Vegetation-Driven Changes in Soil Properties, Enzymatic Activities, and Microbial Communities of Saline–Alkaline Wetlands
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Soil Sample Collection
2.2. Soil Physicochemical and Microbial Analyses
2.3. High-Throughput Sequencing
2.4. Soil Enzymatic Activity Determination
2.5. Data Analysis
3. Results
3.1. Soil Chemical Properties
3.2. Soil Enzymatic Activity
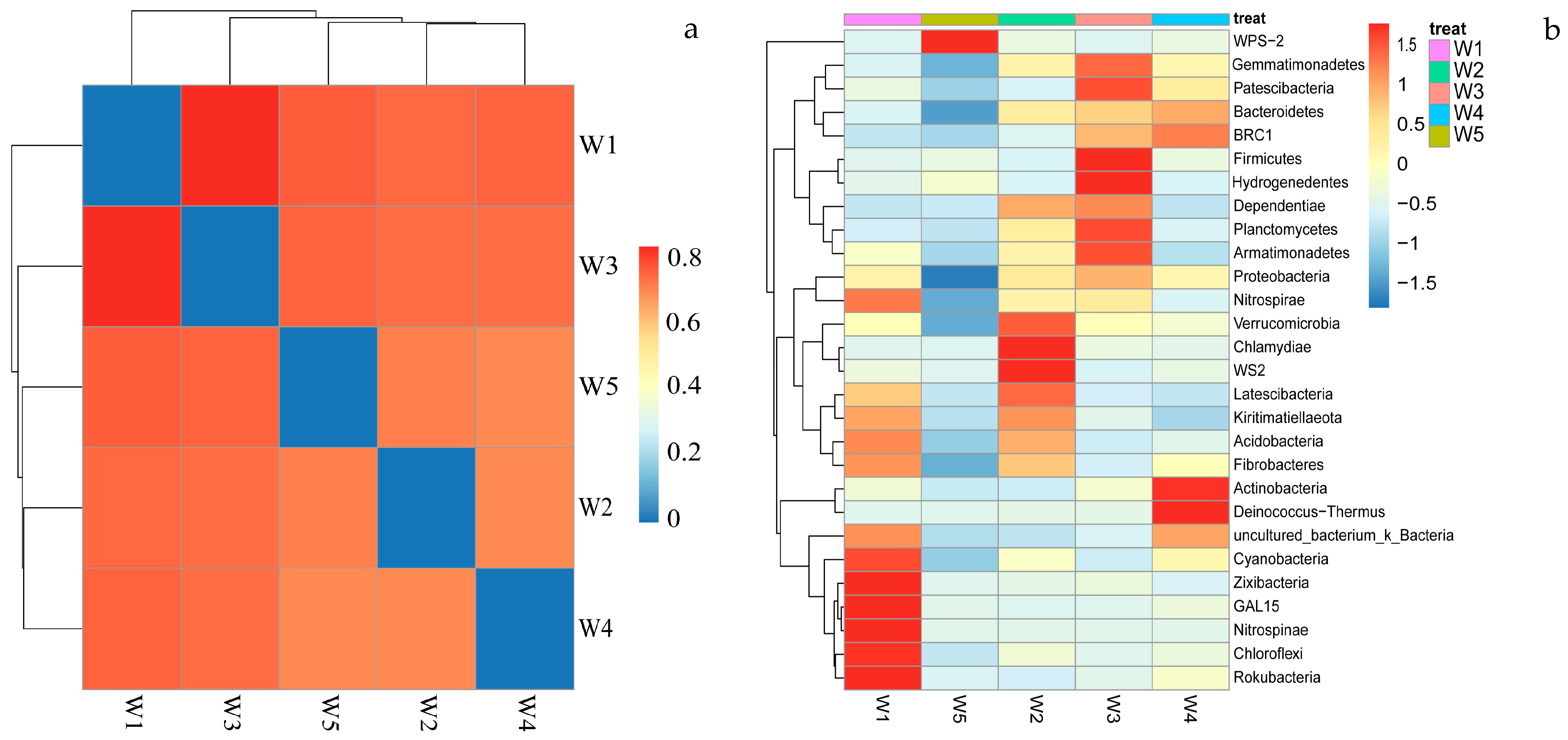
3.3. Composition of Soil Bacterial Community
3.4. Soil Bacterial Diversity
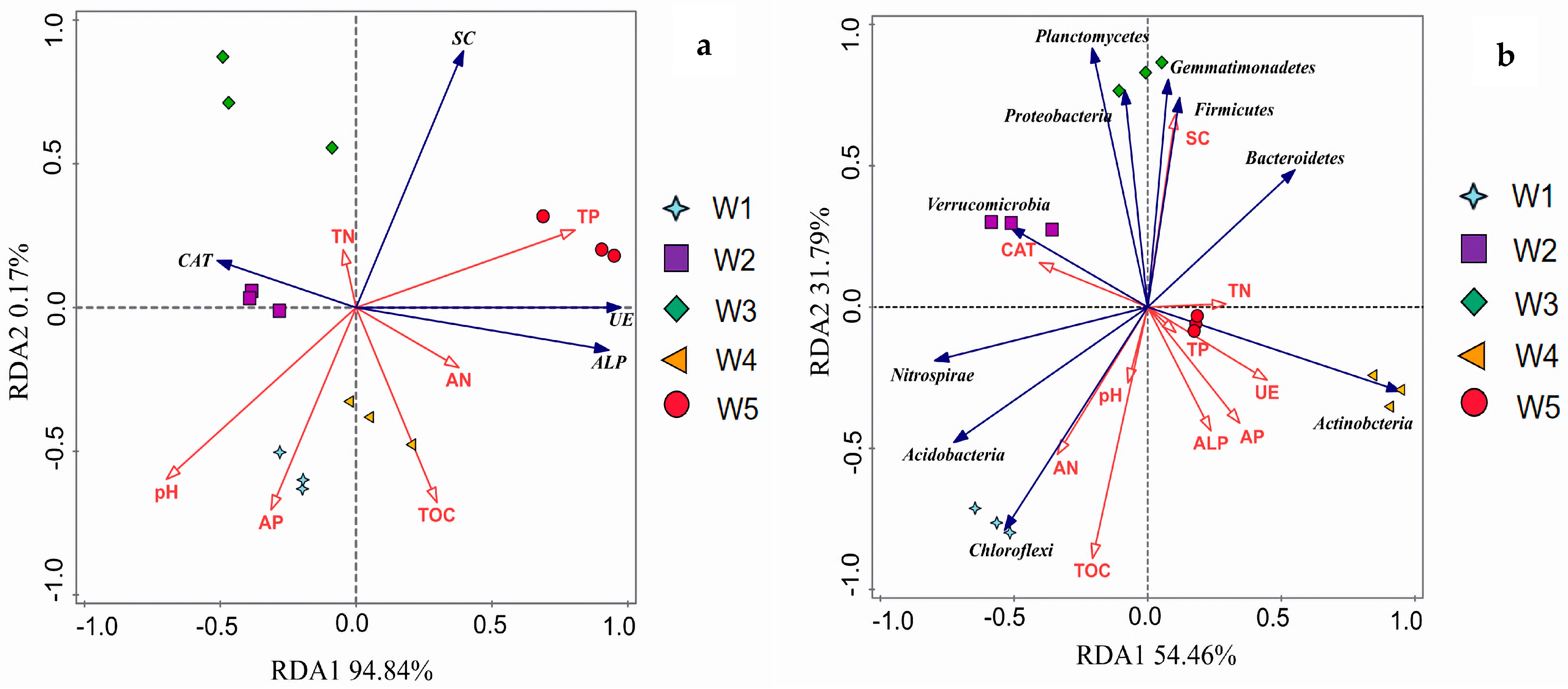
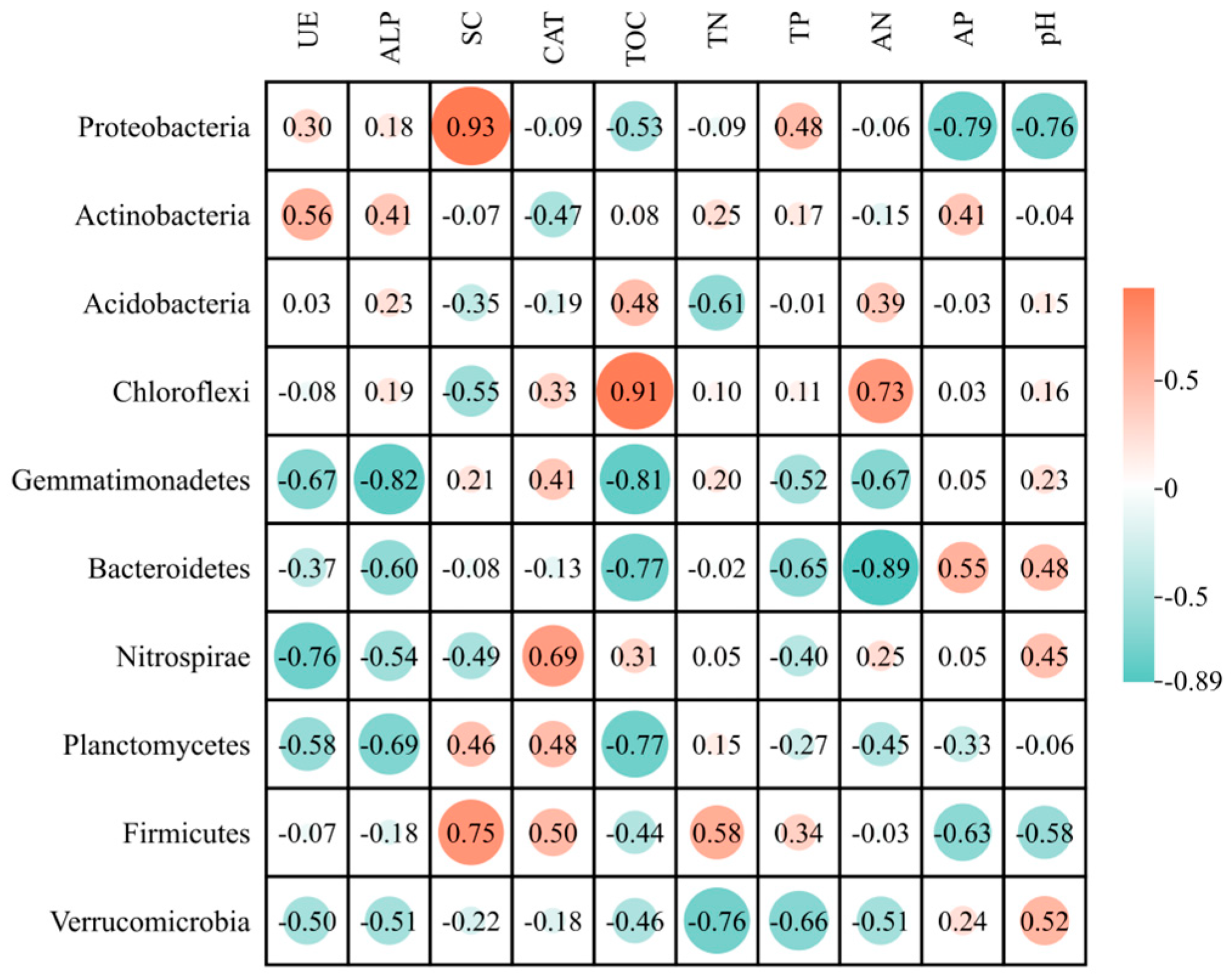
3.5. Correlation Between Soil Bacterial Communities, Enzymes, and Soil Chemical Properties
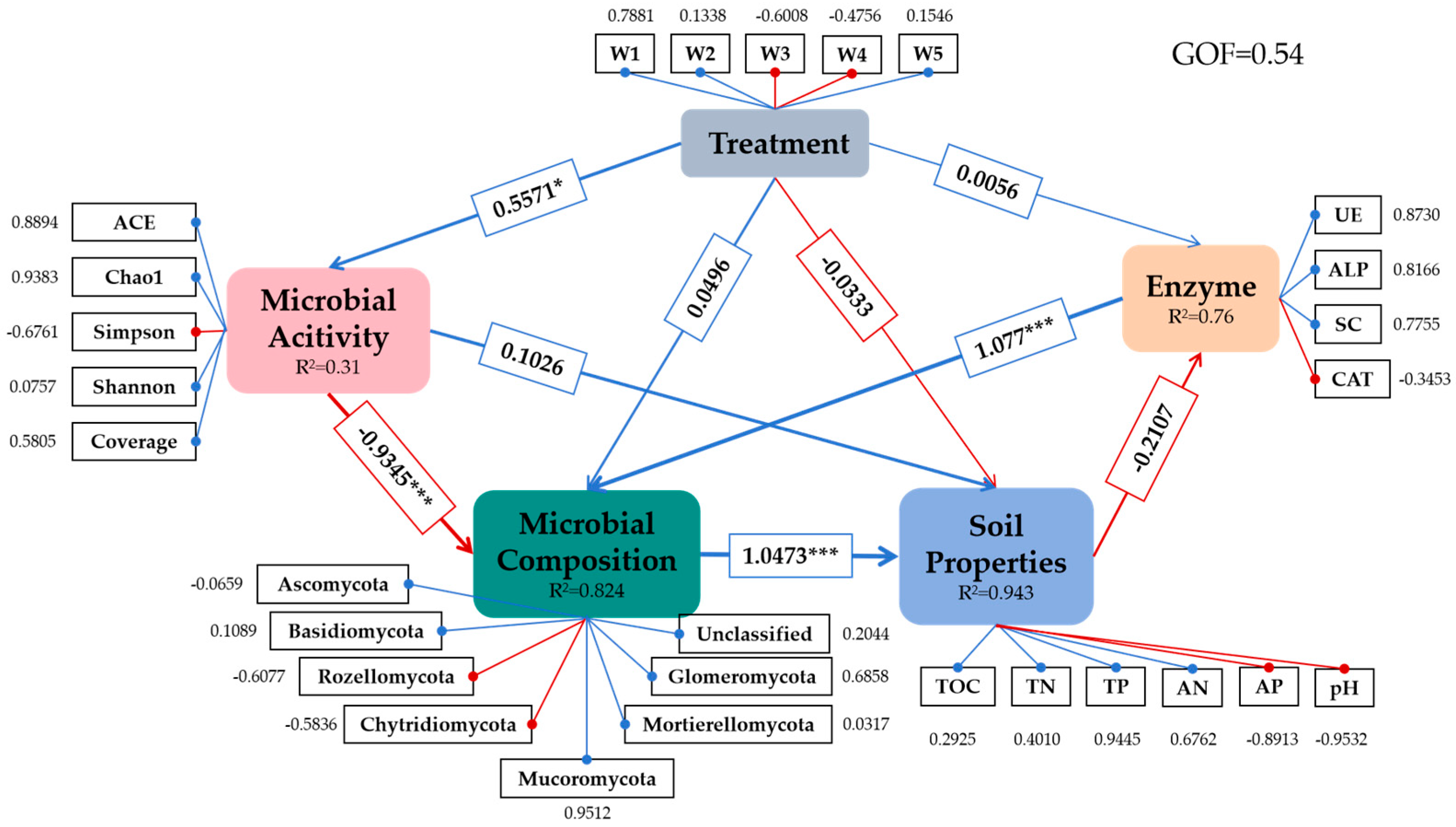
3.6. Integrated Summary of Findings
4. Discussion
4.1. Effects of Vegetation Type on Soil Nutrient Dynamics
4.2. Influence of Soil Bacterial Community Structure on Soil Functional Properties
4.3. Interactions Between Enzyme Activities and Microbial Diversity Across Vegetation Types
4.4. Structural Interdependencies Revealed by PLS-SEM
4.5. Implications for Saline Wetland Restoration and Management
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ding, J.; Yu, S. Structural and Functional Characteristics of Soil Microbial Communities in Forest–Wetland Ecotones: A Case Study of the Lesser Khingan Mountains. Life 2025, 15, 570. [Google Scholar] [CrossRef]
- Wu, D.; Bai, H.; Zhao, C.; Peng, M.; Chi, Q.; Dai, Y.; Gao, F.; Zhang, Q.; Huang, M.; Niu, B. The characteristics of soil microbial co-occurrence networks across a high-latitude forested wetland ecotone in China. Front. Microbiol. 2023, 14, 1160683. [Google Scholar] [CrossRef]
- Wang, H.; Krauss, K.W.; Noe, G.B.; Stagg, C.L.; Swarzenski, C.M.; Duberstein, J.A.; Conner, W.H.; DeAngelis, D.L. Modeling soil porewater salinity response to drought in tidal freshwater forested wetlands. J. Geophys. Res. Biogeosci. 2020, 125, e2018JG004996. [Google Scholar] [CrossRef]
- Wang, H.; Dai, Z.; Trettin, C.C.; Krauss, K.W.; Noe, G.B.; Burton, A.J.; Stagg, C.L.; Ward, E.J. Modeling impacts of drought-induced salinity intrusion on carbon dynamics in tidal freshwater forested wetlands. Ecol. Appl. 2022, 32, e2700. [Google Scholar] [CrossRef] [PubMed]
- Stefanowicz, A.M.; Kapusta, P.; Stanek, M.; Rożek, K.; Rola, K.; Zubek, S. Herbaceous plant species and their combinations positively affect soil microorganisms and processes and modify soil physicochemical properties in a mesocosm experiment. For. Ecol. Manag. 2023, 532, 120826. [Google Scholar] [CrossRef]
- Ren, J.; Li, X.; Zhao, K. Quantitative Analysis of Relationships Between Crack Characteristics and Properties of Soda-saline Soils in Songnen Plain, China. Chin. Geogr. Sci. 2015, 25, 591–601. [Google Scholar] [CrossRef]
- Daba, A. Rehabilitation of soil salinity and sodicity using diverse amendments and plants: A critical review. Discov. Environ. 2025, 3, 53. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, J.; Liu, R.; Tang, Y.; Wu, D.; Jin, R.; Zhu, W. Soil properties, climate, and topography jointly determine plant community characteristics in marsh wetlands. J. Plant Res. 2025, 138, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, L.; Shi, Z.; Yang, Y.; Liu, J. Microbial nutrient limitation and carbon use efficiency in saline-alkali soil amended with biochar: Insights from ecoenzymatic C: N: P stoichiometry. Biochar 2025, 7, 68. [Google Scholar] [CrossRef]
- Song, X.; Su, Y.; Zheng, J.; Zhang, Z.; Liang, Z.; Tang, Z. Study on the effects of salt tolerance type, soil salinity and soil characteristics on the element composition of Chenopodiaceae halophytes. Plants 2022, 11, 1288. [Google Scholar] [CrossRef] [PubMed]
- Tootoonchi, M.; Gettys, L.A.; Ferrell, J.A.; Erickson, J.E.; Bhadha, J.H. Salt tolerance assessment of aquatic and wetland plants: Increased salinity can reshape aquatic vegetation communities. Hydrobiologia 2023, 850, 4575–4587. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Zhao, Z.; Liu, R.; Wang, F.; Zhang, Z.; Yu, Q. Moss Biochar Facilitates Root Colonization of Halotolerant Halomonas salifodinae for Promoting Plant Growth Under Saline–Alkali Stress. Soil Syst. 2025, 9, 73. [Google Scholar] [CrossRef]
- Li, F.; Guo, Y.; Wang, Z.; Mu, Y. Influence of different phytoremediation on soil microbial diversity and community composition in saline-alkaline land. Int. J. Phytoremediation 2022, 24, 507–517. [Google Scholar] [CrossRef]
- Sergey, S. Learning from halophytes: Physiological basis and strategies to improve abiotic stress tolerance in crops. Ann. Bot. 2013, 112, 1209–1221. [Google Scholar] [CrossRef]
- He, Q.; Bertness, M.D. Extreme stresses, niches, and positive species interactions along stress gradients. Ecology 2014, 95, 1437–1443. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, Z.; Qu, Y.; Cao, Y.; Sun, F.; Dai, Y. Analysis of landscape change and its driving mechanism in Chagan Lake National Nature Reserve. Sustainability 2022, 14, 5675. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, G.; Zhao, M.; Wang, M.; Xue, Z.; Liu, B.; Jiang, M. Seed limitation and saline-alkaline stress restrict wetland restoration potential in the Songnen Plain, northeastern China. Ecol. Indic. 2021, 129, 107998. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, H.; Chen, J. Effects of continuous periodic Phragmites harvesting on microbial characteristics and soil multifunctionality in wetlands adjacent to residential and agricultural areas. Environ. Technol. Innov. 2025, 40, 104386. [Google Scholar] [CrossRef]
- Yan, J.; Wang, L.; Hu, Y.; Tsang, Y.F.; Zhang, Y.; Wu, J.; Fu, X.; Sun, Y. Plant litter composition selects different soil microbial structures and in turn drives different litter decomposition pattern and soil carbon sequestration capability. Geoderma 2018, 319, 194–203. [Google Scholar] [CrossRef]
- Lombardi, T.; Bertacchi, A.; Pistelli, L.; Pardossi, A.; Pecchia, S.; Toffanin, A.; Sanmartin, C. Biological and Agronomic Traits of the Main Halophytes Widespread in the Mediterranean Region as Potential New Vegetable Crops. Horticulturae 2022, 8, 195. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, T.; Peñuelas, J.; Sardans, J.; Tan, W.; Wei, X.; Cui, Y.; Cui, Q.; Wu, C.; Liu, L.; et al. Crop residue return sustains global soil ecological stoichiometry balance. Glob. Change Biol. 2023, 29, 2203–2226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xiao, G.Z.; Li, L.B.; Su, F.K.; Yang, J. Optimal Color Development Conditions and Spectrophotometric Wavelength for Determination of Total Phosphorus in Soil by Mo-Sb Anti Colorimetric Method. Soil Fertil. Sci. China 1992, 2, 46–48. [Google Scholar]
- Beck, T. Die Messung der Katalaseaktivitt von Bden. J. Plant Nutr. Soil Sci. 2010, 130, 68–81. [Google Scholar] [CrossRef]
- Shu, S.Y.; Wang, K.L.; Zhang, W.; He, X.Y.; Liu, S.J.; Wei, G.F. Soil alkaline phosphatase activity at different vegetation succession stages in karst peak-cluster depression. Chin. J. Ecol. 2010, 29, 1722–1728. [Google Scholar] [CrossRef]
- Karlson, P.; Nachtigall, M. Ein biologischer Test zur quantitativen Bestimmung der Juvenilhormon-Aktivitt von Insektenextrakten. J. Insect Physiol. 1961, 7, 210–215. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, Q.; Wang, L.; Liu, W.; Liu, X.; Huang, Y.; Christie, P. Response of soil enzymes and microbial communities to root extracts of the alien Alternanthera philoxeroides. Arch. Agron. Soil Sci. 2017, 64, 708–717. [Google Scholar] [CrossRef]
- Zandi, P.; Schnug, E. Reactive oxygen species, antioxidant responses and implications from a microbial modulation perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef]
- Du, X.; Li, M.S.; Song, D.; Yang, J.S.; Liu, H.; Sui, X.; Huo, T.B. Changes in the bacterial community structure and diversity of Chagan Lake sediments, northeastern China. Appl. Ecol. Environ. Res. 2022, 20, 4257–4270. [Google Scholar] [CrossRef]
- Yin, F.; Zhang, F. Reclamation of abandoned saline-alkali soil increased soil microbial diversity and degradation potential. Plant Soil 2022, 477, 521–538. [Google Scholar] [CrossRef]
- Pan, Y.; She, D.; Shi, Z.; Cao, T.; Xia, Y.; Shan, J. Salinity and high pH reduce denitrification rates by inhibiting denitrifying gene abundance in a saline-alkali soil. Sci. Rep. 2023, 13, 2155. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, K.; Dong, L.; Li, J.; Wang, X.; Shangguan, Z.; Qu, B.; Deng, L. Dynamics of litter decomposition rate and soil organic carbon sequestration following vegetation succession on the Loess Plateau, China. Catena 2023, 229, 107225. [Google Scholar] [CrossRef]
- Prommer, J.; Walker, T.W.N.; Wanek, W.; Braun, J.; Zezula, D.; Hu, Y.; Hofhansl, F.; Richter, A. Increased microbial growth, biomass, and turnover drive soil organic carbon accumulation at higher plant diversity. Glob. Change Biol. 2020, 26, 669–681. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Zhu, R.; Chen, N.; Ding, L.; Chen, C. Vegetation richness, species identity and soil nutrients drive the shifts in soil bacterial communities during restoration process. Environ. Microbiol. Rep. 2021, 13, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Sekaran, U.; McCoy, C.; Kumar, S.; Subramanian, S. Soil microbial community structure and enzymatic activity responses to nitrogen management and landscape positions in switchgrass (Panicum virgatum L.). Gcb Bioenergy 2019, 11, 836–851. [Google Scholar] [CrossRef]
- Ma, J.; Wang, G.; Liu, X.; Lei, B.; Xing, G. Effects of Phosphorus Application Levels on Its Uptake and Utilization in Foxtail Millet. Agronomy 2024, 14, 2078. [Google Scholar] [CrossRef]
- Luo, M.; Moorhead, D.L.; Ochoa-Hueso, R.; Mueller, C.W.; Ying, S.C.; Chen, J. Nitrogen loading enhances phosphorus limitation in terrestrial ecosystems with implications for soil carbon cycling. Funct. Ecol. 2022, 36, 2845–2858. [Google Scholar] [CrossRef]
- Araújo, C.A.; Ferreira, P.C.; Pupin, B.; Dias, L.P.; Avalos, J.; Edwards, J.; Hallsworth, J.E.; Rangel, D.E. Osmotolerance as a determinant of microbial ecology: A study of phylogenetically diverse fungi. Fungal Biol. 2019, 124, 273–288. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Pablo, C.H.D.; Mavrodi, O.V.; Weller, D.M.; Thomashow, L.S.; Mavrodi, D.V. Rhizosphere plant-microbe interactions under water stress. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Naylor, D.; McClure, R.; Jansson, J. Trends in microbial community composition and function by soil depth. Microorganisms 2022, 10, 540. [Google Scholar] [CrossRef]
- Nan, L.; Guo, Q.; Cao, S.; Zhan, Z. Diversity of bacterium communities in saline-alkali soil in arid regions of Northwest China. BMC Microbiol. 2022, 22, 11. [Google Scholar] [CrossRef]
- Song, L.; Wang, Y.; Zhang, R.; Yang, S. Microbial mediation of carbon, nitrogen, and sulfur cycles during solid waste decomposition. Microb. Ecol. 2023, 86, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Tong, D.; Li, Z.; Nie, X.; Liu, Y.; Ran, F.; Liao, S. Characteristics of microbial community composition and its relationship with carbon, nitrogen and sulfur in sediments. Sci. Total Environ. 2021, 795, 148848. [Google Scholar] [CrossRef]
- Dong, H.; Sun, H.; Fan, S.; Jiang, L.; Ma, D. Rhizobacterial communities, enzyme activity, and soil properties affect rice seedling’s nitrogen use. Agron. J. 2021, 113, 633–644. [Google Scholar] [CrossRef]
- Zheng, M.M.; Wang, C.; Li, W.X.; Guo, L.; Cai, Z.J.; Wang, B.R.; Chen, J.; Shen, R.F. Changes of acid and alkaline phosphatase activities in long-term chemical fertilization are driven by the similar soil properties and associated microbial community composition in acidic soil. Eur. J. Soil Biol. 2021, 104, 103312. [Google Scholar] [CrossRef]
- Qu, X.; Pan, Y.; Wang, P.; Ran, L.; Qin, G.; Li, Q.; Kang, P. Response of phyllosphere and rhizosphere microbial communities to salt stress of Tamarix chinensis. Plants 2024, 13, 1091. [Google Scholar] [CrossRef]
- Msimbira, L.A.; Smith, D.L. The roles of plant growth promoting microbes in enhancing plant tolerance to acidity and alkalinity stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Vaidya, S.; Dev, K.; Sourirajan, A. Distinct Osmoadaptation Strategies in the Strict Halophilic and Halotolerant Bacteria Isolated from Lunsu Saltwater Body of Northwest Himalayas. Curr. Microbiol. 2018, 75, 888–895. [Google Scholar] [CrossRef]
- Li, X.; Qu, C.; Bian, Y.; Gu, C.; Jiang, X.; Song, Y. New insights into the responses of soil microorganisms to polycyclic aromatic hydrocarbon stress by combining enzyme activity and sequencing analysis with metabolomics. Environ. Pollut. 2019, 255, 113312. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Miller, R.; Deng, S. Dynamic relationships between microbial community, enzyme activity, and soil properties across global ecosystems. Appl. Soil Ecol. 2025, 206, 105843. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, X.; Zhang, Y.; Gu, X.; Nie, H.; Zhu, L. Intercropping of Echinochloa frumentacea with Leguminous Forages Improves Hay Yields, Arbuscular Mycorrhizal Fungi Diversity, and Soil Enzyme Activities in Saline–Alkali Soil. Agronomy 2023, 13, 2356. [Google Scholar] [CrossRef]
- Song, J.; Min, L.; Wu, J.; He, Q.; Chen, F.; Wang, Y.; Nazir, R. Response of the microbial community to phosphate-solubilizing bacterial inoculants on Ulmus chenmoui Cheng in Eastern China. PLoS ONE 2021, 16, e0247309. [Google Scholar] [CrossRef]
- AbdelGawad, H.; Abuelsoud, W.; Madany, M.M.; Selim, S.; Zinta, G.; Mousa, A.S.; Hozzein, W.N. Actinomycetes enrich soil rhizosphere and improve seed quality as well as productivity of legumes by boosting nitrogen availability and metabolism. Biomolecules 2020, 10, 1675. [Google Scholar] [CrossRef]
- Zhou, J.; Miao, Y.; Guo, L.; Zhang, T.; Nie, Z.; Luo, X.; Yang, F.; Wang, Z. Evaluating the Enzyme Activities and Soil Physicochemical Properties of Four Typical Halophytic Communities in Saline-Sodic Soil. Agronomy 2024, 14, 141. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y. Microbial response to environmental stresses: From fundamental mechanisms to biotechnological applications. FEMS Microbiol. Rev. 2020, 44, 348–369. [Google Scholar] [CrossRef]
- Bustos-Caparros, E.; Viver, T.; Gago, J.F.; Rodriguez-Rojas, L.M.; Hatt, J.K.; Venter, S.N.; Fuchs, B.M.; Amann, R.; Bosch, R.; Konstantinidis, K.T.; et al. Ecological success of extreme halophiles subjected to recurrent osmotic disturbances is primarily driven by congeneric species replacement. ISME J. 2024, 18, wrae215. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, L.; Qi, X.; Huang, J.; Han, M.; Wang, C.; Li, X.; Jiang, H. Microbial assembly and stress-tolerance mechanisms in salt-adapted plants along the shore of a Salt Lake: Implications for saline–alkaline soil remediation. Microorganisms 2025, 13, 1942. [Google Scholar] [CrossRef]
- Yin, R.; Deng, H.; Wang, H.-L.; Zhang, B. Vegetation type affects soil enzyme activities and microbial functional diversity following re-vegetation of a severely eroded red soil in sub-tropical China. Catena 2014, 115, 96–103. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Tardy, V.; Spor, A.; Mathieu, O.; Lévèque, J.; Terrat, S.; Plassart, P.; Regnier, T.; Bardgett, R.D.; van der Putten, W.H.; Roggero, P.P.; et al. Shifts in microbial diversity through land use intensity as drivers of carbon mineralization in soil. Soil Biol. Biochem. 2015, 90, 204–213. [Google Scholar] [CrossRef]
- Wang, D.; Lan, Y.; Chen, W.; Liu, Z.; Gao, J.; Cao, D.; Wang, Q.; Mazhang, C.; An, X. Response of bacterial communities, enzyme activities and dynamic changes of soil organic nitrogen fractions to six-year different application levels of biochar retention in Northeast China. Soil Tillage Res. 2024, 240, 106097. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, R.; Petropoulos, E.; Yu, B.; Zhang, J.; Lin, X.; Gao, M.; Feng, Y. Interactive effects of salinity and SOM on the ecoenzymatic activities across coastal soils subjected to a saline gradient. Geoderma 2022, 406, 115519. [Google Scholar] [CrossRef]
- Chauhan, P.; Sharma, N.; Tapwal, A.; Kumar, A.; Verma, G.S.; Meena, M.; Seth, C.S.; Swapnil, P. Soil microbiome: Diversity, benefits and interactions with plants. Sustainability 2023, 15, 14643. [Google Scholar] [CrossRef]
- Qi, X.; Chen, T.; Ding, C.; Chen, X.; He, B.; Hu, W. Diversity of fungal communities in the rhizosphere soil of Tamarix chinensis in saline–alkaline wetland. Environ. Dev. Sustain. 2025, 27, 8693–8709. [Google Scholar] [CrossRef]
- Liu, L.; Liu, D.; Ding, X.; Chen, M.; Zhang, S. Straw incorporation and nitrogen fertilization enhance soil carbon sequestration by altering soil aggregate and microbial community composition in saline-alkali soil. Plant Soil 2024, 498, 341–356. [Google Scholar] [CrossRef]
- Sloey, T.M.; Ellis, V.S.; Kettenring, K.M. Using plant functional traits to inform wetland restoration. Wetlands 2023, 43, 92. [Google Scholar] [CrossRef]
- Farooqi, Z.U.R.; Qadir, A.A.; Alserae, H.; Raza, A.; Mohy-Ud-Din, W. Organic amendment–mediated reclamation and build-up of soil microbial diversity in salt-affected soils: Fostering soil biota for shaping rhizosphere to enhance soil health and crop productivity. Environ. Sci. Pollut. Res. 2023, 30, 109889–109920. [Google Scholar] [CrossRef]
- Sun, T.; Zhou, J.; Shi, L.; Feng, W.; Dippold, M.A.; Zang, H.; Kurganova, I.; de Gerenyu, V.L.; Kalinina, O.; Giani, L.; et al. Microbial growth rates, carbon use efficiency and enzyme activities during post-agricultural soil restoration. Catena 2022, 214, 106226. [Google Scholar] [CrossRef]
- Guo, J.; Kneeshaw, D.; Peng, C.; Wu, Y.; Feng, L.; Qu, X.; Wang, W.; Pan, C.; Feng, H. Positive effects of species mixing on biodiversity of understory plant communities and soil health in forest plantations. Proc. Natl. Acad. Sci. USA 2025, 122, e2418090122. [Google Scholar] [CrossRef]
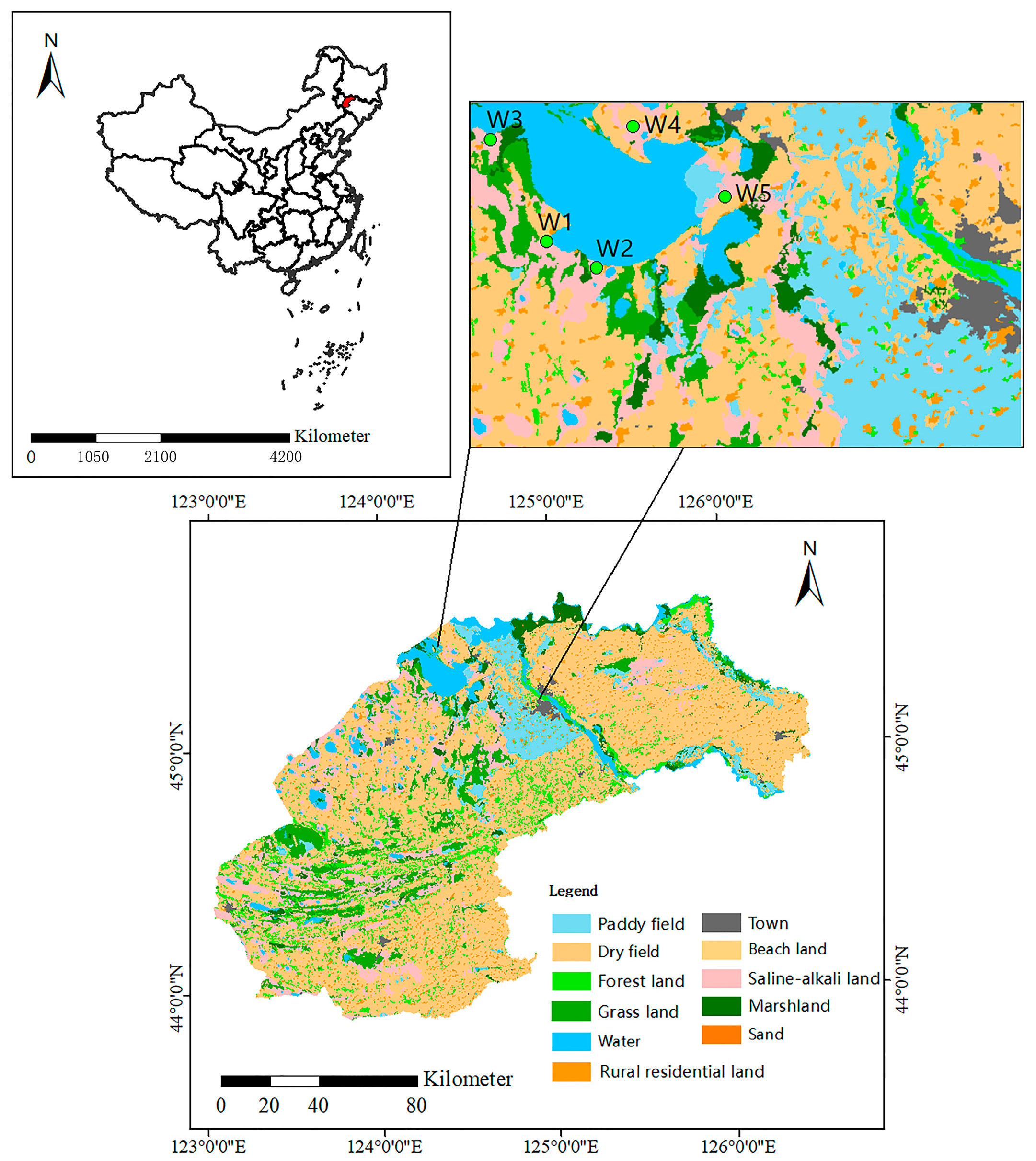
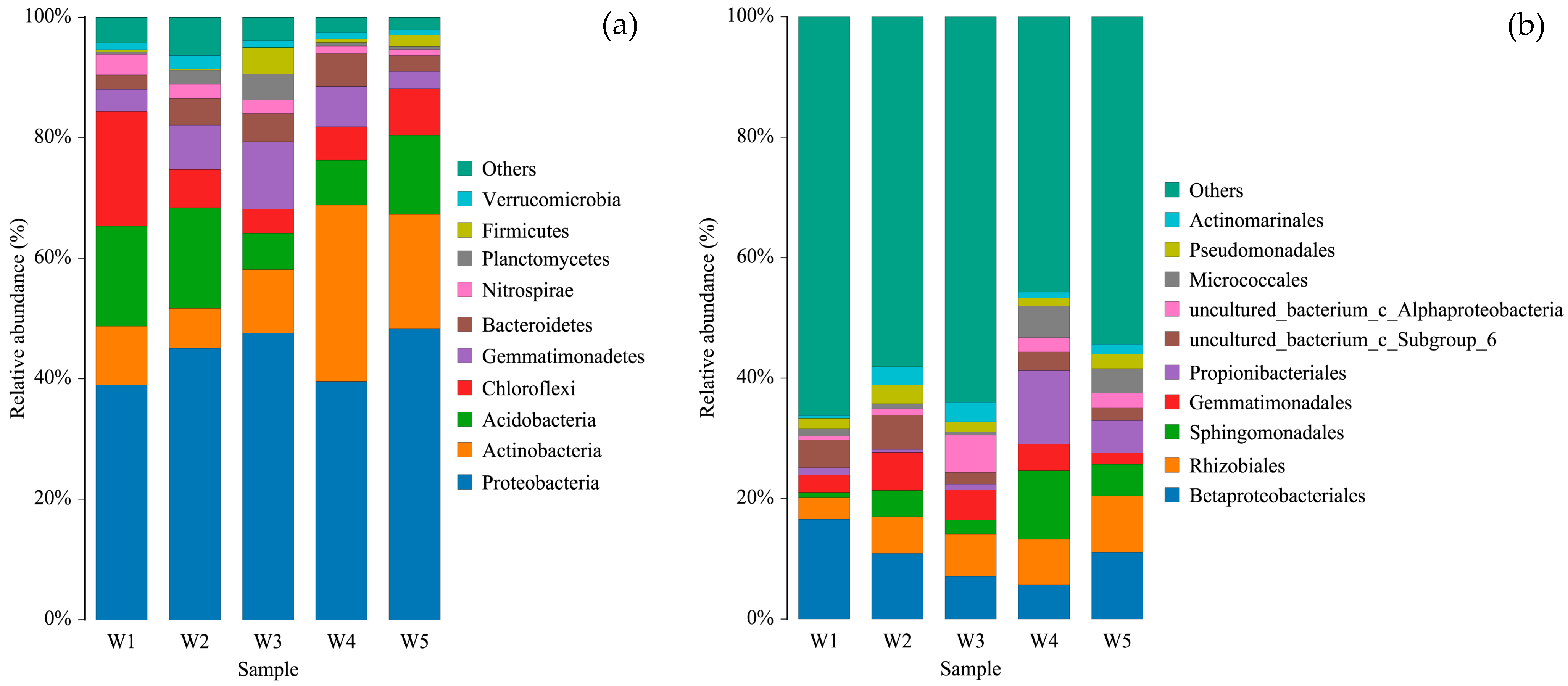
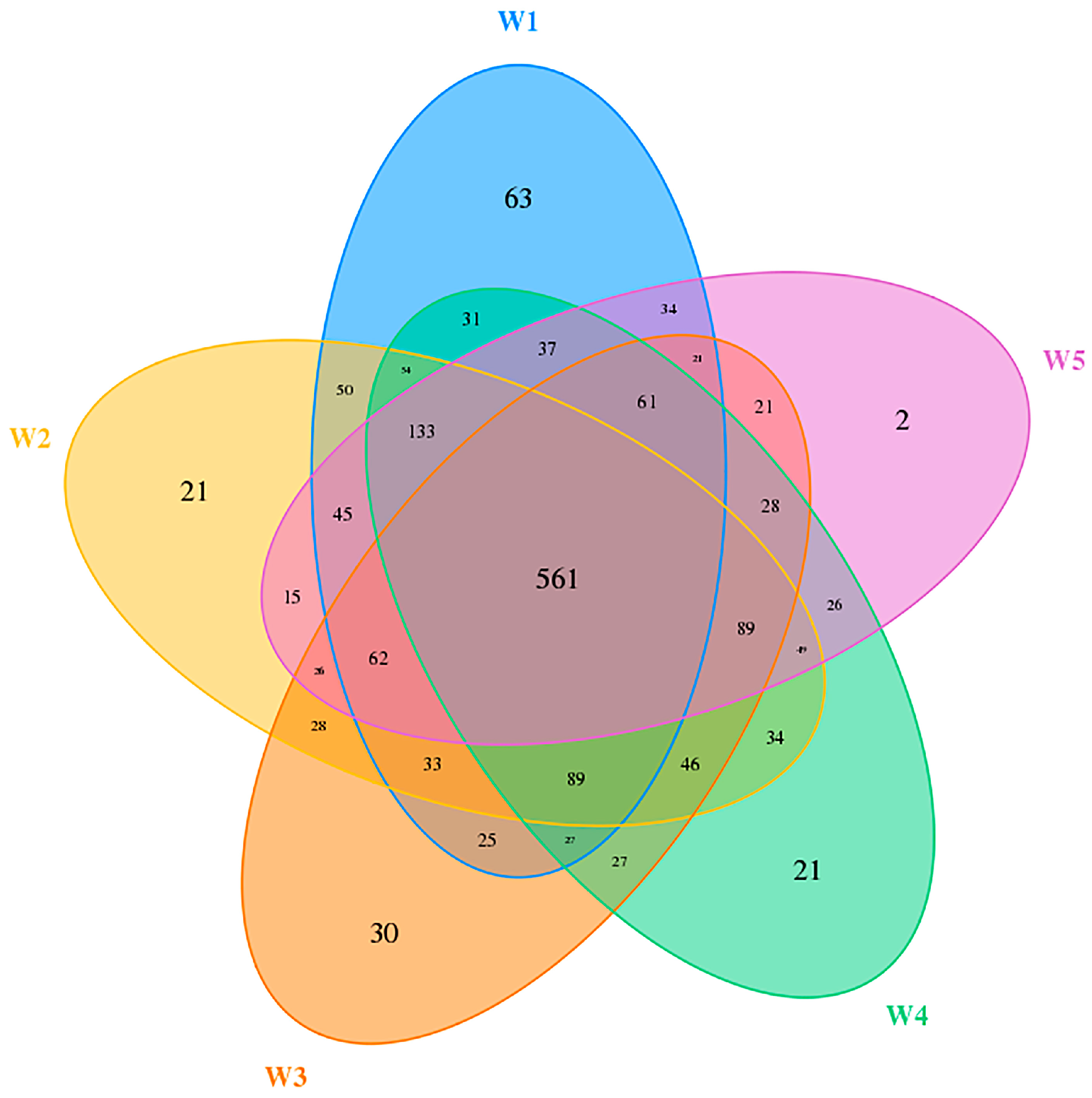
| Sample ID | Dominant Vegetation | pH | EC (dS/m) |
|---|---|---|---|
| W1 | Phragmites communis Trin. | 9.83 ± 0.05 | 3.6 ± 0.04 |
| W2 | Phragmites communis Trin. + Typha orientalis L. | 10.07 ± 0.02 | 2.8 ± 0.05 |
| W3 | Phragmites communis Trin. + Bryum spp. | 9.09 ± 0.10 | 3.2 ± 0.03 |
| W4 | Phragmites communis Trin. + Suaeda salsa (L.) Pall. | 10.12 ± 0.02 | 5.7 ± 0.02 |
| W5 | Echinochloa phyllopogon (Stapf) Koss. | 8.18 ± 0.02 | 1.8 ± 0.03 |
| Sample ID | SOC (g·kg−1) | TN (g·kg−1) | TP (g·kg−1) | AN (mg·kg−1) | AP (mg·kg−1) | pH |
|---|---|---|---|---|---|---|
| W1 | 9.47 ± 0.60 d | 0.58 ± 0.36 bc | 2.16 ± 1.24 c | 4.78 ± 0.40 b | 41.06 ± 0.20 c | 9.83 ± 0.05 c |
| W2 | 2.46 ± 0.25 a | 0.23 ± 0.81 a | 1.50 ± 1.16 a | 1.12 ± 0.12 a | 42.34 ± 0.53 c | 10.07 ± 0.02 d |
| W3 | 2.15 ± 0.08 a | 0.69 ± 1.40 c | 2.16 ± 0.91 c | 2.08 ± 0.35 a | 35.61 ± 1.25 b | 9.09 ± 0.10 b |
| W4 | 4.53 ± 0.16 b | 0.55 ± 0.12 bc | 1.79 ± 1.26 b | 1.28 ± 0.40 a | 48.61 ± 1.43 d | 10.12 ± 0.02 d |
| W5 | 6.17 ± 0.01 c | 0.49 ± 0.70 b | 3.17 ± 1.09 d | 4.14 ± 1.24 b | 33.53 ± 1.59 a | 8.18 ± 0.02 a |
| Sample ID | UE (mg·g−1·d−1) | ALP (mg·g−1·d−1) | SC (mg·g−1·d−1) | CAT (mg·g−1·d−1) |
|---|---|---|---|---|
| W1 | 157.31 ± 2.18 b | 9.29 ± 0.28 b | 3.98 ± 1.73 a | 0.61 ± 0.02 b |
| W2 | 141.85 ± 3.96 b | 3.32 ± 0.16 a | 8.14 ± 0.98 b | 0.43 ± 0.06 a |
| W3 | 121.48 ± 9.70 a | 0.75 ± 0.14 a | 14.35 ± 1.80 c | 0.64 ± 0.02 b |
| W4 | 236.1 ± 16.17 c | 8.64 ± 1.69 b | 5.61 ± 1.18 a | 0.42 ± 0.03 a |
| W5 | 406.67 ± 5.61 d | 23.65 ± 3.55 c | 15.41 ± 0.00 c | 0.40 ± 0.06 a |
| Sample ID | ACE | Chao1 | Simpson | Shannon | Coverage |
|---|---|---|---|---|---|
| W1 | 1483.59 ± 4.56 b | 1510.54 ± 8.68 c | 0.99 ± 0.04 b | 8.8 ± 0.97 b | 0.99 ± 0 b |
| W2 | 1541.05 ± 8.01 c | 1580.49 ± 6.06 c | 0.99 ± 0.01 b | 8.74 ± 1.01 b | 0.99 ± 0 b |
| W3 | 1351.32 ± 5.35 a | 1353.45 ± 4.63 a | 0.99 ± 0.06 b | 8.48 ± 0.27 b | 0.99 ± 0 b |
| W4 | 1505.6 ± 5.90 c | 1565.26 ± 6.17 c | 0.98 ± 0.04 a | 8.14 ± 0.52 a | 0.99 ± 0 b |
| W5 | 1432.79 ± 6.06 b | 1417.49 ± 9.92 b | 1 ± 0.02 b | 8.95 ± 0.19 b | 0.97 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Jiang, S.; Wu, P.; Zhang, X.; Guo, X.; Qu, Y.; Zheng, J.; Xing, Y. Vegetation-Driven Changes in Soil Properties, Enzymatic Activities, and Microbial Communities of Saline–Alkaline Wetlands. Forests 2025, 16, 1468. https://doi.org/10.3390/f16091468
Liu Q, Jiang S, Wu P, Zhang X, Guo X, Qu Y, Zheng J, Xing Y. Vegetation-Driven Changes in Soil Properties, Enzymatic Activities, and Microbial Communities of Saline–Alkaline Wetlands. Forests. 2025; 16(9):1468. https://doi.org/10.3390/f16091468
Chicago/Turabian StyleLiu, Qian, Shan Jiang, Pengbing Wu, Xu Zhang, Xingchi Guo, Ying Qu, Junyan Zheng, and Yuhe Xing. 2025. "Vegetation-Driven Changes in Soil Properties, Enzymatic Activities, and Microbial Communities of Saline–Alkaline Wetlands" Forests 16, no. 9: 1468. https://doi.org/10.3390/f16091468
APA StyleLiu, Q., Jiang, S., Wu, P., Zhang, X., Guo, X., Qu, Y., Zheng, J., & Xing, Y. (2025). Vegetation-Driven Changes in Soil Properties, Enzymatic Activities, and Microbial Communities of Saline–Alkaline Wetlands. Forests, 16(9), 1468. https://doi.org/10.3390/f16091468






