Different Outcomes of Experimental Hepatitis E Virus Infection in Diverse Mouse Strains, Wistar Rats, and Rabbits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inocula
2.2. Animals and Experimental Design
2.3. Inoculation of Animals with a Wild Boar Derived HEV-3 Strain
2.3.1. Inoculation of Mice
2.3.2. Inoculation of Wistar Rats
2.3.3. Inoculation of European Rabbits
2.4. Anti-HEV Antibody ELISA and Quantitative Real-Time RT-PCR
3. Results
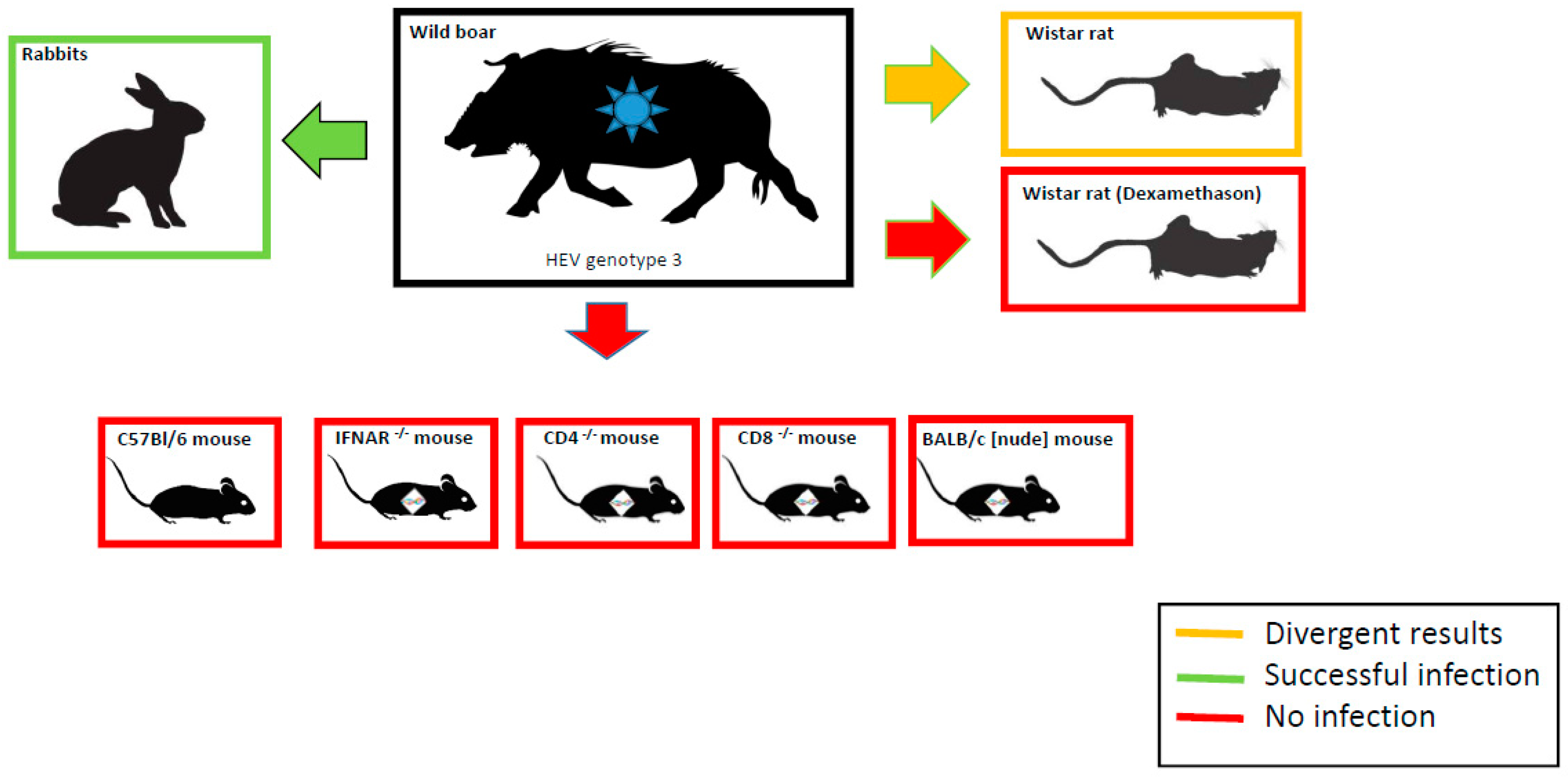
3.1. Inoculation Experiments in Different Mouse Strains
3.2. Infection Trial in Dexamethasone Treated and Non-Treated Wistar Rats
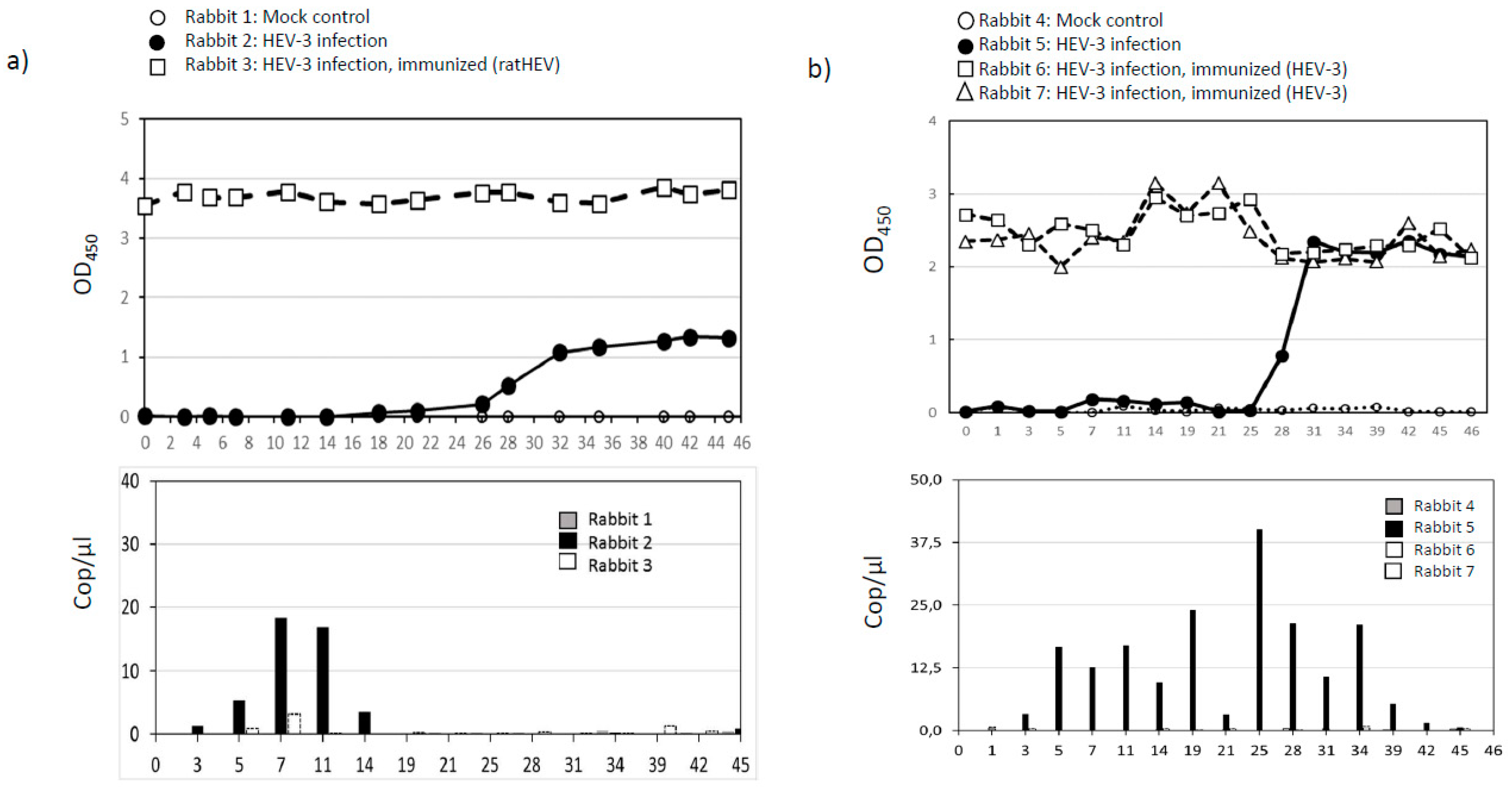
3.3. Infection and Vaccination Trial in Rabbits
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yin, X.; Ambardekar, C.; Lu, Y.; Feng, Z. Distinct Entry Mechanisms for Non enveloped and Quasi-Enveloped Hepatitis E Viruses. J. Virol. 2016, 28, 4232–4242. [Google Scholar] [CrossRef] [PubMed]
- Emerson, S.U.; Purcell, R.H. Hepatitis E virus. Rev. Med. Virol. 2003, 13, 145–154. [Google Scholar] [CrossRef]
- Tam, A.W.; Smith, M.M.; Guerra, M.E.; Huang, C.C.; Bradley, D.W.; Fry, K.E.; Reyes, G.R. Hepatitis E virus (HEV): Molecular cloning and sequencing of the full-length viral genome. Virology 1991, 185, 120–131. [Google Scholar] [CrossRef]
- Emerson, S.U.; Purcell, R.H. Hepatitis E virus. Fields Virol. 2013, 2, 2242–2258. [Google Scholar] [CrossRef]
- Ankcorn, M.J.; Tedder, R.S. Hepatitis E: The current state of play. Transfus. Med. 2017, 27, 84–95. [Google Scholar] [CrossRef]
- Johne, R.; Dremsek, P.; Reetz, J.; Heckel, G.; Hess, M.; Ulrich, R.G. Hepeviridae: An expanding family of vertebrate viruses. Infect. Genet. Evol. 2014, 27, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Spahr, C.; Knauf-Witzens, T.; Vahlenkamp, T.; Ulrich, R.G.; Johne, R. Hepatitis E virus and related viruses in wild, domestic and zoo animals: A review. Zoonoses Public Health 2018, 65, 11–29. [Google Scholar] [CrossRef]
- Purdy, M.A.; Harrison, T.J.; Jameel, S.; Meng, X.J.; Okamoto, H.; Van der Poel, W.H.M.; Smith, D.B. Ictv Report Consortium. ICTV Virus Taxonomy Profile: Hepeviridae. J. Gen. Virol. 2017, 98, 2645–2646. [Google Scholar] [CrossRef] [PubMed]
- Cossaboom, C.M.; Cordoba, L.; Dryman, B.A.; Meng, X.J. Hepatitis E virus in rabbits, Virginia, USA. Emerg. Infect. Dis. 2011, 17, 2047–2049. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Ma, Z.; Harrison, T.J.; Feng, R.; Zhang, C.; Qiao, Z.; Fan, J.; Ma, H.; Li, M.; Song, A.; et al. A novel genotype of hepatitis E virus prevalent among farmed rabbits in China. J. Med. Virol. 2009, 81, 1371–1379. [Google Scholar] [CrossRef]
- Izopet, J.; Dubois, M.; Bertagnoli, S.; Lhomme, S.; Marchandeau, S.; Boucher, S.; Kamar, N.; Abravanel, F.; Guerin, J.L. Hepatitis E virus strains in rabbits and evidence of a closely related strain in humans, France. Emerg. Infect. Dis. 2012, 18, 1274–1281. [Google Scholar] [CrossRef]
- Ryll, R.; Eiden, M.; Heuser, E.; Weinhardt, M.; Ziege, M.; Höper, D.; Groschup, M.H.; Heckel, G.; Johne, R.; Ulrich, R.G. Hepatitis E virus in feral rabbits along a rural-urban transect in Central Germany. Infect. Genet. Evol. 2018, 61, 155–159. [Google Scholar] [CrossRef]
- Hammerschmidt, F.; Schwaiger, K.; Dähnert, L.; Vina-Rodriguez, A.; Höper, D.; Gareis, M.; Groschup, M.H.; Eiden, M. Hepatitis E virus in wild rabbits and European brown hares in Germany. Zoonoses Public Health 2017, 64, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Colson, P.; Borentain, P.; Queyriaux, B.; Kaba, M.; Moal, V.; Gallian, P.; Heyries, L.; Raoult, D.; Gerolami, R. Pig liver sausage as a source of hepatitis E virus transmission to humans. J. Infect. Dis. 2010, 202, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Okada, K.; Takahashi, K.; Mishiro, S. Severe Hepatitis E Virus Infection after Ingestion of Uncooked Liver from a Wild Boar. J. Infect. Dis. 2003, 188, 944. [Google Scholar] [CrossRef]
- Wichmann, O.; Schimanski, S.; Koch, J.; Kohler, M.; Rothe, C.; Plentz, A.; Jilg, W.; Stark, K. Phylogenetic and case-control study on hepatitis E virus infection in Germany. J. Infect. Dis. 2008, 198, 1732–1741. [Google Scholar] [CrossRef]
- Dremsek, P.; Wenzel, J.; Johne, R.; Ziller, M.; Hofmann, J.; Groschup, M.; Werdermann, S.; Mohn, U.; Dorn, S.; Motz, M.; et al. Seroprevalence study in forestry workers from eastern Germany using novel genotype 3- and rat hepatitis E virus-specific immunoglobulin G ELISAs. Med. Microbiol. Immunol. 2012, 201, 189–200. [Google Scholar] [CrossRef]
- Krumbholz, A.; Joel, S.; Dremsek, P.; Neubert, A.; Johne, R.; Durrwald, R.; Walther, M.; Muller, T.H.; Kuhnel, D.; Lange, J.; et al. Seroprevalence of hepatitis E virus (HEV) in humans living in high pig density areas of Germany. Med. Microbiol. Immunol. 2014, 203, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Nimgaonkar, I.; Ding, Q.; Schwartz, R.E.; Ploss, A. Hepatitis E virus: Advances and challenges. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 96–110. [Google Scholar] [CrossRef]
- Kamar, N.; Izopet, J.; Pavio, N.; Aggarwal, R.; Labrique, A.; Wedemeyer, H.; Dalton, H.R. Hepatitis E virus infection. Nat. Rev. Dis. Primers. 2017, 3, 17086. [Google Scholar] [CrossRef] [Green Version]
- Doceul, V.; Bagdassarian, E.; Demange, A.; Pavio, N. Zoonotic Hepatitis E Virus: Classification, Animal Reservoirs and Transmission Routes. Viruses 2016, 8, 270. [Google Scholar] [CrossRef]
- Pavio, N.; Doceul, V.; Bagdassarian, E.; Johne, R. Recent knowledge on hepatitis E virus in Suidae reservoirs and transmission routes to human. Vet. Res. 2017, 48, 78. [Google Scholar] [CrossRef] [PubMed]
- Maneerat, Y.; Clayson, E.T.; Myint, K.S.; Young, G.D.; Innis, B.L. Experimental infection of the laboratory rat with the hepatitis E virus. J. Med. Virol. 1996, 48, 121–128. [Google Scholar] [CrossRef]
- Li, T.C.; Yoshizaki, S.; Ami, Y.; Suzaki, Y.; Yasuda, S.P.; Yoshimatsu, K.; Arikawa, J.; Takeda, N.; Wakita, T. Susceptibility of laboratory rats against genotypes 1, 3, 4, and rat hepatitis E viruses. Vet. Microbiol. 2013, 163, 54–61. [Google Scholar] [CrossRef]
- Purcell, R.H.; Engle, R.E.; Rood, M.P.; Kabrane-Lazizi, Y.; Nguyen, H.T.; Govindarajan, S.; St Claire, M.; Emerson, S.U. Hepatitis E virus in rats, Los Angeles, California, USA. Emerg. Infect. Dis. 2011, 17, 2216–2222. [Google Scholar] [CrossRef]
- Li, T.C.; Ami, Y.; Suzaki, Y.; Takeda, N.; Takaji, W. No evidence for hepatitis E virus genotype 3 susceptibility in rats. Emerg. Infect. Dis. 2013, 19, 1343–1345. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, X.; Zhang, Y.; Ni, Y.; Si, F.; Yu, R.; Dong, S.; Huang, Y.; Li, Z. Infectivity of a genotype 4 hepatitis E virus cDNA clone by intrahepatic inoculation of laboratory rats. Vet. Microbiol. 2013, 166, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhang, W.; Gong, G.; Yuan, C.; Yan, Y.; Yang, S.; Cui, L.; Zhu, J.; Yang, Z.; Hua, X. Experimental infection of Balb/c nude mice with Hepatitis E virus. BMC Infect. Dis. 2009, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Li, T.C.; Suzaki, Y.; Ami, Y.; Tsunemitsu, H.; Miyamura, T.; Takeda, N. Mice are not susceptible to hepatitis E virus infection. J. Vet. Med. Sci. 2008, 70, 1359–1362. [Google Scholar] [CrossRef]
- Allweiss, L.; Gass, S.; Giersch, K.; Groth, A.; Kah, J.; Volz, T.; Rapp, G.; Schöbel, A.; Lohse, A.W.; Polywka, S.; et al. Human liver chimeric mice as a new model of chronic hepatitis E virus infection and preclinical drug evaluation. J. Hepatol. 2016, 64, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, S.; Dai, X.; Shi, C.; Wen, Y.; Zhu, M.; Zhan, S.; Meng, J. Rabbit as a novel animal model for hepatitis E virus infection and vaccine evaluation. PLoS ONE 2012, 7, e51616. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zheng, L.; Liu, Y.; Zhao, C.; Harrison, T.J.; Ma, Y.; Sun, S.; Zhang, J.; Wang, Y. Experimental infection of rabbits with rabbit and genotypes 1 and 4 hepatitis E viruses. PLoS ONE 2010, 5, e9160. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lei, Y.; Liu, L.; Liu, P.; Xia, J.; Zhang, Y.; Zeng, H.; Wang, L.; Wang, L.; Zhuang, H. SPF rabbits infected with rabbit hepatitis E virus isolate experimentally showing the chronicity of hepatitis. PLoS ONE 2014, 9, e99861. [Google Scholar] [CrossRef]
- Cossaboom, C.M.; Cordoba, L.; Sanford, B.J.; Pineyro, P.; Kenney, S.P.; Dryman, B.A.; Wang, Y.; Meng, X.J. Cross-species infection of pigs with a novel rabbit, but not rat, strain of hepatitis E virus isolated in the United States. J. Gen. Virol. 2012, 93, 1687–1695. [Google Scholar] [CrossRef]
- Liu, P.; Bu, Q.N.; Wang, L.; Han, J.; Du, R.J.; Lei, Y.X.; Ouyang, Y.Q.; Li, J.; Zhu, Y.H.; Lu, F.M.; et al. Transmission of hepatitis E virus from rabbits to cynomolgus macaques. Emerg. Infect. Dis. 2013, 19, 559–565. [Google Scholar] [CrossRef]
- Schlosser, J.; Eiden, M.; Vina-Rodriguez, A.; Fast, C.; Dremsek, P.; Lange, E.; Ulrich, R.G.; Groschup, M.H. Natural and experimental hepatitis E virus genotype 3 - infection in European wild boar is transmissible to domestic pigs. Vet. Res. 2014, 45, 121. [Google Scholar] [CrossRef] [PubMed]
- Vina-Rodriguez, A.; Schlosser, J.; Becher, D.; Kaden, V.; Groschup, M.H.; Eiden, M. Hepatitis E virus genotype 3 diversity: Phylogenetic analysis and presence of subtype 3b in wild boar in Europe. Viruses 2015, 7, 2704–2726. [Google Scholar] [CrossRef]
- Toussaint, J.F.; Sailleau, C.; Breard, E.; Zientara, S.; De Clercq, K. Bluetongue virus detection by two real-time RT-qPCRs targeting two different genomic segments. J. Virol. Methods 2007, 140, 115–123. [Google Scholar] [CrossRef]
- Kenney, S.P.; Meng, X.J. Hepatitis E Virus: Animal Models and Zoonosis. Annu. Rev. Anim. Biosci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Li, T.C.; Wakita, T. Small Animal Models of Hepatitis E Virus Infection. Cold Spring Harb. Perspect. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Krawczynski, K.; Meng, X.J.; Rybczynska, J. Pathogenetic elements of hepatitis E and animal models of HEV infection. Virus Res. 2011, 161, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, J.; Vina-Rodriguez, A.; Fast, C.; Groschup, M.H.; Eiden, M. Chronically infected wild boar can transmit genotype 3 hepatitis E virus to domestic pigs. Vet. Microbiol. 2015, 180, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ryll, R.; Bernstein, S.; Heuser, E.; Schlegel, M.; Dremsek, P.; Zumpe, M.; Wolf, S.; Pépin, M.; Bajomi, D.; Müller, G.; et al. Detection of rat hepatitis E virus in wild Norway rats (Rattus norvegicus) and Black rats (Rattus rattus) from 11 European countries. Vet. Microbiol. 2017, 208, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Lack, J.B.; Volk, K.; Van Den Bussche, R.A. Hepatitis E virus genotype 3 in wild rats, United States. Emerg. Infect. Dis. 2012, 18, 1268–1273. [Google Scholar] [CrossRef]
- Kanai, Y.; Miyasaka, S.; Uyama, S.; Kawami, S.; Kato-Mori, Y.; Tsujikawa, M.; Yunoki, M.; Nishiyama, S.; Ikuta, K.; Hagiwara, K. Hepatitis E virus in Norway rats (Rattus norvegicus) captured around a pig farm. BMC Res. Notes 2012, 5, 4. [Google Scholar] [CrossRef]
- Sridhar, S.; Yip, C.C.Y.; Wu, S.; Cai, J.; Zhang, A.J.; Leung, K.H.; Chung, T.W.H.; Chan, J.F.W.; Chan, W.M.; Teng, J.L.L.; et al. Rat Hepatitis E Virus as Cause of Persistent Hepatitis after Liver Transplant. Emerg. Infect. Dis. 2018, 24, 2241–2250. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, S.J.; Choi, S.H.; Kang, Y.J.; Kim, K.H. Dexamethasone treatment decreases replication of viral hemorrhagic septicemia virus in Epithelioma papulosum cyprini cells. Arch. Virol. 2017, 162, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Eddy, J.L.; Krukowski, K.; Janusek, L.; Mathews, H.L. Glucocorticoids regulate natural killer cell function epigenetically. Cell. Immunol. 2014, 290, 120–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, H.Y.; Müller, N.; Herold, M.J.; van den Brandt, J.; Reichardt, H.M. Glucocorticoids exert opposing effects on macrophage function dependent on their concentration. Immunology 2007, 122, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Gong, W.; Song, W.T.; Fu, H.; Wang, L.; Li, M.; Wang, L.; Zhuang, H. Different susceptibility and pathogenesis of rabbit genotype 3 hepatitis E virus (HEV-3) and human HEV-3 (JRC-HE3) in SPF rabbits. Vet. Microbiol. 2017, 207, 1–6. [Google Scholar] [CrossRef]
- Liu, P.; Du, R.; Wang, L.; Han, J.; Liu, L.; Zhang, Y.; Xia, J.; Lu, F.; Zhuang, H. Management of hepatitis E virus (HEV) zoonotic transmission: Protection of rabbits against HEV challenge following immunization with HEV 239 vaccine. PLoS ONE 2014, 9, e87600. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cheng, X.; Dai, X.; Dong, C.; Xu, M.; Liang, J.; Dong, M.; Purdy, M.A.; Meng, J. Rabbit and human hepatitis E virus strains belong to a single serotype. Virus Res. 2013, 176, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Simmonds, P.; Izopet, J.; Oliveira-Filho, E.F.; Ulrich, R.G.; Johne, R.; Koenig, M.; Jameel, S.; Harrison, T.J.; Meng, X.-J.; et al. Proposed reference sequences for Hepatitis E virus subtypes. J. Gen. Virol. 2016, 97, 537–542. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlosser, J.; Dähnert, L.; Dremsek, P.; Tauscher, K.; Fast, C.; Ziegler, U.; Gröner, A.; Ulrich, R.G.; Groschup, M.H.; Eiden, M. Different Outcomes of Experimental Hepatitis E Virus Infection in Diverse Mouse Strains, Wistar Rats, and Rabbits. Viruses 2019, 11, 1. https://doi.org/10.3390/v11010001
Schlosser J, Dähnert L, Dremsek P, Tauscher K, Fast C, Ziegler U, Gröner A, Ulrich RG, Groschup MH, Eiden M. Different Outcomes of Experimental Hepatitis E Virus Infection in Diverse Mouse Strains, Wistar Rats, and Rabbits. Viruses. 2019; 11(1):1. https://doi.org/10.3390/v11010001
Chicago/Turabian StyleSchlosser, Josephine, Lisa Dähnert, Paul Dremsek, Kerstin Tauscher, Christine Fast, Ute Ziegler, Albrecht Gröner, Rainer G Ulrich, Martin H Groschup, and Martin Eiden. 2019. "Different Outcomes of Experimental Hepatitis E Virus Infection in Diverse Mouse Strains, Wistar Rats, and Rabbits" Viruses 11, no. 1: 1. https://doi.org/10.3390/v11010001
APA StyleSchlosser, J., Dähnert, L., Dremsek, P., Tauscher, K., Fast, C., Ziegler, U., Gröner, A., Ulrich, R. G., Groschup, M. H., & Eiden, M. (2019). Different Outcomes of Experimental Hepatitis E Virus Infection in Diverse Mouse Strains, Wistar Rats, and Rabbits. Viruses, 11(1), 1. https://doi.org/10.3390/v11010001




