Differential Susceptibility and Innate Immune Response of Aedes aegypti and Aedes albopictus to the Haitian Strain of the Mayaro Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus
2.2. Mosquito Colonies
2.3. Oral Infection and Dissection
2.4. Saliva Collection and Titration
2.5. Detection of MAYV in Mosquito Tissues by Real-Time PCR
2.6. Immune Gene Expression Analysis by Real-Time PCR
2.7. Statistical Analyses
3. Results
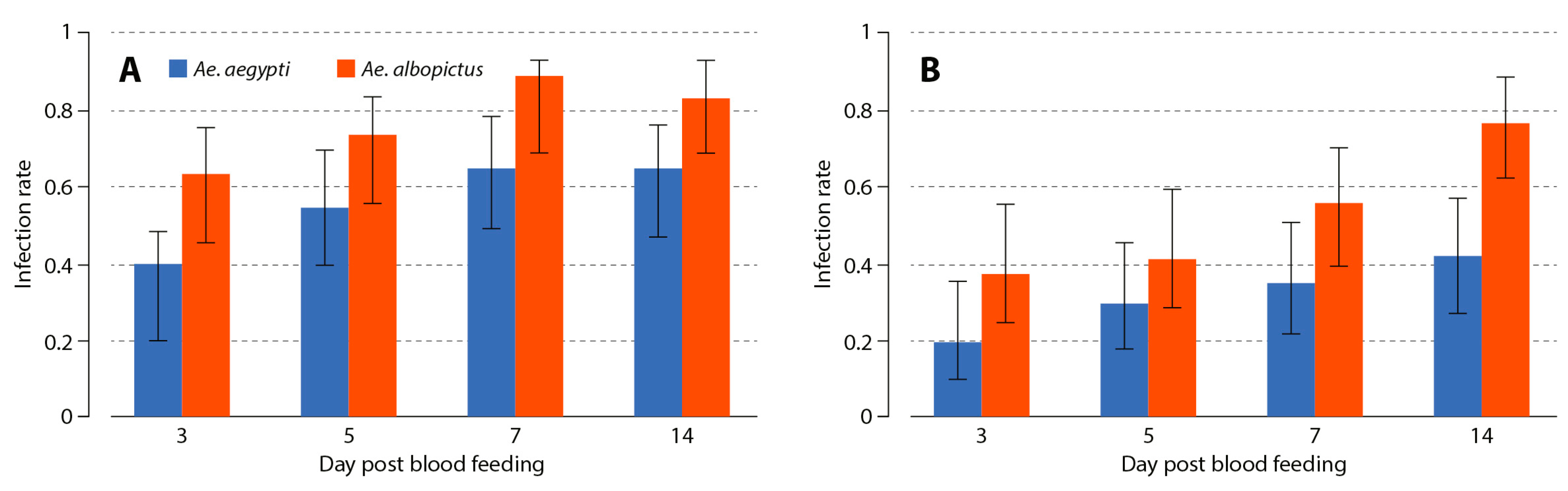
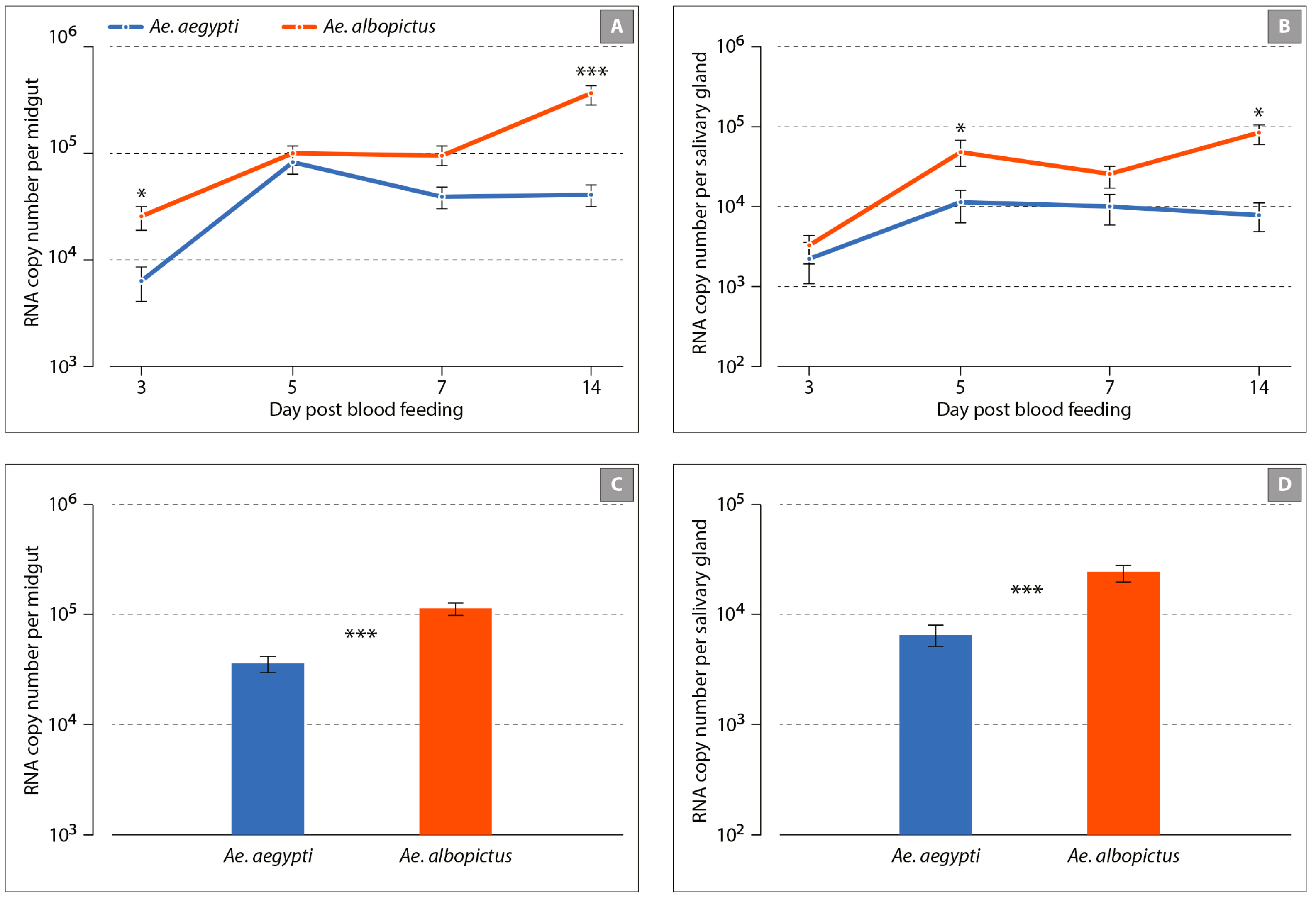
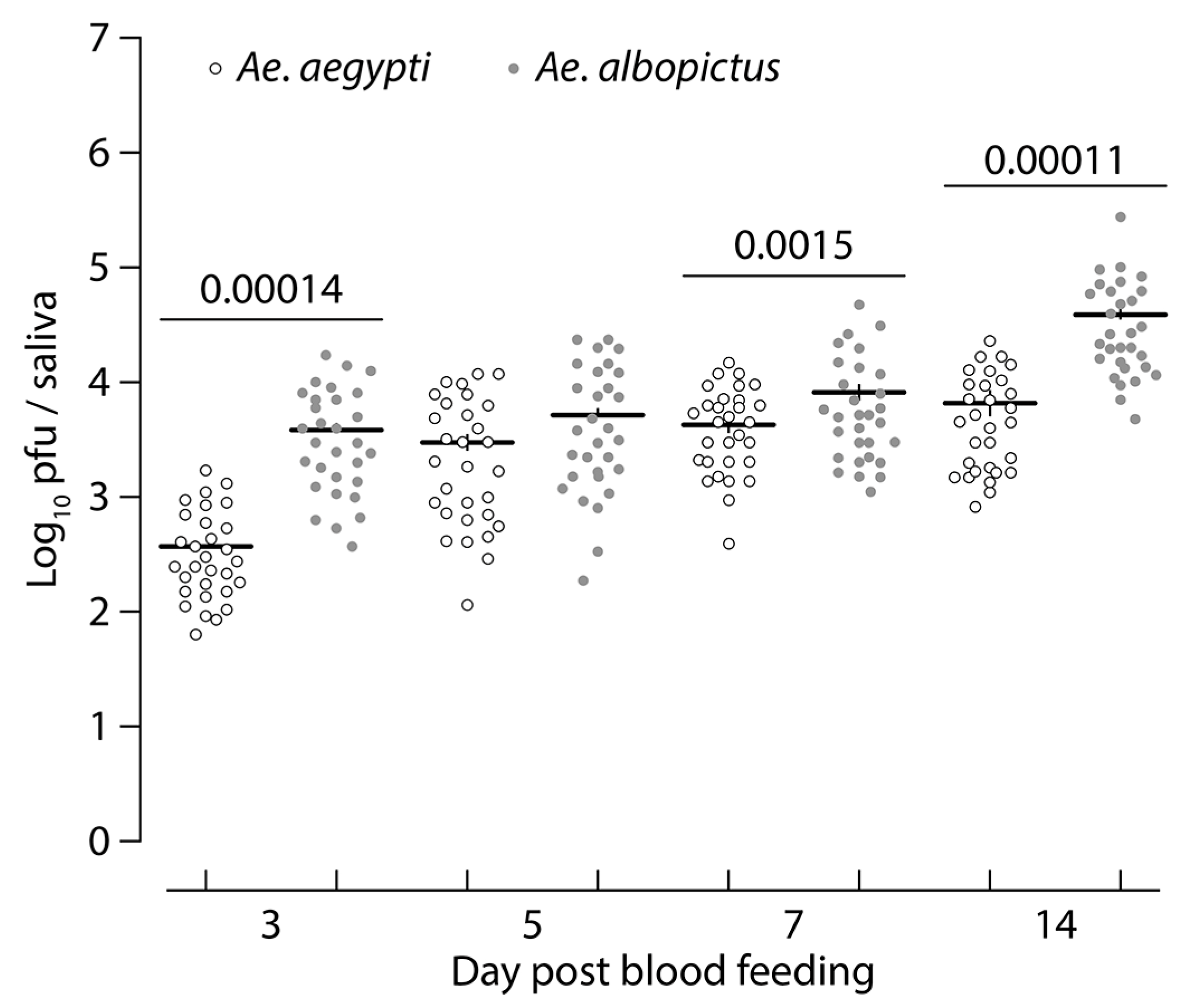
3.1. Aedes albopictus and Aedes aegypti Infection Dynamics to MAYV
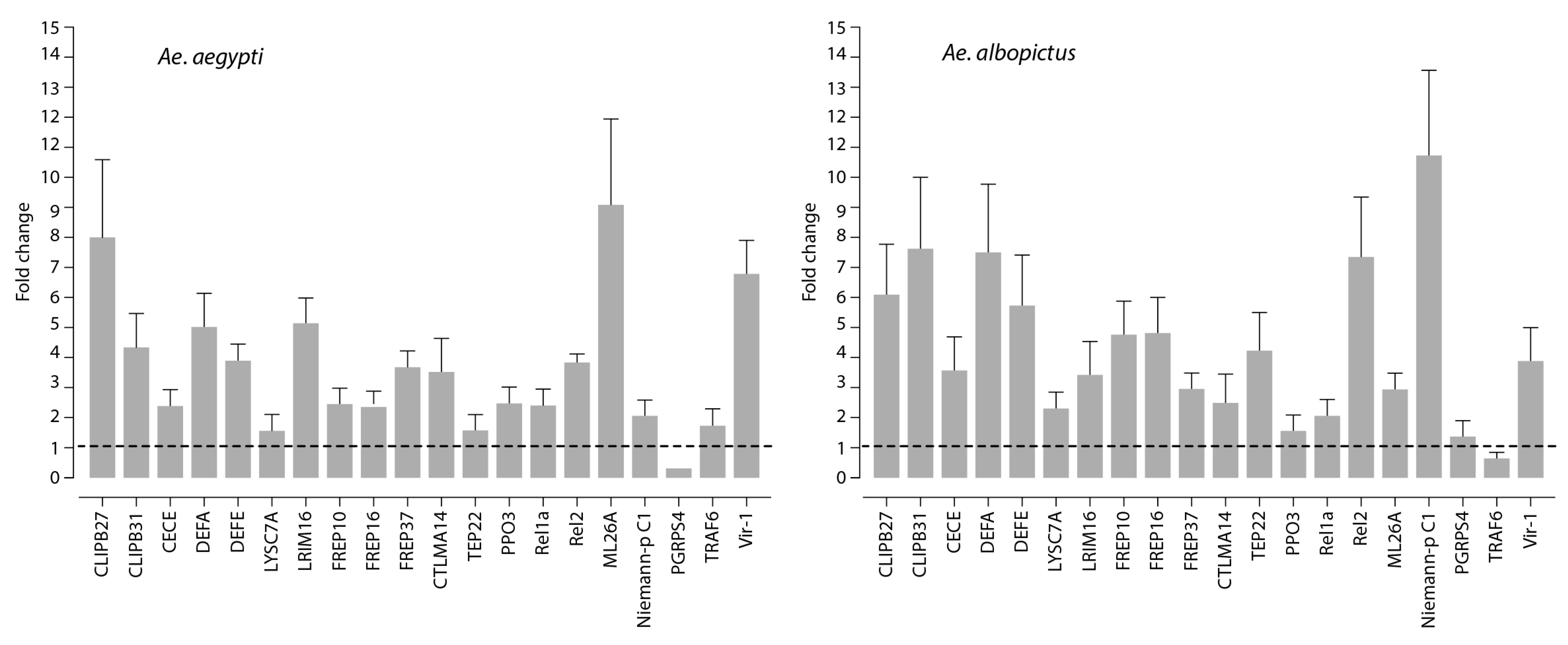
3.2. Innate Immune Response to Mayaro Virus Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anderson, C.R.; Downs, W.G.; Wattley, G.H.; Ahin, N.W.; Reese, A.A. Mayaro virus: A new human disease agent. II. Isolation from blood of patients in Trinidad, B.W.I. Am. J. Trop. Med. Hyg. 1957, 6, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Phillips, P.; Lehmann, D.; Spooner, V.; Barker, J.; Tulloch, S.; Sungu, M.; Canil, K.; Pratt, R.; Lupiwa, T.; Alpers, M. Viruses associated with acute lower respiratory tract infections in children from the eastern highlands of Papua New Guinea (1983–1985). Southeast. Asian J. Trop. Med. Public Health 1990, 21, 373–382. [Google Scholar] [PubMed]
- Tesh, R.B.; Watts, D.M.; Russell, K.L.; Damodaran, C.; Calampa, C.; Cabezas, C.; Ramirez, G.; Vasquez, B.; Hayes, C.G.; Rossi, C.A.; et al. Mayaro virus disease: An emerging mosquito-borne zoonosis in tropical South America. Clin. Infect. Dis. 1999, 28, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.T.; Vedovello, D.; Estofolete, C.; Malossi, C.D.; Araujo, J.P., Jr.; Nogueira, M.L. Complete Genome Sequence of Mayaro Virus Imported from the Amazon Basin to Sao Paulo State, Brazil. Genome Announc. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Hoch, A.L.; Peterson, N.E.; LeDuc, J.W.; Pinheiro, F.P. An outbreak of Mayaro virus disease in Belterra, Brazil. III. Entomological and ecological studies. Am. J. Trop. Med. Hyg. 1981, 30, 689–698. [Google Scholar] [CrossRef]
- Pinheiro, F.P.; Hoch, A.L.; Gomes, M.; Roberts, D.R. Oropouche virus. IV. Laboratory transmission by Culicoides paraensis. Am. J. Trop. Med. Hyg. 1981, 30, 172–176. [Google Scholar] [CrossRef]
- Lednicky, J.; de Rochars, V.M.; Elbadry, M.; Loeb, J.; Telisma, T.; Chavannes, S.; Anilis, G.; Cella, E.; Ciccozzi, M.; Okech, B.; et al. Mayaro Virus in Child with Acute Febrile Illness, Haiti, 2015. Emerg. Infect. Dis. 2016, 22, 2000–2002. [Google Scholar] [CrossRef]
- Hassing, R.J.; Leparc-Goffart, I.; Blank, S.N.; Thevarayan, S.; Tolou, H.; van Doornum, G.; van Genderen, P.J. Imported Mayaro virus infection in the Netherlands. J. Infect. 2010, 61, 343–345. [Google Scholar] [CrossRef]
- Receveur, M.C.; Grandadam, M.; Pistone, T.; Malvy, D. Infection with Mayaro virus in a French traveller returning from the Amazon region, Brazil, January, 2010. Euro Surveill. 2010, 15, 19563. [Google Scholar]
- Neumayr, A.; Gabriel, M.; Fritz, J.; Günther, S.; Hatz, C.; Schmidt-Chanasit, J.; Blum, J. Mayaro virus infection in traveler returning from Amazon Basin, northern Peru. Emerg. Infect. Dis. 2012, 18, 695. [Google Scholar] [CrossRef]
- Powers, A.M. Chikungunya. Clin. Lab. Med. 2010, 30, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Auguste, A.J.; Liria, J.; Forrester, N.L.; Giambalvo, D.; Moncada, M.; Long, K.C.; Moron, D.; de Manzione, N.; Tesh, R.B.; Halsey, E.S.; et al. Evolutionary and Ecological Characterization of Mayaro Virus Strains Isolated during an Outbreak, Venezuela, 2010. Emerg. Infect. Dis. 2015, 21, 1742–1750. [Google Scholar] [CrossRef] [PubMed]
- Santiago, F.W.; Halsey, E.S.; Siles, C.; Vilcarromero, S.; Guevara, C.; Silvas, J.A.; Ramal, C.; Ampuero, J.S.; Aguilar, P.V. Long-Term Arthralgia after Mayaro Virus Infection Correlates with Sustained Pro-inflammatory Cytokine Response. PLoS Negl. Trop. Dis. 2015, 9, e0004104. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar] [CrossRef]
- Mourao, M.P.; Bastos Mde, S.; de Figueiredo, R.P.; Gimaque, J.B.; Galusso Edos, S.; Kramer, V.M.; de Oliveira, C.M.; Naveca, F.G.; Figueiredo, L.T. Mayaro fever in the city of Manaus, Brazil, 2007–2008. Vector Borne Zoonotic Dis. 2012, 12, 42–46. [Google Scholar] [CrossRef]
- Smith, G.C.; Francy, D.B. Laboratory studies of a Brazilian strain of Aedes albopictus as a potential vector of Mayaro and Oropouche viruses. J. Am. Mosq. Control. Assoc. 1991, 7, 89–93. [Google Scholar]
- Long, K.C.; Ziegler, S.A.; Thangamani, S.; Hausser, N.L.; Kochel, T.J.; Higgs, S.; Tesh, R.B. Experimental transmission of Mayaro virus by Aedes aegypti. Am. J. Trop. Med. Hyg. 2011, 85, 750–757. [Google Scholar] [CrossRef]
- Wiggins, K.; Eastmond, B.; Alto, B.W. Transmission potential of Mayaro virus in Florida Aedes aegypti and Aedes albopictus mosquitoes. Med. Vet. Entomol. 2018, 32, 436–442. [Google Scholar] [CrossRef]
- Waterhouse, R.M.; Kriventseva, E.V.; Meister, S.; Xi, Z.; Alvarez, K.S.; Bartholomay, L.C.; Barillas-Mury, C.; Bian, G.; Blandin, S.; Christensen, B.M. Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science 2007, 316, 1738–1743. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Q.; Zhou, J.; Xie, W.; Chen, C.; Wang, Z.; Yang, H.; Cui, J. Zika virus evades interferon-mediated antiviral response through the co-operation of multiple nonstructural proteins in vitro. Cell Discov. 2017, 3, 17006. [Google Scholar] [CrossRef]
- Fros, J.; Pijlman, G. Alphavirus infection: Host cell shut-off and inhibition of antiviral responses. Viruses 2016, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Aubry, F.; Nougairede, A.; de Fabritus, L.; Querat, G.; Gould, E.A.; de Lamballerie, X. Single-stranded positive-sense RNA viruses generated in days using infectious subgenomic amplicons. J. Gen. Virol. 2014, 95, 2462. [Google Scholar] [CrossRef] [PubMed]
- Atieh, T.; Baronti, C.; de Lamballerie, X.; Nougairède, A. Simple reverse genetics systems for Asian and African Zika viruses. Sci. Rep. 2016, 6, 39384. [Google Scholar] [CrossRef] [PubMed]
- Surasombatpattana, P.; Hamel, R.; Patramool, S.; Luplertlop, N.; Thomas, F.; Desprès, P.; Briant, L.; Yssel, H.; Missé, D. Dengue virus replication in infected human keratinocytes leads to activation of antiviral innate immune responses. Infect. Genet. Evol. 2011, 11, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Manokaran, G.; Finol, E.; Wang, C.; Gunaratne, J.; Bahl, J.; Ong, E.Z.; Tan, H.C.; Sessions, O.M.; Ward, A.M.; Gubler, D.J. Dengue subgenomic RNA binds TRIM25 to inhibit interferon expression for epidemiological fitness. Science 2015, 350, 217–221. [Google Scholar] [CrossRef] [PubMed]
- John Fox and Sanford Weisberg. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 8 October 2019).
- Russel, P.M.; Brewer, B.J.; Klaere, S.; Bouckaert, R.R. Model selection and parameter inference in phylogenetics using Nested Sampling. Syst. Biol. 2018, 68, 219–233. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 8 October 2019).
- Tabachnick, W.; Wallis, G.; Aitken, T.H.; Miller, B.; Amato, G.; Lorenz, L.; Powell, J.R.; Beaty, B.J. Oral infection of Aedes aegypti with yellow fever virus: Geographic variation and genetic considerations. Am. J. Trop. Med. Hyg. 1985, 34, 1219–1224. [Google Scholar] [CrossRef]
- Bosio, C.F.; Beaty, B.J.; Black, W., 4th. Quantitative genetics of vector competence for dengue-2 virus in Aedes aegypti. Am. J. Trop. Med. Hyg. 1998, 59, 965–970. [Google Scholar] [CrossRef]
- Zouache, K.; Fontaine, A.; Vega-Rua, A.; Mousson, L.; Thiberge, J.-M.; Lourenco-De-Oliveira, R.; Caro, V.; Lambrechts, L.; Failloux, A.-B. Three-way interactions between mosquito population, viral strain and temperature underlying chikungunya virus transmission potential. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141078. [Google Scholar] [CrossRef]
- Chouin-Carneiro, T.; Vega-Rua, A.; Vazeille, M.; Yebakima, A.; Girod, R.; Goindin, D.; Dupont-Rouzeyrol, M.; Lourenço-de-Oliveira, R.; Failloux, A.-B. Differential susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika virus. PLoS Negl. Trop. Dis. 2016, 10, e0004543. [Google Scholar] [CrossRef]
- Kanost, M.R.; Jiang, H. Clip-domain serine proteases as immune factors in insect hemolymph. Curr. Opin. Insect. Sci. 2015, 11, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Fuchs, J.F.; Mayhew, G.F.; Helen, E.Y.; Christensen, B.M. Tissue-enriched expression profiles in Aedes aegypti identify hemocyte-specific transcriptome responses to infection. Insect Biochem. Mol. Biol. 2012, 42, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Ramirez, J.L.; Dimopoulos, G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008, 4, e1000098. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Souza-Neto, J.; Xi, Z.; Kokoza, V.; Shin, S.W.; Dimopoulos, G.; Raikhel, A. Transcriptome analysis of Aedes aegypti transgenic mosquitoes with altered immunity. PLoS Pathog. 2011, 7, e1002394. [Google Scholar] [CrossRef] [PubMed]
- Anglero-Rodriguez, Y.I.; MacLeod, H.; Kang, S.; Carlson, J.; Jupatanakul, N.; Dimopoulos, G. Aedes aegypti Molecular Responses to Zika Virus: Modulation of Infection by the Toll and Jak/Stat Immune Pathways and Virus Host Factors. Front Microbiol. 2017, 8, 2050. [Google Scholar] [CrossRef]
- Dong, Y.; Aguilar, R.; Xi, Z.; Warr, E.; Mongin, E.; Dimopoulos, G. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog. 2006, 2, e52. [Google Scholar] [CrossRef]
- Waterhouse, R.M.; Povelones, M.; Christophides, G.K. Sequence-structure-function relations of the mosquito leucine-rich repeat immune proteins. BMC Genom. 2010, 11, 531. [Google Scholar] [CrossRef]
- Colpitts, T.M.; Cox, J.; Vanlandingham, D.L.; Feitosa, F.M.; Cheng, G.; Kurscheid, S.; Wang, P.; Krishnan, M.N.; Higgs, S.; Fikrig, E. Alterations in the Aedes aegypti transcriptome during infection with West Nile, dengue and yellow fever viruses. PLoS Pathog. 2011, 7, e1002189. [Google Scholar] [CrossRef]
- Behura, S.K.; Gomez-Machorro, C.; Harker, B.W.; Debruyn, B.; Lovin, D.D.; Hemme, R.R.; Mori, A.; Romero-Severson, J.; Severson, D.W. Global cross-talk of genes of the mosquito Aedes aegypti in response to dengue virus infection. PLoS Negl. Trop. Dis. 2011, 5, e1385. [Google Scholar] [CrossRef]
- Dong, Y.; Dimopoulos, G. Anopheles fibrinogen-related proteins provide expanded pattern recognition capacity against bacteria and malaria parasites. J. Biol. Chem. 2009, 284, 9835–9844. [Google Scholar] [CrossRef]
- Rancès, E.; Yixin, H.Y.; Woolfit, M.; McGraw, E.A.; O’Neill, S.L. The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog. 2012, 8, e1002548. [Google Scholar] [CrossRef] [PubMed]
- Agaisse, H.; Perrimon, N. The roles of JAK/STAT signaling in Drosophila immune responses. Immunol. Rev. 2004, 198, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Jupatanakul, N.; Sim, S.; Dimopoulos, G. Aedes aegypti ML and Niemann-Pick type C family members are agonists of dengue virus infection. Dev. Comp. Immunol. 2014, 43, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Suyama, K.; Buchanan, J.; Zhu, A.J.; Scott, M.P. A Drosophila model of the Niemann-Pick type C lysosome storage disease: Dnpc1a is required for molting and sterol homeostasis. Development 2005, 132, 5115–5124. [Google Scholar] [CrossRef]
- Chang, T.-Y.; Chang, C.C.; Ohgami, N.; Yamauchi, Y. Cholesterol sensing, trafficking, and esterification. Annu. Rev. Cell Dev. Biol. 2006, 22, 129–157. [Google Scholar] [CrossRef]
- Niwa, H. Wnt: What’s needed to maintain pluripotency? Nat. Cell Biol. 2011, 13, 1024. [Google Scholar] [CrossRef]
- Gruber, A.; Manček, M.; Wagner, H.; Kirschning, C.J.; Jerala, R. Structural model of MD-2 and functional role of its basic amino acid clusters involved in cellular lipopolysaccharide recognition. J. Biol. Chem. 2004, 279, 28475–28482. [Google Scholar] [CrossRef]
- Starostina, E.; Xu, A.; Lin, H.; Pikielny, C.W. A Drosophila protein family implicated in pheromone perception is related to Tay-Sachs GM2-activator protein. J. Biol. Chem. 2009, 284, 585–594. [Google Scholar] [CrossRef]
- Horackova, J.; Rudenko, N.; Golovchenko, M.; Grubhoffer, L. Der-p2 (Dermatophagoides pteronyssinus) allergen-like protein from the hard tick Ixodes ricinus—a novel member of ML (MD-2-related lipid-recognition) domain protein family. Parasitology 2010, 137, 1139–1149. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diop, F.; Alout, H.; Diagne, C.T.; Bengue, M.; Baronti, C.; Hamel, R.; Talignani, L.; Liegeois, F.; Pompon, J.; Vargas, R.E.M.; et al. Differential Susceptibility and Innate Immune Response of Aedes aegypti and Aedes albopictus to the Haitian Strain of the Mayaro Virus. Viruses 2019, 11, 924. https://doi.org/10.3390/v11100924
Diop F, Alout H, Diagne CT, Bengue M, Baronti C, Hamel R, Talignani L, Liegeois F, Pompon J, Vargas REM, et al. Differential Susceptibility and Innate Immune Response of Aedes aegypti and Aedes albopictus to the Haitian Strain of the Mayaro Virus. Viruses. 2019; 11(10):924. https://doi.org/10.3390/v11100924
Chicago/Turabian StyleDiop, Fodé, Haoues Alout, Cheikh Tidiane Diagne, Michèle Bengue, Cécile Baronti, Rodolphe Hamel, Loïc Talignani, Florian Liegeois, Julien Pompon, Ronald E Morales Vargas, and et al. 2019. "Differential Susceptibility and Innate Immune Response of Aedes aegypti and Aedes albopictus to the Haitian Strain of the Mayaro Virus" Viruses 11, no. 10: 924. https://doi.org/10.3390/v11100924
APA StyleDiop, F., Alout, H., Diagne, C. T., Bengue, M., Baronti, C., Hamel, R., Talignani, L., Liegeois, F., Pompon, J., Vargas, R. E. M., Nougairède, A., & Missé, D. (2019). Differential Susceptibility and Innate Immune Response of Aedes aegypti and Aedes albopictus to the Haitian Strain of the Mayaro Virus. Viruses, 11(10), 924. https://doi.org/10.3390/v11100924








