Cross-Protection of Inactivated Rabies Vaccines for Veterinary Use against Bat Lyssaviruses Occurring in Europe
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus
2.2. Vaccine
2.3. Animals
2.4. In Vivo Experiments
2.5. Virus Titrations and Preparation of Challenge Doses
2.6. Vaccine Protection Study
2.7. Virus Neutralizing Antibody (VNA) Induction in Vaccinated Unchallenged Mice
2.8. Fluorescent Antibody Test
2.9. Rabies Serological Assays
2.10. Statistical Analysis
3. Results
3.1. Development of Clinical Disease in Unvaccinated Mice
3.2. Vaccination-Challenge Study
3.2.1. Intracerebral Challenge
Protection
Rabies Antigen Detection in Mice Brains
3.2.2. Intramuscular Challenge
Protection
Rabies Antigen Detection in Mice Brains
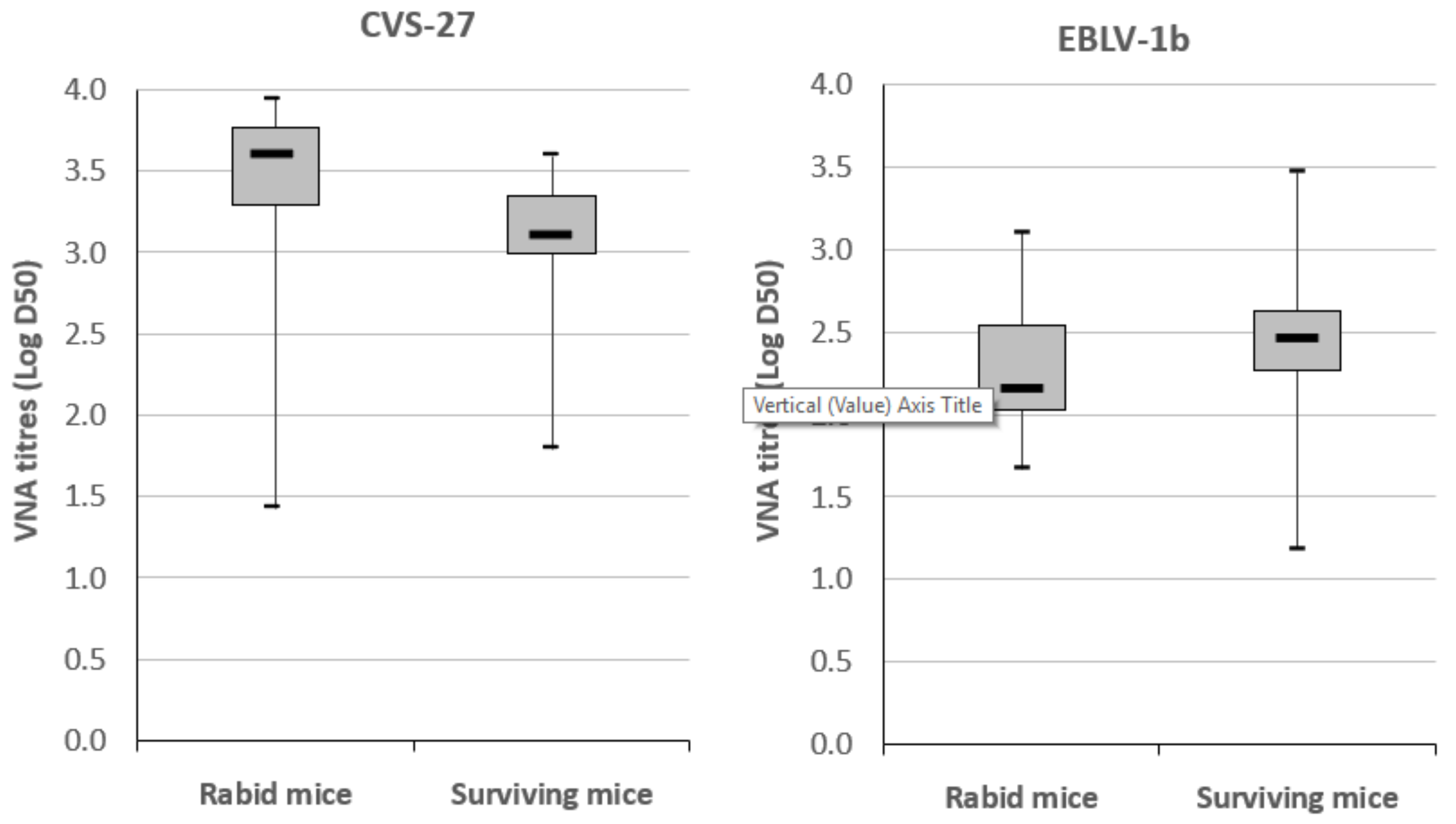
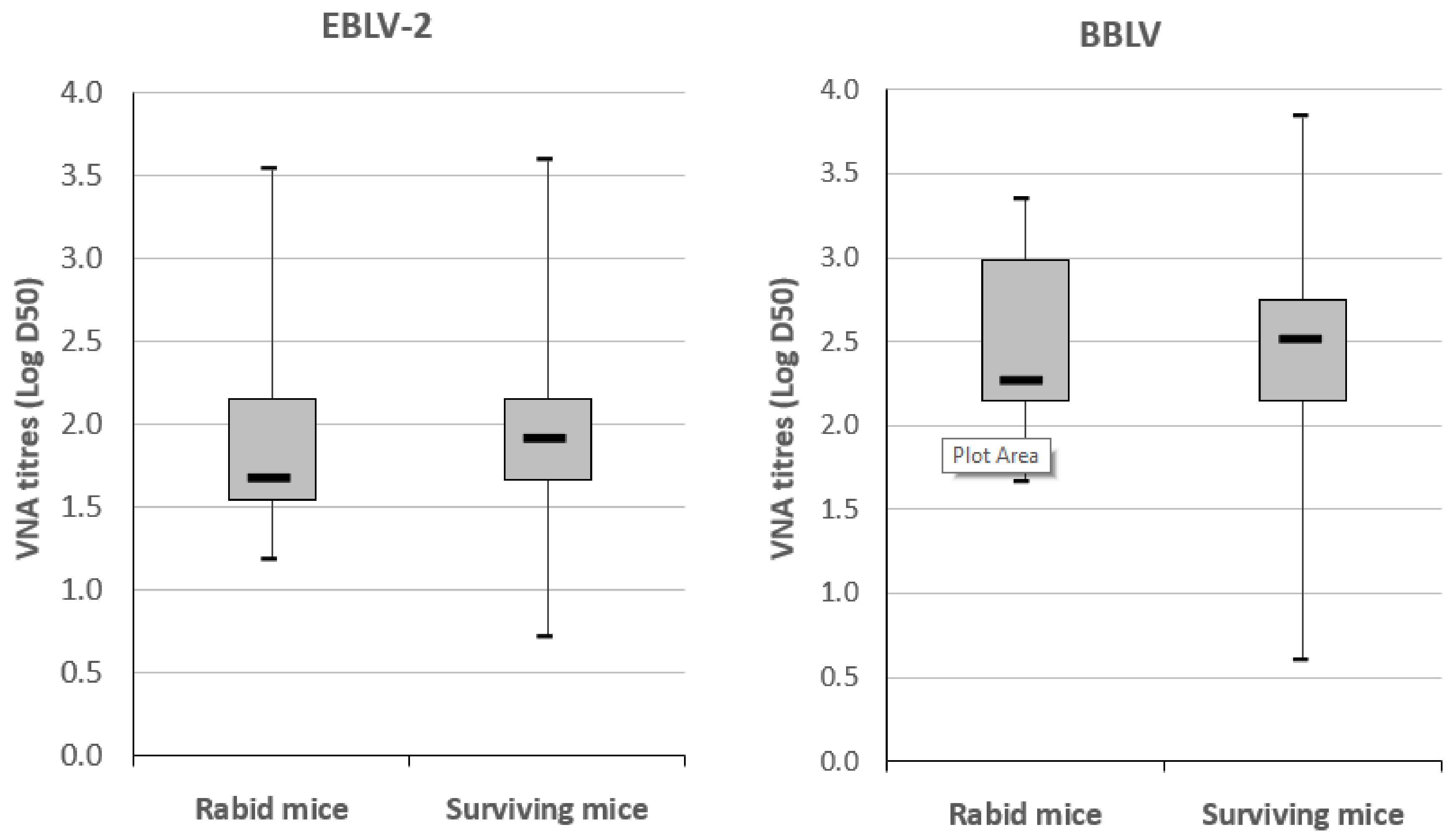
3.2.3. Virus Neutralizing Antibody Response in Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mueller, T.; Freuling, C.M.; Wysocki, P.; Roumiantzeff, M.; Freney, J.; Mettenleiter, T.C.; Vos, A. Terrestrial rabies control in the European Union: Historical achievements and challenges ahead. Vet. J. 2015, 203, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Cliquet, F.; Picard-Meyer, E.; Robardet, E. Rabies in Europe: What are the risks? Expert Rev. Anti-Infect. Ther. 2014, 12, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Shipley, R.; Wright, E.; Selden, D.; Wu, G.; Aegerter, J.; Fooks, A.R.; Banyard, A.C. Bats and Viruses: Emergence of Novel Lyssaviruses and Association of Bats with Viral Zoonoses in the EU. Trop. Med. Infect. Dis. 2019, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Banyard, A.C.; Evans, J.S.; Luo, T.R.; Fooks, A.R. Lyssaviruses and Bats: Emergence and Zoonotic Threat. Viruses 2014, 6, 2974–2990. [Google Scholar] [CrossRef] [PubMed]
- McElhinney, L.M.; Marston, D.A.; Leech, S.; Freuling, C.M.; van der Poel, W.H.M.; Echevarria, J.; Vázquez-Moron, S.; Horton, D.L.; Müller, T.; Fooks, A.R. Molecular Epidemiology of Bat Lyssaviruses in Europe: Molecular Epidemiology of EBLV. Zoonoses Public Health 2013, 60, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Picard-Meyer, E.; Robardet, E.; Arthur, L.; Larcher, G.; Harbusch, C.; Servat, A.; Cliquet, F. Bat Rabies in France: A 24-Year Retrospective Epidemiological Study. PLoS ONE 2014, 9, e98622. [Google Scholar] [CrossRef]
- Vázquez-Morón, S.; Juste, J.; Ibáñez, C.; Ruiz-Villamor, E.; Avellon, A.; Vera, M.; Echevarría, J.E. Endemic Circulation of European Bat Lyssavirus Type 1 in Serotine Bats, Spain. Emerg. Infect. Dis. 2008, 14, 1263–1266. [Google Scholar] [CrossRef]
- Moldal, T.; Vikøren, T.; Cliquet, F.; Marston, D.A.; van der Kooij, J.; Madslien, K.; Ørpetveit, I. First detection of European bat lyssavirus type 2 (EBLV-2) in Norway. BMC Vet. Res. 2017, 13, 216. [Google Scholar] [CrossRef]
- Freuling, C. Novel Lyssavirus in Natterer’s Bat, Germany. Emerg. Infect. Dis. 2011, 17, 1519. [Google Scholar] [CrossRef]
- Picard-Meyer, E.; Servat, A.; Robardet, E.; Moinet, M.; Borel, C.; Cliquet, F. Isolation of Bokeloh bat lyssavirus in Myotis nattereri in France. Arch. Virol. 2013, 158, 2333–2340. [Google Scholar] [CrossRef]
- Smreczak, M.; Orłowska, A.; Marzec, A.; Trębas, P.; Müller, T.; Freuling, C.M.; Żmudziński, J.F. Bokeloh bat lyssavirus isolation in a Natterer’s bat, Poland. Zoonoses Public Health 2018, 65, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, N.A.; Morón, S.V.; Berciano, J.M.; Nicolás, O.; López, C.A.; Juste, J.; Nevado, C.R.; Setién, Á.A.; Echevarría, J.E. Novel Lyssavirus in Bat, Spain. Emerg. Infect. Dis. 2013, 19, 793–795. [Google Scholar] [CrossRef]
- Picard-Meyer, E.; Beven, V.; Hirchaud, E.; Guillaume, C.; Larcher, G.; Robardet, E.; Servat, A.; Blanchard, Y.; Cliquet, F. Lleida Bat Lyssavirus isolation in Miniopterus schreibersii in France. Zoonoses Public Health 2018, 66, 254–258. [Google Scholar] [CrossRef]
- Nokireki, T.; Tammiranta, N.; Kokkonen, U.-M.; Kantala, T.; Gadd, T. Tentative novel lyssavirus in a bat in Finland. Transbound. Emerg. Dis. 2018, 65, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Dacheux, L.; Larrous, F.; Mailles, A.; Boisseleau, D.; Delmas, O.; Biron, C.; Bouchier, C.; Capek, I.; Muller, M.; Ilari, F.; et al. European Bat Lyssavirus Transmission among Cats, Europe. Emerg. Infect. Dis. 2009, 15, 280–284. [Google Scholar] [CrossRef]
- Müller, T.; Cox, J.; Peter, W.; Schäfer, R.; Johnson, N.; McElhinney, L.M.; Geue, J.L.; Tjørnehøj, K.; Fooks, A.R. Spill-over of European Bat Lyssavirus Type 1 into a Stone Marten (Martes foina) in Germany. J. Vet. Med. Ser. B 2004, 51, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Ronsholt, L. A new case of European Bat Lyssavirus (EBL) infection in Danish sheep. Rabies Bull. Eur. 2002, 26, 15. [Google Scholar]
- Fooks, A.R.; Brookes, S.M.; Johnson, N.; McElhinney, L.M.; Hutson, A.M.; Brookes, S.; McElhinney, L. European bat lyssaviruses: An emerging zoonosis. Epidemiol. Infect. 2003, 131, 1029–1039. [Google Scholar] [CrossRef]
- Nolden, T.; Banyard, A.C.; Finke, S.; Fooks, A.R.; Hanke, D.; Höper, D.; Höper, D.; Horton, D.L.; Mettenleiter, T.C.; Müller, T.; et al. Comparative studies on the genetic, antigenic and pathogenic characteristics of Bokeloh bat lyssavirus. J. Gen. Virol. 2014, 95, 1647–1653. [Google Scholar] [CrossRef]
- Brookes, S.M.; Parsons, G.; Johnson, N.; McElhinney, L.M.; Fooks, A.R. Rabies human diploid cell vaccine elicits cross-neutralising and cross-protecting immune responses against European and Australian bat lyssaviruses. Vaccine 2005, 23, 4101–4109. [Google Scholar] [CrossRef]
- Nokireki, T.; Jakava-Viljanen, M.; Virtala, A.-M.; Sihvonen, L. Efficacy of rabies vaccines in dogs and cats and protection in a mouse model against European bat lyssavirus type 2. Acta Vet. Scand. 2017, 59, 64. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, C.A.; Kuzmin, I.V.; Blanton, J.D.; Weldon, W.C.; Manangan, J.S.; Rupprecht, C.E. Efficacy of rabies biologics against new lyssaviruses from Eurasia. Virus Res. 2005, 111, 44–54. [Google Scholar] [CrossRef] [PubMed]
- European Bat Lyssavirus 2 Isolate RV1332 Glycoprotein (G.) mRNA, Partial Cds. 25 July 2016. Available online: http://www.ncbi.nlm.nih.gov/nuccore/GU936871.1 (accessed on 22 August 2019).
- Bokeloh Bat Lyssavirus Isolate 129700, Complete Genome. 8 December 2015. Available online: http://www.ncbi.nlm.nih.gov/nuccore/KC169985.1 (accessed on 22 August 2019).
- Wilbur, L.; Aubert, M.F. Laboratory techniques in rabies: The NIH test for potency. In Laboratory Techniques in Rabies, 4th ed.; World Health Organization: Geneva, Switzerland, 1996; pp. 360–368. [Google Scholar]
- Council of Europe. Rabies Vaccine (Inactivated) for Veterinary Use, Monograph 0451. In European Pharmacopoeia, 8th ed.; Council of Europe: Strasbourg, France, 2013. [Google Scholar]
- Daas, A.; Bruckner, L.; Milne, C. EDQM biological reference preparation for rabies vaccine (inactivated) for veterinary use. Pharmeuropa Bio. Sci. Notes 2015, 2015, 57–72. [Google Scholar]
- European Commission. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes; European Commission: Brussels, Belgium, 2010; pp. 33–79. [Google Scholar]
- Officiel de la République Française. Décret n 2013-118 Du 1er Février 2013 Relatif à La Protection des Animaux Utilisés à Des Fins Scientifiques; Journal Officiel de la République Française: Paris, France, 2013; p. 2199. [Google Scholar]
- Bruckner, L.; Cussler, K.; Halder, M.; Barrat, J.; Castle, P.; Duchow, K.; Gatewood, D.M.; Gibert, R.; Groen, J.; Knapp, B.; et al. Three Rs approaches in the quality control of inactivated rabies vaccines. The report and recommendations of ECVAM workshop 48. Altern. Lab. Anim. ATLA 2003, 31, 429–454. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, C.E.; Fooks, A.R.; Abela-Ridder, B. The Direct Fluorescent Antibody Test. In Laboratory Techniques in Rabies; World Health Organization: Geneva, Switzerland, 2019; pp. 108–123. [Google Scholar]
- Wasniewski, M.; Barrat, J.; Combes, B.; Guiot, A.L.; Cliquet, F. Use of filter paper blood samples for rabies antibody detection in foxes and raccoon dogs. J. Virol. Methods 2014, 204, 11–16. [Google Scholar] [CrossRef]
- Servat, A.; Picard-Meyer, E.; Robardet, E.; Muizniece, Z.; Must, K.; Cliquet, F. Evaluation of a rapid immunochromatographic diagnostic test for the detection of rabies from brain material of European mammals. Biologicals 2012, 40, 61–66. [Google Scholar] [CrossRef]
- Cliquet, F.; Aubert, M.; Sagné, L. Development of a fluorescent antibody virus neutralisation test (FAVN test) for the quantitation of rabies-neutralising antibody. J. Immunol. Methods 1998, 212, 79–87. [Google Scholar] [CrossRef]
- Wasniewski, M.; Fanka, R.; Muller, T.; Sabeta, C.; Cliquet, F. Production and calibration of the second batch of OIE anti-rabies positive reference serum: -EN- -FR- Production et étalonnage du second lot de sérum de référence positif de l’OIE pour la rage -ES- Producción y calibración del segundo lote de suero positivo antirrábico de referencia de la OIE. Rev. Sci. Tech. OIE 2017, 36, 779–788. [Google Scholar]
- Picard-Meyer, E.; De Garam, C.P.; Schereffer, J.L.; Marchal, C.; Robardet, E.; Cliquet, F. Cross-Platform Evaluation of Commercial Real-Time SYBR Green RT-PCR Kits for Sensitive and Rapid Detection of European Bat Lyssavirus Type 1. BioMed Res. Int. 2015, 2015, 839518. [Google Scholar] [CrossRef]
- World Health Organization. WHO Expert Consultation on Rabies: Third Report; WHO technical report series; World Health Organization: Geneva, Switzerland, 2018; 183p. [Google Scholar]
- World Health Organization. WHO Expert Consultation on Rabies: First Report; WHO technical report series; World Health Organization: Geneva, Switzerland, 2005; 88p. [Google Scholar]
- Lafon, M.; Bourhy, H.; Sureau, P. Immunity against the European bat rabies (Duvenhage) virus induced by rabies vaccines: An experimental study in mice. Vaccine 1988, 6, 362–368. [Google Scholar] [CrossRef]
- Servat, A.; Kempff, S.; Brogat, V.; Litaize, E.; Schereffer, J.-L.; Cliquet, F. A step forward in the quality control testing of inactivated rabies vaccines—Extensive evaluation of European vaccines by using alternative methods to the in vivo potency tests. Altern. Lab. Anim. ATLA. 2015, 43, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.P.; Kipper, M.J.; Mallapragada, S.K.; Wannemuehler, M.J.; Narasimhan, B.; Wilson-Welder, J.H.; Wilson-Welder, J.H. Vaccine adjuvants: Current challenges and future approaches. J. Pharm. Sci. 2009, 98, 1278–1316. [Google Scholar]
- Taylor, E.; Banyard, A.C.; Bourhy, H.; Cliquet, F.; Ertl, H.; Fehlner-Gardiner, C.; Horton, D.L.; Mani, R.S.; Müller, T.; Rupprecht, C.E.; et al. Avoiding preventable deaths: The scourge of counterfeit rabies vaccines. Vaccine 2019, 37, 2285–2287. [Google Scholar] [CrossRef] [PubMed]

| Survivorship after Challenge | |||||
|---|---|---|---|---|---|
| Virus | IC Route n (%) | IM Route n (%) | |||
| 50MLD50 | 2MLD50 | 50MLD50 | 2MLD50 | ||
| CVS | Control group | 0/5 (0) | 1/5 (20) | 0/5 (0) | 4/5 (80) |
| Vaccine 5 UI | 5/7 * (71) | 5/5 * (100) | 4/7 * (57) | 8/8 (100) | |
| p-value | 0.028 | 0.048 | 0.081 | 0.385 | |
| Vaccine 1 UI | 6/7 * (86) | 8/8 (100) | 3/8 (38) | 8/8 (100) | |
| p-value | 0.015 | 0.007 | 0.231 | 0.385 | |
| EBLV-1b | Control group | 0/5 (0) | 0/5 (0) | 0/5 (0) | 2/5 (40) |
| Vaccine 5 UI | 0/7 * (0) | 0/7 * (0) | 3/7 * (43) | 7/7 * (100) | |
| p-value | 1 | 1 | 0.205 | 0.045 | |
| Vaccine 1 UI | 0/7 * (0) | 1/8 (13) | 3/8 (38) | 6/8 (75) | |
| p-value | 1 | 1 | 0.231 | 0.293 | |
| EBLV-2 | Control group | 0/5 (0) | 0/5 (0) | 2/5 (40) | nc |
| Vaccine 5 UI | 1/8 (13) | 3/8 (38) | 8/8 (100) | nc | |
| p-value | 1 | 0.231 | 0.035 | / | |
| Vaccine 1 UI | 0/8 (0) | 2/8 (25) | 7/8 (88) | nc | |
| p-value | 1 | 0.487 | 0.216 | / | |
| BBLV | Control group | 0/5 (0) | 2/5 (40) | nc | nc |
| Vaccine 5 UI | 4/8 (50) | 7/7 * (100) | nc | nc | |
| p-value | 0.105 | 0.046 | / | / | |
| Vaccine 1 UI | 2/7 * (29) | 8/8 (100) | nc | nc | |
| p-value | 0.47 | 0.036 | / | / | |
| Mean VNA (IU/mL) | |||
|---|---|---|---|
| Challenge Virus | Immunization 5 IU/Dose | Immunization 1 IU/Dose | p-Value |
| CVS-27 | 75.4 (13.8–287) | 43.0 (2–165) | 0.007 |
| EBLV-1b | 48.2 (6.7–244) | 21.8 (1.3–35.4) | 0.232 |
| EBLV-2 | 9.2 (0.5–33) | 7.2 (0.1–29.4) | 0.133 |
| BBLV | 21.4 (1.3–81.1) | 11.2 (0.1–35.4) | 0.115 |
| Mean VNA (IU/mL) | |||
|---|---|---|---|
| Challenge Virus | Immunization 5 IU/Dose | Immunization 1 IU/Dose | p-Value |
| CVS-27 | 63.2 (0.9–125.6) | 167.2 (41.6–287) | nc |
| EBLV-1b | 15.9 (3.8–45.6) | 16 (6.6–34.6) | 0.422 |
| EBLV-2 | 18.9 (2.7–97.5) | 4.4 (1.2–10.9) | 0.056 |
| BBLV | 41.2 (1.7–81.1) | 11.5 (5.1–35.4) | 0.151 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Servat, A.; Wasniewski, M.; Cliquet, F. Cross-Protection of Inactivated Rabies Vaccines for Veterinary Use against Bat Lyssaviruses Occurring in Europe. Viruses 2019, 11, 936. https://doi.org/10.3390/v11100936
Servat A, Wasniewski M, Cliquet F. Cross-Protection of Inactivated Rabies Vaccines for Veterinary Use against Bat Lyssaviruses Occurring in Europe. Viruses. 2019; 11(10):936. https://doi.org/10.3390/v11100936
Chicago/Turabian StyleServat, Alexandre, Marine Wasniewski, and Florence Cliquet. 2019. "Cross-Protection of Inactivated Rabies Vaccines for Veterinary Use against Bat Lyssaviruses Occurring in Europe" Viruses 11, no. 10: 936. https://doi.org/10.3390/v11100936
APA StyleServat, A., Wasniewski, M., & Cliquet, F. (2019). Cross-Protection of Inactivated Rabies Vaccines for Veterinary Use against Bat Lyssaviruses Occurring in Europe. Viruses, 11(10), 936. https://doi.org/10.3390/v11100936




