.; Tuyishime, M.; Pollara, J.J.; Ferrari, G.; Soriano-Sarabia, N.; Margolis, D.M. Interleukin-15-stimulated natural killer cells clear HIV-1-infected cells following latency reversal ex vivo. J. Virol. 2018, 92, e00235-18. [Google Scholar] [CrossRef]
- Grossman, W.J.; Verbsky, J.W.; Barchet, W.; Colonna, M.; Atkinson, J.P.; Ley, T.J. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity 2004, 21, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Grossman, W.J.; Verbsky, J.W.; Tollefsen, B.L.; Kemper, C.; Atkinson, J.P.; Ley, T.J. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood 2004, 104, 2840–2848. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simões, R.D.; LaVoy, A.; Dean, G.A. Effects of Regulatory T Cell Depletion on NK Cell Responses against Listeria monocytogenes in Feline Immunodeficiency Virus Infected Cats. Viruses 2019, 11, 984. https://doi.org/10.3390/v11110984
Simões RD, LaVoy A, Dean GA. Effects of Regulatory T Cell Depletion on NK Cell Responses against Listeria monocytogenes in Feline Immunodeficiency Virus Infected Cats. Viruses. 2019; 11(11):984. https://doi.org/10.3390/v11110984
Chicago/Turabian StyleSimões, Rita D., Alora LaVoy, and Gregg A. Dean. 2019. "Effects of Regulatory T Cell Depletion on NK Cell Responses against Listeria monocytogenes in Feline Immunodeficiency Virus Infected Cats" Viruses 11, no. 11: 984. https://doi.org/10.3390/v11110984
APA StyleSimões, R. D., LaVoy, A., & Dean, G. A. (2019). Effects of Regulatory T Cell Depletion on NK Cell Responses against Listeria monocytogenes in Feline Immunodeficiency Virus Infected Cats. Viruses, 11(11), 984. https://doi.org/10.3390/v11110984




