Methyl-Beta-Cyclodextrin-Induced Macropinocytosis Results in Increased Infection of Sf21 Cells by Bombyx Mori Nucleopolyhedrovirus
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Bacmids, and Viruses
2.2. MβCD Treatments and BmNPV Infections
2.3. Comparison of Viral One-Step Growth Curves
2.4. Electron Microscopy Analysis
2.5. Drug Treatments of Sf21 Cells
2.6. Incubation Time Points and Infection Assay
2.7. Analysis of the Endocytic Pathway by Which BmNPV Enters Sf21 Cells
2.8. Flow cytometry and Western Blotting
2.9. Q-PCR
2.10. Statistical Analysis
3. Results
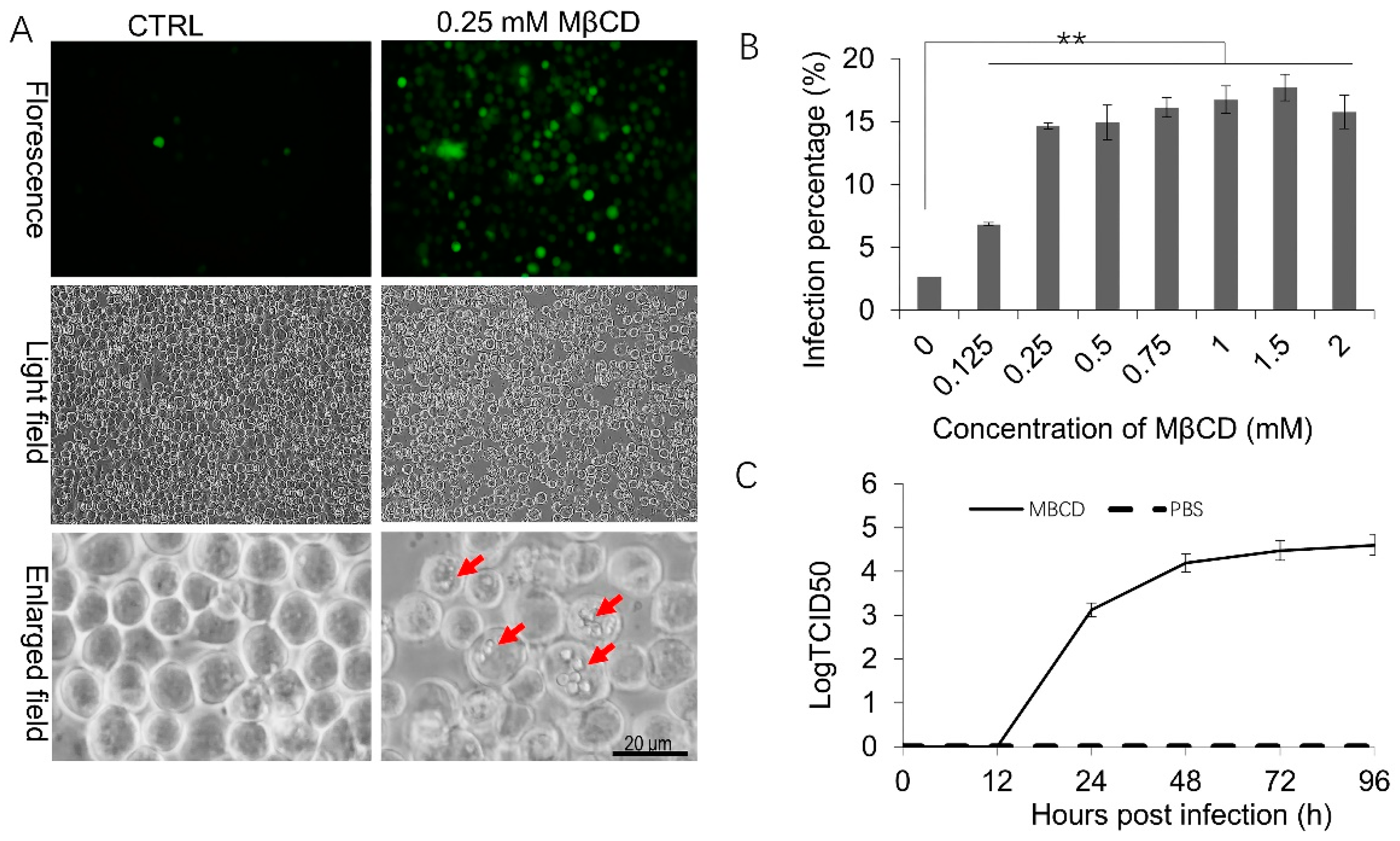
3.1. Incubation with a Low Concentration of MβCD Facilitates BmNPV Infection in Sf21 Cells
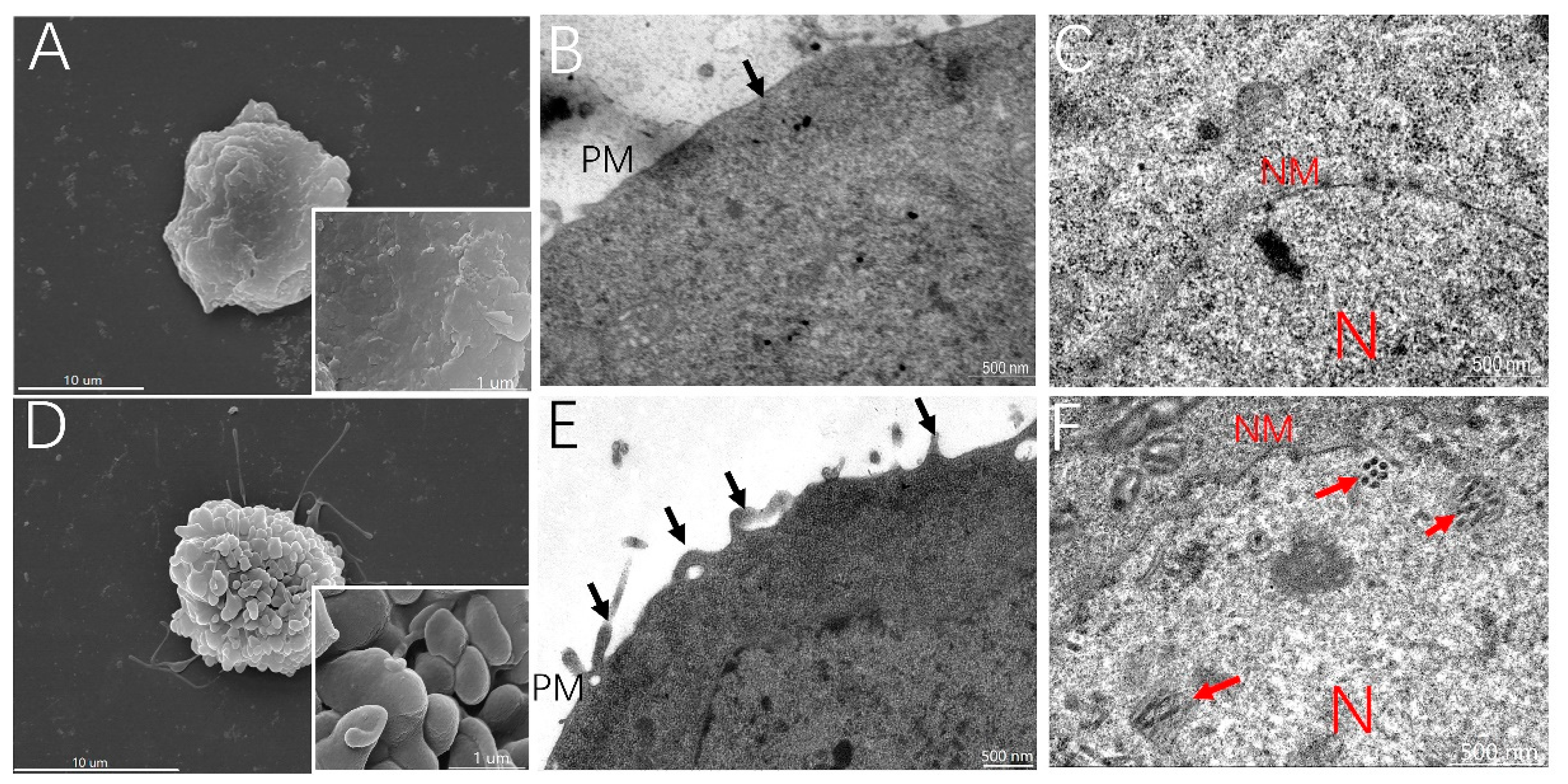
3.2. MβCD Incubation Activates Membrane Ruffling, Which Results in BmNPV Infection in Sf21 Cells
3.3. Inhibition of Membrane Ruffling Greatly Reduces the MβCD-Induced Increase in BmNPV Infection of Sf21 Cells
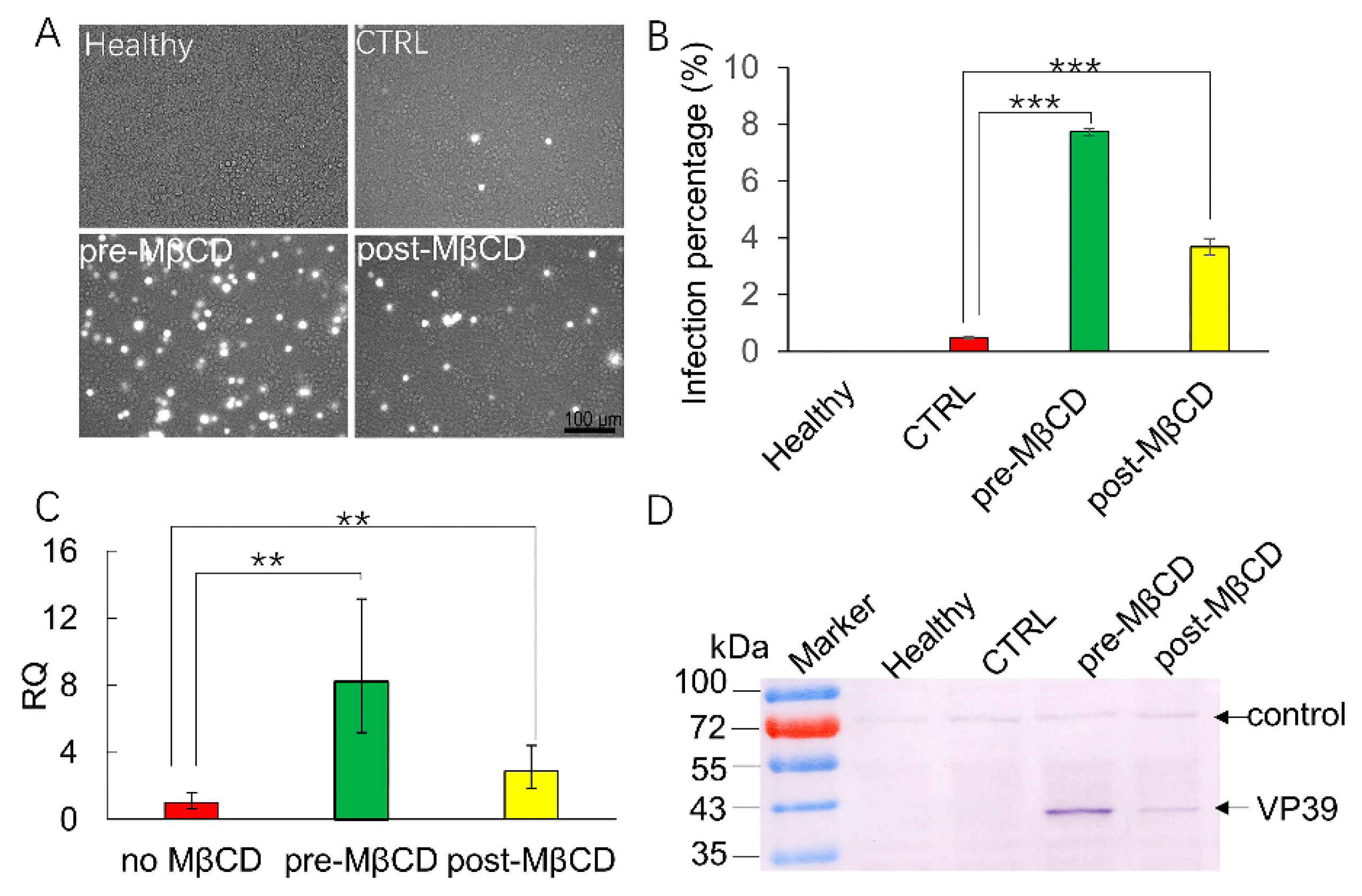
3.4. MβCD Incubation Post Viral Entry Increases BmNPV Infection of Sf21 Cells
3.5. BmNPV Entry into Sf21 Cells Via CME
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rohrmann, G.F. Baculovirus Molecular Biology [Internet], 4th ed.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2019. [Google Scholar]
- Jehle, J.A.; Blissard, G.W.; Bonning, B.C.; Cory, J.S.; Herniou, E.A.; Rohrmann, G.F.; Theilmann, D.A.; Thiem, S.M.; Vlak, J.M. On the classification and nomenclature of baculoviruses: A proposal for revision. Arch. Virol. 2006, 151, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Gomi, S.; Majima, K.; Maeda, S. Sequence analysis of the genome of Bombyx mori nucleopolyhedrovirus. J. Gen. Virol. 1999, 80, 1323–1337. [Google Scholar] [CrossRef] [PubMed]
- Katou, Y.; Ikeda, M.; Kobayashi, M. Abortive replication of Bombyx mori nucleopolyhedrovirus in Sf9 and High Five cells: Defective nuclear transport of the virions. Virology 2006, 347, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, M.; Takaya, K.; Katsuma, S.; Ote, M.; Tanaka, S.; Kamita, S.G.; Kang, W.; Shimada, T.; Kobayashi, M. Expression profiling of baculovirus genes in permissive and nonpermissive cell lines. Biochem. Biophys. Res. Commun. 2004, 323, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Kamita, S.G.; Kondo, A. Host range expansion of Autographa californica nuclear polyhedrosis virus (NPV) following recombination of a 0.6-kilobase-pair DNA fragment originating from Bombyx mori NPV. J. Virol. 1993, 67, 6234–6238. [Google Scholar]
- Argaud, O.; Croizier, L.; Lopez-Ferber, M.; Croizier, G. Two key mutations in the host-range specificity domain of the p143 gene of Autographa californica nucleopolyhedrovirus are required to kill Bombyx mori larvae. J. Gen. Virol. 1998, 79, 931–935. [Google Scholar] [CrossRef]
- Croizier, G.; Croizier, L.; Argaud, O.; Poudevigne, D. Extension of Autographa californica nuclear polyhedrosis virus host range by interspecific replacement of a short DNA sequence in the p143 helicase gene. Proc. Natl. Acad. Sci. USA 1994, 91, 48–52. [Google Scholar] [CrossRef]
- Kamita, S.G.; Maeda, S. Sequencing of the putative DNA helicase-encoding gene of the Bombyx mori nuclear polyhedrosis virus and fine-mapping of a region involved in host range expansion. Gene 1997, 190, 173–179. [Google Scholar] [CrossRef]
- Xu, Y.P.; Gu, L.Z.; Lou, Y.H.; Cheng, R.L.; Xu, H.J.; Wang, W.B.; Zhang, C.X. A baculovirus isolated from wild silkworm encompasses the host ranges of Bombyx mori nucleopolyhedrosis virus and Autographa californica multiple nucleopolyhedrovirus in cultured cells. J. Gen. Virol. 2012, 93, 2480–2489. [Google Scholar] [CrossRef]
- Cossart, P.; Helenius, A. Endocytosis of viruses and bacteria. Cold Spring Harb. Perspect. Biol. 2014, 6, a016972. [Google Scholar] [CrossRef]
- Dong, S.; Wang, M.; Qiu, Z.; Deng, F.; Vlak, J.M.; Hu, Z.; Wang, H. Autographa californica multicapsid nucleopolyhedrovirus efficiently infects Sf9 cells and transduces mammalian cells via direct fusion with the plasma membrane at low pH. J. Virol. 2010, 84, 5351–5359. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, C.; Kaname, Y.; Taguwa, S.; Abe, T.; Fukuhara, T.; Tani, H.; Moriishi, K.; Matsuura, Y. Baculovirus GP64-mediated entry into mammalian cells. J. Virol. 2012, 86, 2610–2620. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hao, B.; Cheng, C.; Liang, F.; Shen, X.; Cheng, X. Entry of Bombyx mori nucleopolyhedrovirus into BmN cells by cholesterol-dependent macropinocytic endocytosis. Biochem. Biophys. Res. Commun. 2014, 453, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, C.; Tang, X.; Liu, L.; Nan, W.; Shen, X.; Hao, B. Transport Via Macropinocytic Vesicles is Crucial for Productive Infection with Bombyx Mori Nucleopolyhedrovirus. Viruses 2019, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.; Helenius, A. Virus entry by macropinocytosis. Nat. Cell Boil. 2009, 11, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Grimmer, S.; van Deurs, B.; Sandvig, K. Membrane ruffling and macropinocytosis in A431 cells require cholesterol. J. Cell Sci. 2002, 115, 2953–2962. [Google Scholar] [PubMed]
- Huang, J.; Liu, N.; Shen, X.; Hao, B. Preincubation with a low concentration of methyl-beta-cyclodextrin enhances baculovirus expression system productivity. Biotechnol. Lett. 2019, 41, 921–928. [Google Scholar] [CrossRef]
- Huang, J.; Hao, B.; Sun, X.; Deng, F.; Wang, H.; Hu, Z. Construction of the Bac-to-Bac System of Bombyx mori Nucleopolyhedrovirus. Virol. Sin. 2007, 22, 218–225. [Google Scholar]
- Huang, J.; Li, J.; Cheng, C.; Tang, X.; Shen, X.; Hao, B. An amino acid duplication/insertion in the Bm126 gene of Bombyx mori nucleopolyhedrovirus alters viral gene expression as shown by differential gene expression analysis. Arch. Virol. 2019, 164, 831–838. [Google Scholar] [CrossRef]
- Ridley, A.J.; Paterson, H.F.; Johnston, C.L.; Diekmann, D.; Hall, A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 1992, 70, 401–410. [Google Scholar] [CrossRef]
- Liberali, P.; Ramo, P.; Pelkmans, L. Protein kinases: Starting a molecular systems view of endocytosis. Annu. Rev. Cell Dev. Biol. 2008, 24, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Nishida, E.; Koyasu, S.; Yahara, I.; Sakai, H. Protein kinase C-dependent and -independent pathways in the growth factor-induced cytoskeletal reorganization. J. Biol. Chem. 1989, 264, 15565–15568. [Google Scholar] [PubMed]
- Mercer, J.; Schelhaas, M.; Helenius, A. Virus entry by endocytosis. Annu. Rev. Biochem. 2010, 79, 803–833. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Rothberg, K.G.; Anderson, R.G. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 1993, 123, 1107–1117. [Google Scholar] [CrossRef]
- Sanchez, E.G.; Quintas, A.; Perez-Nunez, D.; Nogal, M.; Barroso, S.; Carrascosa, A.L.; Revilla, Y. African swine fever virus uses macropinocytosis to enter host cells. PLoS Pathog. 2012, 8, 1002754. [Google Scholar] [CrossRef]
- Meier, O.; Boucke, K.; Hammer, S.V.; Keller, S.; Stidwill, R.P.; Hemmi, S.; Greber, U.F. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J. Cell Biol. 2002, 158, 1119–1131. [Google Scholar] [CrossRef]
- Vaughn, J.L.; Goodwin, R.H.; Tompkins, G.J.; McCawley, P. The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera; Noctuidae). In Vitro 1977, 13, 213–217. [Google Scholar] [CrossRef]
- Au, S.; Wu, W.; Zhou, L.; Theilmann, D.A.; Pante, N. A new mechanism for nuclear import by actin-based propulsion used by a baculovirus nucleocapsid. J. Cell Sci. 2016, 129, 2905–2911. [Google Scholar] [CrossRef]
- Ohkawa, T.; Volkman, L.E.; Welch, M.D. Actin-based motility drives baculovirus transit to the nucleus and cell surface. J. Cell Biol. 2010, 190, 187–195. [Google Scholar] [CrossRef]
- Mercer, J.; Helenius, A. Gulping rather than sipping: Macropinocytosis as a way of virus entry. Curr. Opin. Microbiol. 2012, 15, 490–499. [Google Scholar] [CrossRef]
- Marechal, V.; Prevost, M.C.; Petit, C.; Perret, E.; Heard, J.M.; Schwartz, O. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J. Virol. 2001, 75, 11166–11177. [Google Scholar] [CrossRef] [PubMed]
- Lozach, P.Y.; Huotari, J.; Helenius, A. Late-penetrating viruses. Curr. Opin. Virol. 2011, 1, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Miller, L.K. Differential requirements for baculovirus late expression factor genes in two cell lines. J. Virol. 1995, 69, 6265–6272. [Google Scholar] [PubMed]
- Lu, A.; Miller, L.K. Species-specific effects of the hcf-1 gene on baculovirus virulence. J. Virol. 1996, 70, 5123–5130. [Google Scholar]
- Hefferon, K.L. Characterization of HCF-1, a determinant of Autographa californica multiple nucleopolyhedrovirus host specificity. Insect Mol. Biol. 2003, 12, 651–658. [Google Scholar] [CrossRef]
- Tachibana, A.; Hamajima, R.; Tomizaki, M.; Kondo, T.; Nanba, Y.; Kobayashi, M.; Yamada, H.; Ikeda, M. HCF-1 encoded by baculovirus AcMNPV is required for productive nucleopolyhedrovirus infection of non-permissive Tn368 cells. Sci. Rep. 2017, 7, 3807. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Li, C.; Fan, F.; Liu, N.; Boadi, F.; Shen, X.; Hao, B. Methyl-Beta-Cyclodextrin-Induced Macropinocytosis Results in Increased Infection of Sf21 Cells by Bombyx Mori Nucleopolyhedrovirus. Viruses 2019, 11, 937. https://doi.org/10.3390/v11100937
Huang J, Li C, Fan F, Liu N, Boadi F, Shen X, Hao B. Methyl-Beta-Cyclodextrin-Induced Macropinocytosis Results in Increased Infection of Sf21 Cells by Bombyx Mori Nucleopolyhedrovirus. Viruses. 2019; 11(10):937. https://doi.org/10.3390/v11100937
Chicago/Turabian StyleHuang, Jinshan, Chenya Li, Fengxiu Fan, Na Liu, Frank Boadi, Xingjia Shen, and Bifang Hao. 2019. "Methyl-Beta-Cyclodextrin-Induced Macropinocytosis Results in Increased Infection of Sf21 Cells by Bombyx Mori Nucleopolyhedrovirus" Viruses 11, no. 10: 937. https://doi.org/10.3390/v11100937
APA StyleHuang, J., Li, C., Fan, F., Liu, N., Boadi, F., Shen, X., & Hao, B. (2019). Methyl-Beta-Cyclodextrin-Induced Macropinocytosis Results in Increased Infection of Sf21 Cells by Bombyx Mori Nucleopolyhedrovirus. Viruses, 11(10), 937. https://doi.org/10.3390/v11100937




