Evaluating the Potential of Cuscuta japonica as Biological Control Agent for Derris trifoliata Management in Mangrove Forests
Abstract
1. Introduction
- (1)
- Does C. japonica successfully infect D. trifoliata and have significant negative effects?
- (2)
- Will C. japonica also infect the mangrove covered by D. trifoliata and cause significant negative effects?
2. Materials and Methods
2.1. Plant Material Preparation
2.2. Experimental Design
2.3. Sampling and Analysis
2.4. Statistical Analysis
3. Results
3.1. Survival Rates of Cuscuta japonica and Hosts
3.2. Haustoria on Different Host Species
3.3. Infection Responses of Host Species
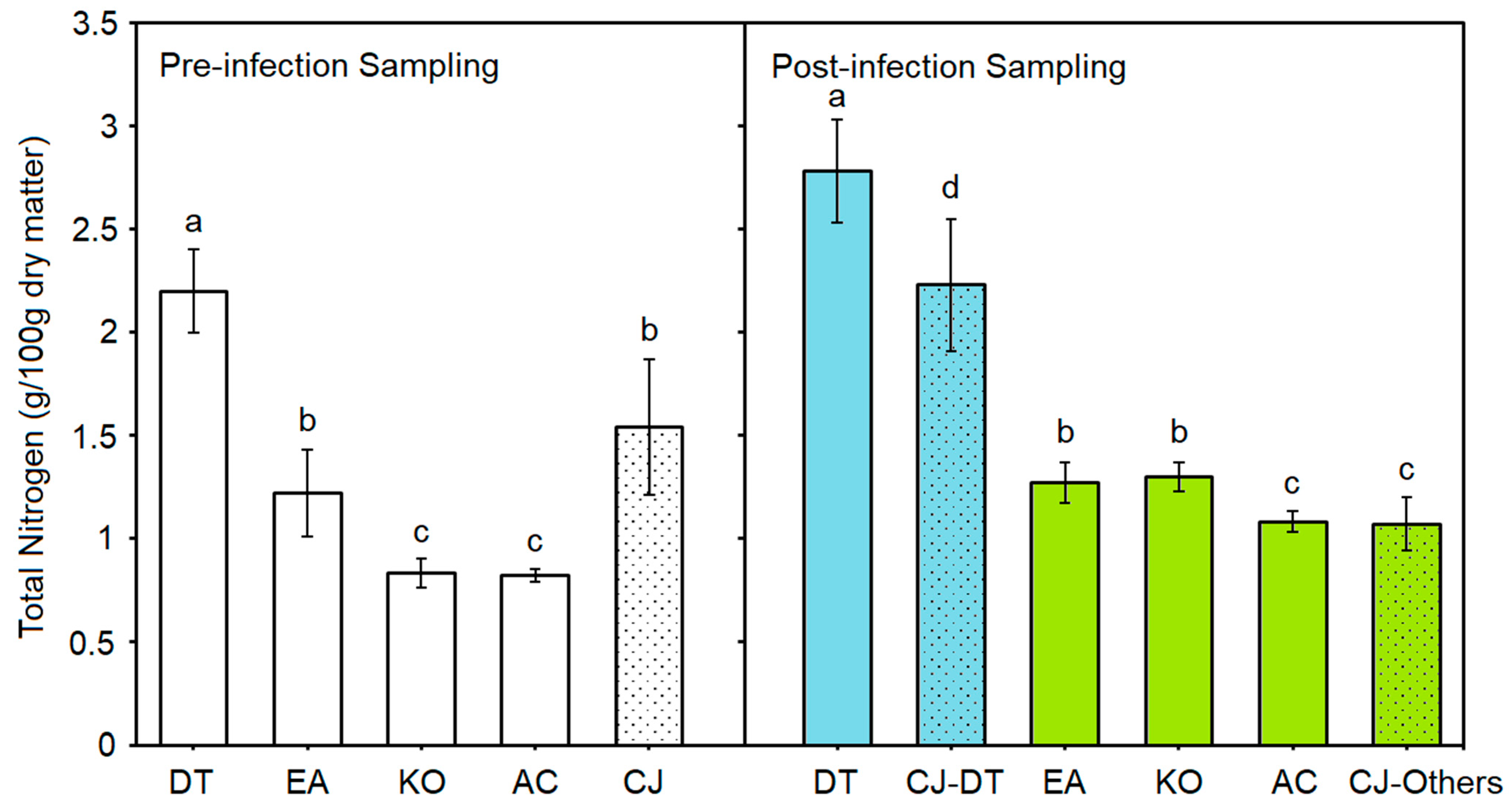
3.4. Other Special Features in Chemical Analysis
4. Discussion
4.1. Infection and Effects of Cuscuta on Different Host Species
4.2. Risk Assessment and Control of Cuscuta japonica in Mangroves
4.3. The Potential of the Proposed Method and Further Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeWalt, S.J.; Schnitzer, S.A.; Chave, J.; Bongers, F.; Burnham, R.J.; Cai, Z.; Chuyong, G.; Clark, D.B.; Ewango, C.E.N.; Gerwing, J.J.; et al. Annual rainfall and seasonality predict pan-tropical patterns of liana density and basal area. Biotropica 2010, 42, 309–317. [Google Scholar] [CrossRef]
- Parolari, A.J.; Paul, K.; Griffing, A.; Condit, R.; Perez, R.; Aguilar, S.; Schnitzer, S.A. Liana abundance and diversity increase with rainfall seasonality along a precipitation gradient in Panama. Ecography 2020, 43, 25–33. [Google Scholar] [CrossRef]
- Prayudha, B.; Siregar, V.; Ulumuddin, Y.I.; Prasetyo, L.B.; Agus, S.B.; Suyadi; Suyarso; Salatalohi, A.; Anggraini, K. Mangrove forest encroachment by Nypa frutican, Derris trifoliata, and Acanthus spp. in Segara Anakan Lagoon. IOP Conf. Ser. Earth Environ. Sci. 2023, 1251, 012017. [Google Scholar] [CrossRef]
- Matthews, E.R.; Schmit, J.P.; Campbell, J.P. Climbing vines and forest edges affect tree growth and mortality in temperate forests of the U.S. Mid-Atlantic States. For. Ecol. Manag. 2016, 374, 166–173. [Google Scholar] [CrossRef]
- Schnitzer, S.A.; DeFilippis, D.M.; Visser, M.; Estrada-Villegas, S.; Rivera-Camaña, R.; Bernal, B.; Peréz, S.; Valdéz, A.; Valdéz, S.; Aguilar, A.; et al. Local canopy disturbance as an explanation for long-term increases in liana abundance. Ecol. Lett. 2021, 24, 2635–2647. [Google Scholar] [CrossRef]
- Campbell, M.J.; Edwards, W.; Magrach, A.; Alamgir, M.; Porolak, G.; Mohandass, D.; Laurance, W.F. Edge disturbance drives liana abundance increase and alteration of liana-host tree interactions in tropical forest fragments. Ecol. Evol. 2018, 8, 4237–4251. [Google Scholar] [CrossRef]
- Paul, G.S.; Yavitt, J.B. Tropical vine growth and the effects on forest succession: A review of the ecology and management of tropical climbing plants. Bot. Rev. 2011, 77, 11–30. [Google Scholar] [CrossRef]
- Becknell, J.; Vargas, G.G.; Wright, L.; Woods, N.-F.; Medvigy, D.; Powers, J. Increasing liana abundance and associated reductions in tree growth in secondary seasonally dry tropical forest. Front. For. Glob. Change 2022, 5, 838357. [Google Scholar] [CrossRef]
- Biswas, S.R.; Choudhury, J.K.; Nishat, A.; Rahman, M.M. Do invasive plants threaten the Sundarbans mangrove forest of Bangladesh? For. Ecol. Manag. 2007, 245, 1–9. [Google Scholar] [CrossRef]
- Polyium, U.; Thaisong, P.N. Phytochemical and nutritional values of local plants in the Phraek Nam Daeng Community Samut Songkhram Province Thailand. AMM 2018, 879, 101–107. [Google Scholar] [CrossRef]
- Raju, A.J.S.; Kumar, R. Pollination ecology of Derris trifoliata (Fabaceae), a mangrove associate in Coringa Mangrove Forest, Andhra Pradesh, India. J. Threat. Taxa 2016, 8, 8788–8796. [Google Scholar] [CrossRef]
- Zhang, Y.; Xin, K.; Liao, B.; Ai, X.; Sheng, N. The genetic and environmental adaptation of the associated liana species Derris trifoliata Lour. (Legum.) mangroves. Forests 2021, 12, 1375. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, K.; Liu, L.; Myint, S.W.; Wang, S.; Cao, J.; Wu, Z. Estimating and mapping mangrove biomass dynamic change using WorldView-2 images and digital surface models. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2020, 13, 2123–2134. [Google Scholar] [CrossRef]
- Sheng, N.; Xin, K.; Zhang, C.; Hua, G. Dispersal pattern of Derris trifoliata in mangroves. J. Hainan Norm. Univ. (Nat. Sci.) 2021, 34, 148–153. [Google Scholar]
- Aluri, J.S.R.; Kumar, R.; Chappidi, P. Reproductive biology of mangrove plants Clerodendrum inerme, Derris trifoliata, Suaeda maritima, Suaeda monoica, Suaeda nudiflora. Transylv. Rev. Syst. Ecol. Res. 2016, 18, 31–68. [Google Scholar] [CrossRef]
- Huang, X.; Zhong, C.; Chen, S.; Liu, Y.; Liang, S. Mangrove forest threatened by Derris trifoliata. Wetl. Sci. Manag. 2015, 11, 26–29. [Google Scholar] [CrossRef]
- Lyu, T.; Zhong, S.; Jiang, W.; Ling, Z.; Chu, A. Effects of Deris trfoliata on mangrove degradation in the Lianzhou Bay of Guangxi based on China’s high-resolution remote sensing data. Wetl. Sci. 2025, 23, 11–21. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, B.; Yang, L.; Jiang, Z.; Xin, K. Research summary on native associated liana species Derris trifoliata in mangrove forests. Wetl. Sci. 2022, 20, 421–426. [Google Scholar] [CrossRef]
- Huang, D.; Sun, X.; Guo, X.; Gou, Z. Risk analysis of mangrove associate plant Derris trifoliate in Hainan. Trop. For. 2019, 47, 62–65. [Google Scholar] [CrossRef]
- Li, L.; Lin, J. The distribution and preventing strategy of Derris trifoliate in Hainan Dongzhaigang National Nature Reserve. Trop. For. 2019, 47, 36–38. [Google Scholar] [CrossRef]
- Těšitel, J.; Cirocco, R.M.; Facelli, J.M.; Watling, J.R. Native parasitic plants: Biological control for plant invasions? Appl. Veg. Sci. 2020, 23, 464–469. [Google Scholar] [CrossRef]
- Wang, Y.; Bu, H.; Gu, X.; Liu, W.; Wang, X. Integrated omic analysis provides insights into how Cuscuta australis inhibits the growth and reproduction of Xanthium spinosum. BMC Plant Biol. 2025, 25, 657. [Google Scholar] [CrossRef]
- Cirocco, R.M.; Facelli, J.M.; Watling, J.R. The impact of a native hemiparasite on a major invasive shrub is affected by host size at time of infection. J. Exp. Bot. 2020, 71, 3725–3734. [Google Scholar] [CrossRef] [PubMed]
- Zan, Q.; Wang, B.; Wang, Y.; Zhang, J.; Liao, W.; Li, M. The harm caused by Mikania micrantha and its control by Cuscuta campestris. Chin. J. Plant Ecol. 2003, 27, 822–828. [Google Scholar] [CrossRef]
- Koch, A.M.; Binder, C.; Sanders, I.R. Does the generalist parasitic plant Cuscuta campestris selectively forage in heterogeneous plant communities? New Phytol. 2004, 162, 147–155. [Google Scholar] [CrossRef]
- Furuhashi, T.; Katsuhisa, F.; Weckwerth, W. The parasitic mechanism of the holostemparasitic plant Cuscuta. J. Plant Interact. 2011, 6, 207–219. [Google Scholar] [CrossRef]
- Yu, H.; Liu, J.; He, W.-M.; Miao, S.-L.; Dong, M. Cuscuta australis restrains three exotic invasive plants and benefits native species. Biol. Invasions 2011, 13, 747–756. [Google Scholar] [CrossRef]
- Li, J.; Yang, B.; Yan, Q.; Zhang, J.; Yan, M.; Li, M. Effects of a native parasitic plant on an exotic invader decrease with increasing host age. AoBP 2015, 7, plv031. [Google Scholar] [CrossRef]
- Wu, A.P.; Zhong, W.; Yuan, J.R.; Qi, L.Y.; Chen, F.L.; Liang, Y.S.; He, F.F.; Wang, Y.H. The factors affecting a native obligate parasite, Cuscuta australis, in selecting an exotic weed, Humulus scandens, as its host. Sci. Rep. 2019, 9, 511. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jin, Z.; Song, W. Do native parasitic plants cause more damage to exotic invasive hosts than native non-invasive hosts? An implication for biocontrol. PLoS ONE 2012, 7, e34577. [Google Scholar] [CrossRef][Green Version]
- Yu, H.; Yu, F.H.; Miao, S.L.; Dong, M. Holoparasitic Cuscuta campestris suppresses invasive Mikania micrantha and contributes to native community recovery. Biol. Conserv. 2008, 141, 2653–2661. [Google Scholar] [CrossRef]
- Wang, W.B.; Gao, F.F.; Shao, M.N.; Liu, M.C.; Zhai, H.F.; Qu, B.; Feng, Y.L. First record of field dodder (Cuscuta campestris) parasitizing invasive buffalobur (Solanum rostratum). J. Plant Pathol. 2020, 102, 703–707. [Google Scholar] [CrossRef]
- Wang, H.; Ze, S.; Ji, M.; Zhao, N.; Hu, L.; Xie, S. Impacts of different fertilizer processing on Cuscuta Reflexa infecting Mikania micrantha. Jiangsu Agri. Sci. 2020, 48, 98–101. [Google Scholar] [CrossRef]
- Wang, W.; Gao, F.; Feng, W.; Wu, Q.; Feng, Y. The native stem holoparasitic Cuscuta japonica suppresses the invasive plant Ambrosia trifida and related mechanisms in different light conditions in northeast China. Front. Plant Sci. 2022, 13, 904326. [Google Scholar] [CrossRef]
- Li, Q.; Xiao, H. Study on the allelochemicals of inhibiting Mikania micrantha growth from Cuscuta japonica. Ecol. Environ. 2012, 21, 434–439. [Google Scholar] [CrossRef]
- Jiang, H.; Fang, F.; Guo, S. Influences of parasitism by Cuscuta japonica plants on eco-physiological characteristics of Solidago canadensis. Acta Ecol. Sin. 2008, 28, 399–406. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Xue, Y.; Liang, Q.; Tao, Y.; Wu, H.; Jiang, W. Phenomenon and mechanisms of Sonneratia apetala introduction and spread promoting excessive growth of Derris trifoliata. Forests 2024, 15, 525. [Google Scholar] [CrossRef]
- Facelli, E.; Wynn, N.; Tsang, H.T.; Watling, J.R.; Facelli, J.M. Defence responses of native and invasive plants to the native generalist vine parasite Cassytha pubescens—anatomical and functional studies. Aust. J. Bot. 2020, 68, 300–309. [Google Scholar] [CrossRef]
- Jeschke, W.D.; Räth, N.; Bäumel, P.; Czygan, F.-C.; Proksch, P. Modelling the flow and partitioning of carbon and nitrogen in the holoparasite Cuscuta reflexa Roxb. and its host Lupinus albus L.: I. Methods for estimating net flows. J. Exp. Bot. 1994, 45, 791–800. [Google Scholar] [CrossRef]
- Zagorchev, L.; Stöggl, W.; Teofanova, D.; Li, J.; Kranner, I. Plant parasites under pressure: Effects of abiotic stress on the interactions between parasitic plants and their hosts. Int. J. Mol. Sci. 2021, 22, 7418. [Google Scholar] [CrossRef] [PubMed]
- Jhu, M.Y.; Sinha, N.R. Cuscuta species: Model organisms for haustorium development in stem holoparasitic plants. Front. Plant Sci. 2022, 13, 1086384. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, W.D.; Bäumel, P.; Räth, N.; Czygan, F.-C.; Proksch, P. Modelling of the flows and partitioning of carbon and nitrogen in the holoparasite Cuscuta reflexa Roxb. and its host Lupinus albus L.: II. Flows between host and parasite and within the parasitized host. J. Exp. Bot. 1994, 45, 801–812. [Google Scholar] [CrossRef]
- Gilbert, A. The role of root nodules in plant health and growth. RRJ Bot. Sci. 2023, 12, 2. [Google Scholar]
- Ferguson, B.J.; Mens, C.; Hastwell, A.H.; Zhang, M.; Su, H.; Jones, C.H.; Chu, X.; Gresshoff, P.M. Legume nodulation: The host controls the party. Plant Cell Environ. 2018, 42, 41–51. [Google Scholar] [CrossRef]
- Nikam, S.S.; Pawar, S.B.; Kanade, M.B. Study of Cuscuta reflexa Roxb. with reference to host diversity, anatomy and biochemistry. Cent. Euro. J. Exp. Biol. 2014, 3, 6–12. [Google Scholar]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2010. [Google Scholar]
- Wingler, A.; Roitsch, T. Metabolic regulation of leaf senescence: Interactions of sugar signalling with biotic and abiotic stress responses. Plant Biol. 2008, 10, 50–62. [Google Scholar] [CrossRef]
- Hock, S.M.; Wiecko, G.; Knezevic, S.Z. Glyphosate dose affected control of field dodder (Cuscuta campestris) in the tropics. Weed Technol. 2008, 22, 151–155. [Google Scholar] [CrossRef]
- Du, X.; Huang, M.; Ma, Y.; Guo, C.; Liang, H.; Tian, H.; Jiang, X.; Nong, G. Occurrence and Growth Dynamics of Semen cuscutae in Gardens of Guangxi and Evaluation of Herbicides for Its Control. J. South. Agric. 2012, 42, 748–751. [Google Scholar]
- Li, H.; Ouyang, M.; Zeng, T.; Wu, C.; Liu, J.; Liu, Y. The research on the mangrove resources in Dajiaoshan Seaside Park, Nansha, Guangzhou. J. Guangdong Univ. Educ. 2019, 39, 43–47. [Google Scholar] [CrossRef]
- David, A.S.; Lake, E.C. Biological control of vines: A review of past efforts, evaluation, and future directions. Biol. Control 2023, 183, 105257. [Google Scholar] [CrossRef]
- Sheng, N. Spatial Distribution Characteristics of Derris trifoliata in Mangroves and Its Influencing Factors. Master’s Thesis, Hainan Normal University, Haikou, China, April 2021. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |



| Species | Experimental Group | Individuals | No. of Sampling | Weight per Sample |
|---|---|---|---|---|
| Hosts: | ||||
| Derris trifoliata | Control | 30 | 6 | 45–50 g |
| Infection | 30 | 6 | ||
| Excoecaria agallocha | Control | 10 | 3 | |
| Infection | 10 | 3 | ||
| Kandelia obovata | Control | 10 | 3 | |
| Infection | 10 | 3 | ||
| Aegiceras corniculatum | Control | 10 | 3 | |
| Infection | 10 | 3 | ||
| Parasite: | ||||
| Cuscuta japonica | Infecting D. trifoliata | Not Available | 6 | 90–100 g |
| Infecting others | Not Available | 6 | ||
| Moisture | Nitrogen | Phosphorous | Potassium | Soluble Sugar | Soluble Protein | |
|---|---|---|---|---|---|---|
| Host Species | ||||||
| F3, 22 | 89.610 | 889.591 | 125.824 | 8.534 | 6.432 | 213.865 |
| p | 0.000 | 0.000 | 0.000 | 0.001 | 0.003 | 0.000 |
| Infection | ||||||
| F1, 22 | 17.792 | 23.975 | 21.718 | 13.397 | 9.114 | 1.820 |
| p | 0.000 | 0.000 | 0.000 | 0.001 | 0.006 | 0.191 |
| Host Species × Infection | ||||||
| F3, 22 | 2.602 | 15.055 | 18.159 | 13.785 | 13.686 | 7.968 |
| p | 0.078 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 |
| R2 | 0.933 | 0.992 | 0.956 | 0.820 | 0.797 | 0.968 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Xue, Y.; Liu, W. Evaluating the Potential of Cuscuta japonica as Biological Control Agent for Derris trifoliata Management in Mangrove Forests. Forests 2025, 16, 1250. https://doi.org/10.3390/f16081250
Wu H, Xue Y, Liu W. Evaluating the Potential of Cuscuta japonica as Biological Control Agent for Derris trifoliata Management in Mangrove Forests. Forests. 2025; 16(8):1250. https://doi.org/10.3390/f16081250
Chicago/Turabian StyleWu, Huiying, Yunhong Xue, and Wenai Liu. 2025. "Evaluating the Potential of Cuscuta japonica as Biological Control Agent for Derris trifoliata Management in Mangrove Forests" Forests 16, no. 8: 1250. https://doi.org/10.3390/f16081250
APA StyleWu, H., Xue, Y., & Liu, W. (2025). Evaluating the Potential of Cuscuta japonica as Biological Control Agent for Derris trifoliata Management in Mangrove Forests. Forests, 16(8), 1250. https://doi.org/10.3390/f16081250







