Schmallenberg Disease—A Newly Emerged Culicoides-Borne Viral Disease of Ruminants
Abstract
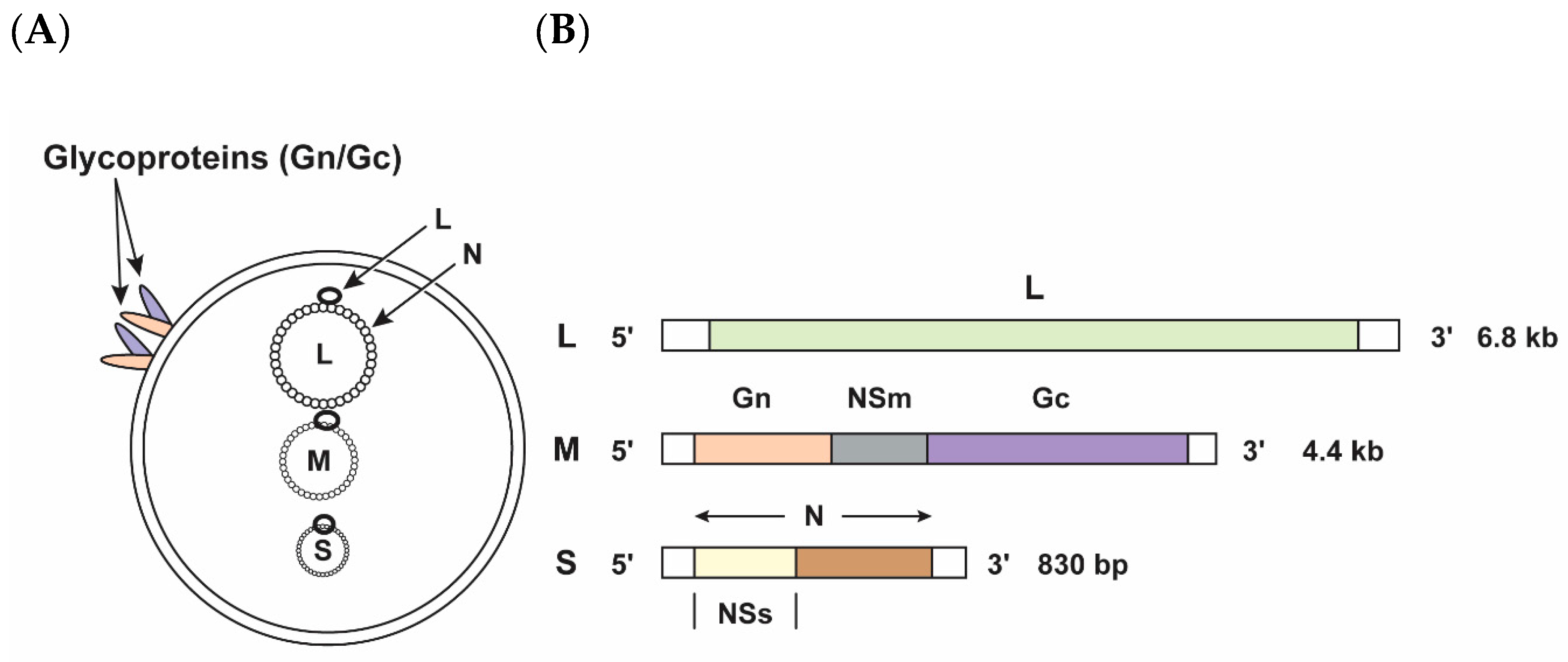
:1. Discovery and Genomic Structure of the Virus
2. Epidemiology
3. Clinical and Pathological Findings
4. Immunity
5. Diagnosis
6. Surveillance and Vaccination
7. Inactivated Vaccines
8. Modified-Live and Subunit Vaccines
9. Potential for Re-Emergence of SBV
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hoffmann, B.; Scheuch, M.; Höper, D.; Jungblut, R.; Holsteg, M.; Schirrmeier, H.; Eschbaumer, M.; Goller, K.V.; Wernike, K.; Fischer, M.; et al. Novel Orthobunyavirus in Cattle, Europe, 2011. Emerg. Infect. Dis. 2012, 18, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Goller, K.V.; Höper, D.; Schirrmeier, H.; Mettenleiter, T.C.; Beer, M. Schmallenberg Virus as Possible Ancestor of Shamonda Virus. Emerg. Infect. Dis. 2012, 18, 1644. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.M. Orthobunyaviruses: Recent genetic and structural insights. Nat. Rev. Microbiol. 2014, 12, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.; Schnettler, E.; Caporale, M.; Murgia, C.; Barry, G.; McFarlane, M.; McGregor, E.; Piras, I.M.; Shaw, A.; Lamm, C.; et al. Schmallenberg virus pathogenesis, tropism and interaction with the innate immune system of the host. PLoS Pathog. 2013, 9, e1003133. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.M.; Blakqori, G.; van Knippenberg, I.C.; Koudriakova, E.; Li, P.; McLees, A.; Shi, X.; Szemiel, A.M. Establishment of a reverse genetics system for Schmallenberg virus, a newly emerged orthobunyavirus in Europe. J. Gen. Virol. 2013, 94, 851–859. [Google Scholar] [CrossRef]
- Epstein, P.R. Chikungunya Fever resurgence and global warming. Am. J. Trop. Med. Hyg. 2007, 76, 403–404. [Google Scholar] [CrossRef]
- Conraths, F.J.; Gethmann, J.M.; Staubach, C.; Mettenleiter, T.C.; Beer, M.; Hoffmann, B. Epidemiology of bluetongue virus serotype 8, Germany. Emerg. Infect. Dis. 2009, 15, 433–435. [Google Scholar] [CrossRef]
- Luttikholt, S.; Veldhuis, A.; van den Brom, R.; Moll, L.; Lievaart-Peterson, K.; Peperkamp, K.; van Schaik, G.; Vellema, P. Risk factors for malformations and impact on reproductive performance and mortality rates of Schmallenberg virus in sheep flocks in the Netherlands. PLoS ONE 2014, 9, e100135. [Google Scholar] [CrossRef]
- Sedda, L.; Rogers, D.J. The influence of the wind in the Schmallenberg virus outbreak in Europe. Sci. Rep. 2013, 3, 3361. [Google Scholar] [CrossRef]
- Balmer, S.; Vögtlin, A.; Thür, B.; Büchi, M.; Abril, C.; Houmard, M.; Danuser, J.; Schwermer, H. Serosurveillance of Schmallenberg virus in Switzerland using bulk tank milk samples. Prev. Vet. Med. 2014, 116, 370–379. [Google Scholar] [CrossRef]
- Elbers, A.R.W.; Elbers, A.R.; Stockhofe, N.; Stockhofe, N.; van der Poel, W.H.M.; van der Poel, W.H. Schmallenberg virus antibodies in adult cows and maternal antibodies in calves. Emerg. Infect. Dis. 2014, 20, 901–902. [Google Scholar] [CrossRef] [PubMed]
- Claine, F.; Coupeau, D.; Wiggers, L.; Muylkens, B.; Kirschvink, N. Schmallenberg virus among female lambs, Belgium, 2012. Emerg. Infect. Dis. 2013, 19, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Conraths, F.J.; Kämer, D.; Teske, K.; Hoffmann, B.; Mettenleiter, T.C.; Beer, M. Reemerging Schmallenberg virus infections, Germany, 2012. Emerg. Infect. Dis. 2013, 19, 513–514. [Google Scholar] [CrossRef] [PubMed]
- Claine, F.; Coupeau, D.; Wiggers, L.; Muylkens, B.; Kirschvink, N. Schmallenberg virus infection of ruminants: Challenges and opportunities for veterinarians. Vet. Med. 2015, 6, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Delooz, L.; Saegerman, C.; Quinet, C.; Petitjean, T.; De Regge, N.; Cay, B. Resurgence of Schmallenberg Virus in Belgium after 3 Years of Epidemiological Silence. Transbound. Emerg. Dis. 2017, 64, 1641–1642. [Google Scholar] [CrossRef] [PubMed]
- Gache, K.; Zientara, S.; Collin, E.; Authié, E.; Dion, F.; Garin, E.; Zanella, G.; Calavas, D. Spatial and temporal patterns of Schmallenberg virus in France in 2016. Vet. Rec. 2018, 182, 575. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.E.; Tarlinton, R.E.; Lovatt, F.; Baylis, M.; Carson, A.; Duncan, J.S. Survey to determine the farm-level impact of Schmallenberg virus during the 2016–2017 United Kingdom lambing season. Vet. Rec. 2018, 183, 690. [Google Scholar] [CrossRef] [PubMed]
- Molini, U.; Capobianco Dondona, A.; Hilbert, R.; Monaco, F. Antibodies against Schmallenberg virus detected in cattle in the Otjozondjupa region, Namibia. J. S. Afr. Vet. Assoc. 2018, 89, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Oluwayelu, D.; Wernike, K.; Adebiyi, A.; Cadmus, S.; Beer, M. Neutralizing antibodies against Simbu serogroup viruses in cattle and sheep, Nigeria, 2012–2014. BMC Vet. Res. 2018, 14, 277. [Google Scholar] [CrossRef]
- Sibhat, B.; Ayelet, G.; Gebremedhin, E.Z.; Skjerve, E.; Asmare, K. Seroprevalence of Schmallenberg virus in dairy cattle in Ethiopia. Acta Trop. 2018, 178, 61–67. [Google Scholar] [CrossRef]
- Abutarbush, S.M.; La Rocca, A.; Wernike, K.; Beer, M.; Al Zuraikat, K.; Al Sheyab, O.M.; Talafha, A.Q.; Steinbach, F. Circulation of a Simbu Serogroup Virus, Causing Schmallenberg Virus-Like Clinical Signs in Northern Jordan. Transbound. Emerg. Dis. 2017, 64, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, H.; Hoffmann, B.; Turan, N.; Cizmecigil, U.Y.; Richt, J.A.; van der Poel, W.H.M. Detection and partial sequencing of Schmallenberg virus in cattle and sheep in Turkey. Vector Borne Zoonotic Dis. 2014, 14, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Azkur, A.K.; Albayrak, H.; Risvanli, A.; Pestil, Z.; Ozan, E.; Yılmaz, O.; Tonbak, S.; Cavunt, A.; Kadı, H.; Macun, H.C.; et al. Antibodies to Schmallenberg virus in domestic livestock in Turkey. Trop. Anim. Health Prod. 2013, 45, 1825–1828. [Google Scholar] [CrossRef] [PubMed]
- Chaintoutis, S.C.; Kiossis, E.; Giadinis, N.D.; Brozos, C.N.; Sailleau, C.; Viarouge, C.; Breard, E.; Papanastassopoulou, M.; Zientara, S.; Papadopoulos, O.; et al. Evidence of Schmallenberg virus circulation in ruminants in Greece. Trop. Anim. Health Prod. 2014, 46, 251–255. [Google Scholar] [CrossRef]
- Rasmussen, L.D.; Kristensen, B.; Kirkeby, C.; Rasmussen, T.B.; Belsham, G.J.; Bødker, R.; Bøtner, A. Culicoids as vectors of Schmallenberg virus. Emerg. Infect. Dis. 2012, 18, 1204–1206. [Google Scholar] [CrossRef]
- De Regge, N.; Deblauwe, I.; De Deken, R.; Vantieghem, P.; Madder, M.; Geysen, D.; Smeets, F.; Losson, B.; van den Berg, T.; Cay, A.B. Detection of Schmallenberg virus in different Culicoides spp. by real-time RT-PCR. Transbound. Emerg. Dis. 2012, 59, 471–475. [Google Scholar] [CrossRef]
- Goffredo, M.; Monaco, F.; Capelli, G.; Quaglia, M.; Federici, V.; Catalani, M.; Montarsi, F.; Polci, A.; Pinoni, C.; Calistri, P.; et al. Schmallenberg virus in Italy: A retrospective survey in Culicoides stored during the bluetongue Italian surveillance program. Prev. Vet. Med. 2013, 111, 230–236. [Google Scholar] [CrossRef]
- Elbers, A.R.W.; Elbers, A.R.; Meiswinkel, R.; Meiswinkel, R.; van Weezep, E.; van Weezep, E.; Sloet van Oldruitenborgh-Oosterbaan, M.M.; Sloet van Oldruitenborgh-Oosterbaan, M.M.; Kooi, E.A.; Kooi, E.A. Schmallenberg virus in Culicoides spp. biting midges, the Netherlands, 2011. Emerg. Infect. Dis. 2013, 19, 106–109. [Google Scholar] [CrossRef]
- Veronesi, E.; Henstock, M.; Gubbins, S.; Batten, C.; Manley, R.; Barber, J.; Hoffmann, B.; Beer, M.; Attoui, H.; Mertens, P.P.C.; et al. Implicating Culicoides biting midges as vectors of Schmallenberg virus using semi-quantitative RT-PCR. PLoS ONE 2013, 8, e57747. [Google Scholar] [CrossRef]
- Kęsik-Maliszewska, J.; Larska, M.; Collins, Á.B.; Rola, J. Post-Epidemic Distribution of Schmallenberg Virus in Culicoides Arbovirus Vectors in Poland. Viruses 2019, 11, 447. [Google Scholar] [CrossRef]
- Bilk, S.; Schulze, C.; Fischer, M.; Beer, M.; Hlinak, A.; Hoffmann, B. Organ distribution of Schmallenberg virus RNA in malformed newborns. Vet. Microbiol. 2012, 159, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Wernike, K.; Holsteg, M.; Schirrmeier, H.; Hoffmann, B.; Beer, M. Natural Infection of Pregnant Cows with Schmallenberg Virus—A Follow-Up Study. PLoS ONE 2014, 9, e98223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wernike, K.; Eschbaumer, M.; Schirrmeier, H.; Blohm, U.; Breithaupt, A.; Hoffmann, B.; Beer, M. Oral exposure, reinfection and cellular immunity to Schmallenberg virus in cattle. Vet. Microbiol. 2013, 165, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Wernike, K.; Beer, M.; Hoffmann, B. Infectious Schmallenberg Virus from Bovine Semen, Germany. Emerg. Infect. Dis. 2014, 20, 338–339. [Google Scholar] [CrossRef]
- Rapid spread and association of Schmallenberg virus with ruminant abortions and foetal death in Austria in 2012/2013. Prev. Vet. Med. 2014, 116, 350–359. [CrossRef]
- Natural intrauterine infection with Schmallenberg virus in malformed newborn calves. Emerg. Infect. Dis. 2014, 20, 1327–1330. [CrossRef]
- De Regge, N. Akabane, Aino and Schmallenberg virus-where do we stand and what do we know about the role of domestic ruminant hosts and Culicoides vectors in virus transmission and overwintering? Curr. Opin. Virol. 2017, 27, 15–30. [Google Scholar] [CrossRef]
- Larska, M.; Krzysiak, M.K.; Kęsik-Maliszewska, J.; Rola, J. Cross-sectional study of Schmallenberg virus seroprevalence in wild ruminants in Poland at the end of the vector season of 2013. BMC Vet. Res. 2014, 10, 967. [Google Scholar] [CrossRef] [Green Version]
- Schulz, C.; Beer, M.; Hoffmann, B. Schmallenberg virus infection in South American camelids: Field and experimental investigations. Vet. Microbiol. 2015, 180, 171–179. [Google Scholar] [CrossRef]
- García-Bocanegra, I.; Cano-Terriza, D.; Vidal, G.; Rosell, R.; Paniagua, J.; Jiménez-Ruiz, S.; Expósito, C.; Rivero-Juarez, A.; Arenas, A.; Pujols, J. Monitoring of Schmallenberg virus in Spanish wild artiodactyls, 2006–2015. PLoS ONE 2017, 12, e0182212. [Google Scholar] [CrossRef] [Green Version]
- Zhai, S.L.; Lv, D.H.; Wen, X.H.; Zhu, X.L.; Yang, Y.Q.; Chen, Q.L.; Wei, W.K. Preliminary serological evidence for Schmallenberg virus infection in China. Trop. Anim. Health Prod. 2018, 50, 449–453. [Google Scholar] [CrossRef]
- Rasekh, M.; Sarani, A.; Hashemi, S.H. Detection of Schmallenberg virus antibody in equine population of Northern and Northeast of Iran. Vet. World 2018, 11, 30–33. [Google Scholar] [CrossRef]
- Sailleau, C.; Boogaerts, C.; Meyrueix, A.; Laloy, E.; Breard, E.; Viarouge, C.; Desprat, A.; Vitour, D.; Doceul, V.; Boucher, C.; et al. Schmallenberg virus infection in dogs, France, 2012. Emerg. Infect. Dis. 2013, 19, 1896–1898. [Google Scholar] [CrossRef]
- Poskin, A.; Van Campe, W.; Mostin, L.; Cay, B.; De Regge, N. Experimental Schmallenberg virus infection of pigs. Vet. Microbiol. 2014, 170, 398–402. [Google Scholar] [CrossRef]
- Wernike, K.; Eschbaumer, M.; Breithaupt, A.; Hoffmann, B.; Beer, M. Schmallenberg virus challenge models in cattle: Infectious serum or culture-grown virus? Vet. Res. 2012, 43, 84. [Google Scholar] [CrossRef] [Green Version]
- Afonso, A.; Abrahantes, J.C.; Conraths, F.; Veldhuis, A.; Elbers, A.; Roberts, H.; Van der Stede, Y.; Méroc, E.; Gache, K.; Richardson, J. The Schmallenberg virus epidemic in Europe-2011–2013. Prev. Vet. Med. 2014, 116, 391–403. [Google Scholar] [CrossRef]
- Helmer, C.; Eibach, R.; Tegtmeyer, P.C.; Humann-Ziehank, E.; Ganter, M. Survey of Schmallenberg virus (SBV) infection in German goat flocks. Epidemiol. Infect. 2013, 141, 2335–2345. [Google Scholar] [CrossRef] [Green Version]
- Lievaart-Peterson, K.; Luttikholt, S.; Peperkamp, K.; Van den Brom, R.; Vellema, P. Schmallenberg disease in sheep or goats: Past, present and future. Vet. Microbiol. 2015, 181, 147–153. [Google Scholar] [CrossRef]
- Endalew, A.D.; Morozov, I.; Davis, A.S.; Gaudreault, N.N.; Wernike, K.; Bawa, B.; Ruder, M.G.; Drolet, B.S.; McVey, D.S.; Shivanna, V.; et al. Virological and Serological Responses of Sheep and Cattle to Experimental Schmallenberg Virus Infection. Vector Borne Zoonotic Dis. 2018, 18, 697–703. [Google Scholar] [CrossRef]
- Tsuda, T.; Yoshida, K.; Ohashi, S.; Yanase, T.; Yanase, T.; Sueyoshi, M.; Kamimura, S.; Misumi, K.; Hamana, K.; Sakamoto, H.; et al. Arthrogryposis, hydranencephaly and cerebellar hypoplasia syndrome in neonatal calves resulting from intrauterine infection with Aino virus. Vet. Res. 2004, 35, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Herder, V.; Wohlsein, P.; Peters, M.; Hansmann, F.; Baumgartner, W. Salient lesions in domestic ruminants infected with the emerging so-called Schmallenberg virus in Germany. Vet. Pathol. 2012, 49, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Doceul, V.; Lara, E.; Sailleau, C.; Belbis, G.; Richardson, J.; Breard, E.; Viarouge, C.; Dominguez, M.; Hendrikx, P.; Calavas, D.; et al. Epidemiology, molecular virology and diagnostics of Schmallenberg virus, an emerging orthobunyavirus in Europe. Vet. Res. 2013, 44, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wernike, K.; Holsteg, M.; Sasserath, M.; Beer, M. Schmallenberg virus antibody development and decline in a naturally infected dairy cattle herd in Germany, 2011–2014. Vet. Microbiol. 2015, 181, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Claine, F.; Coupeau, D.; Wiggers, L.; Muylkens, B.; Kirschvink, N. Modelling the evolution of Schmallenberg virus seroprevalence in a sheep flock after natural infection. Prev. Vet. Med. 2018, 154, 132–138. [Google Scholar] [CrossRef]
- Peperkamp, N.H.; Luttikholt, S.J.; Dijkman, R.; Vos, J.H.; Junker, K.; Greijdanus, S.; Roumen, M.P.; van Garderen, E.; Meertens, N.; van Maanen, C.; et al. Ovine and Bovine Congenital Abnormalities Associated with Intrauterine Infection with Schmallenberg Virus. Vet. Pathol. 2015, 52, 1057–1066. [Google Scholar] [CrossRef] [Green Version]
- Van der Poel, W.H.M. Diagnostics for Schmallenberg virus. Vet. Rec. 2012, 171, 294–295. [Google Scholar] [CrossRef]
- De Regge, N.; van den Berg, T.; Georges, L.; Cay, B. Diagnosis of Schmallenberg virus infection in malformed lambs and calves and first indications for virus clearance in the fetus. Vet. Microbiol. 2013, 162, 595–600. [Google Scholar] [CrossRef]
- Breard, E.; Lara, E.; Comtet, L.; Viarouge, C.; Doceul, V.; Desprat, A.; Vitour, D.; Pozzi, N.; Cay, A.B.; De Regge, N.; et al. Validation of a commercially available indirect ELISA using a nucleocapside recombinant protein for detection of Schmallenberg virus antibodies. PLoS ONE 2013, 8, e53446. [Google Scholar] [CrossRef]
- Humphries, D.; Burr, P. Schmallenberg virus milk antibody ELISA. Vet. Rec. 2012, 171, 511–512. [Google Scholar]
- Van der Poel, W.H.M.; Cay, B.; Zientara, S.; Steinbach, F.; Valarcher, J.F.; Botner, A.; Mars, M.H.; Hakze-van der Honing, R.; Schirrmeier, H.; Beer, M. Limited interlaboratory comparison of Schmallenberg virus antibody detection in serum samples. Vet. Rec. 2014, 174, 380. [Google Scholar] [CrossRef]
- Tarlinton, R.; Daly, J.; Dunham, S.; Kydd, J. The challenge of Schmallenberg virus emergence in Europe. Vet. J. 2012, 194, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Gerhauser, I.; Weigand, M.; Hahn, K.; Herder, V.; Wohlsein, P.; Habierski, A.; Varela, M.; Palmarini, M.; Baumgartner, W. Lack of schmallenberg virus in ruminant brain tissues archived from 1961 to 2010 in Germany. J. Comp. Pathol. 2014, 150, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Gache, K.; Touratier, A.; Bournez, L.; Zientara, S.; Bronner, A.; Dion, F.; Garin, E.; Calavas, D. Detection of Schmallenberg virus in France since 2012. Vet. Rec. 2017, 180, 24. [Google Scholar] [CrossRef] [PubMed]
- Sohier, C.; Deblauwe, I.; Van Loo, T.; Hanon, J.B.; Cay, A.B.; De Regge, N. Evidence of extensive renewed Schmallenberg virus circulation in Belgium during summer of 2016—Increase in arthrogryposis-hydranencephaly cases expected. Transbound. Emerg. Dis. 2017, 64, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Hechinger, S.; Wernike, K.; Beer, M. Single immunization with an inactivated vaccine protects sheep from Schmallenberg virus infection. Vet. Res. 2014, 45, 79. [Google Scholar] [CrossRef] [Green Version]
- Wernike, K.; Nikolin, V.M.; Hechinger, S.; Hoffmann, B.; Beer, M. Inactivated Schmallenberg virus prototype vaccines. Vaccine 2013, 31, 3558–3563. [Google Scholar] [CrossRef]
- Anonymous Merck Animal Health. Available online: http://wwwmerck-animal-healthcom/news/2013-5-21aspx. (accessed on 12 November 2019).
- Anonymous Merial. Available online: http://merialcom/en/press-releases/merial-receives-approval-for-new-vaccine-to-prevent-schmallenberg-disease-in-livestock/ (accessed on 12 November 2019).
- Kraatz, F.; Wernike, K.; Hechinger, S.; König, P.; Granzow, H.; Reimann, I.; Beer, M. Deletion Mutants of Schmallenberg Virus Are Avirulent and Protect from Virus Challenge. J. Virol. 2015, 89, 1825–1837. [Google Scholar] [CrossRef] [Green Version]
- Varela, M.; Pinto, R.M.; Caporale, M.; Piras, I.M.; Taggart, A.; Seehusen, F.; Hahn, K.; Janowicz, A.; de Souza, W.M.; Baumgärtner, W.; et al. Mutations in the Schmallenberg Virus Gc Glycoprotein Facilitate Cellular Protein Synthesis Shutoff and Restore Pathogenicity of NSs Deletion Mutants in Mice. J. Virol. 2016, 90, 5440–5450. [Google Scholar] [CrossRef] [Green Version]
- Endalew, A.; Faburay, B.; Trujillo, J.D.; Gaudreault, N.N.; Davis, A.S.; Shivanna, V.; Sunwoo, S.Y.; Ma, W.; Drolet, B.S.; McVey, D.S.; et al. Immunogenicity and Efficacy of Schmallenberg Virus Envelope Glycoprotein Subunit Vaccines. J. Vet. Sci. 2019. [Google Scholar] [CrossRef]
- Wernike, K.; Aebischer, A.; Roman-Sosa, G.; Beer, M. The N-terminal domain of Schmallenberg virus envelope protein Gc is highly immunogenic and can provide protection from infection. Sci. Rep. 2017, 7, 42500. [Google Scholar] [CrossRef] [Green Version]
- Boshra, H.Y.; Charro, D.; Lorenzo, G.; Sánchez, I.; Lazaro, B.; Brun, A.; Abrescia, N.G.A. DNA vaccination regimes against Schmallenberg virus infection in IFNAR-/- mice suggest two targets for immunization. Antivir. Res. 2017, 141, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Wernike, K.; Mundt, A.; Link, E.K.; Aebischer, A.; Schlotthauer, F.; Sutter, G.; Fux, R.; Beer, M. N-terminal domain of Schmallenberg virus envelope protein Gc delivered by recombinant equine herpesvirus type 1 and modified vaccinia virus Ankara: Immunogenicity and protective efficacy in cattle. Vaccine 2018, 36, 5116–5123. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Schmallenberg virus vaccine. Vet. Rec. 2015, 177, 321. [Google Scholar]
- Kono, R.; Hirata, M.; Kaji, M.; Goto, Y.; Ikeda, S.; Yanase, T.; Kato, T.; Tanaka, S.; Tsutsui, T.; Imada, T.; et al. Bovine epizootic encephalomyelitis caused by Akabane virus in southern Japan. BMC Vet. Res. 2008, 4, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkland, P.D. Akabane virus infection. Rev. Sci. Tech. 2015, 34, 403–410. [Google Scholar] [CrossRef]
- Sailleau, C.; Breard, E.; Viarouge, C.; Vitour, D.; Romey, A.; Garnier, A.; Fablet, A.; Lowenski, S.; Gorna, K.; Caignard, G.; et al. Re-Emergence of Bluetongue Virus Serotype 8 in France, 2015. Transbound. Emerg. Dis. 2017, 64, 998–1000. [Google Scholar] [CrossRef]
- Meroc, E.; Poskin, A.; Van Loo, H.; Van Driessche, E.; Czaplicki, G.; Quinet, C.; Riocreux, F.; De Regge, N.; Caij, B.; van den Berg, T.; et al. Follow-up of the Schmallenberg Virus Seroprevalence in Belgian Cattle. Transbound. Emerg. Dis. 2015, 62, e80–e84. [Google Scholar] [CrossRef]
- Collins, Á.B.; Barrett, D.; Doherty, M.L.; Larska, M.; Mee, J.F. Post-epidemic Schmallenberg virus circulation: Parallel bovine serological and Culicoides virological surveillance studies in Ireland. BMC Vet. Res. 2016, 12, 234. [Google Scholar] [CrossRef] [Green Version]
- Stokes, J.E.; Baylis, M.; Duncan, J.S. A freedom from disease study: Schmallenberg virus in the south of England in 2015. Vet. Rec. 2016, 179, 435. [Google Scholar] [CrossRef] [Green Version]
- Stavrou, A.; Daly, J.M.; Maddison, B.; Gough, K.; Tarlinton, R. How is Europe positioned for a re-emergence of Schmallenberg virus? Vet. J. 2017, 230, 45–51. [Google Scholar] [CrossRef]
| Type of Vaccine | Host Species Evaluated | DIVA Compatibility | References | ||
|---|---|---|---|---|---|
| Mice | Cattle | Sheep | |||
| Inactivated vaccines: | |||||
| Binary ethylenimine inactivated | NO | YES | YES | NO | [65,66] |
| Bovilis SBV (MSD Animal Health) | NO | YES | YES | NO | [67] |
| Zulvac SBV (Zoetis) | NO | YES | YES | NO | [75] |
| SBVvax (Merial) | NO | YES | YES | NO | [68] |
| Genetically modified live virus vaccines: | |||||
| Recombinant NSm and/or NSs deletion mutants | YES | YES | YES | YES | [69] |
| DNA vaccines: | |||||
| SBV Gc (N-terminal) | YES | NO | NO | YES | [72] |
| SBV Nucleoprotein | YES | NO | NO | YES | [73] |
| SBV Gn (ectodomain), SBV Gc (ectodomain 1 and 2) | YES | NO | NO | YES | [73] |
| Virus-vectored vaccines: | |||||
| Recombinant Equine Herpes Virus 1, Gc (N-terminal) | NO | YES | NO | YES | [74] |
| Modified Vaccinia Virus Ankara, Gc (N-terminal) | YES | NO | YES | YES | [74] |
| Recombinant subunit Vaccines: | |||||
| Baculovirus-expressed Gc or Gc/Gn | NO | YES | NO | YES | [71] |
| Gc (N-terminal), HEK cells | YES | YES | NO | YES | [72] |
| Gc + Gn linked ectodomains, HEK cells | YES | YES | NO | YES | [72] |
| Gc (N-terminal) of SBV and Akabane, HEK cells | YES | YES | NO | YES | [72] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endalew, A.D.; Faburay, B.; Wilson, W.C.; Richt, J.A. Schmallenberg Disease—A Newly Emerged Culicoides-Borne Viral Disease of Ruminants. Viruses 2019, 11, 1065. https://doi.org/10.3390/v11111065
Endalew AD, Faburay B, Wilson WC, Richt JA. Schmallenberg Disease—A Newly Emerged Culicoides-Borne Viral Disease of Ruminants. Viruses. 2019; 11(11):1065. https://doi.org/10.3390/v11111065
Chicago/Turabian StyleEndalew, Abaineh D., Bonto Faburay, William C. Wilson, and Juergen A. Richt. 2019. "Schmallenberg Disease—A Newly Emerged Culicoides-Borne Viral Disease of Ruminants" Viruses 11, no. 11: 1065. https://doi.org/10.3390/v11111065
APA StyleEndalew, A. D., Faburay, B., Wilson, W. C., & Richt, J. A. (2019). Schmallenberg Disease—A Newly Emerged Culicoides-Borne Viral Disease of Ruminants. Viruses, 11(11), 1065. https://doi.org/10.3390/v11111065







