Current Rabies Vaccines Do Not Confer Protective Immunity against Divergent Lyssaviruses Circulating in Europe
Abstract
Funding
Conflicts of Interest
References
- Fooks, A.R.; Cliquet, F.; Finke, S.; Freuling, C.; Hemachudha, T.; Mani, R.S.; Müller, T.; Nadin-Davis, S.; Picard-Meyer, E.; Wilde, H.; et al. Rabies. Nat. Rev. Dis. Primers 2017, 3. [Google Scholar] [CrossRef]
- Cleaveland, S.; Hampson, K. Rabies elimination research: juxtaposing optimism, pragmatism and realism. Proc. Biol. Sci. 2017, 284, 20171880. [Google Scholar] [CrossRef] [PubMed]
- Banyard, A.C.; Fooks, A.R. The impact of novel lyssavirus discovery. Microbiol. Aust. 2017, 38, 18–21. [Google Scholar] [CrossRef]
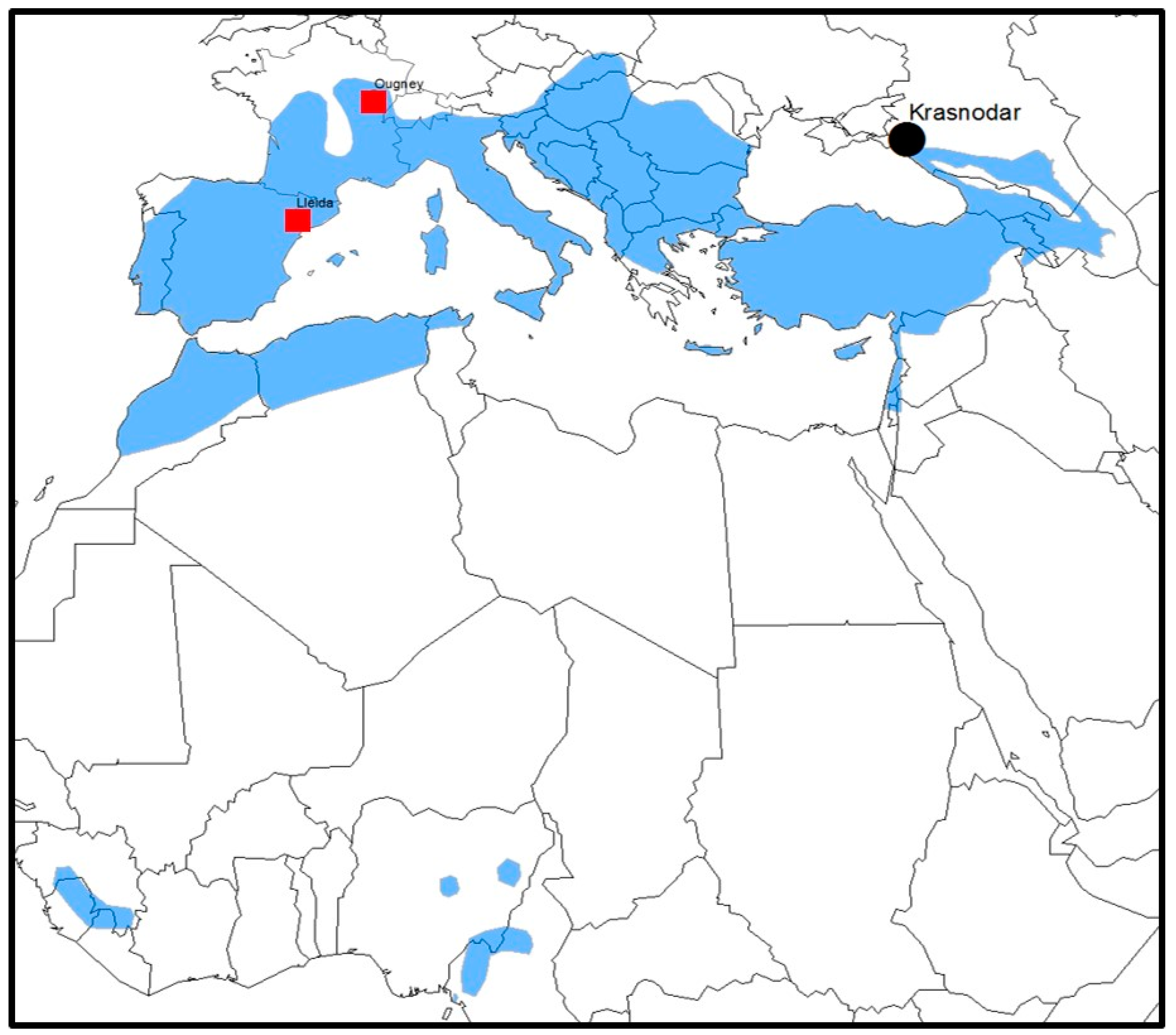
- Banyard, A.C.; Selden, D.; Wu, G.; Thorne, L.; Jennings, D.; Marston, D.; Finke, S.; Freuling, C.M.; Mueller, T.; Echevarria, J.E.; et al. Isolation, antigenicity and immunogenicity of Lleida bat lyssavirus. J. Gen. Virol. 2018, 99, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Vans, J.S.; Horton, D.L.; Easton, A.J.; Fooks, A.R.; Banyard, A.C. Rabies virus vaccines: is there a need for a pan-lyssavirus vaccine? Vaccine 2012, 30, 7447–7454. [Google Scholar] [CrossRef] [PubMed]
- Badrane, H.; Bahloul, C.; Perrin, P.; Tordo, N. Evidence of two Lyssavirus phylogroups with distinct pathogenicity and immunogenicity. J. Virol. 2001, 75, 3268–3276. [Google Scholar] [CrossRef] [PubMed]
- Schatz, J.; Fooks, A.R.; McElhinney, L.; Horton, D.; Echevarria, J.; Vazquez-Moron, S.; Kooi, E.A.; Rasmussen, T.B.; Müller, T.; Freuling, C.M. Bat Rabies Surveillance in Europe. Zoonoses Public Health 2013, 60, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Malerczyk, C.; Freuling, C.; Gniel, D.; Giesen, A.; Selhorst, T.; Muller, T. Cross-neutralization of antibodies induced by vaccination with Purified Chick Embryo Cell Vaccine (PCECV) against different Lyssavirus species. Hum. Vaccines Immunother. 2014, 10, 2799–2804. [Google Scholar] [CrossRef] [PubMed]
- Picard-Meyer, E.; Beven, V.; Hirchaud, E.; Guillaume, C.; Larcher, G.; Robardet, E.; Servat, A.; Blanchard, Y.; Cliquet, F. Lleida Bat Lyssavirus isolation in Miniopterus schreibersii in France. Zoonoses Public Health 2019, 66, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, C.A.; Kuzmin, I.V.; Blanton, J.D.; Weldon, W.C.; Manangan, J.S.; Rupprecht, C.E. Efficacy of rabies biologics against new lyssaviruses from Eurasia. Virus Res. 2005, 111, 44–54. [Google Scholar] [CrossRef] [PubMed]
- García-Mudarra, J.L.; Ibáñez, C.; Juste, J. The Straits of Gibraltar: barrier or bridge to Ibero-Moroccan bat diversity? Biol. J. Linn. Soc. Lond. 2009, 96, 434–450. [Google Scholar] [CrossRef]
| Lyssavirus Species | Phylogroup | Bat Species Most Often Associated with Lyssavirus Infection | Human Cases | Countries Reporting Lyssavirus in Bats |
|---|---|---|---|---|
| Aravan lyssavirus (ARAV) | I | Myotis blythi | No | Kyrgyzstan |
| Australian bat lyssavirus (ABLV) | I | Pteropus alecto Pteropus scapulatus Pteropus poliocephalus Pteropus conspicillatus Saccolaimus flaviventris | Yes, three | Australia |
| Bokeloh bat lyssavirus (BBLV) | I | Myotis nattereri | No | Germany, France, Poland |
| Duvenhage lyssavirus (DUVV) | I | Miniopterus sp Nycteris thebaica | Yes, three | South Africa, Kenya Zimbabwe |
| European bat 1 Lyssavirus (EBLV-1) | I | Eptesicus serotinus Eptesicus isabellinus Vespertilio murinus Pipistrellus nathusii Pipistrellus pipistrellus | Yes, two | France, Germany, The Netherlands, Poland, Denmark, Spain, Ukraine, Russia, Hungary |
| European bat 2 lyssavirus (EBLV-2) | I | Myotis daubentonii Myotis dasycneme | Yes, two | The Netherlands, Switzerland, United Kingdom, Germany, Finland, Norway, Denmark |
| Gannoruwa bat lyssavirus (GBLV) | I | Pteropus medius | No | Sri Lanka |
| Irkut lyssavirus (IRKV) | I | Murina leucogaster | Yes, one | Russian Federation, China |
| Kotolahti Bat Lyssavirus (KBLV)$ | I | Myotis brandtii | No | Finland |
| Khujand lyssavirus (KHUV) | I | Myotis mystacinus | No | Tajikistan |
| Lagos bat lyssavirus (LBV) | II | Eidolon helvum Rousettus aegyptiacus Micropteropus pussilus Nycteris gambiensis Epomophorus wahlbergi | No | Nigeria, Senegal, Ghana, Guinea, Kenya, France (ex-Togo or Egypt), Central African Republic, South Africa |
| Lleida bat lyssavirus (LLEBV) | III | Miniopterus schreibersii | No | Spain, France |
| Rabies lyssavirus (RABV) | I | Multiple | Yes, 59,000/year. Mostly transmitted by dogs | North and South America |
| Shimoni bat lyssavirus (SHIBV) | II | Hipposideros commersoni | No | Kenya |
| Taiwan bat lyssavirus (TWBLV)$ | I | Pipistrellus abramus | No | Taiwan |
| West Caucasian bat lyssavirus (WCBV) | III | Miniopterus schreibersii | No | Russian Federation |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echevarría, J.E.; Banyard, A.C.; McElhinney, L.M.; Fooks, A.R. Current Rabies Vaccines Do Not Confer Protective Immunity against Divergent Lyssaviruses Circulating in Europe. Viruses 2019, 11, 892. https://doi.org/10.3390/v11100892
Echevarría JE, Banyard AC, McElhinney LM, Fooks AR. Current Rabies Vaccines Do Not Confer Protective Immunity against Divergent Lyssaviruses Circulating in Europe. Viruses. 2019; 11(10):892. https://doi.org/10.3390/v11100892
Chicago/Turabian StyleEchevarría, Juan E., Ashley C. Banyard, Lorraine M. McElhinney, and Anthony R. Fooks. 2019. "Current Rabies Vaccines Do Not Confer Protective Immunity against Divergent Lyssaviruses Circulating in Europe" Viruses 11, no. 10: 892. https://doi.org/10.3390/v11100892
APA StyleEchevarría, J. E., Banyard, A. C., McElhinney, L. M., & Fooks, A. R. (2019). Current Rabies Vaccines Do Not Confer Protective Immunity against Divergent Lyssaviruses Circulating in Europe. Viruses, 11(10), 892. https://doi.org/10.3390/v11100892





