Biopolymer-Based Solutions for Sustainable Wood Modification: A Review of Current Advancements
Abstract
1. Introduction
2. Wood Modification Techniques with Biopolymers
2.1. Furfurylation
2.1.1. Process Description
2.1.2. Changes in Material Properties
2.2. Polylactic Acid (PLA)
2.2.1. Process Description
2.2.2. Changes in Material Properties
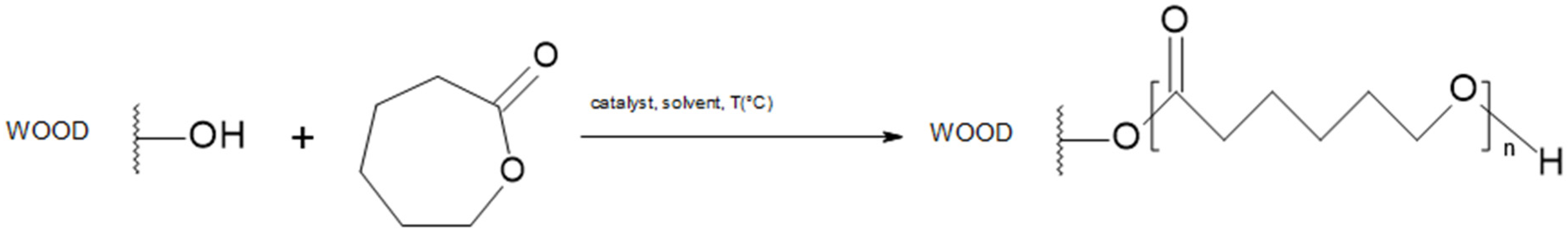
2.3. Polycaprolactone (PCL)
2.3.1. Process Description
2.3.2. Changes in Material Properties
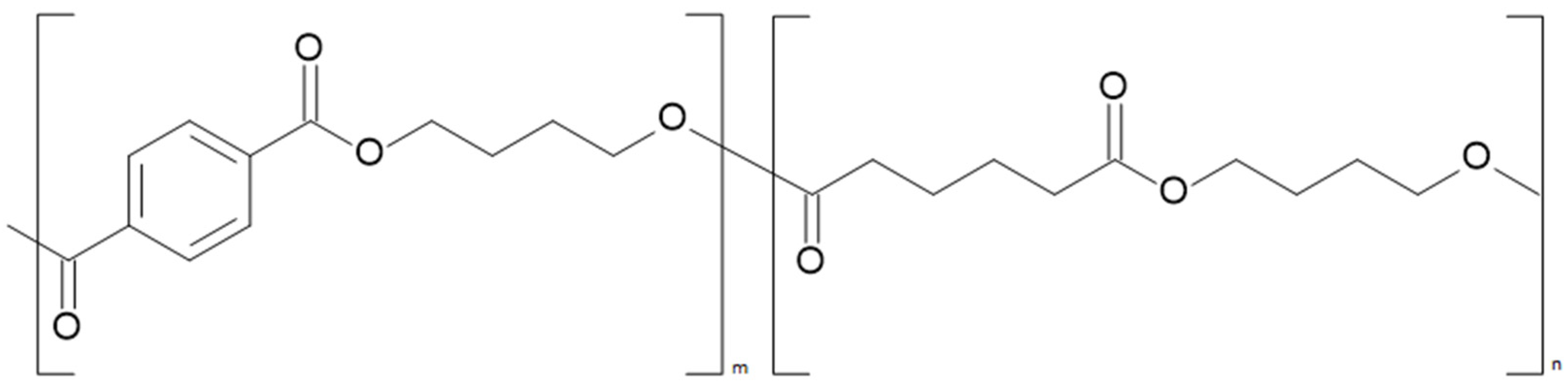
2.4. Polybutylene Adipate Terephthalate (PBAT)
2.4.1. Process Description
2.4.2. Changes in Material Properties
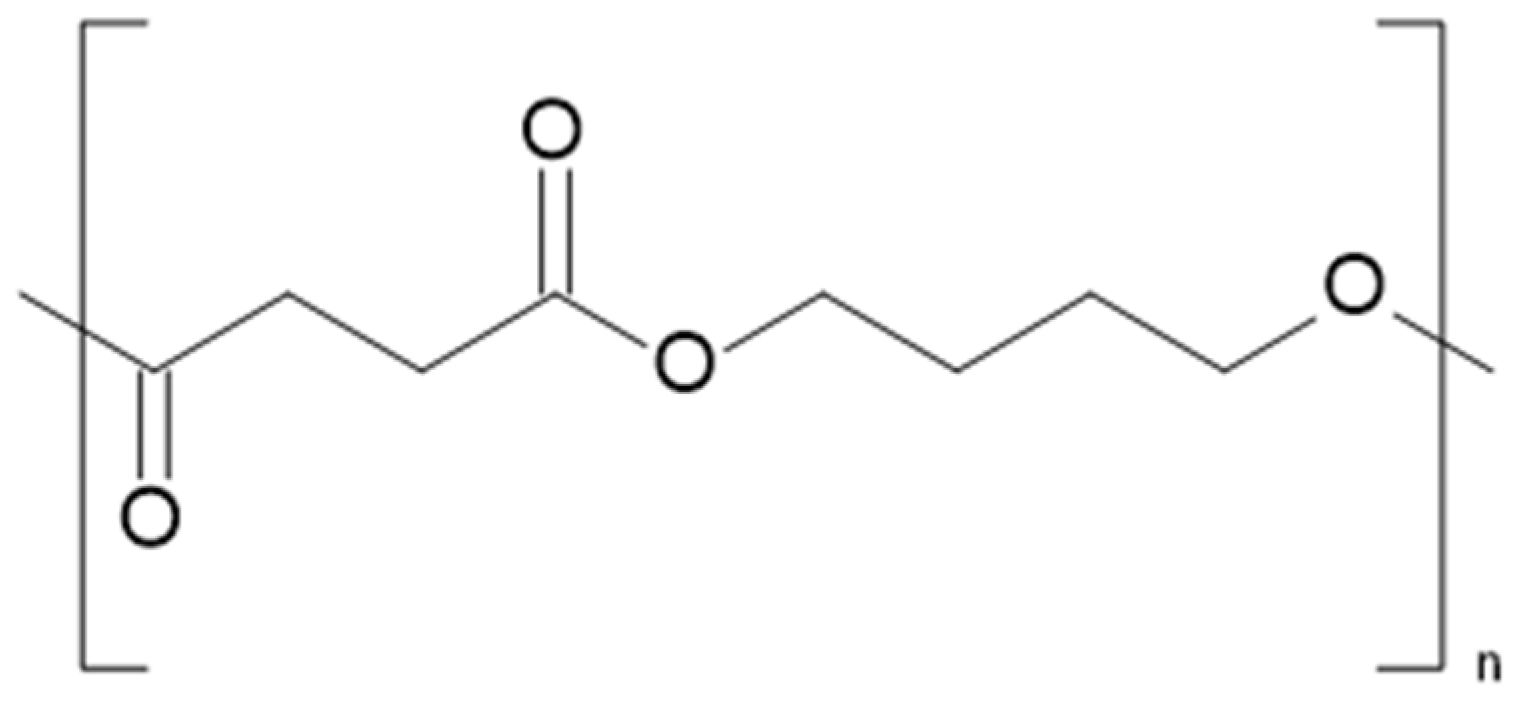
2.5. Poly(Butylene Succinate) (PBS)
2.5.1. Process Description
2.5.2. Changes in Material Properties
2.6. Zein
2.6.1. Process Description
2.6.2. Changes in Material Properties
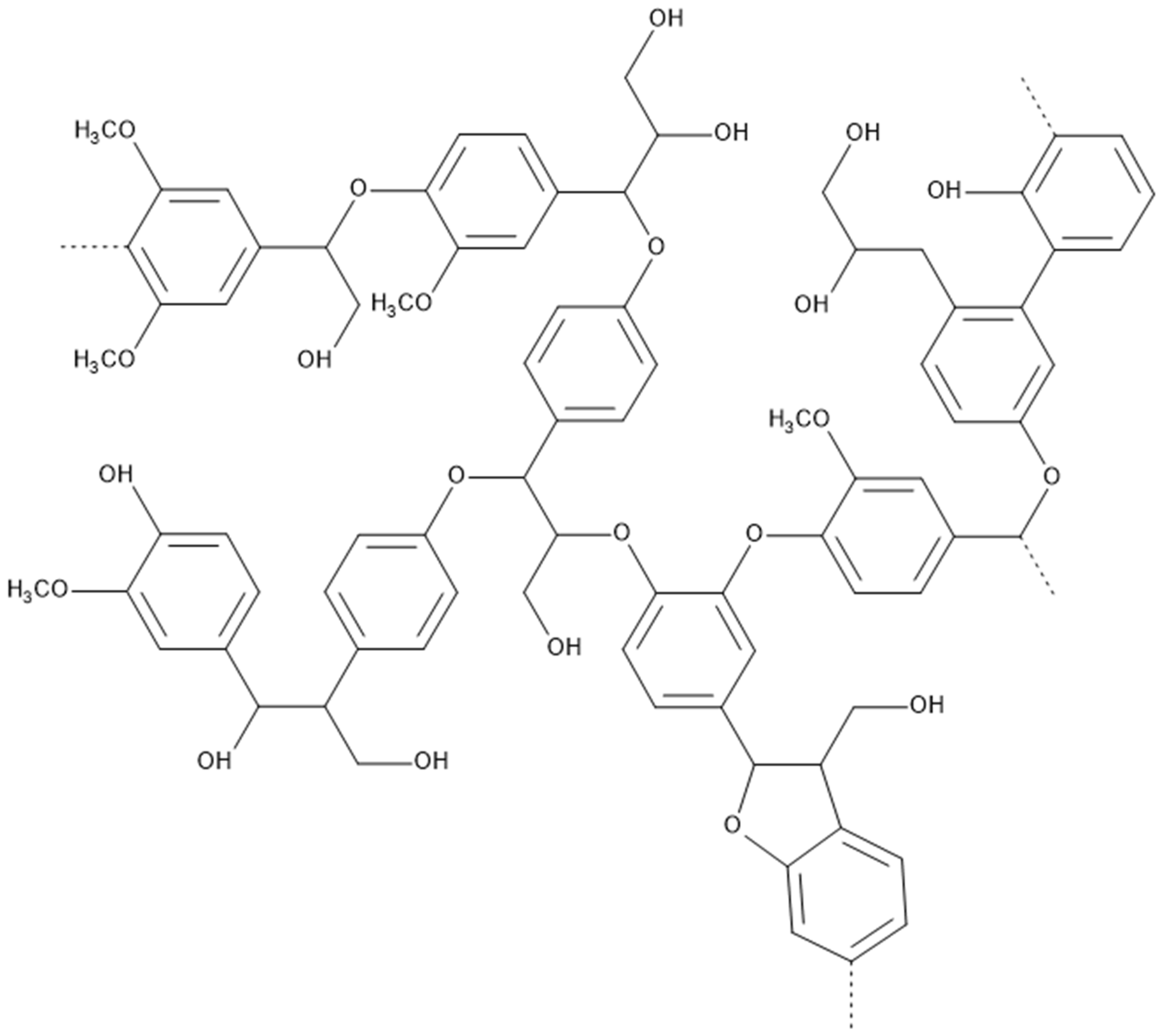
2.7. Lignin
2.7.1. Process Description
2.7.2. Changes in Material Properties
2.8. Tannin
2.8.1. Process Description
2.8.2. Changes in Material Properties
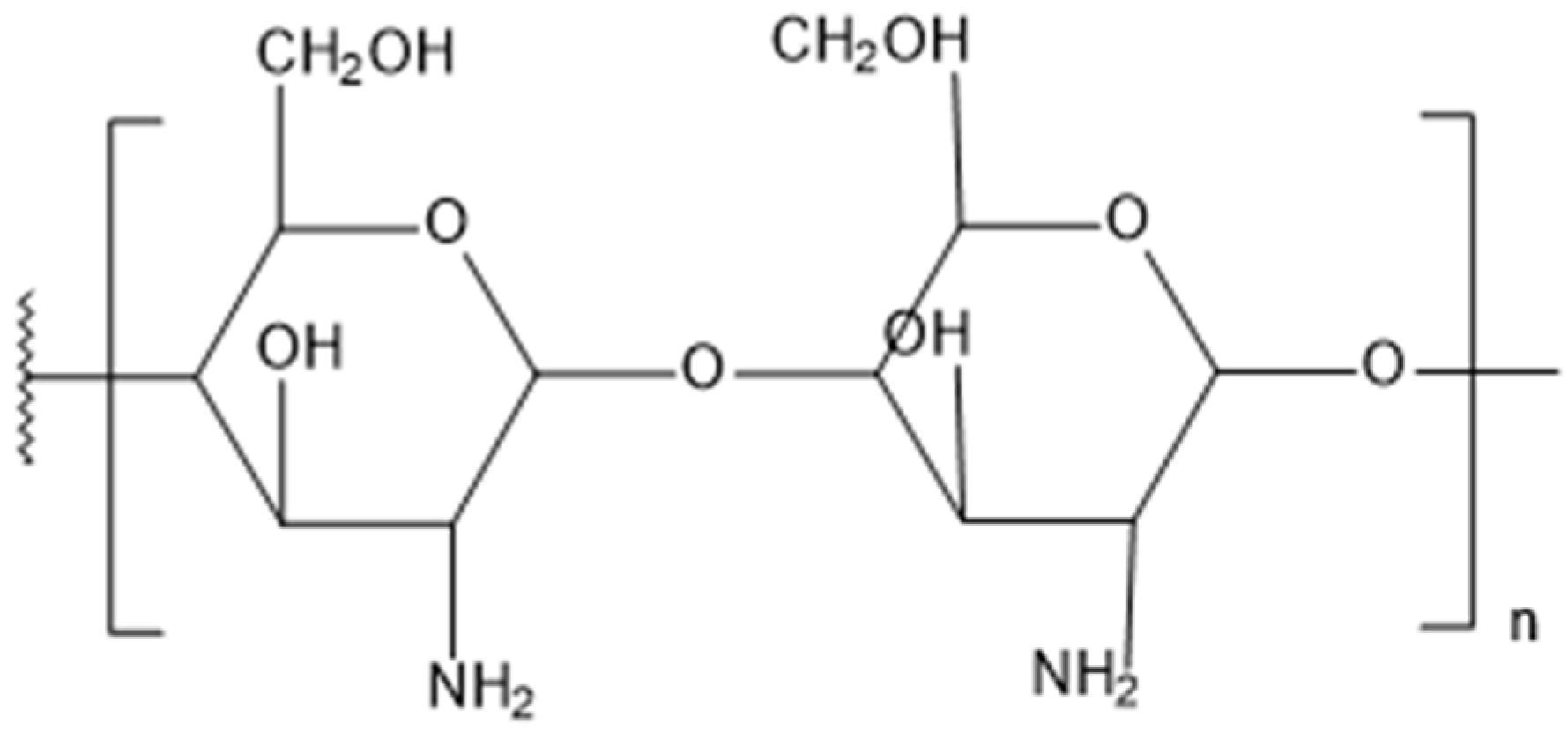
2.9. Chitosan
2.9.1. Process Description
2.9.2. Changes in Material Properties
2.10. Alginate
2.10.1. Process Description
2.10.2. Changes in Material Properties
2.11. Natural Gums
2.11.1. Process Description
2.11.2. Changes in Material Properties
2.12. Fatty Acids
2.12.1. Process Description
2.12.2. Changes in Material Properties
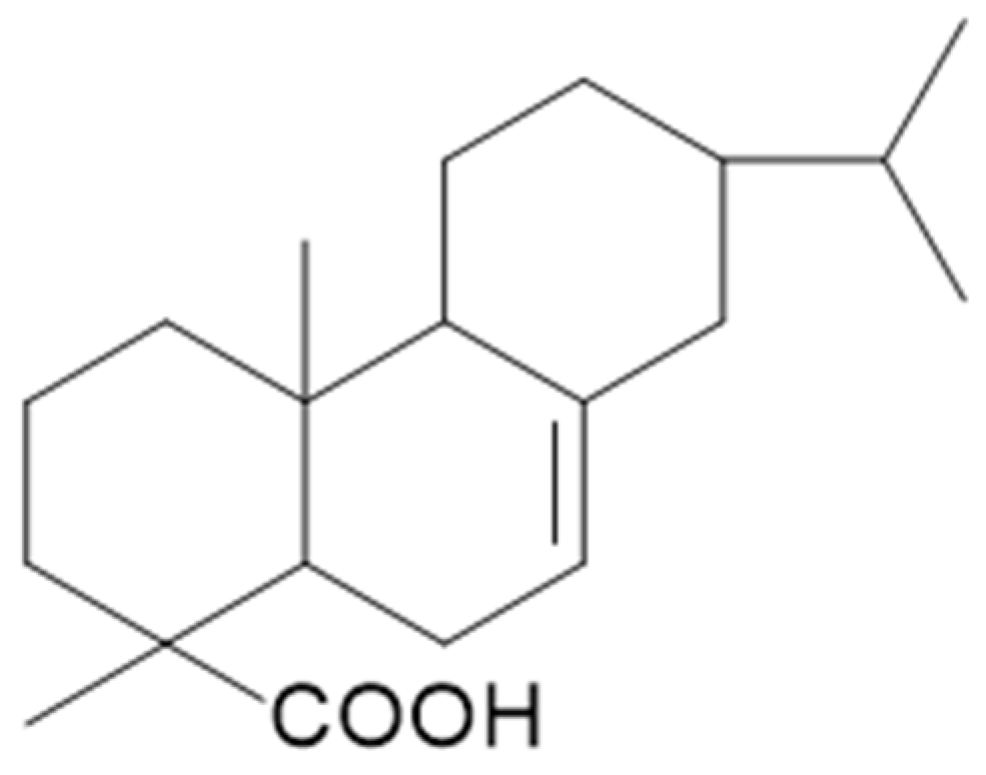
2.13. Rosin
2.13.1. Process Description
2.13.2. Changes in Material Properties
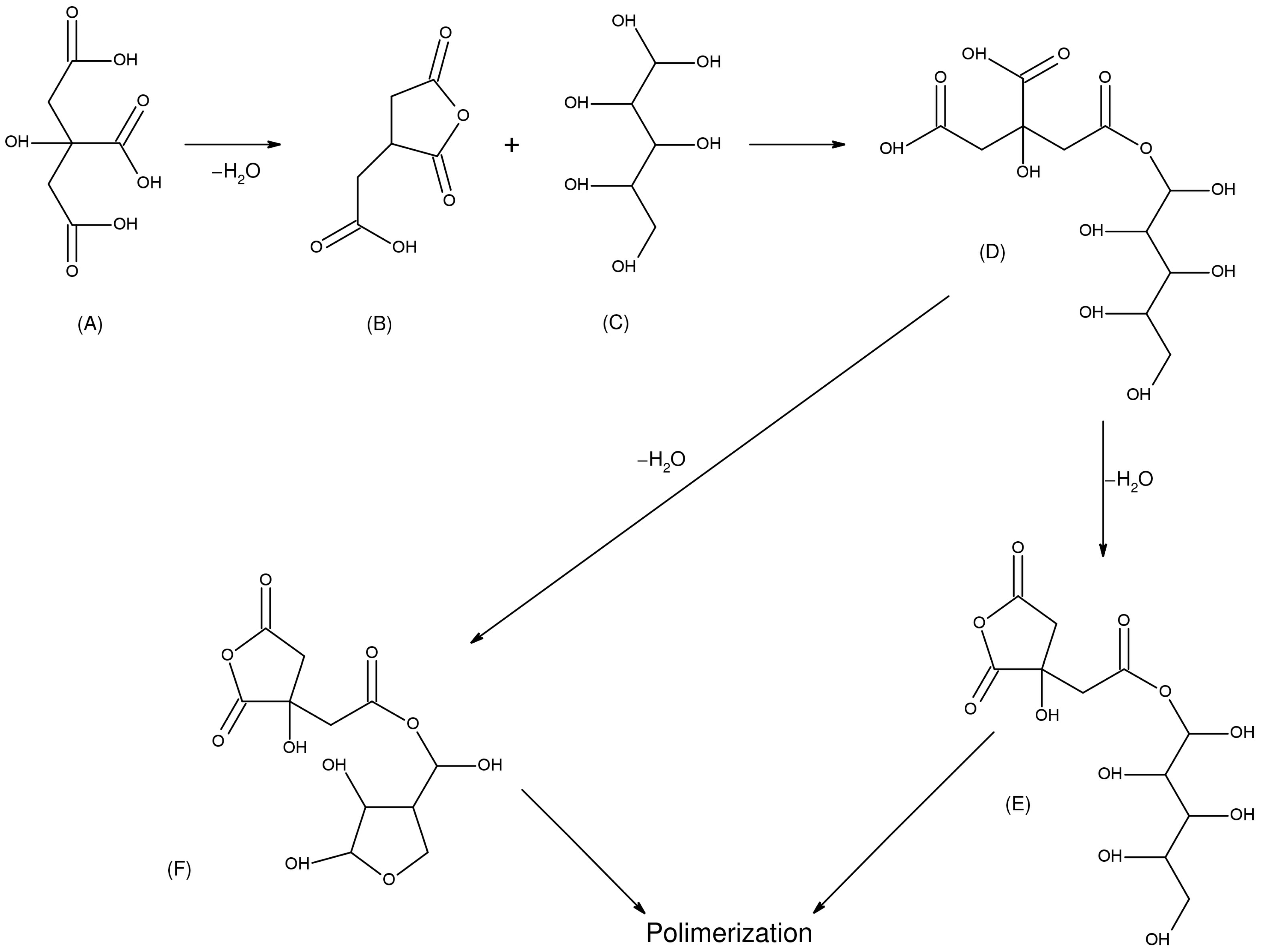
2.14. Sorbitol and Citric Acid
2.14.1. Process Description
2.14.2. Changes in Material Properties
3. Comparative Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of Plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, A. Wood Products and Green Chemistry. Ann. For. Sci. 2016, 73, 185–203. [Google Scholar] [CrossRef]
- Chandra, R.; Rustgi, R. Biodegradable Polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Patachia, S.; Croitoru, C. 14—Biopolymers for Wood Preservation. In Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials; Pacheco-Torgal, F., Ivanov, V., Karak, N., Jonkers, H., Eds.; Woodhead Publishing: Sawston, Cambridg, UK, 2016; pp. 305–332. ISBN 978-0-08-100214-8. [Google Scholar]
- Herrera, R.; Franco, L.; Rodríguez-Galán, A.; Puiggalí, J. Characterization and Degradation Behavior of Poly(Butylene Adipate-Co-Terephthalate)s. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 4141–4157. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Li, S.; Li, J. The Combined Effects of Copper Sulfate and Rosin Sizing Agent Treatment on Some Physical and Mechanical Properties of Poplar Wood. Constr. Build. Mater. 2013, 40, 33–39. [Google Scholar] [CrossRef]
- Jian, J.; Xiangbin, Z.; Xianbo, H. An Overview on Synthesis, Properties and Applications of Poly(Butylene-Adipate-Co-Terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Goldstein, I. Impregnating Solutions and Method of Impregnation Therewith. U.S. Patent 2,909,450, 20 October 1959. [Google Scholar]
- Stamm, A.J. Dimensional Stabilization of Wood with Furfuryl Alcohol Resin. In Wood Technology: Chemical Aspects; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1977; Volume 43, pp. 141–149. ISBN 978-0-8412-0373-0. [Google Scholar]
- Lande, S.; Westin, M.; Schneider, M. Properties of Furfurylated Wood. Scand. J. For. Res. 2004, 19, 22–30. [Google Scholar] [CrossRef]
- Pilgård, A.; Treu, A.; van Zeeland, A.; Gosselink, R.; Westin, M. Toxic Hazard and Chemical Analysis of Leachates from Furfurylated Wood. Environ. Toxicol. Chem. 2010, 29, 1918–1924. [Google Scholar] [CrossRef]
- Sandberg, D.; Kutnar, A.; Karlsson, O.; Jones, D. Wood Modification Technologies: Principles, Sustainability, and the Need for Innovation, 1st ed.; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-1-351-02822-6. [Google Scholar]
- Schneider, M.H. New Cell Wall and Cell Lumen Wood Polymer Composites. Wood Sci. Technol. 1995, 29, 121–127. [Google Scholar] [CrossRef]
- Our Story. 2025. Available online: https://kebony.com/about/our-story/ (accessed on 12 June 2025).
- Jones, D.; Sandberg, D.; Goli, G.; Todaro, L. (Eds.) Wood Modification in Europe: A State-of-the-Art About Processes, Products and Applications; Firenze University Press: Florence, Italy, 2019. [Google Scholar]
- Ringman, R.; Pilgård, A.; Richter, K. Brown Rot Gene Expression and Regulation in Acetylated and Furfurylated Wood: A Complex Picture. Holzforschung 2020, 74, 391–399. [Google Scholar] [CrossRef]
- Esteves, B.; Nunes, L.; Pereira, H. Properties of Furfurylated Wood (Pinus Pinaster). Eur. J. Wood Prod. 2011, 69, 521–525. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Ren, D.; Yu, Y.; Yu, Y. Wood Modification with Furfuryl Alcohol Catalysed by a New Composite Acidic Catalyst. Wood Sci. Technol. 2015, 49, 845–856. [Google Scholar] [CrossRef]
- Westin, M.; Rapp, A.; Nilsson, T. Field Test of Resistance of Modified Wood to Marine Borers. Wood Mater. Sci. Eng. 2006, 1, 34–38. [Google Scholar] [CrossRef]
- Mantanis, G.; Lykidis, C. Evaluation of Weathering of Furfurylated Wood Decks after a 3-Year Outdoor Exposure in Greece. Drv. Ind. 2015, 66, 115–122. [Google Scholar] [CrossRef]
- Acosta, A.P.; Schulz, H.R.; Barbosa, K.T.; Zanol, G.S.; Gallio, E.; Delucis, R.d.A.; Gatto, D.A.; Acosta, A.P.; Schulz, H.R.; Barbosa, K.T.; et al. Dimensional Stability and Colour Responses of Pinus Elliottii Wood Subjected to Furfurylation Treatments. Maderas. Cienc. Tecnol. 2020, 22, 303–310. [Google Scholar] [CrossRef]
- Sejati, P.S.; Imbert, A.; Gérardin-Charbonnier, C.; Dumarçay, S.; Fredon, E.; Masson, E.; Nandika, D.; Priadi, T.; Gérardin, P. Tartaric Acid Catalyzed Furfurylation of Beech Wood. Wood Sci. Technol. 2017, 51, 379–394. [Google Scholar] [CrossRef]
- Dong, Y.; Qin, Y.; Wang, K.; Yan, Y.; Zhang, S.; Li, J.; Zhang, S. Assessment of the Performance of Furfurylated Wood and Acetylated Wood: Comparison among Four Fast-Growing Wood Species. BioResources 2016, 11, 3679–3690. [Google Scholar] [CrossRef]
- Yang, T.; Ma, E.; Cao, J. Synergistic Effects of Partial Hemicellulose Removal and Furfurylation on Improving the Dimensional Stability of Poplar Wood Tested under Dynamic Condition. Ind. Crops Prod. 2019, 139, 111550. [Google Scholar] [CrossRef]
- Dong, Y.; Yan, Y.; Zhang, S.; Li, J.; Wang, J. Flammability and Physical–Mechanical Properties Assessment of Wood Treated with Furfuryl Alcohol and Nano-SiO2. Eur. J. Wood Prod. 2015, 73, 457–464. [Google Scholar] [CrossRef]
- Martha, R.; Mubarok, M.; Batubara, I.; Rahayu, I.S.; Setiono, L.; Darmawan, W.; Akong, F.O.; George, B.; Gérardin, C.; Gérardin, P. Effect of Furfurylation Treatment on Technological Properties of Short Rotation Teak Wood. J. Mater. Res. Technol. 2021, 12, 1689–1699. [Google Scholar] [CrossRef]
- Dongre, P.; Driscoll, M.; Amidon, T.; Bujanovic, B. Lignin-Furfural Based Adhesives. Energies 2015, 8, 7897–7914. [Google Scholar] [CrossRef]
- Lande, S.; Westin, M.; Schneider, M. Development of Modified Wood Products Based on Furan Chemistry. Mol. Cryst. Liq. Cryst. 2008, 484, 1/[367]–12/[378]. [Google Scholar] [CrossRef]
- Lande, S.; Eikenes, M.; Westin, M.; Schneider, M.H. Furfurylation of Wood: Chemistry, Properties, and Commercialization. In Development of Commercial Wood Preservatives; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2008; Volume 982, pp. 337–355. ISBN 978-0-8412-3951-7. [Google Scholar]
- Larsson-Brelid, P. Benchmarking and State-of-the-Art Report for Modified Wood; SP Technical Research Institute of Sweden: Borås, Sweden, 2013. [Google Scholar]
- EN 350:2016; Durability of Wood and Wood-Based Products. Testing and Classification of the Durability to Biological Agents of Wood and Wood-Based Materials. European Committee for Standardization: Brussels, Belgium, 2016.
- Lande, S.; Westin, M.; Schneider, M.H. Eco-efficient Wood Protection. Manag. Environ. Qual. Int. J. 2004, 15, 529–540. [Google Scholar] [CrossRef]
- Gérardin, P. New Alternatives for Wood Preservation Based on Thermal and Chemical Modification of Wood—A Review. Ann. For. Sci. 2016, 73, 559–570. [Google Scholar] [CrossRef]
- Hadi, Y.S.; Herliyana, E.N.; Mulyosari, D.; Abdillah, I.B.; Pari, R.; Hiziroglu, S. Termite Resistance of Furfuryl Alcohol and Imidacloprid Treated Fast-Growing Tropical Wood Species as Function of Field Test. Appl. Sci. 2020, 10, 6101. [Google Scholar] [CrossRef]
- Hadi, Y.S.; Mulyosari, D.; Herliyana, E.N.; Pari, G.; Arsyad, W.O.M.; Abdillah, I.B.; Gérardin, P. Furfurylation of Wood from Fast-Growing Tropical Species to Enhance Their Resistance to Subterranean Termite. Eur. J. Wood Prod. 2021, 79, 1007–1015. [Google Scholar] [CrossRef]
- Thygesen, L.G.; Beck, G.; Nagy, N.E.; Alfredsen, G. Cell Wall Changes during Brown Rot Degradation of Furfurylated and Acetylated Wood. Int. Biodeterior. Biodegrad. 2021, 162, 105257. [Google Scholar] [CrossRef]
- Slevin, C.R.; Westin, M.; Lande, S.; Cragg, S. Laboratory and Marine Trials of Resistance of Furfurylated Wood to Marine Borers: Eighth European Conference on Wood Modification. In Proceedings of the Eighth European Conference on Wood Modification, Helsinki, Finland, 26–27 October 2015; Hughes, M., Rautkari, L., Uimonen, T., Militz, H., Junge, B., Eds.; Aalto University: Espoo, Finland, 2015; pp. 464–471. [Google Scholar]
- Martin, L.S.; Jelavić, S.; Cragg, S.M.; Thygesen, L.G. Furfurylation Protects Timber from Degradation by Marine Wood Boring Crustaceans. Green Chem. 2021, 23, 8003–8015. [Google Scholar] [CrossRef]
- Westin, M.; Larsson-Brelid, P.; Nilsson, T.; Rapp, A.; Dickerson, J.; Lande, S.; Cragg, S. Marine Borer Resistance of Acetylated and Furfurylated Wood—Results from up to 16 Years of Field Exposure Marine Borer Resistance of Acetylated and Furfurylated Wood—Results from up to 16 Years of Field Exposure; International Research Group on Wood Protection: Stockholm, Sweden, 2016; IRG/WP 16-40756. [Google Scholar]
- Ringman, R.; Pilgård, A.; Richter, K. In Vitro Oxidative and Enzymatic Degradation of Modified Wood. Int. Wood Prod. J. 2015, 6, 36–39. [Google Scholar] [CrossRef]
- Ringman, R.; Pilgård, A.; Brischke, C.; Richter, K. Mode of Action of Brown Rot Decay Resistance in Modified Wood: A Review. Holzforschung 2014, 68, 239–246. [Google Scholar] [CrossRef]
- Choura, M.; Belgacem, N.M.; Gandini, A. Acid-Catalyzed Polycondensation of Furfuryl Alcohol: Mechanisms of Chromophore Formation and Cross-Linking. Macromolecules 1996, 29, 3839–3850. [Google Scholar] [CrossRef]
- Acosta, A.P.; Beltrame, R.; Missio, A.L.; de Avila Delucis, R.; Gatto, D.A. Juvenile and Mature Woods from Pine Subjected to in Situ Polymerization with Furfuryl Alcohol. Wood Mater. Sci. Eng. 2022, 17, 151–156. [Google Scholar] [CrossRef]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic Acid Technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Vink, E.T.H.; Rábago, K.R.; Glassner, D.A.; Gruber, P.R. Applications of Life Cycle Assessment to NatureWorksTM Polylactide (PLA) Production. Polym. Degrad. Stab. 2003, 80, 403–419. [Google Scholar] [CrossRef]
- Gupta, A.P.; Kumar, V. New Emerging Trends in Synthetic Biodegradable Polymers—Polylactide: A Critique. Eur. Polym. J. 2007, 43, 4053–4074. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(Lactic Acid) Modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Fink, H.-P.; Ganster, J. Novel Thermoplastic Composites from Commodity Polymers and Man-Made Cellulose Fibers. Macromol. Symp. 2006, 244, 107–118. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and Biodegradation of Poly(Lactide). Appl. Microbiol. Biotechnol. 2006, 72, 244–251. [Google Scholar] [CrossRef]
- Altun, Y.; Doğan, M.; Bayramlı, E. Effect of Alkaline Treatment and Pre-Impregnation on Mechanical and Water Absorbtion Properties of Pine Wood Flour Containing Poly (Lactic Acid) Based Green-Composites. J. Polym. Environ. 2013, 21, 850–856. [Google Scholar] [CrossRef]
- Madhav Gondaliya, A.; Foster, K.; Johan Foster, E. Polylactic Acid/Wood-Based in Situ Polymerized Densified Composite Material. Mater. Adv. 2023, 4, 5633–5642. [Google Scholar] [CrossRef]
- Avinc, O.; Khoddami, A. Overview of Poly(Lactic Acid) (PLA) Fibre: Part I: Production, Properties, Performance, Environmental Impact, and End-Use Applications of Poly(Lactic Acid) Fibres. Fibre Chem. 2009, 41, 391–401. [Google Scholar] [CrossRef]
- Li, X.; Lin, Y.; Liu, M.; Meng, L.; Li, C. A Review of Research and Application of Polylactic Acid Composites. J. Appl. Polym. Sci. 2023, 140, e53477. [Google Scholar] [CrossRef]
- Noël, M.; Fredon, E.; Mougel, E.; Masson, D.; Masson, E.; Delmotte, L. Lactic Acid/Wood-Based Composite Material. Part 1: Synthesis and Characterization. Bioresour. Technol. 2009, 100, 4711–4716. [Google Scholar] [CrossRef]
- Noel, M. Elaboration d’un Matériau Composite Innovant à Base de Bois et de Bio-Polymère D’acide Lactique; Université Henri Poincaré: Nancy, France, 2008; p. 263. [Google Scholar]
- Noël, M.; Mougel, E.; Fredon, E.; Masson, D.; Masson, E. Lactic Acid/Wood-Based Composite Material. Part 2: Physical and Mechanical Performance. Bioresour. Technol. 2009, 100, 4717–4722. [Google Scholar] [CrossRef]
- Báder, M.; Németh, R. Hosszirányban tömörített faanyagok kezelése tejsavval = Lactic-acid treatment of longitudinally compressed wood. Gradus 2019, 6, 59–66. [Google Scholar]
- Albertsson, A.-C.; Varma, I.K. Recent Developments in Ring Opening Polymerization of Lactones for Biomedical Applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef]
- Abdollahi, M.; Bairami Habashi, R.; Mohsenpour, M. Poly(ε-Caprolactone) Chains Grafted from Lignin, Hydroxymethylated Lignin and Silica/Lignin Hybrid Macroinitiators: Synthesis and Characterization of Lignin- Based Thermoplastic Copolymers. Ind. Crops Prod. 2019, 130, 547–557. [Google Scholar] [CrossRef]
- Olsén, P.; Herrera, N.; Berglund, L.A. Polymer Grafting Inside Wood Cellulose Fibers by Improved Hydroxyl Accessibility from Fiber Swelling. Biomacromolecules 2020, 21, 597–603. [Google Scholar] [CrossRef]
- Ermeydan, M.A.; Cabane, E.; Hass, P.; Koetz, J.; Burgert, I. Fully Biodegradable Modification of Wood for Improvement of Dimensional Stability and Water Absorption Properties by Poly(ε-Caprolactone) Grafting into the Cell Walls. Green Chem. 2014, 16, 3313–3321. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Suzuki, T. Hydrolysis of Copolyesters Containing Aromatic and Aliphatic Ester Blocks by Lipase. J. Appl. Polym. Sci. 1981, 26, 441–448. [Google Scholar] [CrossRef]
- Ermeydan, M.A.; Cambazoğlu, M.D.; Tomak, E. A Methodological Approach to ε-Caprolactone Modification of Wood. J. Wood Chem. Technol. 2022, 42, 286–296. [Google Scholar] [CrossRef]
- Akahori, S.; Osawa, Z. Preparation and Biodegradation of Polycaprolactone-Paper Composites. Polym. Degrad. Stab. 1994, 45, 261–265. [Google Scholar] [CrossRef]
- Gonultas, O.; Ermeydan, M.; Candan, Z. Main Chemical Components and Ftir Analysis of Chemically Modified Paulownia, Poplar, and Eucalyptus Wood by ε-Caprolactone. J. Adv. Technol. Sci. 2017, 6, 698–704. [Google Scholar]
- Ermeydan, M.; Gonultas, O.; Candan, Z. Chemical Modification of Paulownia, Poplar, and Eucalyptus Wood by ε-Caprolactone Grafting Inside Cell Walls to Improve Wood Properties. J. Adv. Technol. Sci. 2017, 6, 323–330. [Google Scholar]
- Ermeydan, M.; Babacan, M.; Dizman Tomak, E. Poly(ε-Caprolactone) Grafting into Scots Pine Wood: Improvement on the Dimensional Stability, Weathering and Decay Resistance. Cellulose 2021, 28, 5827–5841. [Google Scholar] [CrossRef]
- Cambazoglu, M.; Tomak, E.D.; Ermeydan, M.A. Natural Weathering of Spruce Wood Chemically Modified by Re-Used ε-Caprolactone Solution. Color. Technol. 2023, 139, 265–275. [Google Scholar] [CrossRef]
- Dizman Tomak, E.; Can, A.; Ermeydan, M. Biodegradability of Poly (Ɛ-Caprolactone) Modifed Wood by Decaying Fungi. J. Polym. Environ. 2023, 31, 4097–4111. [Google Scholar] [CrossRef]
- Ermeydan, M.A.; Kartal, Z.N.; Tomak, E.D. Effect of Process Variations of Polycaprolactone Modification on Wood Durability, Dimensional Stability and Boron Leaching. Holzforschung 2019, 73, 847–858. [Google Scholar] [CrossRef]
- Wang, H.-M.; Wang, B.; Yuan, T.-Q.; Zheng, L.; Shi, Q.; Wang, S.-F.; Song, G.-Y.; Sun, R.-C. Tunable, UV-Shielding and Biodegradable Composites Based on Well-Characterized Lignins and Poly(Butylene Adipate-Co-Terephthalate). Green Chem. 2020, 22, 8623–8632. [Google Scholar] [CrossRef]
- Ratshoshi, B.K.; Farzad, S.; Görgens, J.F. A Techno-Economic Study of Polybutylene Adipate Terephthalate (PBAT) Production from Molasses in an Integrated Sugarcane Biorefinery. Food Bioprod. Process. 2024, 145, 11–20. [Google Scholar] [CrossRef]
- Nagarajan, V.; Misra, M.; Mohanty, A.K. New Engineered Biocomposites from Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) (PHBV)/Poly(Butylene Adipate-Co-Terephthalate) (PBAT) Blends and Switchgrass: Fabrication and Performance Evaluation. Ind. Crops Prod. 2013, 42, 461–468. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Cividanes, L.S.; Gouveia, R.F.; Lona, L.M.F. An Overview on Properties and Applications of Poly(Butylene Adipate-Co-Terephthalate)–PBAT Based Composites. Polym. Eng. Sci. 2019, 59, E7–E15. [Google Scholar] [CrossRef]
- Lee, S.H.; Lim, S.W.; Lee, K.H. Properties of Potentially Biodegradable Copolyesters of (Succinic Acid–1,4-Butanediol)/(Dimethyl Terephthalate–1,4-Butanediol). Polym. Int. 1999, 48, 861–867. [Google Scholar] [CrossRef]
- Witt, U.; Müller, R.-J.; Deckwer, W.-D. Biodegradation Behavior and Material Properties of Aliphatic/Aromatic Polyesters of Commercial Importance. J. Environ. Polym. Degrad. 1997, 5, 81–89. [Google Scholar] [CrossRef]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.R.; Gruter, G.-J.M.; Coelho, J.F.J.; Silvestre, A.J.D. Biobased Polyesters and Other Polymers from 2,5-Furandicarboxylic Acid: A Tribute to Furan Excellency. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Díaz, A.; Katsarava, R.; Puiggalí, J. Synthesis, Properties and Applications of Biodegradable Polymers Derived from Diols and Dicarboxylic Acids: From Polyesters to Poly(Ester Amide)s. Int. J. Mol. Sci. 2014, 15, 7064–7123. [Google Scholar] [CrossRef]
- Kathuria, A.; Zhang, S. Sustainable and Repulpable Barrier Coatings for Fiber-Based Materials for Food Packaging: A Review. Front. Mater. 2022, 9, 929501. [Google Scholar] [CrossRef]
- Oh, J.; Shin, N.; Kim, S.; Lee, Y.; Shin, Y.; Choi, S.; Joo, J.C.; Jeon, J.-M.; Yoon, J.-J.; Bhatia, S.K.; et al. Discovery of a Novel Bacillus Sp. JO01 for the Degradation of Poly(Butylene Adipate-Co-Terephthalate)( PBAT) and Its Inhibition by PBAT Monomers. J. Microbiol. Biotechnol. 2025, 35, e2408051. [Google Scholar] [CrossRef]
- Oksman, K.; Aitomäki, Y.; Mathew, A.P.; Siqueira, G.; Zhou, Q.; Butylina, S.; Tanpichai, S.; Zhou, X.; Hooshmand, S. Review of the Recent Developments in Cellulose Nanocomposite Processing. Compos. Part A Appl. Sci. Manuf. 2016, 83, 2–18. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Pinheiro, I.F.; Gouveia, R.F.; Thim, G.P.; Lona, L.M.F. Functionalized Cellulose Nanocrystals as Reinforcement in Biodegradable Polymer Nanocomposites. Polym. Compos. 2018, 39, E9–E29. [Google Scholar] [CrossRef]
- Pinheiro, I.F.; Morales, A.R.; Mei, L.H. Polymeric Biocomposites of Poly (Butylene Adipate-Co-Terephthalate) Reinforced with Natural Munguba Fibers. Cellulose 2014, 21, 4381–4391. [Google Scholar] [CrossRef]
- Wikipedia Polybutylene Adipate Terephthalate. Available online: https://en.wikipedia.org/w/index.php?title=Polybutylene_adipate_terephthalate&oldid=1232501009 (accessed on 24 February 2025).
- BASF Ecovio® (PBAT, PLA)—Certified Compostable Polymer with Bio-Based Content 2025. Available online: https://plastics-rubber.basf.com/global/en/performance_polymers/products/ecovio (accessed on 12 March 2025).
- BASF Ecoflex® (PBAT): The Original Since 1998—Certified Compostable Biopolymer 2025. Available online: https://plastics-rubber.basf.com/global/en/performance_polymers/products/ecoflex (accessed on 12 March 2025).
- Lee, H.S.; Cho, D.; Han, S.O. Effect of Natural Fiber Surface Treatments on the Interfacial and Mechanical Properties of Henequen/Polypropylene Biocomposites. Macromol. Res. 2008, 16, 411–417. [Google Scholar] [CrossRef]
- Yu, W.; Qiu, R.; Lei, W.; Chen, Y. Effects of Modification on Properties of Wood Flour/PBAT Biocomposites. Polymers 2025, 17, 555. [Google Scholar] [CrossRef]
- Marques, M.F.V.; Lunz, J.N.; Aguiar, V.O.; Grafova, I.; Kemell, M.; Visentin, F.; Sartori, A.; Grafov, A. Thermal and Mechanical Properties of Sustainable Composites Reinforced with Natural Fibers. J. Polym. Environ. 2015, 23, 251–260. [Google Scholar] [CrossRef]
- Pereira da Silva, J.S.; Farias da Silva, J.M.; Soares, B.G.; Livi, S. Fully Biodegradable Composites Based on Poly(Butylene Adipate-Co-Terephthalate)/Peach Palm Trees Fiber. Compos. Part B Eng. 2017, 129, 117–123. [Google Scholar] [CrossRef]
- Georgiopoulos, P.; Kontou, E.; Christopoulos, A. Short-Term Creep Behavior of a Biodegradable Polymer Reinforced with Wood-Fibers. Compos. Part B Eng. 2015, 80, 134–144. [Google Scholar] [CrossRef]
- Yu, W.; Qiu, R.; Li, M.; Lei, W. Effects of Wood Content and Modification on Properties of Wood Flour/Polybutylene Adipate Terephthalate Biocomposites. Molecules 2023, 28, 8057. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Wang, Y.; Zhu, W.; Lan, D.; Song, Y.-M. Flexible Poly(Butylene Adipate-Co-Butylene Terephthalate) Enabled High-Performance Polylactide/Wood Fiber Biocomposite Foam. Ind. Crops Prod. 2023, 204, 117381. [Google Scholar] [CrossRef]
- Li, M.; Lei, W.; Yu, W. FDM 3D Printing and Properties of WF/PBAT/PLA Composites. Molecules 2024, 29, 5087. [Google Scholar] [CrossRef]
- Yu, S.; Wang, H.-M.; Xiong, S.-J.; Zhou, S.-J.; Wang, H.-H.; Yuan, T.-Q. Sustainable Wood-Based Poly(Butylene Adipate-Co-Terephthalate) Biodegradable Composite Films Reinforced by a Rapid Homogeneous Esterification Strategy. ACS Sustain. Chem. Eng. 2022, 10, 14568–14578. [Google Scholar] [CrossRef]
- Witt, U.; Einig, T.; Yamamoto, M.; Kleeberg, I.; Deckwer, W.D.; Müller, R.J. Biodegradation of Aliphatic-Aromatic Copolyesters: Evaluation of the Final Biodegradability and Ecotoxicological Impact of Degradation Intermediates. Chemosphere 2001, 44, 289–299. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Laforsch, C.; Greiner, A.; Agarwal, S. Fate of So-Called Biodegradable Polymers in Seawater and Freshwater. Glob. Chall. 2017, 1, 1700048. [Google Scholar] [CrossRef]
- Fujimaki, T. Processability and Properties of Aliphatic Polyesters, ‘BIONOLLE’, Synthesized by Polycondensation Reaction. Polym. Degrad. Stab. 1998, 59, 209–214. [Google Scholar] [CrossRef]
- Ishioka, R.; Kitakuni, E.; Ichikawa, Y. Aliphatic Polyesters: “Bionolle”. In Biopolymers Online; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; ISBN 978-3-527-60003-8. [Google Scholar]
- Barletta, M.; Genovesi, A.; Desole, M.P.; Gisario, A. Melt Processing of Biodegradable Poly(Butylene Succinate) (PBS)—A Critical Review. Clean Technol. Environ. Policy 2025, 27, 683–725. [Google Scholar] [CrossRef]
- Tserki, V.; Matzinos, P.; Pavlidou, E.; Vachliotis, D.; Panayiotou, C. Biodegradable Aliphatic Polyesters. Part I. Properties and Biodegradation of Poly(Butylene Succinate-Co-Butylene Adipate). Polym. Degrad. Stab. 2006, 91, 367–376. [Google Scholar] [CrossRef]
- Papaspyrides, C.D. Unsaturated Polyester Resin Technology: Possible Improvement in Hydrolysis Resistance. Master’s Thesis, Loughborough University of Technology, Loughborough, UK, 1976. [Google Scholar]
- Barletta, M.; Aversa, C.; Ayyoob, M.; Gisario, A.; Hamad, K.; Mehrpouya, M.; Vahabi, H. Poly(Butylene Succinate) (PBS): Materials, Processing, and Industrial Applications. Prog. Polym. Sci. 2022, 132, 101579. [Google Scholar] [CrossRef]
- Wang, H.; Ji, J.; Zhang, W.; Zhang, Y.; Jiang, J.; Wu, Z.; Pu, S.; Chu, P.K. Biocompatibility and Bioactivity of Plasma-Treated Biodegradable Poly(Butylene Succinate). Acta Biomater. 2009, 5, 279–287. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Hayase, N.; Yano, H.; Kudoh, E.; Tsutsumi, C.; Ushio, K.; Miyahara, Y.; Tanaka, S.; Nakagawa, K. Isolation and Characterization of Poly(Butylene Succinate-Co-Butylene Adipate)-Degrading Microorganism. J. Biosci. Bioeng. 2004, 97, 131–133. [Google Scholar] [CrossRef]
- Noël, M.; Grigsby, W.J.; Ormondroyd, G.A.; Spear, M.J. Influence of Water and Humidity on Chemically Modifying Wood with Polybutylene Succinate Bio-Polyester. Int. Wood Prod. J. 2016, 7, 80–88. [Google Scholar] [CrossRef]
- Lee, S.-H.; Ohkita, T. Mechanical and Thermal Flow Properties of Wood Flour–Biodegradable Polymer Composites. J. Appl. Polym. Sci. 2003, 90, 1900–1905. [Google Scholar] [CrossRef]
- Ndazi, B.; Tesha, J.V.; Bisanda, E.T.N. Some Opportunities and Challenges of Producing Bio-Composites from Non-Wood Residues. J. Mater. Sci. 2006, 41, 6984–6990. [Google Scholar] [CrossRef]
- Tserki, V.; Matzinos, P.; Panayiotou, C. Novel Biodegradable Composites Based on Treated Lignocellulosic Waste Flour as Filler. Part II. Development of Biodegradable Composites Using Treated and Compatibilized Waste Flour. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1231–1238. [Google Scholar] [CrossRef]
- Yue, X.; Xu, Y.; Gou, N.; Li, H. Synthesis of Cellulose-Based Macromolecular Coupling Agent and Its Utilization in Poly (Butylene Succinate)/Wood Flour Composites. Polym. Plast. Technol. Eng. 2015, 54, 639–646. [Google Scholar] [CrossRef]
- Valente, M.; Tirillò, J.; Quitadamo, A.; Santulli, C. Paper Fiber Filled Polymer. Mechanical Evaluation and Interfaces Modification. Compos. Part B Eng. 2017, 110, 520–529. [Google Scholar] [CrossRef]
- Calabia, B.P.; Ninomiya, F.; Yagi, H.; Oishi, A.; Taguchi, K.; Kunioka, M.; Funabashi, M. Biodegradable Poly(Butylene Succinate) Composites Reinforced by Cotton Fiber with Silane Coupling Agent. Polymers 2013, 5, 128–141. [Google Scholar] [CrossRef]
- Weng, F.; Liu, X.; Koranteng, E.; Ma, N.; Wu, Z.; Wu, Q. Structure and Properties of a Compatible Wood-Plastic Composite Prepared by Using Poly(Butylene Succinate)-Based Polyurethane Prepolymer. Polym. Compos. 2019, 40, 4694–4703. [Google Scholar] [CrossRef]
- Mazzanti, V.; Mollica, F.; El Kissi, N. Rheological and Mechanical Characterization of Polypropylene-Based Wood Plastic Composites. Polym. Compos. 2016, 37, 3460–3473. [Google Scholar] [CrossRef]
- Sahoo, S.; Misra, M.; Mohanty, A.K. Enhanced Properties of Lignin-Based Biodegradable Polymer Composites Using Injection Moulding Process. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1710–1718. [Google Scholar] [CrossRef]
- Sahoo, S.; Misra, M.; Mohanty, A.K. Biocomposites From Switchgrass and Lignin Hybrid and Poly(Butylene Succinate) Bioplastic: Studies on Reactive Compatibilization and Performance Evaluation. Macromol. Mater. Eng. 2014, 299, 178–189. [Google Scholar] [CrossRef]
- Park, C.-W.; Youe, W.-J.; Han, S.-Y.; Park, J.-S.; Lee, E.-A.; Park, J.-Y.; Kwon, G.-J.; Kim, S.-J.; Lee, S.-H. Influence of Lignin and Polymeric Diphenylmethane Diisocyante Addition on the Properties of Poly(Butylene Succinate)/Wood Flour Composite. Polymers 2019, 11, 1161. [Google Scholar] [CrossRef]
- Kozłowska, A.; Gorący, K.; El Fray, M. Biodegradable Polymer Composites Based on Poly(Butylene Succinate) Copolyesters and Wood Flour. Polymers 2025, 17, 883. [Google Scholar] [CrossRef] [PubMed]
- Noël, M.; Grigsby, W.; Volkmer, T. Investigating the Viscoelastic Properties and Mechanical Performance of Wood Modifi Ed by Biopolyester Treatments. J. Renew. Mater. 2014, 2, 291–305. [Google Scholar] [CrossRef]
- Noël, M.; Grigsby, W.J.; Volkmer, T. Evaluating The Extent of Bio-Polyester Polymerization in Solid Wood by Thermogravimetric Analysis. J. Wood Chem. Technol. 2015, 35, 325–336. [Google Scholar] [CrossRef]
- Noël, M.; Grigsby, W.; Vitkeviciute, I.; Volkmer, T. Modifying Wood with Bio-Polyesters: Analysis and Performance. Int. Wood Prod. J. 2015, 6, 14–20. [Google Scholar] [CrossRef]
- Grosse, C.; Spear, M.; Curling, S.; Noël, M.; Rautkari, L.; Uimonen, T.; Gérardin, P. Dynamic Mechanical Thermal Analysis of Wood Modified with Bio-Polyesters. In Proceedings of the 9th European Conference on Wood Modification, Arnhem, The Netherlands, 17–18 September 2018. [Google Scholar]
- Shin, N.; Kim, S.H.; Oh, J.; Kim, S.; Lee, Y.; Shin, Y.; Choi, S.; Bhatia, S.K.; Kim, Y.-G.; Yang, Y.-H. Reproducible Polybutylene Succinate (PBS)-Degrading Artificial Consortia by Introducing the Least Type of PBS-Degrading Strains. Polymers 2024, 16, 651. [Google Scholar] [CrossRef]
- Bher, A.; Mayekar, P.C.; Auras, R.A.; Schvezov, C.E. Biodegradation of Biodegradable Polymers in Mesophilic Aerobic Environments. Int. J. Mol. Sci. 2022, 23, 12165. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Morreale, M. Green Composites: A Brief Review. Compos. Part A Appl. Sci. Manuf. 2011, 42, 579–588. [Google Scholar] [CrossRef]
- Kim, H.-S.; Yang, H.-S.; Kim, H.-J. Biodegradability and Mechanical Properties of Agro-Flour–Filled Polybutylene Succinate Biocomposites. J. Appl. Polym. Sci. 2005, 97, 1513–1521. [Google Scholar] [CrossRef]
- Wang, Y.; Rakotonirainy, A.M.; Padua, G.W. Thermal Behavior of Zein-Based Biodegradable Films. Starch–Stärke 2003, 55, 25–29. [Google Scholar] [CrossRef]
- Murray, R.K. Harper’s Biochemistry, 24th ed.; Appleton & Lange: London, UK, 1998; ISBN 978-0-8385-3611-7. [Google Scholar]
- Shukla, R.; Cheryan, M. Zein: The Industrial Protein from Corn. Ind. Crops Prod. 2001, 13, 171–192. [Google Scholar] [CrossRef]
- Bräuer, S.; Meister, F.; Gottlöber, R.-P.; Nechwatal, A. Preparation and Thermoplastic Processing of Modified Plant Proteins. Macromol. Mater. Eng. 2007, 292, 176–183. [Google Scholar] [CrossRef]
- Croitoru, C.; Patachia, S.; Lunguleasa, A. A Mild Method of Wood Impregnation with Biopolymers and Resins Using 1-Ethyl-3-Methylimidazolium Chloride as Carrier. Chem. Eng. Res. Des. 2015, 93, 257–268. [Google Scholar] [CrossRef]
- Movaffagh, J.; Nourollahian, T.; Khalatbari, S.; Amiri, N.; Bazzaz, B.S.F.; Kalalinia, F. Fabrication of Zein-Chitosan-Zein Sandwich-Like Nanofibers Containing Teicoplanin as a Local Antibacterial Drug Delivery System. J. Pharm. Innov. 2023, 18, 911–922. [Google Scholar] [CrossRef]
- Yemenicioğlu, A. Chapter 41—Zein and Its Composites and Blends with Natural Active Compounds: Development of Antimicrobial Films for Food Packaging. In Antimicrobial Food Packaging; Barros-Velázquez, J., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 503–513. ISBN 978-0-12-800723-5. [Google Scholar]
- Torres-Giner, S.; Ocio, M.J.; Lagaron, J.M. Novel Antimicrobial Ultrathin Structures of Zein/Chitosan Blends Obtained by Electrospinning. Carbohydr. Polym. 2009, 77, 261–266. [Google Scholar] [CrossRef]
- Han, Y.-L.; Xu, Q.; Lu, Z.-Q.; Wang, J.-Y. Preparation of Transparent Zein Films for Cell Culture Applications. Colloids Surf. B Biointerfaces 2014, 120, 55–62. [Google Scholar] [CrossRef]
- Zhang, Z.; Chao, T.; Li, N.; Jin, Y.; Cui, L.; Wang, P. Fabrication of Hydrophobic Surface from Zein-Based Nanostructure and Rosin to Functionalize Cotton Textiles. Ind. Crops Prod. 2024, 215, 118650. [Google Scholar] [CrossRef]
- Oh, M.; Ma, Q.; Simsek, S.; Bajwa, D.; Jiang, L. Comparative Study of Zein- and Gluten-Based Wood Adhesives Containing Cellulose Nanofibers and Crosslinking Agent for Improved Bond Strength. Int. J. Adhes. Adhes. 2019, 92, 44–57. [Google Scholar] [CrossRef]
- Santoni, I.; Pizzo, B. Evaluation of Alternative Vegetable Proteins as Wood Adhesives. Ind. Crops Prod. 2013, 45, 148–154. [Google Scholar] [CrossRef]
- Johansson, P.; Mjörnell, K. Biopolymerer Som Transportskydd För Byggnadsmaterial. Förstudie. (Biopolymers as Protection during Transport of Construction Materials); SP Technical Research Institute of Sweden: Borås, Sweden, 2007. [Google Scholar]
- Croitoru, C.; Patachia, S.; Lunguleasa, A. Solutions for Wood Impregnation, Based on Natural Polymers, Method of Preparation and Process for Application. RO Patent Application RO126930-A0, 30 December 2011. [Google Scholar]
- Argos, P.; Pedersen, K.; Marks, M.D.; Larkins, B.A. A Structural Model for Maize Zein Proteins. J. Biol. Chem. 1982, 257, 9984–9990. [Google Scholar] [CrossRef] [PubMed]
- Choktaweesap, N.; Arayanarakul, K.; Aht-ong, D.; Meechaisue, C.; Supaphol, P. Electrospun Gelatin Fibers: Effect of Solvent System on Morphology and Fiber Diameters. Polym. J. 2007, 39, 622–631. [Google Scholar] [CrossRef]
- Karthäuser, J.; Biziks, V.; Mai, C.; Militz, H. Lignin and Lignin-Derived Compounds for Wood Applications—A Review. Molecules 2021, 26, 2533. [Google Scholar] [CrossRef] [PubMed]
- Melro, E.; Filipe, A.; Sousa, D.; Medronho, B.; Romano, A. Revisiting Lignin: A Tour through Its Structural Features, Characterization Methods and Applications. New J. Chem. 2021, 45, 6986–7013. [Google Scholar] [CrossRef]
- Chirkova, J.; Andersone, I.; Irbe, I.; Spince, B.; Andersons, B. Lignins as Agents for Bio-Protection of Wood. Holzforschung 2011, 65, 497–502. [Google Scholar] [CrossRef]
- Herrera, R.; Poohphajai, F.; Sandak, A.; Gordobil, O. Simultaneous Improvement of Surface Wettability and UV Resistance of Wood with Lignin-Based Treatments. Polymers 2023, 15, 3409. [Google Scholar] [CrossRef]
- Leng, W.; Wang, J.; He, S.; Wang, X.; Zhai, S.; Li, W.; Quan, H.; Lu, B.; Shi, J.; Hafez, I.; et al. Dimensionally Stable and Durable Wood by Lignin Impregnation. Int. J. Biol. Macromol. 2024, 268, 131684. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, W.; Yu, F.; Wang, Y.; Xiao, Z.; Xie, Y. Improved Dimensional Stability and Mechanical Properties of Rubberwood via Modification with Maleated Lignin and Densification. Holzforschung 2023, 77, 170–183. [Google Scholar] [CrossRef]
- Broda, M. Natural Compounds for Wood Protection against Fungi—A Review. Molecules 2020, 25, 3538. [Google Scholar] [CrossRef]
- Bonfatti Júnior, E.A.; De Barros, J.M.R.; Silva, G.F.; Lengowski, E.C. A Comparative Analysis of Tannin and Commercial Fire Retardants in Wood Fire Protection. Forests 2024, 15, 951. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins: Prospectives and Actual Industrial Applications. Biomolecules 2019, 9, 344. [Google Scholar] [CrossRef]
- Cesprini, E.; Baccini, R.; Urso, T.; Zanetti, M.; Tondi, G. Quebracho-Based Wood Preservatives: Effect of Concentration and Hardener on Timber Properties. Coatings 2022, 12, 568. [Google Scholar] [CrossRef]
- Mubarok, M.; Gérardin-Charbonnier, C.; Azadeh, E.; Obounou Akong, F.; Dumarçay, S.; Pizzi, A.; Gérardin, P. Modification of Wood by Tannin-Furfuryl Alcohol Resins–Effect on Dimensional Stability, Mechanical Properties and Decay Durability. J. Renew. Mater. 2023, 11, 505–521. [Google Scholar] [CrossRef]
- Dorini Falavinha, J.V.; Gérardin, P.; Gonzales De Cademartori, P.H.; Gérardin-Charbonnier, C. Chestnut Tannin/Furfuryl Alcohol Copolymers for Beech Wood Chemical Modification. Polymers 2025, 17, 1159. [Google Scholar] [CrossRef]
- Tondi, G.; Thevenon, M.F.; Mies, B.; Standfest, G.; Petutschnigg, A.; Wieland, S. Impregnation of Scots Pine and Beech with Tannin Solutions: Effect of Viscosity and Wood Anatomy in Wood Infiltration. Wood Sci. Technol. 2013, 47, 615–626. [Google Scholar] [CrossRef]
- Verly Lopes, D.J.; Barnes, H.M.; dos Santos Bobadilha, G. Influence of Heat Treatment and Tannin Impregnation on Boron Depletion and Wood Durability. Forests 2020, 11, 201. [Google Scholar] [CrossRef]
- Tondi, G.; Wieland, S.; Lemenager, N.; Petutschnigg, A.; Pizzi, A.; Thevenon, M.-F. Efficacy of Tannin in Fixing Boron in Wood: Fungal and Termite Resistance. BioResources 2012, 7, 1238–1252. [Google Scholar] [CrossRef]
- Bernardi, A.C.; Popoff, O. Durability of Pinus Elliottii Wood Impregnated with Quebracho Colorado (Schinopsis Balansae) Bio-Protectives Extracts and CCA. Maderas. Cienc. Tecnol. 2009, 11, 107–115. [Google Scholar] [CrossRef][Green Version]
- Tondi, G.; Wieland, S.; Wimmer, T.; Thevenon, M.F.; Pizzi, A.; Petutschnigg, A. Tannin-Boron Preservatives for Wood Buildings: Mechanical and Fire Properties. Eur. J. Wood Prod. 2012, 70, 689–696. [Google Scholar] [CrossRef]
- Perçin, O.; Doruk, Ş.; Altunok, M. Effects of Impregnation and Heat Treatment on Some Physical and Mechanical Properties of Wood Material. Politek. Derg. 2023, 26, 1421–1429. [Google Scholar] [CrossRef]
- Casado-Sanz, M.M.; Silva-Castro, I.; Ponce-Herrero, L.; Martín-Ramos, P.; Martín-Gil, J.; Acuña-Rello, L. White-Rot Fungi Control on Populus Spp. Wood by Pressure Treatments with Silver Nanoparticles, Chitosan Oligomers and Propolis. Forests 2019, 10, 885. [Google Scholar] [CrossRef]
- Khademibami, L.; Barnes, H.; Jeremic, D.; Shmulsky, R.; Bourne, K.; Fatemi, S. Antifungal Activity and Fire Resistance Properties of Nano-Chitosan Treated Wood. Bioresources 2020, 15, 5926–5939. [Google Scholar] [CrossRef]
- Basturk, M. Heat Applied Chitosan Treatment on Hardwood Chips to Improve Physical and Mechanical Properties of Particleboard. BioResources 2012, 7, 4858–4866. [Google Scholar] [CrossRef]
- Eikenes, M.; Alfredsen, G.; Christensen, B.E.; Militz, H.; Solheim, H. Comparison of Chitosans with Different Molecular Weights as Possible Wood Preservatives. J. Wood Sci. 2005, 51, 387–394. [Google Scholar] [CrossRef]
- Papadopoulos, A.N.; Foti, D.; Kyzas, G.Z. Sorption Behavior of Water Vapor of Wood Treated by Chitosan Polymer. Eur. J. Wood Prod. 2020, 78, 483–491. [Google Scholar] [CrossRef]
- Kayan, G.Ö.; Kayan, A. Composite of Natural Polymers and Their Adsorbent Properties on the Dyes and Heavy Metal Ions. J. Polym. Environ. 2021, 29, 3477–3496. [Google Scholar] [CrossRef]
- Torr, K.M.; Singh, A.P.; Franich, R.A. Improving Stiffness of Lignocellulosics Through Cell Wall Modification with Chitosan-Melamine Co-Polymers. N. Z. J. For. Sci. 2006, 36, 87. [Google Scholar]
- Khademibami, L.; Jeremic, D.; Shmulsky, R.; Barnes, H.M. Chitosan Oligomers and Related Nanoparticles as Environmentally Friendly Wood Preservatives. BioResources 2020, 15, 2800–2817. [Google Scholar] [CrossRef]
- Larn⊘y, E.; Dantz, S.; Eikenes, M.; Militz, H. Screening of Properties of Modified Chitosan-Treated Wood. Wood Mater. Sci. Eng. 2006, 1, 59–68. [Google Scholar] [CrossRef]
- Woźniak, M.; Gromadzka, K.; Kwaśniewska-Sip, P.; Cofta, G.; Ratajczak, I. Chitosan–Caffeine Formulation as an Ecological Preservative in Wood Protection. Wood Sci. Technol. 2022, 56, 1851–1867. [Google Scholar] [CrossRef]
- Walsh-Korb, Z.; Stelzner, I.; dos Santos Gabriel, J.; Eggert, G.; Avérous, L. Morphological Study of Bio-Based Polymers in the Consolidation of Waterlogged Wooden Objects. Materials 2022, 15, 681. [Google Scholar] [CrossRef]
- Bi, Z.; Fang, S.; Gao, Q.; Lei, Y.; Morrell, J.J.; Yan, L. Improvement of Mould Resistance of Wood with Cinnamaldehyde Chitosan Emulsion. Wood Sci. Technol. 2022, 56, 187–204. [Google Scholar] [CrossRef]
- Alfredsen, G.; Eikenes, M.; Militz, H.; Solheim, H. Screening of Chitosan against Wood-Deteriorating Fungi. Scand. J. For. Res. 2004, 19, 4–13. [Google Scholar] [CrossRef]
- Singh, T. Scoping Antifungal Activities of Various Forms of Chitosan Oligomers and Their Potential Fixation in Wood. Maced. J. Chem. Chem. Eng. 2013, 7, 1175–1180. [Google Scholar]
- Istriana, N.; Priadi, T. The Resistance of Modified Manii Wood with Boric Acid and Chitosan/Glycerol and Heating Against Fungi and Termites. IOP Conf. Ser. Earth Environ. Sci. 2021, 891, 012010. [Google Scholar] [CrossRef]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Villani, E.; Popescu, C.-M.; Jancelewicz, M.; Stagno, V.; Capuani, S.; Broda, M. Sodium Alginate as a Green Consolidant for Waterlogged Wood—A Preliminary Study. Forests 2025, 16, 325. [Google Scholar] [CrossRef]
- Zhang, M.; Li, H.; Wang, C.; Wang, Z.; Liu, D.; Yang, T.; Deng, Z.; Yuan, G. Performance Enhancement of the Poplar Wood Composites Biomimetic Mineralized by CaCO3. ACS Omega 2022, 7, 29465–29474. [Google Scholar] [CrossRef]
- Sahoo, D.R.; Biswal, T. Alginate and Its Application to Tissue Engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- He, L.; Bao, G.; Yu, X.; Zhang, X.; Jin, X.; Yu, Z.; He, Y.; Zhang, R.; Qin, D. A Green and Eco-Friendly Method to Enhance Bamboo Flame Resistance via Calcium Alginate Assisted in-Situ Mineralization of Hydroxyapatite. Chem. Eng. J. 2024, 485, 149765. [Google Scholar] [CrossRef]
- Chen, F. Study on Mechanical and Thermal Performance of Building Energy-Saving Wall with Insulation Materials. E3S Web Conf. 2021, 242, 02005. [Google Scholar] [CrossRef]
- Lacoste, C.; El Hage, R.; Bergeret, A.; Corn, S.; Lacroix, P. Sodium Alginate Adhesives as Binders in Wood Fibers/Textile Waste Fibers Biocomposites for Building Insulation. Carbohydr. Polym. 2018, 184, 1–8. [Google Scholar] [CrossRef]
- Song, M.; Kim, D.; Jeon, S. Bamboo–Alginate Composite as a Sustainable Structural Material. ACS Sustain. Chem. Eng. 2023, 11, 3486–3493. [Google Scholar] [CrossRef]
- Meng, D.; Long, W.; Sun, J.; Li, H.; Wang, Z.; Gu, X.; Zhang, S. Eco-Friendly Fabrication of a Delignified Wood-calcium Alginate Aerogel with Improved Mechanical Properties for Efficient Thermal Insulation and Flame Retardancy. Int. J. Biol. Macromol. 2025, 287, 138561. [Google Scholar] [CrossRef] [PubMed]
- Rofii, M.N.; Widyorini, R.; Lukmandaru, G. Effects of Wood Modification Using Natural Resin on Wood Quality and Bonding Properties. Wood Res. J. 2019, 10, 48–52. [Google Scholar] [CrossRef]
- Mahmoud Kia, M.; Tarmian, A.; Karimi, A.N.; Gholamiyan, H.; Abdulkhani, A.; Mastri Farahani, M.R. The Efficiency of Pistacia Atlantica Gum for Increasing Resistance of Rapeseed Oil-Heat Treated Wood to Fungal Attacks. Maderas. Cienc. Tecnol. 2020, 22, 457–466. [Google Scholar] [CrossRef]
- Broda, M.; Hill, C.A.S. Conservation of Waterlogged Wood—Past, Present and Future Perspectives. Forests 2021, 12, 1193. [Google Scholar] [CrossRef]
- Vilela, C.; Rua, R.; Silvestre, A.J.D.; Gandini, A. Polymers and Copolymers from Fatty Acid-Based Monomers. Ind. Crops Prod. 2010, 32, 97–104. [Google Scholar] [CrossRef]
- Schmid, K.M. Chapter 4—Lipid Metabolism in Plants. In Biochemistry of Lipids, Lipoproteins and Membranes, 7th ed.; Ridgway, N.D., McLeod, R.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 121–159. ISBN 978-0-12-824048-9. [Google Scholar]
- Lee, S.H.; Ashaari, Z.; Lum, W.C.; Abdul Halip, J.; Ang, A.F.; Tan, L.P.; Chin, K.L.; Md Tahir, P. Thermal Treatment of Wood Using Vegetable Oils: A Review. Constr. Build. Mater. 2018, 181, 408–419. [Google Scholar] [CrossRef]
- Mastouri, A.; Efhamisisi, D.; Shirmohammadli, Y.; Oladi, R. Physicochemical Properties of Thermally Treated Poplar Wood in Silicone and Rapeseed Oils: A Comparative Study. J. Build. Eng. 2021, 43, 102511. [Google Scholar] [CrossRef]
- Honary, L.A.T. Soybean Oil Impregnation Wood Preservative Process and Products. U.S. Patent 6641927B1, 4 November 2003. [Google Scholar]
- Lyon, F.; Thevenon, M.-F.; Hwang, W.-J.; Imamura, Y.; Gril, J.; Pizzi, A. Effect of an Oil Heat Treatment on the Leachability and Biological Resistance of Boric Acid Impregnated Wood. Ann. For. Sci. 2007, 64, 673–678. [Google Scholar] [CrossRef]
- Chauke, N.P.; Mukaya, H.E.; Nkazi, D.B. Chemical Modifications of Castor Oil: A Review. Sci. Prog. 2019, 102, 199–217. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiao, Z.; Liu, X.; Kang, D.; Dong, W.; Lin, Q.; Zhang, A. Research Progress of Tung Oil/UV Photocomposite Curing Material. J. Renew. Mater. 2022, 11, 1661–1686. [Google Scholar] [CrossRef]
- Wang, J.Y.; Cooper, P.A. Effect of Oil Type, Temperature and Time on Moisture Properties of Hot Oil-Treated Wood. Holz Roh Werkst. 2005, 63, 417–422. [Google Scholar] [CrossRef]
- Tjeerdsma, B.; Swager, P.; Horstman, B.; Holleboom, B.; Homan, W. Process Development of Treatment of Wood with Modified Hot Oil. In Proceedings of the European Conference on Wood Modification 2005, Gottingen, Germany, 6–7 October 2005. [Google Scholar]
- Sidorova, E. Combination of Heating and Preservative Impregnation of Wood for Outdoor Exposure. Ph.D. Thesis, Luleå University of Technology, Luleå, Switzerland, 2016. [Google Scholar]
- Bak, M.; Németh, R.; Tolvaj, L. The Colour Change of Oil-Heat-Treated Timber During Weathering. Óbuda Univ. E-Bull. 2012, 3, 339–345. [Google Scholar]
- Dobrowolska, E. Properties of a Densified Wood Surface Impregnated with Rapeseed Oil. Ann. WULS For. Wood Technol. 2023, 124, 139–152. [Google Scholar] [CrossRef]
- Dastoorian, F.; Ghasemi, M.; Abedini, R.; Amininasab, S.M. Effect of Poplar Wood Impregnation with Epoxidized Soybean Oil on the Set Recovery. Iran. J. Wood Pap. Ind. 2020, 11, 381–394. [Google Scholar]
- Jebrane, M.; Cai, S.; Sandstrom, C.; Terziev, N. The Reactivity of Linseed and Soybean Oil with Different Epoxidation Degree towards Vinyl Acetate and Impact of the Resulting Copolymer on the Wood Durability. Express Polym. Lett. 2017, 11, 383–395. [Google Scholar] [CrossRef]
- Mohebby, B.; Kevily, H.; Kazemi-Najafi, S. Oleothermal Modification of Fir Wood with a Combination of Soybean Oil and Maleic Anhydride and Its Effects on Physico-Mechanical Properties of Treated Wood. Wood Sci. Technol. 2014, 48, 797–809. [Google Scholar] [CrossRef]
- Eriksson, D.; Geladi, P.; Ulvcrona, T. Near-Infrared Spectroscopy for the Quantification of Linseed Oil Uptake in Scots Pine (Pinus sylvestris L.). Wood Mater. Sci. Eng. 2011, 6, 170–176. [Google Scholar] [CrossRef]
- Geladi, P.; Eriksson, D.; Ulvcrona, T. Data Analysis of Hyperspectral NIR Image Mosaics for the Quantification of Linseed Oil Impregnation in Scots Pine Wood. Wood Sci. Technol. 2014, 48, 467–481. [Google Scholar] [CrossRef]
- Ulvcrona, T.; Lindberg, H.; Bergsten, U. Impregnation of Norway Spruce (Picea abies L. Karst.) Wood by Hydrophobic Oil and Dispersion Patterns in Different Tissues. For. Int. J. For. Res. 2006, 79, 123–134. [Google Scholar] [CrossRef][Green Version]
- Ulvcrona, T.; Bergsten, U. Possibilities for compositional tailoring of Norway spruce (Picea abies L. Karst.) wood using a hydrophobic oil impregnation process. Holz Roh Werkst. 2007, 65, 167–169. [Google Scholar] [CrossRef]
- Olsson, T.; Megnis, M.; Varna, J.; Lindberg, H. Measurement of the Uptake of Linseed Oil in Pine by the Use of an X-Ray Microdensitometry Technique. J. Wood Sci. 2001, 47, 275–281. [Google Scholar] [CrossRef]
- Megnis, M.; Olsson, T.; Varna, J.; Lindberg, H. Mechanical Performance of Linseed Oil Impregnated Pine as Correlated to the Take-up Level. Wood Sci. Technol. 2002, 36, 1–18. [Google Scholar] [CrossRef]
- Fadl, N.A.; Basta, A.H. Enhancement of the Dimensional Stability of Natural Wood by Impregnates. Pigment. Resin. Technol. 2005, 34, 72–87. [Google Scholar] [CrossRef]
- Liu, Z.; Wen, L.; Wang, X.; Zhang, Y.; Cai, L. Leachability of ACQ-D after Three Different Preservative Treatments. Wood Res. 2020, 65, 591–604. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Xu, G.; Tu, X.W.; Liu, X.Y.; Wu, Z. Efficacy of Linseed Oil-Treated Wood to Improve Hydrophobicity, Dimensional Stability, and Thermostability. Wood Res. 2021, 66, 777–788. [Google Scholar] [CrossRef]
- Pelit, H.; Arısüt, U. Roughness, Wettability, and Morphological Properties of Impregnated and Densified Wood Materials. BioResources 2023, 18, 429–446. [Google Scholar] [CrossRef]
- Eckeveld, A.V.; Homan, W.J.; Militz, H. Increasing the Water Repellency of Scots Pine Sapwood. Holzforsch. Holzverwert. 2001, 6, 113–115. [Google Scholar]
- Epmeier, H.; Westin, M.; Rapp, A. Differently Modified Wood: Comparison of Some Selected Properties. Scand. J. For. Res. 2004, 19, 31–37. [Google Scholar] [CrossRef]
- Humar, M.; Lesar, B. Efficacy of Linseed- and Tung-Oil-Treated Wood against Wood-Decay Fungi and Water Uptake. Int. Biodeterior. Biodegrad. 2013, 85, 223–227. [Google Scholar] [CrossRef]
- Fredriksson, M.; Wadsö, L.; Ulvcrona, T. Moisture Sorption and Swelling of Norway Spruce [Picea abies (L.) Karst.] Impregnated with Linseed Oil. Wood Mater. Sci. Eng. 2010, 5, 135–142. [Google Scholar] [CrossRef]
- Hassan, B.; Mankowski, M.E.; Kirker, G.T. Evaluation of Heartwood Extracts Combined with Linseed Oil as Wood Preservatives in Field Tests in Southern Mississippi, USA. Insects 2021, 12, 803. [Google Scholar] [CrossRef]
- Przewloka, S.R.; Ahmed, B.; Vinden, P.; French, J.; Hann, J.A. Biodeterioration of Treated Pinus Radiata Timber by Australian Decay Fungi and the Termite Coptotermes Acinaciformis in Laboratory Bioassays and Field Conditions. Holzforschung 2007, 61, 207–213. [Google Scholar] [CrossRef]
- Bansal, R.; Mamatha, N.; Kumar, R.; Pandey, K.K. Fungal Resistance of Hevea Brasiliensis (Rubberwood) Treated with Nano-ZnO and Nano-CuO Dispersed Linseed Oil and Paraffin Wax Nanoemulsion. Eur. J. Wood Prod. 2024, 82, 1095–1109. [Google Scholar] [CrossRef]
- Temiz, A.; Terziev, N.; Eikenes, M.; Hafren, J. Effect of Accelerated Weathering on Surface Chemistry of Modified Wood. Appl. Surf. Sci. 2007, 253, 5355–5362. [Google Scholar] [CrossRef]
- Can, A.; Sivrikaya, H.; Tascioglu, C. Determination of Metal Corrosion in Wood Treated with New-Generation Water-Borne Preservatives. Drewno 2020, 63, 59–68. [Google Scholar] [CrossRef]
- Kaya, A.I. Combined Effects of Linseed Oil and Heat Treatment on the Properties of Cypress and Maple Wood Part 1: Water Absorption, Mechanical Properties, and Sound Absorption Capacity. BioResources 2023, 18, 2940. [Google Scholar] [CrossRef]
- Pelit, H.; Emiroglu, F. Effect of Water Repellents on Hygroscopicity and Dimensional Stability of Densified Fir and Aspen Woods. Drv. Ind. 2020, 71, 29–40. [Google Scholar] [CrossRef]
- Pelit, H.; Emiroglu, F. Density, Hardness and Strength Properties of Densified Fir and Aspen Woods Pretreated with Water Repellents. Holzforschung 2021, 75, 358–367. [Google Scholar] [CrossRef]
- Dubey, M.K.; Pang, S.; Chauhan, S.; Walker, J. Dimensional Stability, Fungal Resistance and Mechanical Properties of Radiata Pine after Combined Thermo-Mechanical Compression and Oil Heat-Treatment. Holzforschung 2016, 70, 793–800. [Google Scholar] [CrossRef]
- Vähäoja, P.; Piltonen, P.; Hyvönen, A.; Niinimäki, J.; Jalonen, J.; Kuokkanen, T. Biodegradability Studies of Certain Wood Preservatives in Groundwater as Determined by the Respirometric Bod Oxitop Method. Water Air Soil Pollut. 2005, 165, 313–324. [Google Scholar] [CrossRef]
- Cirule, D.; Andersone, I.; Kuka, E.; Andersons, B. Recent Research on Linseed Oil Use in Wood Protection—A Review. Science 2024, 6, 54. [Google Scholar] [CrossRef]
- Choi, S.; Lee, K.U.; Kwon, O.; Choi, W.; Lee, Y.; Cho, S.-M.; Choi, J.W.; Yang, I. Evaluating the Applicability of Wood Treated with Fatty Acid as a Raw Material for Exterior Uses. Korean Chem. Eng. Res. 2025, 63, 108–122. [Google Scholar] [CrossRef]
- Kwon, O.; Choi, Y.S.; Kim, D.; Choi, W.; Lee, Y.; Kim, K.; Choi, J.W.; Yang, I. Weatherproof-properties Evaluation of Castor Oil-impregnated Wood Using a Vacuum-pressure Method. Korean Chem. Eng. Res. 2023, 61, 302–311. [Google Scholar] [CrossRef]
- Kwon, O.; Choi, Y.S.; Choi, W.; Lee, Y.; Choi, J.; Choi, J.W.; Yang, I. Decay Resistance and Dimensional Stability of Wood Impregnated with Castor Oil Using a Pressure Treatment. Holzforschung 2023, 77, 879–888. [Google Scholar] [CrossRef]
- Ahmed, S.; Fatima, R.; Nisar, M.S.; Hassan, B. Evaluation of Castor Bean Oil on Acacia Nilotica as Wood Preservative against Odontotermes obesus (Ramb.) (Termitidae: Isoptera). Int. Wood Prod. J. 2014, 5, 5–10. [Google Scholar] [CrossRef]
- Rapp, A.O. Review on Heat Treatments of Wood. In Proceedings of the Special Seminar, Antibes, France, 9 February 2001; pp. 1–66. [Google Scholar]
- Bal, B.C. Physical Properties of Beech Wood Thermally Modified in Hot Oil and in Hot Air at Various Temperatures. Maderas. Cienc. Tecnol. 2015, 17, 789–798. [Google Scholar] [CrossRef]
- Yin, W.; Shan, L.; Lu, H.; Zheng, Y.; Han, Z.; Tian, Y. Impact Resistance of Oil-Immersed Lignum Vitae. Sci. Rep. 2016, 6, 30090. [Google Scholar] [CrossRef]
- Xue, Q.; Dong, Y.; Zhao, Y.; Deng, H.; Shi, J.; Zhan, X. Physical-Mechanical Properties and Weathering Performance of Poplar and Spruce Wood with Various Tung Oil Loadings. Ind. Crops Prod. 2024, 221, 119399. [Google Scholar] [CrossRef]
- He, L.; Zhang, T.; Zhao, Y.; Hao, T.; Wang, Z.; He, Z.; Yi, S. Synergistic Enhancement of Hygroscopicity and Micromechanical Properties of Wood Cell Walls through Joint Tung Oil and Thermal Modification. Constr. Build. Mater. 2024, 420, 135640. [Google Scholar] [CrossRef]
- He, L.; Zhang, T.; Zhao, Y.; Zhao, X.; Hao, T.; Xu, K.; Wang, T.; He, Z.; Yi, S. Contribution of Tung Oil to the Resistance of Heat-Induced Wood Shrinkage during Thermal Modification. Ind. Crops Prod. 2023, 204, 117359. [Google Scholar] [CrossRef]
- He, L.; Zhang, T.; Zhao, Y.; Gao, J.; Zhang, Y.; Yang, Y.; He, Z.; Yi, S. Effect of Natural Tung Oil on Wood Shrinkage during the Thermal Modification Process. J. Clean. Prod. 2022, 379, 134450. [Google Scholar] [CrossRef]
- Mildenberg, R.; Zander, M.; Collin, G. Hydrocarbon Resins; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-3-527-61464-6. [Google Scholar]
- Mansouri, H.R.; Pizzi, A.; Leban, J.M.; Delmotte, L.; Lindgren, O.; Vaziri, M. Causes for the Improved Water Resistance in Pine Wood Linear Welded Joints. J. Adhes. Sci. Technol. 2011, 25, 1987–1995. [Google Scholar] [CrossRef]
- Yao, X.; Zheng, L. Development Potential of Rosin Sizing Agent. Chem. Technol. Mark. 2000, 10, 21. [Google Scholar]
- Pizzi, A. A New Approach to Non-Toxic, Wide-Spectrum, Ground-Contact Wood Preservatives. Part I. Approach and Reaction Mechanisms. Holzforschung 1993, 47, 253–260. [Google Scholar] [CrossRef]
- Pizzi, A. A New Approach to Non-Toxic, Wide-Spectrum, Ground-Contact Wood Preservatives. Part II. Accelerated and Long-Term Field Tests. Holzforschung 1993, 47, 343–348. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, W.; Hughes, M.; Wu, M.; Zhang, S.; Li, J. Various Polymeric Monomers Derived from Renewable Rosin for the Modification of Fast-Growing Poplar Wood. Compos. Part B Eng. 2019, 174, 106902. [Google Scholar] [CrossRef]
- Shu-jun, L.I.; Thi-thanh-hien, N.; Shi-yan, H.A.N.; Jian, L.I. Application of Rosin in Wood Preservation. Chem. Ind. For. Prod. 2011, 31, 117–121. [Google Scholar]
- Dong, Y.; Yan, Y.; Wang, K.; Li, J.; Zhang, S.; Xia, C.; Shi, S.Q.; Cai, L. Improvement of Water Resistance, Dimensional Stability, and Mechanical Properties of Poplar Wood by Rosin Impregnation. Eur. J. Wood Prod. 2016, 74, 177–184. [Google Scholar] [CrossRef]
- McKerrell, H.; Roger, E.; Varsanyi, A. The Acetone/Rosin Method for Conservation of Waterlogged Wood. Stud. Conserv. 1972, 17, 111–125. [Google Scholar] [CrossRef]
- Giachi, G.; Capretti, C.; Donato, I.D.; Macchioni, N.; Pizzo, B. New Trials in the Consolidation of Waterlogged Archaeological Wood with Different Acetone-Carried Products. J. Archaeol. Sci. 2011, 38, 2957–2967. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Li, J.; Li, S. Effects of Water-Borne Rosin on the Fixation and Decay Resistance of Copper-Based Preservative Treated Wood. BioResources 2012, 7, 3573–3584. [Google Scholar] [CrossRef]
- Yang, M.; Chen, X.; Lin, H.; Han, C.; Zhang, S. A Simple Fabrication of Superhydrophobic Wood Surface by Natural Rosin Based Compound via Impregnation at Room Temperature. Eur. J. Wood Prod. 2018, 76, 1417–1425. [Google Scholar] [CrossRef]
- Dahlen, J.; Nicholas, D.D.; Schultz, T.P. Water Repellency and Dimensional Stability of Southern Pine Decking Treated with Waterborne Resin Acids. J. Wood Chem. Technol. 2008, 28, 47–54. [Google Scholar] [CrossRef]
- Pizzi, A.; Mansouri, H.R.; Leban, J.M.; Delmotte, L.; Pichelin, F. Enhancing the Exterior Performance of Wood Joined by Linear and Rotational Welding. J. Adhes. Sci. Technol. 2011, 25, 2717–2730. [Google Scholar] [CrossRef]
- Aşçi, T.; Keskïn, H. Effect of Impregnation with Boron Compound Doped Rosin to the Combustion Resistance of Oriental Beech Wood (Fagus Orientalis Lipsky). Politek. Derg. 2021, 24, 655–662. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Jian, L. Water Repellent Characteristics of Wood Treated with the Mixture of Rosin and Copper Sulfate. Sci. Technol. J. Agric. Rural Dev. (Ha Noi Vietnam) 2014, 11, 64–69. [Google Scholar]
- Nguyen, T.T.H.; Li, S.; Li, J.; Liang, T. Micro-Distribution and Fixation of a Rosin-Based Micronized-Copper Preservative in Poplar Wood. Int. Biodeterior. Biodegrad. 2013, 83, 63–70. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Tran, V.C.; Li, S.; Li, J. Effects of Rosin-Aluminum Sulfate Treatment on the Leachability, Color Stability, and Decay Resistance of Wood Treated with a Boron-Based Preservative. BioResources 2019, 15, 172–186. [Google Scholar] [CrossRef]
- Nguyen, T.T.H. Effects of Rosin Sizing Agent on the Fixation of Boron in Styrax Tonkinensis Wood. J. For. Sci. Technol. 2017, 5, 133–139. [Google Scholar]
- Nguyen, T.T.H.; Tran, N.; Minh, T.; Trinh, H.; Le, X.; Nguyen, T. Evaluation of Weathering Performance of Rosin-Copper Based Treated Wood. J. Renew. Mater. 2022, 10, 2765–2780. [Google Scholar] [CrossRef]
- Kurkowiak, K.; Mayer, A.K.; Emmerich, L.; Militz, H. Investigations of the Chemical Distribution in Sorbitol and Citric Acid (SorCA) Treated Wood—Development of a Quality Control Method on the Basis of Electromagnetic Radiation. Forests 2022, 13, 151. [Google Scholar] [CrossRef]
- Larnøy, E.; Karaca, A.; Gobakken, L.R.; Hill, C.A.S. Polyesterification of Wood Using Sorbitol and Citric Acid under Aqueous Conditions. Int. Wood Prod. J. 2018, 9, 66–73. [Google Scholar] [CrossRef]
- Mubarok, M.; Militz, H.; Dumarçay, S.; Gérardin, P. Beech Wood Modification Based on in Situ Esterification with Sorbitol and Citric Acid. Wood Sci. Technol. 2020, 54, 479–502. [Google Scholar] [CrossRef]
- Beck, G. Leachability and Decay Resistance of Wood Polyesterified with Sorbitol and Citric Acid. Forests 2020, 11, 650. [Google Scholar] [CrossRef]
- Treu, A.; Nunes, L.; Larnøy, E. Macrobiological Degradation of Esterified Wood with Sorbitol and Citric Acid. Forests 2020, 11, 776. [Google Scholar] [CrossRef]
- Kurkowiak, K.; Emmerich, L.; Militz, H. Sorption Behavior and Swelling of Citric Acid and Sorbitol (SorCA) Treated Wood. Holzforschung 2021, 75, 1136–1149. [Google Scholar] [CrossRef]
- Kurkowiak, K.; Wu, M.; Emmerich, L.; Militz, H. Fire-Retardant Properties of Wood Modified with Sorbitol, Citric Acid and a Phosphorous-Based System. Holzforschung 2023, 77, 38–44. [Google Scholar] [CrossRef]
- Kurkowiak, K.; Hentges, D.; Dumarçay, S.; Gérardin, P.; Militz, H. Understanding the Mode of Action of Sorbitol and Citric Acid (SorCA) in Wood. Wood Mater. Sci. Eng. 2023, 18, 67–75. [Google Scholar] [CrossRef]
- Kurkowiak, K.; Emmerich, L.; Militz, H. Biological Durability and Wood–Water Interactions of Sorbitol and Citric Acid (SorCA) Modified Wood. J. Wood Sci. 2023, 69, 34. [Google Scholar] [CrossRef]




| Name of Biopolymer | Physical and Mechanical Properties | Wood-Water Relations and Hydrophobic Properties | Durability and Color Properties | Other Remarks |
|---|---|---|---|---|
| FA | Density increases by 16%–40%, improving strength, hardness. Does not consistently increase MOR, MOE, impact resistance. | ASE up to 60%. | DC1 against fungi and insects. Resistance to marine borers above 50% WPG. Darkens wood to brownish. | Non-toxic, non-flammable. No ecotoxicity reported for leachates. Higher thermal stability. Commercial (Kebony™, Nobelwood™). |
| PLA | Brittle, low toughness. Improved bending, compression strength, and hardness without catalyst. With densification, more improved MOR, hardness. | Improved ASE. Lower water uptake. | No visible fungal decay due to acidity and low moisture content. Not inherently biocidal; agents can be added. Darker, more saturated brown color. | Biodegradable, renewable (corn starch). Requires 25%–55% less energy to produce. No commercial solid wood products. |
| PCL | Compression strength parallel to grain did not significantly decrease. | Water absorption decreased by 70%, ASE of 40%. Water contact angle decreased from 105° to 65° after weathering. | Resisted fungal attacks at 15.5% WPG. Color changes less (ΔE* decreased from 8 to 4). | Low-cost base material, waste-free process. No commercial products. No noticeable cell wall deformations. |
| PBAT | Low strength, low heat resistance. High flexibility, processability, tensile strength, elongation at break, ductility. Strengthens PLA–wood foam by 797%. | Enhances dimensional stability in PBAT–PLA–wood fiber blends. | Does not degrade in marine or freshwater. | Environmentally safe and non-toxic. Synthetic, but biodegradable. Increasing market. |
| PBS | Tensile strength increased to 28 MPa, Young’s modulus to 1007 MPa in composites. | EMC reduced to 3.57% with compatibilizer. ASE 60%–70% | No cracks, no mold during one-year weathering. Reduced weight loss against Coriolus versicolor (from 17% to 3.7%). | Biodegradable and compostable. Good processability, thermal stability. Not commercially available for solid wood. |
| Zein | Brinell hardness increased by 10% at 16.22% WPG. | Absorbed ~80% less water than untreated wood. Showed ~80% better dimensional stability. Water contact angle increased from 36.85° to 50.72°. | Exhibited antifungal properties by forming a barrier against moisture and oxygen. Increased wear resistance. | Hydrophobic protein from corn. Non-toxic, combustible. Not commercially available, rarely researched. |
| Lignin | Cell wall elasticity increased by 8.7%, hardness by 10.3%. MOR improved by nearly 54%, MOE by nearly 200%. | Contact angle up to 111.1°. ASE 99.4%. Moisture absorption decreased to 0.55%. | Highest efficacy against brown rot fungi, negligible mass losses. Better UV stability and lower color change. | Not commercially available for modification. Challenges with molecule size, reactivity, and variability. Reduced flammability. |
| Tannin | Compression strength increased by 35% (10% tannin). MOE increased from 99.2 to 120.9 MPa (20% tannin). Brinell hardness increased. | Reduced roughness. Decreased water uptake, increased ASE. High water solubility, historically limited efficacy. | Reduced weight loss against brown rot (0%–29.26%) and white rot (0%–7.94%). | Plant-derived polyphenolic compounds. Natural defense against pathogens. Not widely on market. Leaching loss of 17%–35%. Increased ignition time from 12 s to >75 s, flame time from 140 s to 15–20 s. |
| Chitosan | Average veneer stiffness enhancement of 20%. MOE of heat-modified wood increased by 27%. Mass gain up to 30.54%, volume gain up to 19.55%. | Total sorption reduced by 23.8%, monomolecular sorption by 35.3%. Reduced hygroscopicity, lower EMC. Enhanced hydrophobation. | Total inhibition of white rot, brown rot fungi, and mold (1% conc.). Resistance to termites (0.48% vs. 3.96% WL). 95.8% | Natural, renewable, biodegradable, non-toxic. Structurally comparable to cellulose. Leaching decreased efficacy. Improved fire-retardant activity. |
| Alginate | Tensile strength 1.1 GPa, flexural strength 679 MPa. Tensile, elongation, bending, compressive strength (aerogel) enhanced by 128.4%, 109.1%, 31.8%, 241.7%, respectively. | Diffusivity decreased from 310 to 174 × 10−7 m2/s (100% wood fiber). | Biodegradable. | Natural anionic polysaccharide from brown seaweeds. Low toxicity, biocompatibility. Low thermal conductivity (0.078–0.089 W/mK). Limiting Oxygen Index reached 59.2% (self-extinguishing). Improves thermal stability of lignin. |
| Natural Gums | Gum rosin with n-hexane exhibited high MOR of 9.7 GPa. Bending MOE 10 GPa, MOR 78 MPa with 7.5% gum rosin in petroleum oil. Dry bonding strength 6 MPa. | P. atlantica gum improved moisture exclusion. Gum rosin largely ineffective in reducing water uptake/shrinkage. | P. atlantica gum slightly improved decay resistance. Significantly reduced mold coverage (from 76%–100% to 26%–50%). | From tree trunks or pine resins. |
| Fatty Acids | Hardness on tangential section increased by 200%–350% (rapeseed oil + densification). Tung oil increased impact bending strength by up to 21%–23%. | Rapeseed oil reduced water absorption to 33.07%, volumetric swelling to 7.31%. H-castor oil had lowest moisture uptake (1%) after 2 weeks. Increased dimensional stability. | Treatment with rapeseed oil at 200°C showed best color stability (ΔE* < 12 for 10 months). Full protection against termites/fungi (boric acid + linseed oil). Tung oil boosts wear/impact resistance. | Derived from natural oils, renewable lipids. Chemically bond with cellulose. Oils can polymerize and modify wood. |
| Rosin | Improves mechanical strength of dry-welded joints. 20% rosin increased density to 440 kg/m3, MOR by 12.8%, MOE by 18.9%, compression strength by 31.6%. | Increased ASE to 36%, decreased EMC by 42.7% (20% rosin). Enhanced water repellency. Superhydrophobic surface (157°). | Long-term (25 years) resistance to termites and fungi. Improved leaching resistance and extended combustion period. | Natural adhesive and preservative. Renewable, abundant, low-cost, highly hydrophobic. Not commercially available. |
| SCA | MOE increased slightly (approx. 9%) at optimum conditions. MOR decreased considerably. Increased brittleness (work to max load decreased up to 80%). | Swelling at saturation state lowest (1%–2%). ASE ranged from 23% to 58%. Liquid/vapor water uptake decreased. | Very low leaching at 140 °C curing. Low weight loss when tested against white rot (4.40%), brown rot (1.38%), soft rot (0.46%). No mass loss/attack by subterranean termites (WPG100%). No marine borer attack. | Low-cost, bio-based, non-toxic. No catalyst required, water as by-product. Solution stable, reusable. Forms crosslinked network. Improved fire-retardancy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fodor, F.; Bak, M. Biopolymer-Based Solutions for Sustainable Wood Modification: A Review of Current Advancements. Forests 2025, 16, 1463. https://doi.org/10.3390/f16091463
Fodor F, Bak M. Biopolymer-Based Solutions for Sustainable Wood Modification: A Review of Current Advancements. Forests. 2025; 16(9):1463. https://doi.org/10.3390/f16091463
Chicago/Turabian StyleFodor, Fanni, and Miklós Bak. 2025. "Biopolymer-Based Solutions for Sustainable Wood Modification: A Review of Current Advancements" Forests 16, no. 9: 1463. https://doi.org/10.3390/f16091463
APA StyleFodor, F., & Bak, M. (2025). Biopolymer-Based Solutions for Sustainable Wood Modification: A Review of Current Advancements. Forests, 16(9), 1463. https://doi.org/10.3390/f16091463







