Novel Viruses in Mosquitoes from Brazilian Pantanal
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito Sampling, Processing, Random PCR, and Sequencing
2.2. Genome Assembly, Taxonomic Classification, and Phylogenetic Analysis
2.3. Viral Isolation and Viral-Specific RT-PCR Design
3. Results
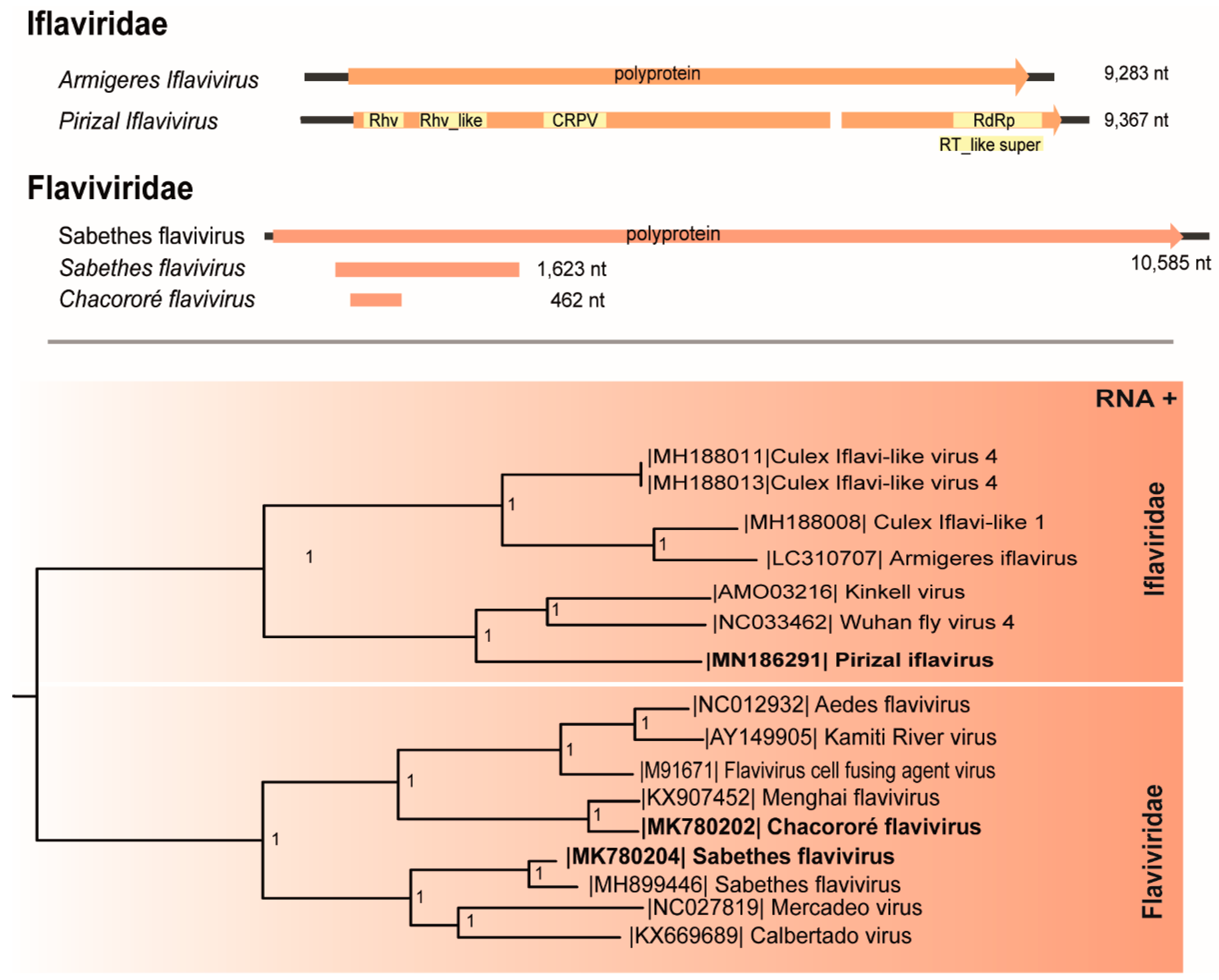
3.1. Iflaviridae
3.2. Flaviviridae
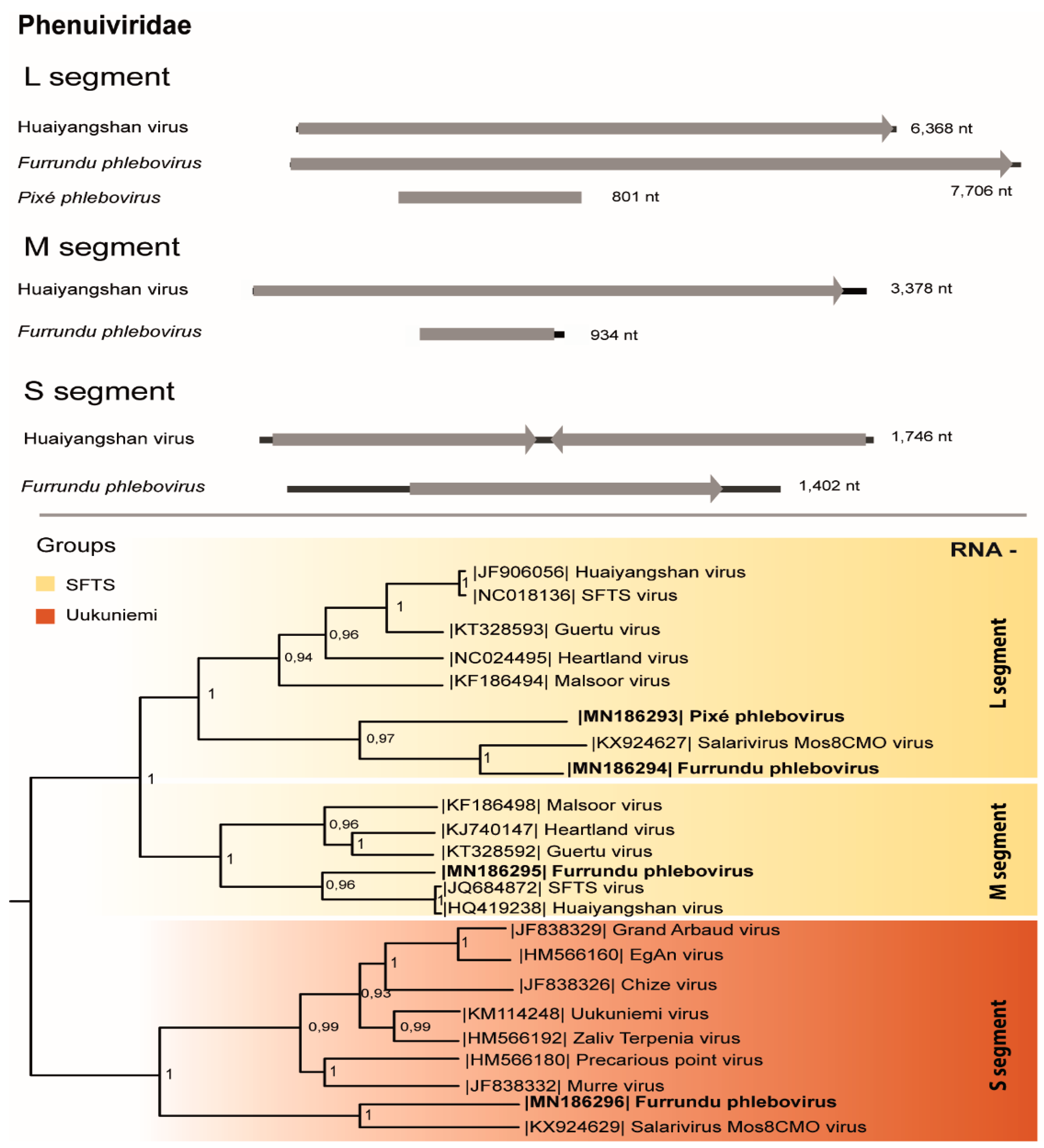
3.3. Phenuiviridae
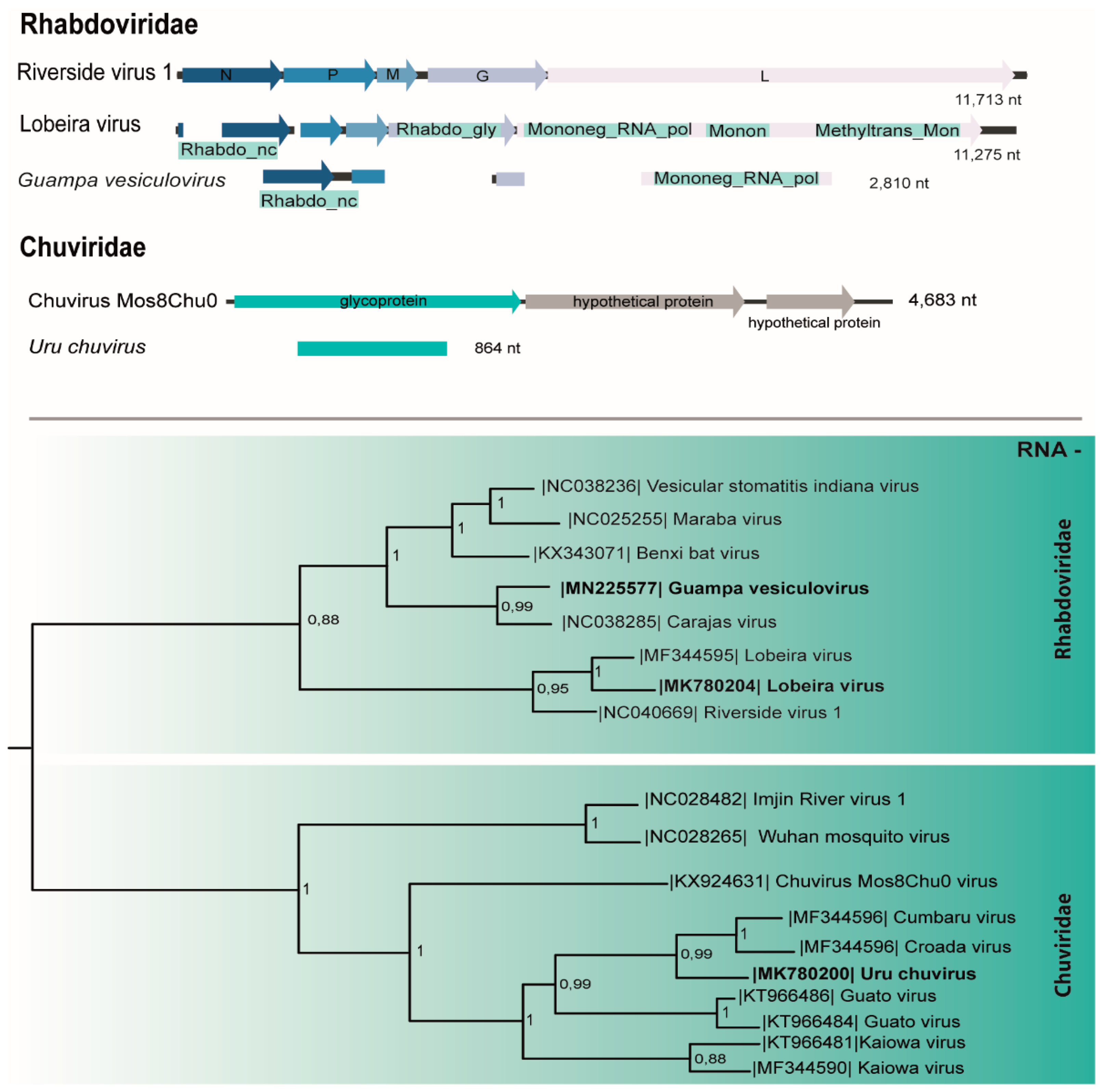
3.4. Rhabdoviridae
3.5. Chuviridae
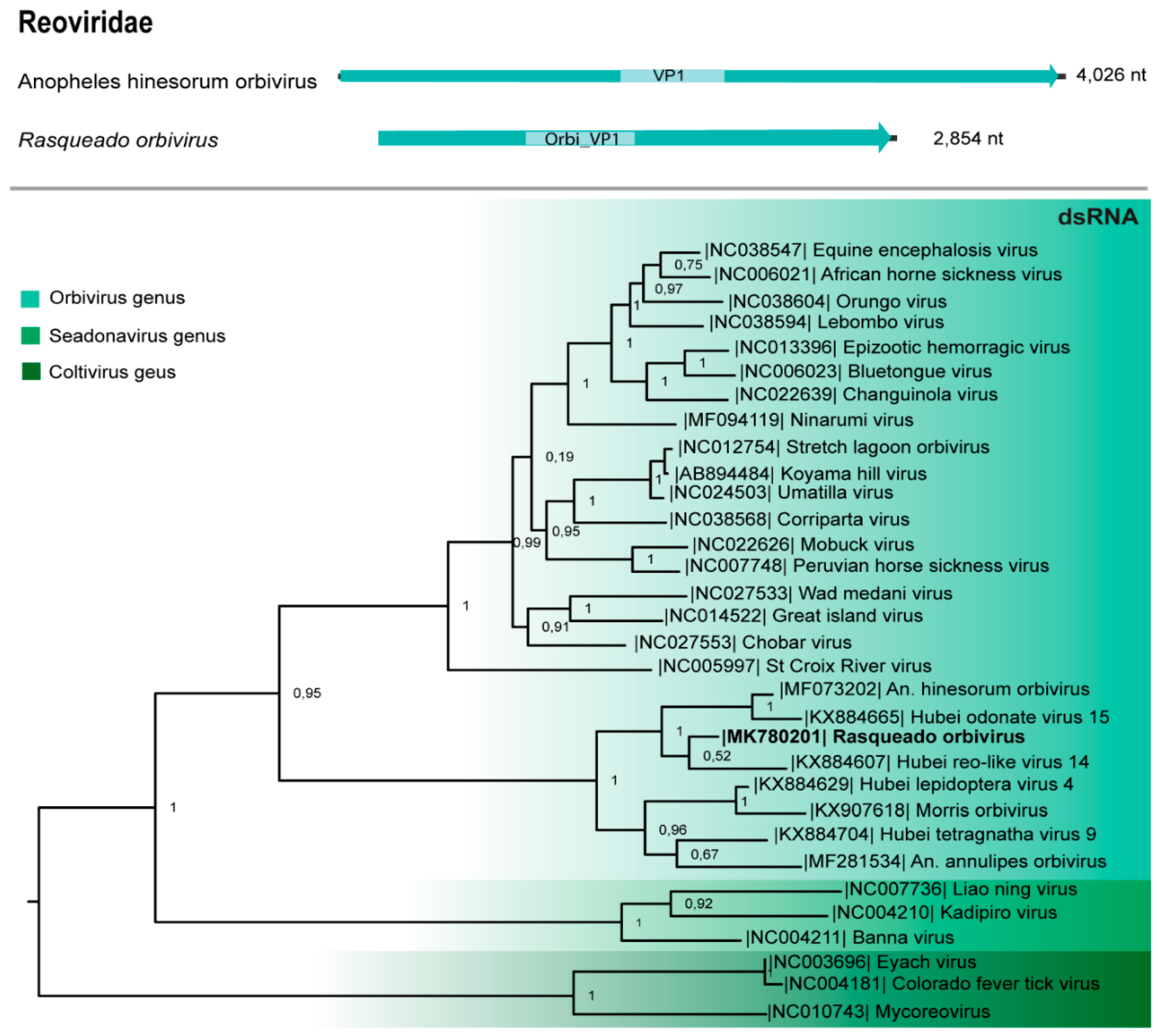
3.6. Reoviridae
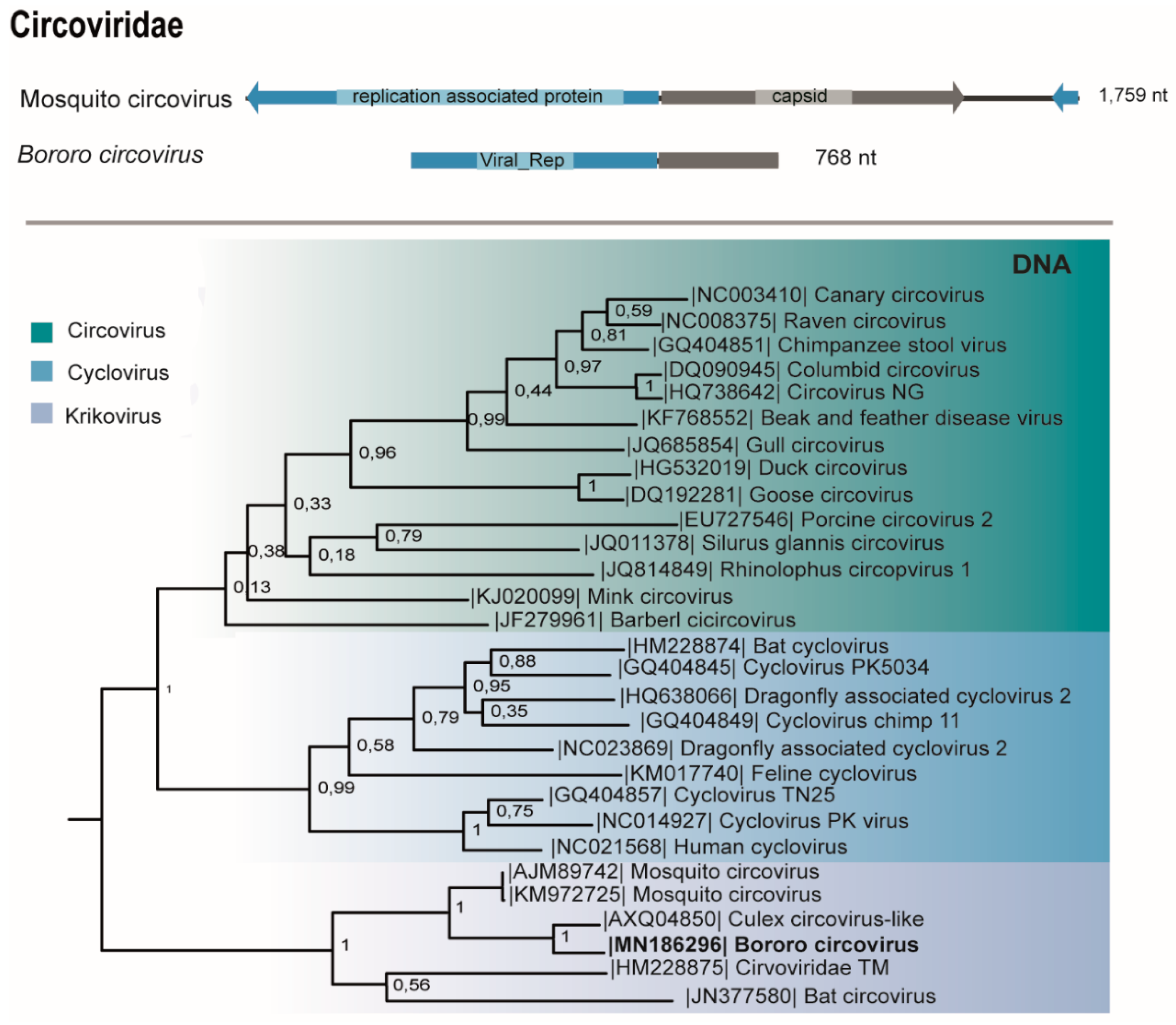
3.7. Circoviridae
3.8. Partitiviridae and Totiviridae
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Junk, W.J.; Piedade, M.T.F.; Lourival, R.; Wittmann, F.; Kandus, P.; Lacerda, L.D.; Bozelli, R.L.; Esteves, F.A.; Nunes da Cunha, C.; Maltchik, L.; et al. Brazilian wetlands: Their definition, delineation, and classification for research, sustainable management, and protection. Aquatic Conserv. Mar. Freshw. Ecosyst. 2014, 24, 5–22. [Google Scholar] [CrossRef]
- Girard, P. Hydrology of surface and ground waters in the Pantanal floodplains. In The Pantanal: Ecology, Biodiversity and Sustainable Management of a Large Neotropical Seasonal Wetland; Junk, W.J., da Silva, C.J., da Cunha, C.N., Wantzen, K.M., Eds.; Pensoft Publishers: Sofia, Bulgaria, 2011; pp. 103–126. [Google Scholar]
- Ministério do Meio Ambiente. Available online: http://www.mma.gov.br/biomas/pantanal (accessed on 20 February 2019).
- Alencar, J.; Lorosa, E.S.; Silva, J.S. Observações sobre padrões alimentares de mosquitoes (Diptera: Culicidae) no Pantanal Mato-Grossense. Neotrop. Entomol. 2005, 34, 681–687. [Google Scholar] [CrossRef]
- Pauvolid-Corrêa, A.; Kenney, J.L.; Couto-Lima, D.; Campos, Z.M.S.; Schatzmayr, H.G.; Nogueira, R.M.R.; Brault, A.C.; Komar, N. Ilheus Virus Isolation in the Pantanal, West-Central Brazil. PLoS Negl. Trop. Dis. 2013, 7, 1–8. [Google Scholar] [CrossRef]
- Pauvolid-Corrêa, A.; Solberg, O.; Couto-Lima, D.; Nogueira, R.M.; Langevin, S.; Komar, N. Novel viruses isolated from mosquitoes in Pantanal, Brazil. Genome Announc. 2016, 4, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Pauvolid-Corrêa, A.; Solber, O.; Kenney, J.; Serra-Freire, N.; Brault, A.; Nogueira, R.; Langevinv, S.; Komar, N. Nhumirim virus, a novel flavivírus isolated from mosquitoes from the Pantanal, Brazil. Arch. Virol. 2016, 160, 21–27. [Google Scholar] [CrossRef]
- Pauvolid-Corrêa, A.; Campos, Z.; Soares, R.; Nogueira, R.M.R.; Komar, N. Neutralizing antibodies for orthobunyaviruses in Pantanal, Brazil. PLoS Negl. Trop. Dis. 2017, 11, 1–11. [Google Scholar] [CrossRef]
- Melo, R.M.; Cavalcanti, R.C.; Villalobos, E.M.C.; Cunha, E.M.S.; Lara, M.C.C.S.H.; Aguiar, D.M. Ocorrência de equídeos soropositivos para os vírus das encefalomielites e anemia infecciosa no estado de Mato Grosso. Arq. Inst. Biol. 2012, 79, 160–175. [Google Scholar] [CrossRef]
- Bolling, B.G.; Weaver, S.C.; Tesh, R.B.; Vasilakis, N. Insect-specific virus discovery: Significance for the arbovirus community. Viruses 2015, 7, 4911–4928. [Google Scholar] [CrossRef]
- Word Health Organization. Neglect Tropical Diseases. Available online: http://www.who.int/ neglected_diseases/vector_ecology/mosquito-borne-diseases (accessed on 21 February 2019).
- Shi, M.; Lin, X.D.; Tian, J.H.; Chien, L.J.; Chen, X.; Li, C.X.; Qin, X.C.; Li, J.; Cao, J.P.; Eden, J.S.; et al. Redefining the invertebrate RNA virosphora. Nature 2016, 540, 539–543. [Google Scholar] [CrossRef]
- Frey, K.G.; Biser, T.; Hamilton, T.; Santos, C.J.; Pimentel, G.; Mokashi, V.P. Bioinformatic Characterization of Mosquito Viromes within the Eastern United States and Puerto Rico: Discovery of Novel Viruses. Evol. Bioinform. Online 2016, 2, 1–12. [Google Scholar] [CrossRef]
- Li, C.X.; Shi, M.; Tian, J.H.; Lin, X.D.; Kang, Y.J.; Chen, L.J.; Qin, X.C.; Xu, J.; Holmes, E.C.; Zhang, Y.Z. Unprecedented genomic diversity of RNA viruses in arthropods reveals the ancestry of negative-sense rna viruses. Elife 2015, 4, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Roundy, C.M.; Azar, S.R.; Rossi, S.J.; Weaver, S.C.; Vasilakis, N. Insect-specificviruses: A historical overview and recent developments. Adv. Virus Res. 2016, 98, 120–146. [Google Scholar] [CrossRef]
- Kenney, J.L.; Brault, A.C. The Role of Environmental, Virological and Vector Interactions in Dictating Biological Transmission of Arthropod-Borne Viruses by Mosquitoes. Adv. Virus Res. 2014, 89, 39–83. [Google Scholar] [CrossRef] [PubMed]
- Vasilakis, N.; Tesha, R.B. Insect-specific viruses and their potential impact on arbovirus transmission. Curr. Opin. Virol. 2015, 15, 69–74. [Google Scholar] [CrossRef]
- Atoni, E.; Wang, Y.; Karungu, S.; Waruhiu, C.; Zohaib, A.; Obanda, V.; Agwanda, B.; Mutua, M.; Xia, H.; Yuan, Z. Metagenomic virome analysis of Culex mosquitoes form Kenya and China. Viruses 2018, 10, 30. [Google Scholar] [CrossRef]
- Pinto, A.Z.L.; Santos, C.M.; Melo, F.L.; Ribeiro, A.L.M.; Morais, R.B.; Slhessarenko, R.D. Novel viruses in salivary glands of mosquitoes from sylvatic Cerrado, Midwestern Brazil. PLoS ONE 2017, 12, 2–16. [Google Scholar] [CrossRef]
- Moraes, O.S.; Cardoso, B.F.; Pacheco, T.A.; Pinto, A.Z.L.; Carvalho, M.S.; Hahn, R.C.; Burlamaqui, T.C.T.; Oliveira, R.S.; Vasconcelos, J.M.; Lemos, P.S.; et al. Natural infection by Culex flavivirus in Culex quinquefasciatus mosquitoes captured in Cuiabá, Mato Grosso mid-western, Brazil. Med. Vet. Entomol. 2019, 33, 1–10. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Pinto, A.Z.L.; Pinheiro, A.; Rodriques, J.S.V.; Melo, F.L.; Silva, L.A.; Ribeiro, B.M.; Slhessarenko, R.D. Viola phlebovirus is a novel Phlebotomus fever serogroup member identified in Lutzomyia (Lutzomyia) longipalpis from Brazilian Pantanal. Parasites Vectors 2018, 11, 2–10. [Google Scholar] [CrossRef]
- Forattini, O.P. Culicidologia Médica. Identificação, Biologia, Epidemiologia; Editora da Universidade de São Paulo: São Paulo, Brazil, 2002. [Google Scholar]
- Coleman, J.; Juhn, J.; James, A.A. Dissection of midgut and salivary glands from Ae. aegypti mosquitoes. J. Vis. 2007, 5, 228. [Google Scholar] [CrossRef]
- Kluge, M.; Campos, F.S.; Tavares, M.; Amorim, D.B.; Valdez, F.P.; Giongo, A.; Franco, A.C. Metagenomic survey of viral diversity obtained from faces of subantartic and south American fur seals. PLoS ONE 2016, 11, 1–24. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneius Basic: An integrates and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFT multiple sequence alignment software v7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2–Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, H. An approximately unbiased test of phylogenetic tree selection. Syst. Biol. 2002, 51, 492–543. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.J. Genomic Characterization, Detection and Molecular Evolution of Arthropod-Borne Viruses of the Family Bunyaviridae. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 2010. [Google Scholar]
- Valles, S.M.; Chen, Y.; Firth, A.E.; Guérin, D.M.A.; Hashimoto, Y.; Herrero, S.; Miranda, J.R.; Ryabov, E. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017, 98, 527–528. [Google Scholar] [CrossRef]
- Webster, C.L.; Longdon, B.; Lewis, S.H.; Obbard, D. Twent-five new viruses associated with Drosophilidae (Diptera). Evol. Bioinform. 2016, 12, 13–25. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, X.; Fan, H.; Zhao, Q.; Zuo, S.; Sun, Q.; Pei, G.; Cheng, S.; An, X.; Wang, Y.; et al. Complete genome sequence of Menghai flavivirus, a novel insectspecific flavivirus from China. Arch. Virol. 2017, 162, 1435–1439. [Google Scholar] [CrossRef]
- Kuno, G.; Chang, G.J.; Tsuchiya, K.R.; Karabastos, N.; Cropp, C.B. Phylogeny of the genus Flavivirus. J. Virol. 1998, 72, 73–83. [Google Scholar]
- Gravina, H.D.; Suzukawa, A.A.; Zanluca, C.; Segovia, F.M.C.; Tschá, M.K.; Silva, A.M.; Faoro, H.; Ribeiro, R.S.; Torres, L.P.M.; Rojas, A.; et al. Identification of insect-specific flaviviruses in areas of Brazil and Paraguay experiencing endemic arbovirus transmission and the description of a novel flavivirus infecting Sabethes belisariori. Virology 2019, 527, 98–106. [Google Scholar] [CrossRef]
- Xu, L.; Wu, J.; Jiang, T.; Qin, S.; Xia, L.; Xingyu, L.; He, B.; Tu, C. Molecular detection and sequence characterization of diverse rhabdoviruses in bats, China. Virus Res. 2018, 244, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Attoui, H.; Mertens, P.P.C.; Becnel, J.; Belaganahalli, S.; Bergoin, M.; Brussaard, C.P. Ninth Report of the International Committee on Taxonomy of Viruses/Family Reoviridae; Elsevier: Amsterdam, The Netherlands, 2011; pp. 541–603. [Google Scholar]
- Sadeghi, M.; Altan, E.; Deng, X.; Barker, C.M.; Fang, L.C.; Delwart, E. Virome of >12 thousand Culex mosquitoes from throughout California. Virology 2018, 523, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Garigliany, M.M.; Börstler, J.; Jost, H.; Badusche, M.; Desmecht, D.; Schmidt-Chanasit, J.; Cadar, D. Characterization of a novel circo-like virus in Aedes vexans mosquitoes from Germany: Evidence for a new genus within the family Circoviridae. J. Gen. Virol. 2015, 96, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Y.I.; Kazlauskas, D.; Iranzo, J.; LucíaSanz, A.; Kuhn, J.H.; Krupovic, M.; Dolja, V.V.; Koonin, E.V. Origins and evolution of the global RNA virome. MBio 2018, 9, 1–31. [Google Scholar] [CrossRef]
- Vasilakis, N.; Castro-Llanos, F.; Widen, S.G.; Aguilar, P.V.; Guzman, H.; Guevara, C.; Fernandez, R.; Auguste, A.J.; Wood, T.G.; Popov, V.; et al. Arboretum and Puerto Almendras viruses: Two novel rhabdoviruses isolated form mosquitoes in Peru. J. Gen. Virol. 2014, 95, 787–792. [Google Scholar] [CrossRef]
- Ajamma, Y.U.; Onchuru, T.O.; Ouso, D.O.; Omondi, D.; Masiga, D.K.; Villinger, J. Vertical transmission of naturally occurring Bunyamwera and insect-specific flavivirus infections in mosquitoes from islands and mainland shores of Lakes Victoria and Baringo in Kenya. PLoS Negl. Trop. Dis. 2018, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wilfert, L.; Long, G.; Legget, H.C.; Schimid-Hempel, P.; Butlin, R.; Martin, S.J.; Boots, M. Deformed wing virus is a recent global epidemic in honeybees driven by Varroa mites. Science 2016, 351, 7–594. [Google Scholar] [CrossRef]
- Charles, J.; Tangudu, C.S.; Hurt, S.L.; Tumescheit, C.; Firth, A.E.; Garcia-Rejon, J.E.; Blivich, B.J. Detection of novel and recognized RNA viruses in mosquitoes and characterization of their in vitro host ranges. J. Gen. Virol. 2018, 99, 1729–1738. [Google Scholar] [CrossRef]
- Haddow, A.D.; Guzman, H.; Popov, V.L.; Wood, T.G.; Widen, S.G.; Haddow, A.D.; Tesh, R.B.; Weaver, S.C. First isolation of Aedes flavivirus in the Western Hemisphere and evidence of vertical transmission in the mosquito Aedes (Stegomyia) albopictus (Diptera: Culicidae). Virology 2013, 440, 134–139. [Google Scholar] [CrossRef]
- Yuan, H.; Xu, P.; Yang, X.; Graham, R.I.; Wilson, K.; Wu, K. Characterization of a novel member of genus Iflavirus in Helicoverpa armigera. J. Invertebr. Pathol. 2017, 144, 65–73. [Google Scholar] [CrossRef]
- Kobayashi, D.; Isawa, H.; Fujita, R.; Murota, K.; Itokawa, K.; Higa, Y.; Katayama, Y.; Sasaki, T.; Mizutani, T.; Iwana, S.; et al. Isolation and characterization of a new iflavirus from Armigeres spp. Mosquitoes in the Philippines. J. Gen. Virol. 2017, 98, 527–528. [Google Scholar] [CrossRef] [PubMed]
- Dilcher, M.; Hassib, L.; Lechner, M.; Wieseke, N.; Middendorf, M.; Marz, M.; Koch, A.; Spiegel, M.; Dobler, G.; Hufert, F.T.; et al. Generic characterization of Tribec Kemerovo virus, two tick-transmitted human-pathogenic Orbiviruses. Virology 2012, 423, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Gao, X.; Liu, B.; Chen, H.; Xiao, J.; Wang, H. Epidemiology and spatial distribution of bluetongue virus in Xinjiang, China. PeerJ 2019, 7, e6514. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.J.; Blasdell, K.R.; Calisher, C.H.; Dietzgen, R.G.; Kondo, H.; Kurath, G.; Longdon, B.; Stone, D.M.; Tesh, R.B.; Tordo, N.; et al. ICTV virus taxonomy profile: Rhabdoviridae. J. Gen. Virol. 2018, 99, 447–448. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.; Moureau, G.; Kitchen, A.; Gould, E.A.; Lamballerie, X.; Holmes, E.C.; Harbach, R.E. Molecular evolution of the insect-specific flaviviruses. J. Gen. Virol. 2012, 93, 34–223. [Google Scholar] [CrossRef] [PubMed]
- Kenney, J.L.; Soldberg, O.D.; Langevin, S.A.; Brault, A.C. Characterization of a novel insect-specific flavivirus from Brazil: Potencial for inhibition of infection of arthropod cells with medically important flaviviruses. J. Gen. Virol. 2014, 95, 2796–2808. [Google Scholar] [CrossRef] [PubMed]
- Rosario, K.; Breitbart, M.; Harrach, B.; Segale, J.; Delwart, E.; Biagini, P.; Varsani, A. Revisiting the taxonomy oh the family Circoviridae: Establishment of the genus Cyclovirus and removal of the genus Gyrovirus. Arch. Virol. 2017, 162, 1447–1463. [Google Scholar] [CrossRef]

| Period | Plots | Pools | Species (n) | Viral Hit | Virus | Genome | Size (nt) | Identity (%) | E-value | Reads | Viral Isolation | Genbank |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rainy | A2; A3 | M05, M06 | Psorophora albigenu (100; 106) | Rhabdoviridae (Dielmovirus) | Lobeira virus | ssRNA- | 11,275 | 100 | 0 | 24,452,482 | Nt | MK780203 |
| Transitional | A1 | M14 | Psorophora albigenu (110) | Iflaviridae (Iflavirus) | Pirizal iflavirus | ssRNA+ | 9367 | 33 | 3e-175 | 10,209,066 | +p1, 300 pb | MN186291 |
| A1 | M22 | Aedes (Ae) aegypti, Ae. Fluviatillis, Ae. crinifer (5) | Flaviviridae (Flavivirus) | Chacororé flavivirus | ssRNA+ | 461 | 55 | 8e-37 | 13,768,232 | nt | MK780202 | |
| Dry | D1 | M25* | Aedes scapularis (7) | Phenuiviridae (Phlebovirus) | Furrundu phlebovirus | ssRNA- | L 7706 | 62 | 0 | 11,579,630 | **500pb | MN186294 |
| M 934 | 30 | 2e-88 | MN186295 | |||||||||
| S 1402 | 40 | 7e-58 | MN186296 | |||||||||
| A3 | M31 | Sabethes gymonothorax (3) | Flaviviridae (Flavivirus) | Sabethes flavivirus | ssRNA- | 1623 | 94 | 6e-95 | 17,431,974 | Nt | MK780204 | |
| Chuviridae (Mivirus) | Uru chuvirus | ssRNA- | 864 | 69 | 3e-150 | MK780200 | ||||||
| Reoviridae (Orbivirus) | Rasqueado orbivirus | dsRNA | 2854 | 38 | 0 | MK780201 | ||||||
| Phenuiviridae (Phlebovirus) | Pixé phlebovirus | ssRNA- | 444 | 39 | 5e-21 | MN186293 | ||||||
| Partitiviridae (Unclassified) | Araticum virus | dsRNA | 1299 | 99 | 0 | MK780207 | ||||||
| A2; A3 | M33 | Coquillettidia albicosta, Coq. shannoni (14) | Rhabdoviridae (Vesiculovirus) | Guampa vesiculovirus | ssRNA- | 2387 | 67 | 2e-75 | 12,498,218 | +p1, 300 pb | MN225577 | |
| A1; A2 | M35* | Aedes scapularis (11) | Phenuiviridae (Phlebovirus) | Furrundu phlebovirus | ssRNA- | L 7706 | 62 | 0 | 10,240,634 | **500pb | MN186294 | |
| M 934 | 30 | 2e-88 | MN186295 | |||||||||
| S 1402 | 40 | 7e-58 | MN186296 | |||||||||
| A1; D1 | M37 | Psorophora. albigenu (100) | Totiviridae (Artivirus) | Murici virus | dsRNA | 907 | 99 | 0 | 13,477,440 | Nt | MK780210 | |
| Rhabdoviridae (Dielmovirus) | Lobeira virus | ssRNA | 450 | 99 | 1e-96 | MK780209 | ||||||
| Partitiviridae (Unclassified) | Araticum virus | dsRNA | 1439 | 99 | 0 | MK780208 | ||||||
| D1 | M38 | Psorophora albigenu (117) | Circoviridae (Krikovirus) | Bororo circovirus | ssDNA | 768 | 78 | 6e-103 | 10,520,776 | **760 pb | MN186292 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maia, L.M.S.; Pinto, A.Z.d.L.; Carvalho, M.S.d.; Melo, F.L.d.; Ribeiro, B.M.; Slhessarenko, R.D. Novel Viruses in Mosquitoes from Brazilian Pantanal. Viruses 2019, 11, 957. https://doi.org/10.3390/v11100957
Maia LMS, Pinto AZdL, Carvalho MSd, Melo FLd, Ribeiro BM, Slhessarenko RD. Novel Viruses in Mosquitoes from Brazilian Pantanal. Viruses. 2019; 11(10):957. https://doi.org/10.3390/v11100957
Chicago/Turabian StyleMaia, Laura Marina Siqueira, Andressa Zelenski de Lara Pinto, Michellen Santos de Carvalho, Fernando Lucas de Melo, Bergmann Morais Ribeiro, and Renata Dezengrini Slhessarenko. 2019. "Novel Viruses in Mosquitoes from Brazilian Pantanal" Viruses 11, no. 10: 957. https://doi.org/10.3390/v11100957
APA StyleMaia, L. M. S., Pinto, A. Z. d. L., Carvalho, M. S. d., Melo, F. L. d., Ribeiro, B. M., & Slhessarenko, R. D. (2019). Novel Viruses in Mosquitoes from Brazilian Pantanal. Viruses, 11(10), 957. https://doi.org/10.3390/v11100957





