The U3 and Env Proteins of Jaagsiekte Sheep Retrovirus and Enzootic Nasal Tumor Virus Both Contribute to Tissue Tropism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Animals
2.3. DNA Constructs and PCR
2.4. Virus Production
2.5. Virus Quantification, Transduction, and Infection
2.6. Western Blotting
2.7. Immunization Protocol to Generate Murine Anti-p27 Serum
2.8. ENTV and JSRV p27 ELISA
2.9. Immunohistochemistry (IHC)
2.10. Statistical Analyses
3. Results
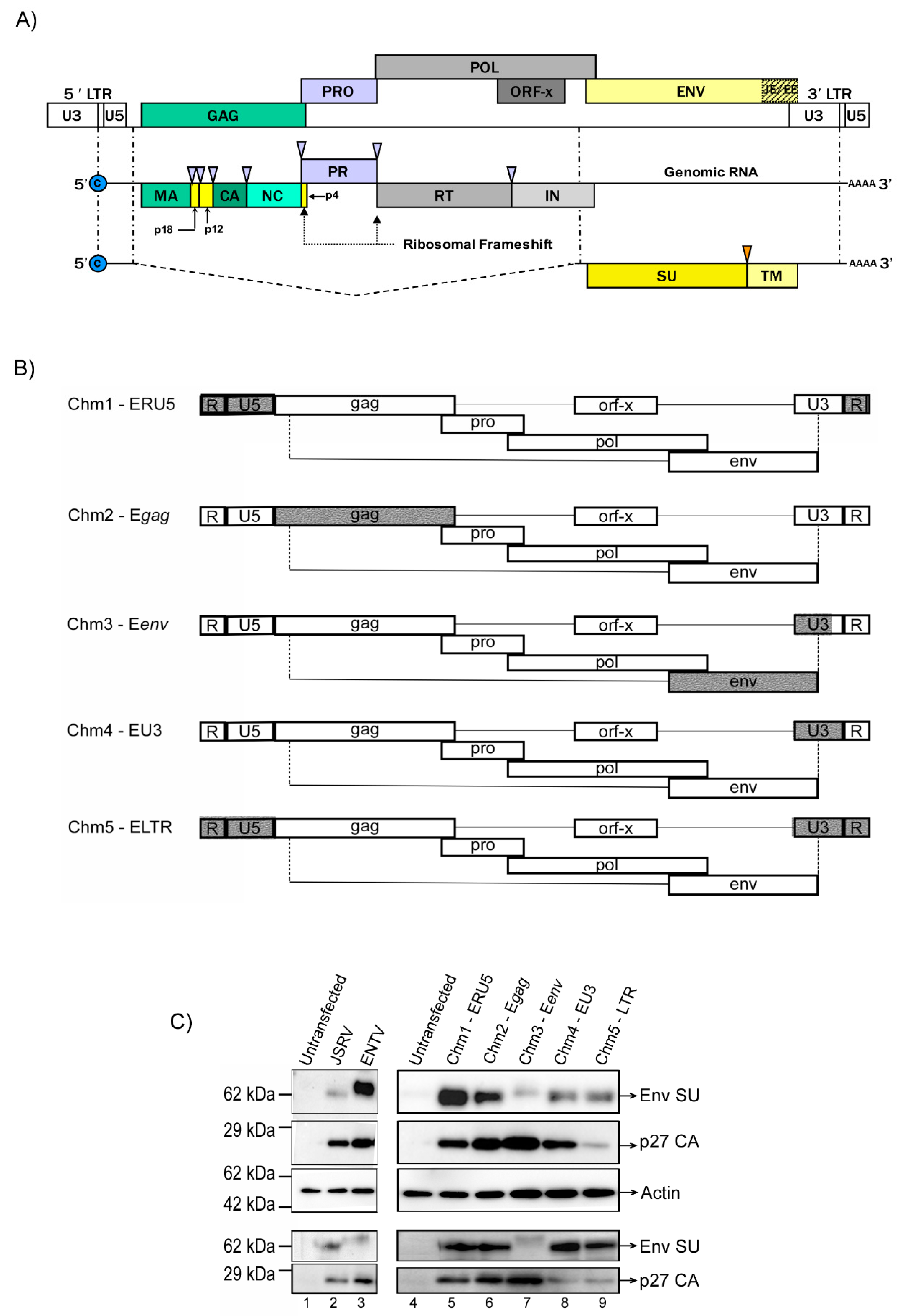
3.1. Construction of JSRV and ENTV Hybrid Viruses
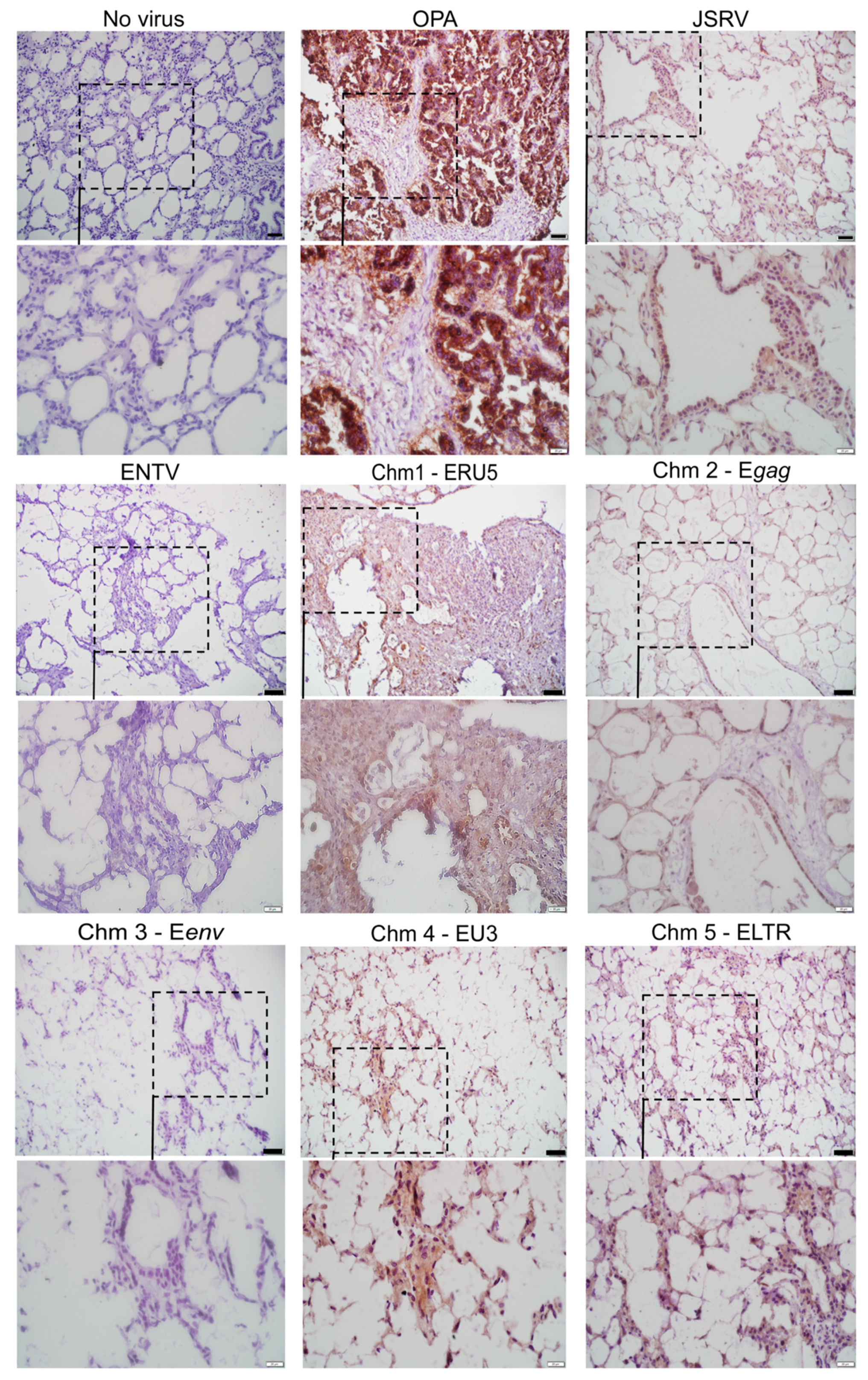
3.2. Jenv-Encoding but Not Eenv-Encoding Chimeras Are Able to Infect Ovine Lung Tissue Slices
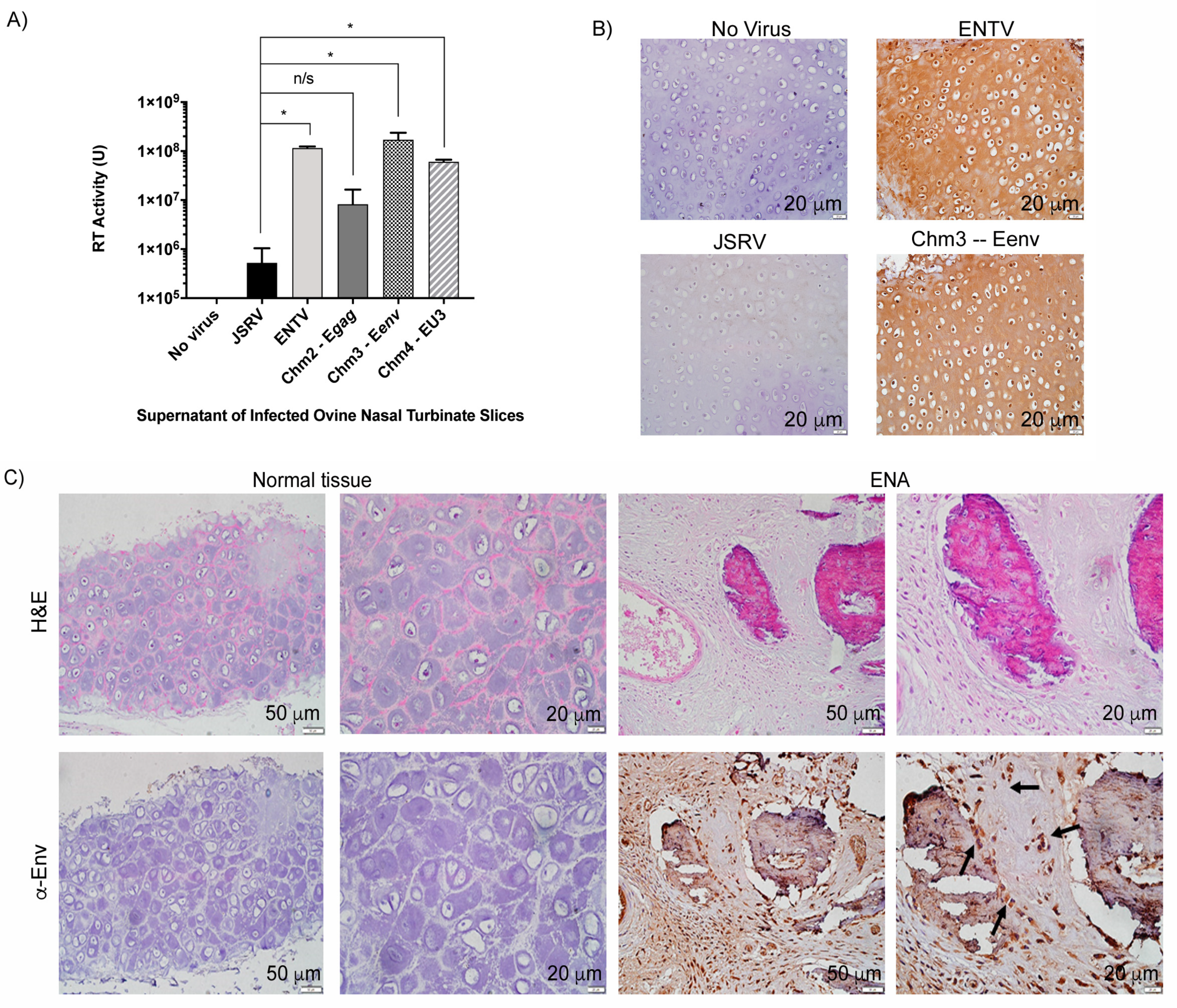
3.3. ENTV Infects Ovine Nasal Turbinate Tissue Slices with Much Greater Efficiency Than JSRV
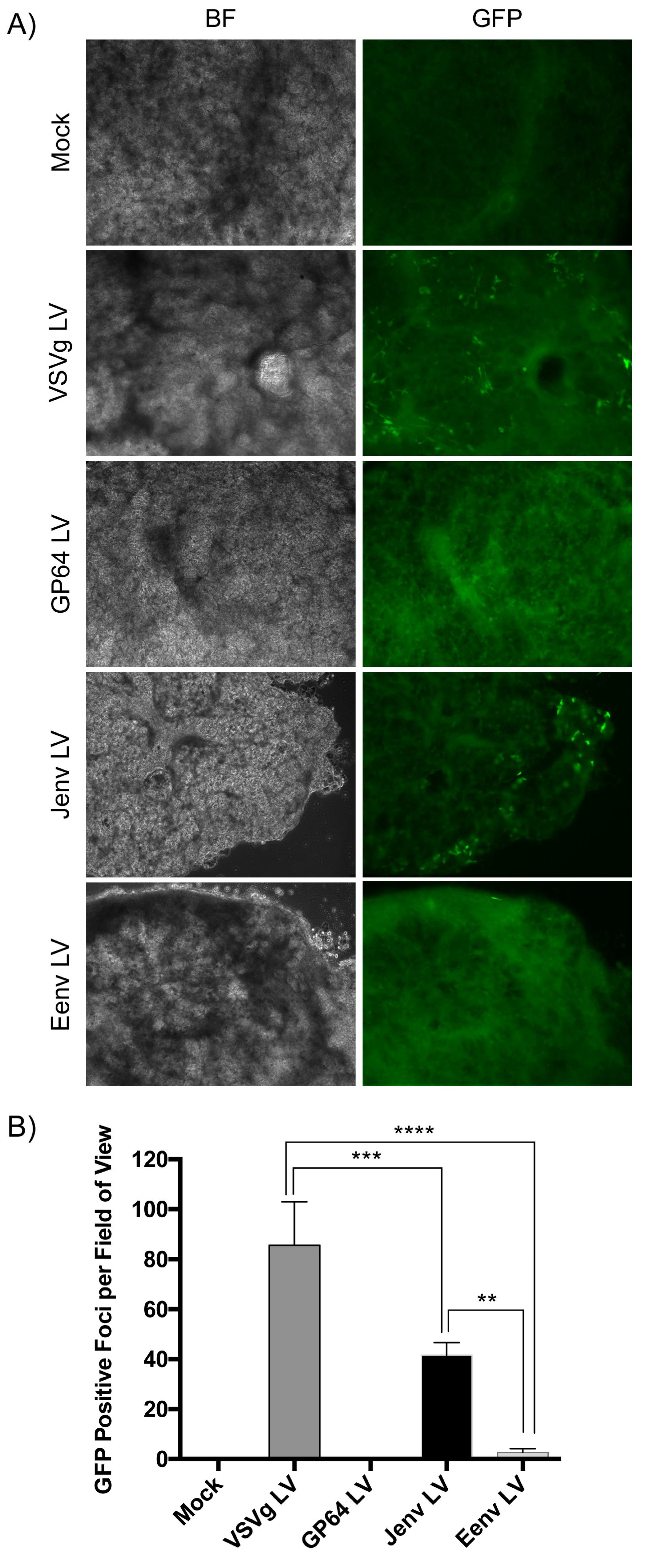
3.4. Jenv Pseudotyped LV Transduces Ovine Lung Tissue Slices with Greater Efficiency Than Eenv Pseudotyped LV
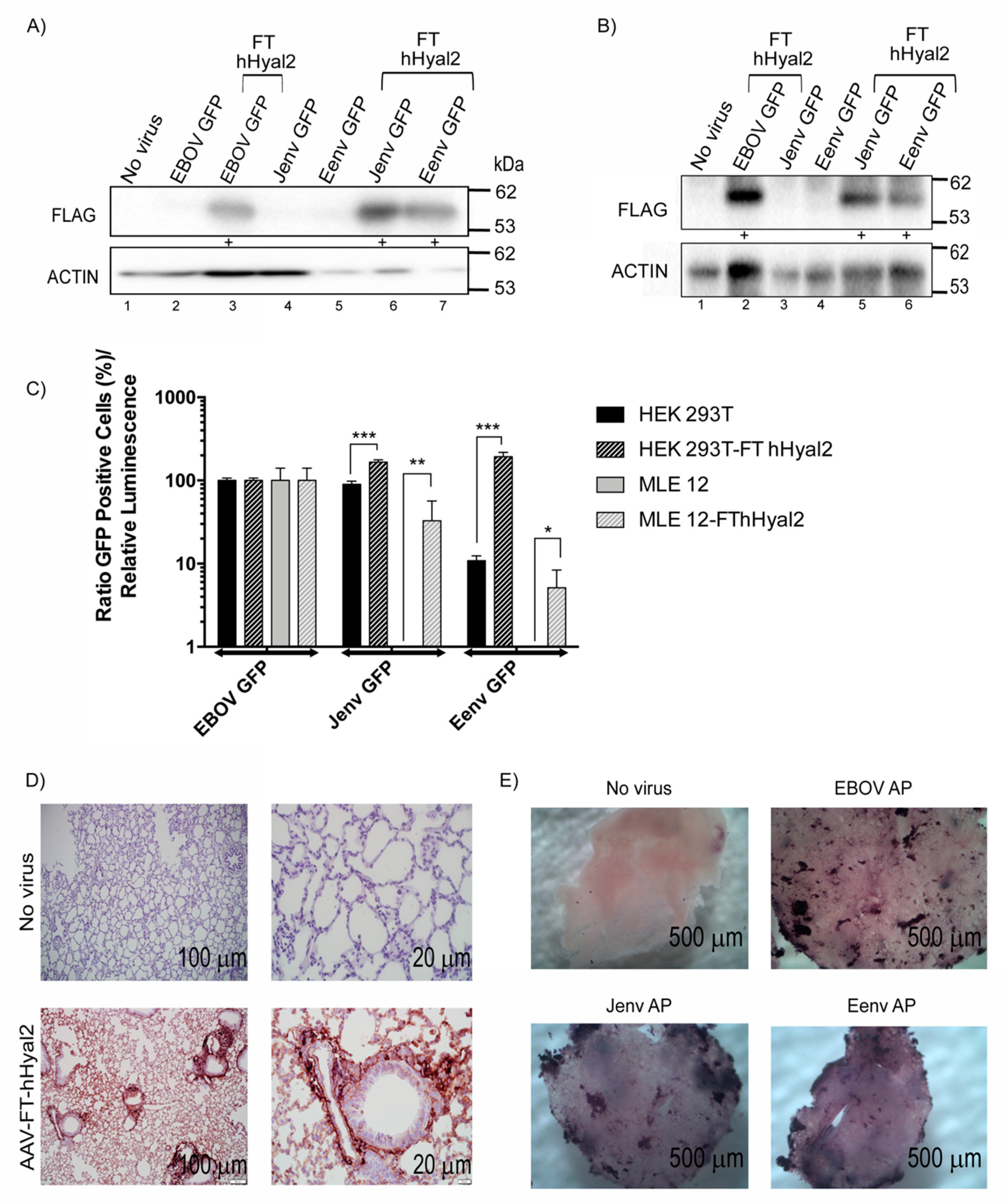
3.5. Eenv-Pseudotyped Lentivector Entry Is Enhanced in Cells Overexpressing hHyal2
3.6. The ENTV LTR Is Significantly more Active in Ovine Primary Chondrocytes Than the JSRV LTR
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Palmarini, M.; Sharp, J.M.; de las Heras, M.; Fan, H. Jaagsiekte sheep retrovirus is necessary and sufficient to induce a contagious lung cancer in sheep. J. Virol. 1999, 73, 6964–6972. [Google Scholar] [PubMed]
- Walsh, S.R.; Linnerth-Petrik, N.M.; Yu, D.L.; Foster, R.A.; Menzies, P.I.; Diaz-Mendez, A.; Chalmers, H.J.; Wootton, S.K. Experimental transmission of enzootic nasal adenocarcinoma in sheep. Vet. Res. 2013, 44, 66. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.K.; Duh, F.M.; Vigdorovich, V.; Danilkovitch-Miagkova, A.; Lerman, M.I.; Miller, A.D. Candidate tumor suppressor hyal2 is a glycosylphosphatidylinositol (gpi)-anchored cell-surface receptor for jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc. Natl. Acad. Sci. USA 2001, 98, 4443–4448. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.; Duh, F.M.; Lerman, M.I.; Miller, A.D. Role of virus receptor hyal2 in oncogenic transformation of rodent fibroblasts by sheep betaretrovirus env proteins. J. Virol. 2003, 77, 2850–2858. [Google Scholar] [CrossRef]
- Wootton, S.; Halbert, C.; Miller, A. Sheep retrovirus structural protein induces lung tumours. Nature 2005, 434, 904–907. [Google Scholar] [CrossRef]
- Wootton, S.; Halbert, C.; Miller, A. Envelope proteins of jaagsiekte sheep retrovirus and enzootic nasal tumor virus induce similar bronchioalveolar tumors in lungs of mice. J. Virol. 2006, 80, 9322–9325. [Google Scholar] [CrossRef]
- Alberti, A.; Murgia, C.; Liu, S.; Mura, M.; Cousens, C.; Sharp, M.; Miller, A.; Palmarini, M. Envelope-induced cell transformation by ovine betaretroviruses. J. Virol. 2002, 76, 5387–5394. [Google Scholar] [CrossRef]
- Murgia, C.; Caporale, M.; Ceesay, O.; Di Francesco, G.; Ferri, N.; Varasano, V.; de las Heras, M.; Palmarini, M. Lung adenocarcinoma originates from retrovirus infection of proliferating type 2 pneumocytes during pulmonary post-natal development or tissue repair. PLoS Pathog. 2011, 7, e1002014. [Google Scholar] [CrossRef]
- Palmarini, M.; Holland, M.; Cousens, C.; Dalziel, R.; Sharp, J. Jaagsiekte retrovirus establishes a disseminated infection of the lymphoid tissues of sheep affected by pulmonary adenomatosis. J. Gen. Virol. 1996, 77, 2991–2998. [Google Scholar] [CrossRef]
- Caporale, M.; Centorame, P.; Giovannini, A.; Sacchini, F.; Di Ventura, M.; De las Heras, M.; Palmarini, M. Infection of lung epithelial cells and induction of pulmonary adenocarcinoma is not the most common outcome of naturally occurring jsrv infection during the commercial lifespan of sheep. Virology 2005, 338, 144–153. [Google Scholar] [CrossRef]
- Walsh, S.R.; Stinson, K.J.; Menzies, P.I.; Wootton, S.K. Development of an antemortem diagnostic test for enzootic nasal tumour virus (entv-1) and detection of neutralizing antibodies in host serum. J. Gen. Virol. 2014, 95, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Dirks, C.; Duh, F.M.; Rai, S.K.; Lerman, M.I.; Miller, A.D. Mechanism of cell entry and transformation by enzootic nasal tumor virus. J. Virol 2002, 76, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.; Linnerth-Petrik, N.; Laporte, A.; Menzies, P.; Foster, R.; Wootton, S. Full-length genome sequence analysis of enzootic nasal tumor virus reveals an unusually high degree of genetic stability. Virus Res. 2010, 151, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.R. Mouse mammary tumor virus molecular biology and oncogenesis. Viruses 2010, 2, 2000–2012. [Google Scholar] [CrossRef] [PubMed]
- Hull, S.; Boris-Lawrie, K. Ru5 of mason-pfizer monkey virus 5’ long terminal repeat enhances cytoplasmic expression of human immunodeficiency virus type 1 gag-pol and nonviral reporter rna. J. Virol. 2002, 76, 10211–10218. [Google Scholar] [CrossRef]
- McGee-Estrada, K.; Fan, H. Comparison of ltr enhancer elements in sheep beta retroviruses: Insights into the basis for tissue-specific expression. Virus Genes 2007, 35, 303–312. [Google Scholar] [CrossRef]
- McGee-Estrada, K.; Palmarini, M.; Fan, H. Hnf-3beta is a critical factor for the expression of the jaagsiekte sheep retrovirus long terminal repeat in type ii pneumocytes but not in clara cells. Virology 2002, 292, 87–97. [Google Scholar] [CrossRef]
- McGee-Estrada, K.; Fan, H. In vivo and in vitro analysis of factor binding sites in jaagsiekte sheep retrovirus long terminal repeat enhancer sequences: Roles of hnf-3, nf-i, and c/ebp for activity in lung epithelial cells. J. Virol. 2006, 80, 332–341. [Google Scholar] [CrossRef]
- Miller, A.D. Hyaluronidase 2 and its intriguing role as a cell-entry receptor for oncogenic sheep retroviruses. Semin Cancer Biol. 2008, 18, 296–301. [Google Scholar] [CrossRef]
- Chow, G.; Knudson, C.; Knudson, W. Human hyaluronidase-2 is localized intracellularly in articular chondrocytes and other cultured cell lines. Osteoarthr. Cartil. 2006, 14, 1312–1314. [Google Scholar] [CrossRef]
- Davey, M.G.; Zoltick, P.W.; Todorow, C.A.; Limberis, M.P.; Ruchelli, E.D.; Hedrick, H.L.; Flake, A.W. Jaagsiekte sheep retrovirus pseudotyped lentiviral vector-mediated gene transfer to fetal ovine lung. Gene Ther. 2012, 19, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Van Hoeven, N.; Miller, A. Improved enzootic nasal tumor virus pseudotype packaging cell lines reveal virus entry requirements in addition to the primary receptor hyal2. J. Virol. 2005, 79, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Steckbeck, J.D.; Kuhlmann, A.S.; Montelaro, R.C. Structural and functional comparisons of retroviral envelope protein c-terminal domains: Still much to learn. Viruses 2014, 6, 284–300. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.R.; de Jong, J.G.; van Vloten, J.P.; Gerpe, M.C.; Santry, L.A.; Wootton, S.K. Truncation of the enzootic nasal tumor virus envelope protein cytoplasmic tail increases env-mediated fusion and infectivity. J. Gen. Virol. 2017, 98, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Côté, M.; Zheng, Y.M.; Albritton, L.M.; Liu, S.L. Fusogenicity of jaagsiekte sheep retrovirus envelope protein is dependent on low ph and is enhanced by cytoplasmic tail truncations. J. Virol. 2008, 82, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Van Lieshout, L.P.; Domm, J.M.; Rindler, T.N.; Frost, K.L.; Sorensen, D.L.; Medina, S.J.; Booth, S.A.; Bridges, J.P.; Wootton, S.K. A novel triple-mutant aav6 capsid induces rapid and potent transgene expression in the muscle and respiratory tract of mice. Mol. Ther. Methods Clin. Dev. 2018, 9, 323–329. [Google Scholar] [CrossRef]
- Rosales Gerpe, M.C.; van Vloten, J.P.; Santry, L.A.; de Jong, J.; Mould, R.C.; Pelin, A.; Bell, J.C.; Bridle, B.W.; Wootton, S.K. Use of precision-cut lung slices as an ex vivo tool for evaluating viruses and viral vectors for gene and oncolytic therapy. Mol. Ther. Methods Clin. Dev. 2018, 10, 245–256. [Google Scholar] [CrossRef]
- Balazs, A.B.; Chen, J.; Hong, C.M.; Rao, D.S.; Yang, L.; Baltimore, D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature 2012, 481, 81–84. [Google Scholar] [CrossRef]
- Bryksin, A.V.; Matsumura, I. Overlap extension pcr cloning: A simple and reliable way to create recombinant plasmids. Biotechniques 2010, 48, 463–465. [Google Scholar] [CrossRef]
- Yu, D.L.; Linnerth-Petrik, N.M.; Halbert, C.L.; Walsh, S.R.; Miller, A.D.; Wootton, S.K. Jaagsiekte sheep retrovirus and enzootic nasal tumor virus promoters drive gene expression in all airway epithelial cells of mice but only induce tumors in the alveolar region of the lungs. J. Virol. 2011, 85, 7535–7545. [Google Scholar] [CrossRef]
- Walsh, S.R.; Gerpe, M.C.; Wootton, S.K. Construction of a molecular clone of ovine enzootic nasal tumor virus. Virol. J. 2016, 13, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wootton, S.; Metzger, M.; Hudkins, K.; Alpers, C.; York, D.; DeMartini, J.; Miller, A. Lung cancer induced in mice by the envelope protein of jaagsiekte sheep retrovirus (jsrv) closely resembles lung cancer in sheep infected with jsrv. Retrovirology 2006, 3, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mould, R.C.; AuYeung, A.W.K.; van Vloten, J.P.; Susta, L.; Mutsaers, A.J.; Petrik, J.J.; Wood, G.A.; Wootton, S.K.; Karimi, K.; Bridle, B.W. Enhancing immune responses to cancer vaccines using multi-site injections. Sci. Rep. 2017, 7, 8322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCormick, A.L.; Thomas, M.S.; Heath, A.W. Immunization with an interferon-gamma-gp120 fusion protein induces enhanced immune responses to human immunodeficiency virus gp120. J. Infect. Dis. 2001, 184, 1423–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, S.M.; Renda, M.; Nam, N.Y.; Klimatcheva, E.; Zhu, Y.; Fisk, J.; Halterman, M.; Rimel, B.J.; Federoff, H.; Pandya, S.; et al. Lentivirus vectors using human and simian immunodeficiency virus elements. J. Virol. 1999, 73, 2832–2840. [Google Scholar]
- Linnerth-Petrik, N.M.; Santry, L.A.; Petrik, J.J.; Wootton, S.K. Opposing functions of akt isoforms in lung tumor initiation and progression. PLoS ONE 2014, 9, e94595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, J.L.; Cao, S.; Maldonado, J.O.; Zhang, W.; Mansky, L.M. Distinct particle morphologies revealed through comparative parallel analyses of retrovirus-like particles. J. Virol. 2016, 90, 8074–8084. [Google Scholar] [CrossRef] [Green Version]
- Marozsan, A.J.; Fraundorf, E.; Abraha, A.; Baird, H.; Moore, D.; Troyer, R.; Nankja, I.; Arts, E.J. Relationships between infectious titer, capsid protein levels, and reverse transcriptase activities of diverse human immunodeficiency virus type 1 isolates. J. Virol. 2004, 78, 11130–11141. [Google Scholar] [CrossRef] [Green Version]
- Hida, D.; Danielson, B.T.; Knudson, C.B.; Knudson, W. Cd44 knock-down in bovine and human chondrocytes results in release of bound hyal2. Matrix Biol. 2015, 48, 42–54. [Google Scholar] [CrossRef] [Green Version]
- Antonioli, E.; Piccinato, C.A.; Nader, H.B.; Cohen, M.; Goldberg, A.C.; Ferretti, M. Modulation of hyaluronan synthesis by the interaction between mesenchymal stem cells and osteoarthritic chondrocytes. Stem Cells Int. 2015, 2015, 640218. [Google Scholar] [CrossRef] [Green Version]
- María, C.; Gerpe, R.; Wootton, S.K. A consistent observation in our laboratory is the inabiltiy of LVs pseudotyped with the EBOV glycoprotein to transduce ovine lung tissue sections. 2017; (Material not intended to publish). [Google Scholar]
- Liu, S.; Miller, A. Transformation of madin-darby canine kidney epithelial cells by sheep retrovirus envelope proteins. J. Virol. 2005, 79, 927–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hull, S.; Fan, H. Mutational analysis of the cytoplasmic tail of jaagsiekte sheep retrovirus envelope protein. J. Virol. 2006, 80, 8069–8080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chesters, P.; Smith, L.; Nair, V. E (xsr) element contributes to the oncogenicity of avian leukosis virus (subgroup j). J. Gen. Virol. 2006, 87, 2685–2692. [Google Scholar] [CrossRef] [PubMed]
- Laimins, L.; Tsichlis, P.; Khoury, G. Multiple enhancer domains in the 3’ terminus of the prague strain of rous sarcoma virus. Nucleic Acids Res. 1984, 12, 6427–6442. [Google Scholar] [CrossRef] [Green Version]
- Hofacre, A.; Fan, H. Jaagsiekte sheep retrovirus biology and oncogenesis. Viruses 2010, 2, 2618–2648. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; An, L.; Liu, G.; Li, X.; Tang, W.; Chen, X. Mouse lung slices: An ex vivo model for the evaluation of antiviral and anti-inflammatory agents against influenza viruses. Antivir. Res. 2015, 120, 101–111. [Google Scholar] [CrossRef]
- Griesenbach, U.; Inoue, M.; Meng, C.; Farley, R.; Chan, M.; Newman, N.K.; Brum, A.; You, J.; Kerton, A.; Shoemark, A.; et al. Assessment of f/hn-pseudotyped lentivirus as a clinically relevant vector for lung gene therapy. Am. J. Respir. Crit. Care Med. 2012, 186, 846–856. [Google Scholar] [CrossRef] [Green Version]
- Cousens, C.; Alleaume, C.; Bijsmans, E.; Martineau, H.M.; Finlayson, J.; Dagleish, M.P.; Griffiths, D.J. Jaagsiekte sheep retrovirus infection of lung slice cultures. Retrovirology 2015, 12, 31. [Google Scholar] [CrossRef] [Green Version]
- Palmarini, M.; Datta, S.; Omid, R.; Murgia, C.; Fan, H. The long terminal repeat of jaagsiekte sheep retrovirus is preferentially active in differentiated epithelial cells of the lungs. J. Virol. 2000, 74, 5776–5787. [Google Scholar] [CrossRef] [Green Version]
- Palmarini, M.; Sharp, J.; Lee, C.; Fan, H. In vitro infection of ovine cell lines by jaagsiekte sheep retrovirus. J. Virol. 1999, 73, 10070–10078. [Google Scholar]
- Pisoni, G.; Moroni, P.; Turin, L.; Bertoni, G. Compartmentalization of small ruminant lentivirus between blood and colostrum in infected goats. Virology 2007, 369, 119–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oskarsson, T.; Hreggvidsdóttir, H.S.; Agnarsdóttir, G.; Matthíasdóttir, S.; Ogmundsdóttir, M.H.; Jónsson, S.R.; Georgsson, G.; Ingvarsson, S.; Andrésson, O.S.; Andrésdóttir, V. Duplicated sequence motif in the long terminal repeat of maedi-visna virus extends cell tropism and is associated with neurovirulence. J. Virol. 2007, 81, 4052–4057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, L.; Ruscetti, S. Tissue tropism of a leukemogenic murine retrovirus is determined by sequences outside of the long terminal repeats. Proc. Natl. Acad. Sci. USA 1986, 83, 3376–3380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruscetti, S.; Wolff, L. Spleen focus-forming virus: Relationship of an altered envelope gene to the development of a rapid erythroleukemia. Curr. Top. Microbiol. Immunol. 1984, 112, 21–44. [Google Scholar]
- Stieler, K.; Schulz, C.; Lavanya, M.; Aepfelbacher, M.; Stocking, C.; Fischer, N. Host range and cellular tropism of the human exogenous gammaretrovirus xmrv. Virology 2010, 399, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Brown, D.; Robinson, H. Influence of env and long terminal repeat sequences on the tissue tropism of avian leukosis viruses. J. Virol. 1988, 62, 4828–4831. [Google Scholar]
- Schulman, H.M.; Ponka, P.; Wilczynska, A.; Gauthier, Y.; Shyamala, G. Transferrin receptor and ferritin levels during murine mammary gland development. Biochim. Biophys. Acta 1989, 1010, 1–6. [Google Scholar] [CrossRef]
- Futran, J.; Kemp, J.D.; Field, E.H.; Vora, A.; Ashman, R.F. Transferrin receptor synthesis is an early event in b cell activation. J. Immunol. 1989, 143, 787–792. [Google Scholar]
- Brekelmans, P.; van Soest, P.; Voerman, J.; Platenburg, P.P.; Leenen, P.J.; van Ewijk, W. Transferrin receptor expression as a marker of immature cycling thymocytes in the mouse. Cell Immunol. 1994, 159, 331–339. [Google Scholar] [CrossRef] [Green Version]
- Ross, S.R. Mouse mammary tumor virus and its interaction with the immune system. Immunol. Res. 1998, 17, 209–216. [Google Scholar] [CrossRef]
- Yanagawa, S.; Kakimi, K.; Tanaka, H.; Murakami, A.; Nakagawa, Y.; Kubo, Y.; Yamada, Y.; Hiai, H.; Kuribayashi, K.; Masuda, T. Mouse mammary tumor virus with rearranged long terminal repeats causes murine lymphomas. J. Virol. 1993, 67, 112–118. [Google Scholar] [PubMed]
- Arnaud, F.; Varela, M.; Spencer, T.E.; Palmarini, M. Coevolution of endogenous betaretroviruses of sheep and their host. Cell Mol. Life Sci. 2008, 65, 3422–3432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armezzani, A.; Arnaud, F.; Caporale, M.; di Meo, G.; Iannuzzi, L.; Murgia, C.; Palmarini, M. The signal peptide of a recently integrated endogenous sheep betaretrovirus envelope plays a major role in eluding gag-mediated late restriction. J. Virol. 2011, 85, 7118–7128. [Google Scholar] [CrossRef] [Green Version]
- Dunlap, K.; Palmarini, M.; Adelson, D.; Spencer, T. Sheep endogenous betaretroviruses (enjsrvs) and the hyaluronidase 2 (hyal2) receptor in the ovine uterus and conceptus. Biol. Reprod. 2005, 73, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Modelski, M.J.; Menlah, G.; Wang, Y.; Dash, S.; Wu, K.; Galileo, D.S.; Martin-DeLeon, P.A. Hyaluronidase 2: A novel germ cell hyaluronidase with epididymal expression and functional roles in mammalian sperm. Biol. Reprod. 2014, 91, 109. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe, L.H.; Wang, L.; Somanathan, S.; Zhi, Y.; Figueredo, J.; Calcedo, R.; Sanmiguel, J.; Desai, R.A.; Chen, C.S.; Johnston, J.; et al. Heparin binding directs activation of t cells against adeno-associated virus serotype 2 capsid. Nat. Med. 2006, 12, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Armezzani, A.; Varela, M.; Spencer, T.E.; Palmarini, M.; Arnaud, F. “Ménage à trois”: The evolutionary interplay between jsrv, enjsrvs and domestic sheep. Viruses 2014, 6, 4926–4945. [Google Scholar] [CrossRef]
- Walsh, S.R.; Stinson, K.J.; Wootton, S.K. Seroconversion of sheep experimentally infected with enzootic nasal tumor virus. BMC Res. Notes 2016, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, Y.F.; Sun, X.L.; Liu, S.Y. Detection of jaagsiekte sheep retrovirus in the peripheral blood during the pre-clinical period of ovine pulmonary adenomatosis. Genet. Mol. Res. 2016, 15, gmr.15038521. [Google Scholar] [CrossRef]
- Kobinger, G.P.; Weiner, D.J.; Yu, Q.C.; Wilson, J.M. Filovirus-pseudotyped lentiviral vector can efficiently and stably transduce airway epithelia in vivo. Nat. Biotechnol. 2001, 19, 225–230. [Google Scholar] [CrossRef]
- Patel, S.; Turner, P.R.; Stubberfield, C.; Barry, E.; Rohlff, C.R.; Stamps, A.; McKenzie, E.; Young, K.; Tyson, K.; Terrett, J.; et al. Hyaluronidase gene profiling and role of hyal-1 overexpression in an orthotopic model of prostate cancer. Int. J. Cancer 2002, 97, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Côté, M.; Kucharski, T.; Liu, S. Enzootic nasal tumor virus envelope requires a very acidic ph for fusion activation and infection. J. Virol. 2008, 82, 9023–9034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clary-Meinesz, C.; Mouroux, J.; Cosson, J.; Huitorel, P.; Blaive, B. Influence of external ph on ciliary beat frequency in human bronchi and bronchioles. Eur. Respir. J. 1998, 11, 330–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- England, R.J.; Homer, J.J.; Knight, L.C.; Ell, S.R. Nasal ph measurement: A reliable and repeatable parameter. Clin. Otolaryngol. Allied Sci. 1999, 24, 67–68. [Google Scholar] [CrossRef] [Green Version]
- Tømmeraas, K.; Melander, C. Kinetics of hyaluronan hydrolysis in acidic solution at various ph values. Biomacromolecules 2008, 9, 1535–1540. [Google Scholar] [CrossRef]
- Duterme, C.; Mertens-Strijthagen, J.; Tammi, M.; Flamion, B. Two novel functions of hyaluronidase-2 (hyal2) are formation of the glycocalyx and control of cd44-erm interactions. J. Biol. Chem. 2009, 284, 33495–33508. [Google Scholar] [CrossRef] [Green Version]
- Andre, B.; Duterme, C.; van Moer, K.; Mertens-Strijthagen, J.; Jadot, M.; Flamion, B. Hyal2 is a glycosylphosphatidylinositol-anchored, lipid raft-associated hyaluronidase. Biochem. Biophys. Res. Commun. 2011, 411, 175–179. [Google Scholar] [CrossRef]
- Bourguignon, L.Y.; Singleton, P.A.; Diedrich, F.; Stern, R.; Gilad, E. Cd44 interaction with na+-h+ exchanger (nhe1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin b activation and breast tumor cell invasion. J. Biol. Chem. 2004, 279, 26991–27007. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosales Gerpe, M.C.; van Lieshout, L.P.; Domm, J.M.; Ingrao, J.C.; Datu, J.; Walsh, S.R.; Yu, D.L.; de Jong, J.; Krell, P.J.; Wootton, S.K. The U3 and Env Proteins of Jaagsiekte Sheep Retrovirus and Enzootic Nasal Tumor Virus Both Contribute to Tissue Tropism. Viruses 2019, 11, 1061. https://doi.org/10.3390/v11111061
Rosales Gerpe MC, van Lieshout LP, Domm JM, Ingrao JC, Datu J, Walsh SR, Yu DL, de Jong J, Krell PJ, Wootton SK. The U3 and Env Proteins of Jaagsiekte Sheep Retrovirus and Enzootic Nasal Tumor Virus Both Contribute to Tissue Tropism. Viruses. 2019; 11(11):1061. https://doi.org/10.3390/v11111061
Chicago/Turabian StyleRosales Gerpe, María C., Laura P. van Lieshout, Jakob M. Domm, Joelle C. Ingrao, Jodre Datu, Scott R. Walsh, Darrick L. Yu, Jondavid de Jong, Peter J. Krell, and Sarah K. Wootton. 2019. "The U3 and Env Proteins of Jaagsiekte Sheep Retrovirus and Enzootic Nasal Tumor Virus Both Contribute to Tissue Tropism" Viruses 11, no. 11: 1061. https://doi.org/10.3390/v11111061
APA StyleRosales Gerpe, M. C., van Lieshout, L. P., Domm, J. M., Ingrao, J. C., Datu, J., Walsh, S. R., Yu, D. L., de Jong, J., Krell, P. J., & Wootton, S. K. (2019). The U3 and Env Proteins of Jaagsiekte Sheep Retrovirus and Enzootic Nasal Tumor Virus Both Contribute to Tissue Tropism. Viruses, 11(11), 1061. https://doi.org/10.3390/v11111061




