Actualized Scope of Forestry Biomass Valorization in Chile: Fostering the Bioeconomy
Abstract
1. Introduction
2. Methodological Approach
3. The Chilean Forestry Industry Landscape
4. Valorization of Forestry Byproducts
4.1. Lignocellulosic
4.1.1. Lignin: Characteristics, Applications, Challenges, and Chilean Advances
4.1.2. Nanocellulose (CNF): Production, Functionalization, and Applications
4.1.3. CNF Production from E. globulus, E. nitens, and P. radiata
4.1.4. CNF Applications
4.1.5. Hemicellulose: Extraction and Use in Food, Pharma, and Remediation
4.2. Phenolic and Bioactive Compounds
4.2.1. Bark Tannins: Extraction and Industrial Applications
4.2.2. Bio-Based Adhesives Replacing Synthetic Resins
4.2.3. Essential Oils and Hydrolates from Eucalyptus and Pinus
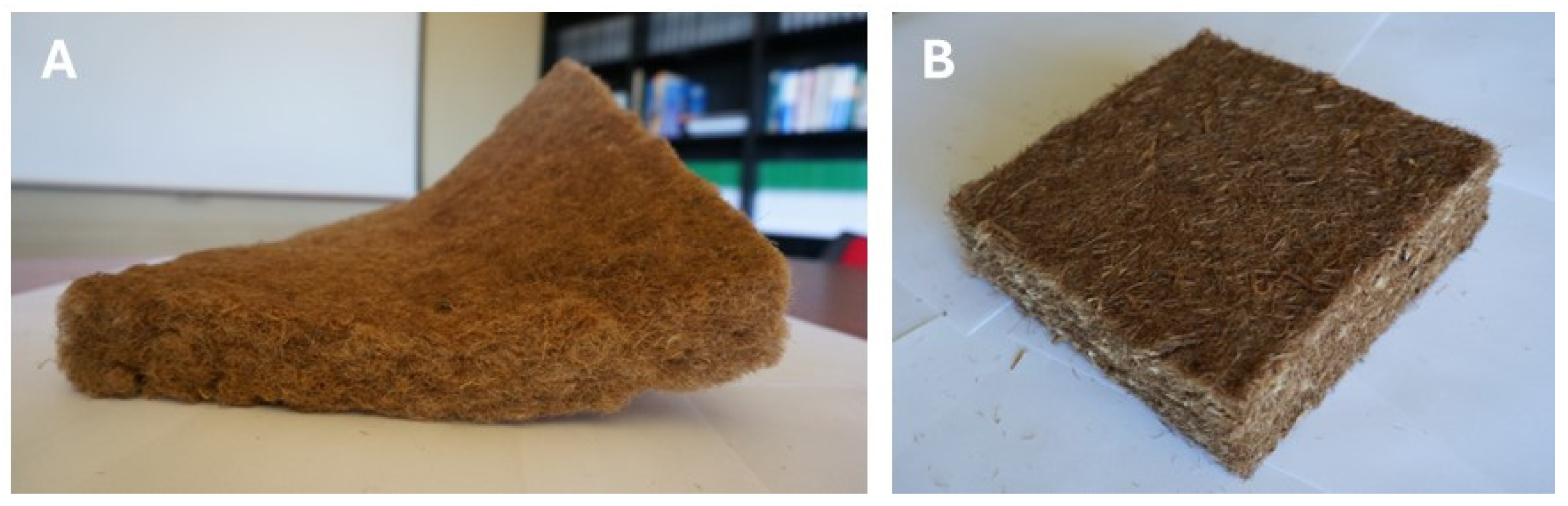
5. Bark Fiber
6. Thermochemical Transformation of Forest Byproducts in Chile
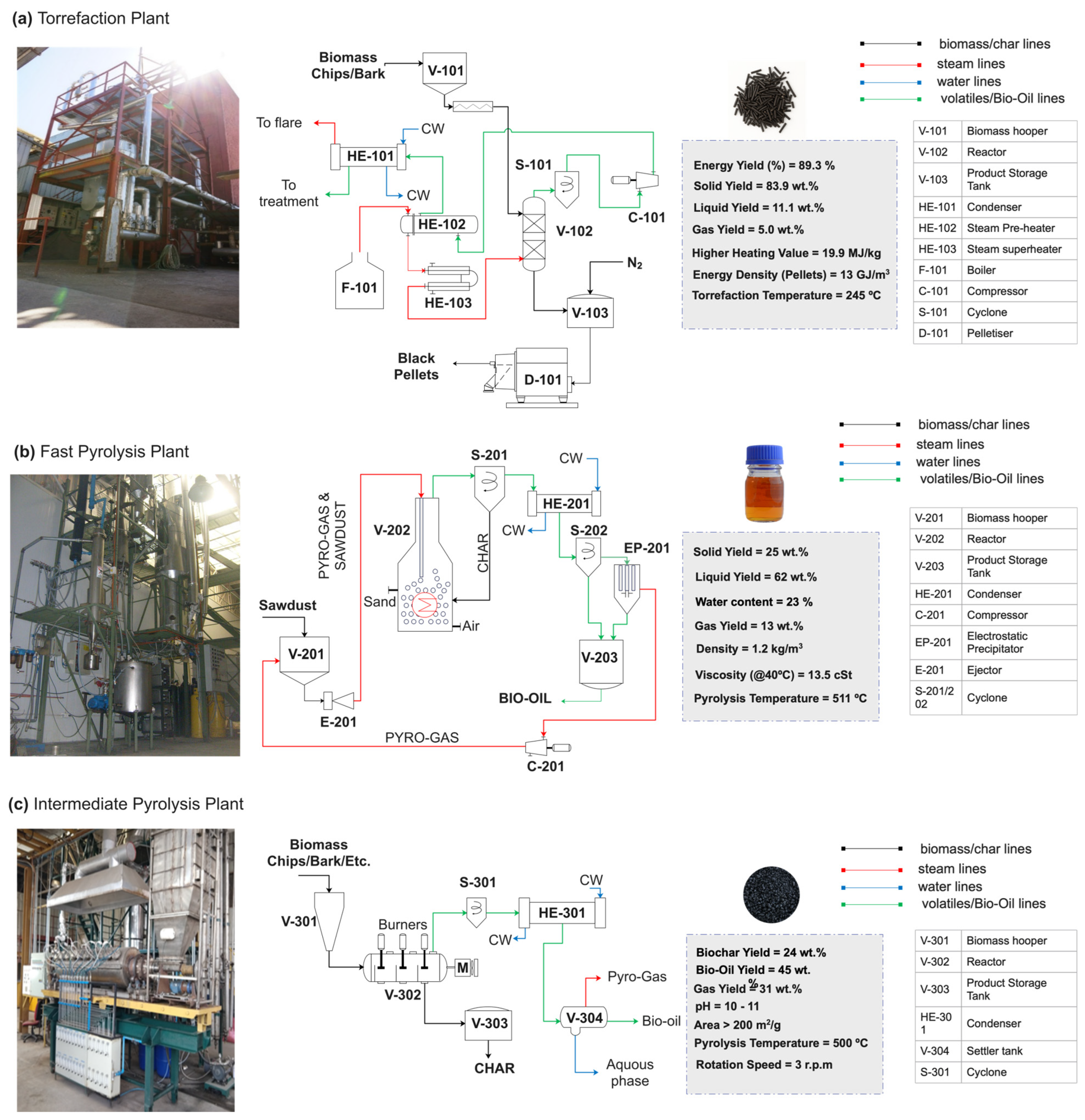
6.1. Torrefaction
6.2. Pyrolysis
6.3. Gasification
7. Sustainability and Regulatory Framework
7.1. Circular Economy Roadmap and Extended Producer Responsibility (REP) Law
7.2. Life Cycle Assessment (LCA) of Bioproducts
8. Barriers to Technology Transfer and Industrial Adoption
9. Research Gaps and Future Directions
9.1. Pilot-Scale Validation and Industrial Scaling
9.2. Functionalization and Performance Optimization
9.3. Standardization and Regulatory Framework
9.4. Integration of Biodiversity and Native Species
9.5. Policy Instruments and Demand Creation
9.6. LCA and Techno-Economic Models for New Products
9.7. Socioeconomic and Regional Development Impacts
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nepal, P.; Prestemon, J.P.; Joyce, L.A.; Skog, K.E. Global Forest Products Markets and Forest Sector Carbon Impacts of Projected Sea Level Rise. Glob. Environ. Change 2022, 77, 102611. [Google Scholar] [CrossRef]
- FAO. Global Forest Products. Facts and Figures 2023; FAO: Rome, Italy, 2024; ISBN 978-92-5-139445-8. [Google Scholar]
- FAO. The State of the World’s Forests 2024; FAO: Rome, Italy, 2024; ISBN 978-92-5-138867-9. [Google Scholar]
- Weiss, G.; Ludvig, A.; Živojinović, I. Embracing the Non-Wood Forest Products Potential for Bioeconomy—Analysis of Innovation Cases across Europe. Land 2023, 12, 305. [Google Scholar] [CrossRef]
- Slavec, A.; Hoeben, A.D.; Moreno-Torres, M.; Primožič, L.; Stern, T. When Intentions Do Not Matter: Climate Change Mitigation and Adaptation Innovations in the Forest-Based Sector. For. Policy Econ. 2023, 157, 103074. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Poblete, P.; Kahler, C.; Bañados, J.; Gysling, J.; Pardo, E.; Soto, D.; Baeza, D.; Catelicán, L.; Hernández, J.; Troncoso, H. Anuario Forestal de Chile 2024, Boletín Estadístico N° 199; Instituto Forestal: Santiago, Chile, 2024. [Google Scholar]
- Cardemil, M. Industria Forestal En Chile. Serie Minutas N° 68-21; Biblioteca del Congreso Nacional de Chile, Departamento de Estudios, Extensión y Publicaciones: Santiago, Chile, 2021; pp. 1–14. [Google Scholar]
- Klein, H.S.; Luna, F.V. Chapter 10: Cellulose. In Brazilian Crops in the Global Market; Springer Nature: Cham, Switzerland, 2023; pp. 269–293. [Google Scholar]
- Barrera, D. Celulosa Chilena: Avances y Perspectivas de Su Comercio Exterior. Oficina de Estudios y Políticas Agrarias (ODEPA); Biblioteca Digital ODEPA: Santiago, Chile, 2018. [Google Scholar]
- González, M.; Morin, V.; Labra, N.; Castro, A. The Cellulose Industry and Its Impact on the Population: From the Social to the Biochemical. Rev. Interam. Ambient. Tur. 2021, 17, 179–188. [Google Scholar] [CrossRef]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hatfield, R.D.; Ralph, S.A.; Christensen, J.H.; et al. Lignins: Natural Polymers from Oxidative Coupling of 4-Hydroxyphenyl-Propanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Dessbesell, L.; Paleologou, M.; Leitch, M.; Pulkki, R.; Xu, C. (Charles) Global Lignin Supply Overview and Kraft Lignin Potential as an Alternative for Petroleum-Based Polymers. Renew. Sustain. Energy Rev. 2020, 123, 109768. [Google Scholar] [CrossRef]
- Ekielski, A.; Mishra, P.K. Lignin for Bioeconomy: The Present and Future Role of Technical Lignin. Int. J. Mol. Sci. 2021, 22, 63. [Google Scholar] [CrossRef]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A Concise Review of Current Lignin Production, Applications, Products and Their Environment Impact. Ind. Crops Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Vásquez-Garay, F.; Carrillo-Varela, I.; Vidal, C.; Reyes-Contreras, P.; Faccini, M.; Mendonça, R.T. A Review on the Lignin Biopolymer and Its Integration in the Elaboration of Sustainable Materials. Sustainability 2021, 13, 2697. [Google Scholar] [CrossRef]
- Sethupathy, S.; Murillo Morales, G.; Gao, L.; Wang, H.; Yang, B.; Jiang, J.; Sun, J.; Zhu, D. Lignin Valorization: Status, Challenges and Opportunities. Bioresour. Technol. 2022, 347, 126696. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose Nanomaterials Review: Structure, Properties and Nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a Tiny Fiber with Huge Applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Volza Grow Global “Exportaciones de Pulpa de Madera Desde Chile”. Available online: https://www.volza.com/p/wood-pulp/export/export-from-chile/ (accessed on 29 April 2025).
- Andrade, A.; Henríquez-Gallegos, S.; Albornoz-Palma, G.; Pereira, M. Effect of the Chemical and Structural Characteristics of Pulps of Eucalyptus and Pinus on the Deconstruction of the Cell Wall during the Production of Cellulose Nanofibrils. Cellulose 2021, 28, 5387–5399. [Google Scholar] [CrossRef]
- Syverud, K.; Chinga-Carrasco, G.; Toledo, J.; Toledo, P.G. A Comparative Study of Eucalyptus and Pinus Radiata Pulp Fibres as Raw Materials for Production of Cellulose Nanofibrils. Carbohydr. Polym. 2011, 84, 1033–1038. [Google Scholar] [CrossRef]
- Henríquez-Gallegos, S.; Albornoz-Palma, G.; Andrade, A.; Soto, C.; Pereira, M. Impact of the Enzyme Charge on the Production and Morphological Features of Cellulose Nanofibrils. Polymers 2021, 13, 3238. [Google Scholar] [CrossRef]
- Andrade, A.; Nuñez, J.; Henríquez-Gallegos, S.; Torres, C.; Mendez-Miranda, A.; Valenzuela-García, E.; Albornoz-Palma, G.; Ortega-Sanhueza, I.; Valerio, O.; Montoya, L.F.; et al. Interaction of Nanofibrillated Cellulose with Lignin Nanoparticles: Effects on CNF-LNP Composite Properties. Carbohydr. Polym. Technol. Appl. 2025, 9, 100651. [Google Scholar] [CrossRef]
- Albornoz-Palma, G.; Henríquez-Gallegos, S.; Ortega-Sanhueza, I.; Teruel-Juanes, R.; Ribes-Greus, A.; Pereira, M. Influence of Hemicellulose and Lignin on the Fibrillation Efficiency and Properties of Cellulose Nanofibrils from Native and Oxidized Eucalyptus Nitens and Pinus Radiata Pulps. Cellulose 2025, 32, 2629–2648. [Google Scholar] [CrossRef]
- Hoeger, I.C.; Nair, S.S.; Ragauskas, A.J.; Deng, Y.; Rojas, O.J.; Zhu, J.Y. Mechanical Deconstruction of Lignocellulose Cell Walls and Their Enzymatic Saccharification. Cellulose 2013, 20, 807–818. [Google Scholar] [CrossRef]
- Aguayo, M.G.; Pérez, A.F.; Reyes, G.; Oviedo, C.; Gacitúa, W.; Gonzalez, R.; Uyarte, O. Isolation and Characterization of Cellulose Nanocrystals from Rejected Fibers Originated in the Kraft Pulping Process. Polymers 2018, 10, 1145. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Figueroa, J.; Alejandro-Martin, S.; Cerda-Leal, F.; Gacitúa, W. Dual Electrospinning of a Nanocomposites Biofilm: Potential Use as an Antimicrobial Barrier. Mater. Today Commun. 2020, 25, 101671. [Google Scholar] [CrossRef]
- Cabrera-Barjas, G.; Becherán, L.; Valdés, O.; Giordano, A.; Segura-del Río, R.; Bravo-Arrepol, G.; Durán-Lara, E.F.; Cea, J.; Berg, A.; Castaños, J.; et al. Effect of Cellulose Nanofibrils on Vancomycin Drug Release from Chitosan Nanocomposite Films. Eur. Polymer. J. 2023, 197, 112371. [Google Scholar] [CrossRef]
- Qaseem, M.F.; Shaheen, H.; Wu, A.M. Cell Wall Hemicellulose for Sustainable Industrial Utilization. Renew. Sustain. Energy Rev. 2021, 144, 110996. [Google Scholar] [CrossRef]
- Reyes, P.; Teixeira Mendonça, R.; Rodríguez, J.; Fardim, P.; Vega, B. Characterization of the Hemicellulosic Fraction Obtained After Pre-Hydrolysis of Pinus Radiata Wood Chips with Hot-Water at Different Initial Ph. J. Chil. Chem. Soc. 2013, 58, 1614–1618. [Google Scholar] [CrossRef]
- Olivares, Y.; Herrera, C.; Seguel, J.; Sepúlveda, C.; Parra, C.; Pecchi, G. Stable Sulfonic MCM-41 Catalyst for Furfural Production from Renewable Resources in a Biphasic System. Catalysts 2023, 13, 1024. [Google Scholar] [CrossRef]
- Valenzuela, R.; Priebe, X.; Troncoso, E.; Ortega, I.; Parra, C.; Freer, J. Fiber Modifications by Organosolv Catalyzed with H2SO4 Improves the SSF of Pinus Radiata. Ind. Crops Prod. 2016, 86, 79–86. [Google Scholar] [CrossRef]
- Singh, A.K.; Upadhyay, S.K.; Singh, S.P. Microbial Bioreactors for Industrial Molecules. In Microbial Bioreactors: An Introduction; Singh, S.P., Upadhyay, S.K., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2023; pp. 1–16. [Google Scholar]
- Mathura, S.R.; Landázuri, A.C.; Mathura, F.; Andrade Sosa, A.G.; Orejuela-Escobar, L.M. Hemicelluloses from Bioresidues and Their Applications in the Food Industry-towards an Advanced Bioeconomy and a Sustainable Global Value Chain of Chemicals and Materials. Sustain. Food Technol. 2024, 2, 183. [Google Scholar] [CrossRef]
- Dax, D.; Chávez, M.S.; Xu, C.; Willför, S.; Mendonça, R.T.; Sánchez, J. Cationic Hemicellulose-Based Hydrogels for Arsenic and Chromium Removal from Aqueous Solutions. Carbohydr. Polym. 2014, 111, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Dax, D.; Soledad Chávez Bastidas, M.; Honorato, C.; Liu, J.; Spoljaric, S.; Seppälä, J.; Mendonça, R.T.; Xu, C.; Willför, S.; Sánchez, J.; et al. Tailor-Made Hemicellulose-Based Hydrogels Reinforced with Nanofibrillated Cellulose. Nord. Pulp Pap. Res. J. 2015, 30, 373–384. [Google Scholar] [CrossRef]
- Sánchez, J.; Dax, D.; Tapiero, Y.; Xu, C.; Willför, S. Bio-Based Hydrogels with Ion Exchange Properties Applied to Remove Cu(II), Cr(VI), and As(V) Ions from Water. Front. Bioeng. Biotechnol. 2021, 9, 656472. [Google Scholar] [CrossRef] [PubMed]
- Encina, L.; Elgueta, E.; Rivas, B.L.; Pereira, M.; Sanhueza, F. Hydrogels Derived from Galactoglucomannan Hemicellulose with Inorganic Contaminant Removal Properties. RSC Adv. 2021, 11, 35960–35972. [Google Scholar] [CrossRef]
- Escobar-Avello, D.; Ferrer, V.; Bravo-Arrepol, G.; Reyes-Contreras, P.; Elissetche, J.P.; Santos, J.; Fuentealba, C.; Cabrera-Barjas, G. Pretreated Eucalyptus Globulus and Pinus Radiata Barks: Potential Substrates to Improve Seed Germination for a Sustainable Horticulture. Forests 2023, 14, 991. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on Tannins: Extraction Processes, Applications and Possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Shirmohammadli, Y.; Efhamisisi, D.; Pizzi, A. Tannins as a Sustainable Raw Material for Green Chemistry: A Review. Ind. Crops Prod. 2018, 126, 316–332. [Google Scholar] [CrossRef]
- Santos, J.; Escobar-Avello, D.; Fuentealba, C.; Cabrera-Barjas, G.; González-Álvarez, J.; Martins, J.M.; Carvalho, L.H. Forest By-Product Valorization: Pilot-Scale Pinus Radiata and Eucalyptus Globulus Bark Mixture Extraction. Forests 2023, 14, 895. [Google Scholar] [CrossRef]
- Cabrera-Barjas, G.; Butto-Miranda, N.; Nesic, A.; Moncada-Basualto, M.; Segura, R.; Bravo-Arrepol, G.; Escobar-Avello, D.; Moeini, A.; Riquelme, S.; Neira-Carrillo, A. Condensed Tannins from Pinus Radiata Bark: Extraction and Their Nanoparticles Preparation in Water by Green Method. Int. J. Biol. Macromol. 2024, 278, 134598. [Google Scholar] [CrossRef]
- García, D.E.; Gavino, J.; Escobar, D.; Cancino, R.A. Maleinated Polyflavonoids and Lignin as Functional Additives for Three Kinds of Thermoplastics. Iran. Polym. J. (Engl. Ed.) 2017, 26, 295–304. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological Application of Tannin-Based Extracts. Molecules 2020, 25, 614. [Google Scholar] [CrossRef] [PubMed]
- Borges-Vilches, J.; Unalan, I.; Aguayo, C.R.; Fernández, K.; Boccaccini, A.R. Multifunctional Chitosan Scaffold Platforms Loaded with Natural Polyphenolic Extracts for Wound Dressing Applications. Biomacromolecules 2023, 24, 5183–5193. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Rivas, D.; Morales, D.V.; Hepp, M.I. Toxicity Evaluation of Pinus Radiata D.Don Bark Wax for Potential Cosmetic Application. Food Chem. Toxicol. 2023, 178, 113896. [Google Scholar] [CrossRef] [PubMed]
- Astuya, A.; Ziehe, J.; Rivera, A.; Ortiz, S.; Ulloa, V.; Roeckel, M.; Aspé, E.; Fernández, K. Antioxidant and Anti-Inflammatory Activities of Pinus Radiata Bark Extract in Salmonid Cell Lines. Aquac. Res. 2017, 48, 3568–3578. [Google Scholar] [CrossRef]
- Vera, N.; Gutiérrez, C.; Williams, P.; Fuentealba, C.; Allende, R.; Ávila–Stagno, J. Low Concentrations of a Polyphenolic Extract from Pine Bark in High–Concentrate Diets Decrease in Vitro Rumen Ammonia Nitrogen but Not Methane Production. J. Appl. Anim. Res. 2021, 49, 413–422. [Google Scholar] [CrossRef]
- García, D.E.; Medina, P.A.; Zúñiga, V.I. Toxicological Features of Maleilated Polyflavonoids from Pinus Radiata (D. Don.) as Potential Functional Additives for Biomaterials Design. Food Chem. Toxicol. 2017, 109, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Vera, M.; Mella, C.; García, Y.; Jiménez, V.A.; Urbano, B.F. Recent Advances in Tannin-Containing Food Biopackaging. Trends Food Sci. Technol. 2023, 133, 28–36. [Google Scholar] [CrossRef]
- Santos, J.; Delgado, N.; Fuentes, J.; Fuentealba, C.; Vega-Lara, J.; García, D.E. Exterior Grade Plywood Adhesives Based on Pine Bark Polyphenols and Hexamine. Ind. Crops Prod. 2018, 122, 340–348. [Google Scholar] [CrossRef]
- Valenzuela, J.; von Leyser, E.; Pizzi, A.; Westermeyer, C.; Gorrini, B. Industrial Production of Pine Tannin-Bonded Particleboard and MDF. Eur. J. Wood Wood Prod. 2012, 70, 735–740. [Google Scholar] [CrossRef]
- Soto, R.; Freer, J.; Baeza, J. Evidence of Chemical Reactions between Di- and Poly-Glycidyl Ether Resins and Tannins Isolated from Pinus Radiata D. Don Bark. Bioresour. Technol. 2005, 96, 95–101. [Google Scholar] [CrossRef]
- Vera, M.; Silva, C.; Li, N.; García, Y.; Jiménez, V.A.; Urbano, B.F. Laccase-mediated Polymerization of Tannins from a Pine Bark Extract: Toward an Eco-friendly Valorization of Forest Wastes. J. Appl. Polym. Sci. 2024, 141, 55437. [Google Scholar] [CrossRef]
- Montoya, L.F.; Flores, J.; Ramírez, J.; Rojas, D.; Oñate, Á.; Fernández, K.; Jaramillo, A.F.; Miranda, C.; Melendrez, M.F. New Sustainable Intumescent Coating Based on Polyphenols Obtained from Wood Industry Waste. Coatings 2024, 14, 1004. [Google Scholar] [CrossRef]
- Jaramillo, A.F.; Montoya, L.F.; Prabhakar, J.M.; Sanhueza, J.P.; Fernández, K.; Rohwerder, M.; Rojas, D.; Montalba, C.; Melendrez, M.F. Formulation of a Multifunctional Coating Based on Polyphenols Extracted from the Pine Radiata Bark and Functionalized Zinc Oxide Nanoparticles: Evaluation of Hydrophobic and Anticorrosive Properties. Prog. Org. Coat. 2019, 135, 191–204. [Google Scholar] [CrossRef]
- Matamala, G.; Smeltzer, W.; Droguett, G. Comparison of Steel Anticorrosive Protection Formulated with Natural Tannins Extracted from Acacia and from Pine Bark. Corros. Sci. 2000, 42, 1351–1362. [Google Scholar] [CrossRef]
- Ferrer-Villasmil, V.; Fuentealba, C.; Reyes-Contreras, P.; Rubilar, R.; Cabrera-Barjas, G.; Bravo-Arrepol, G.; Escobar-Avello, D. Extracted Eucalyptus Globulus Bark Fiber as a Potential Substrate for Pinus Radiata and Quillaja Saponaria Germination. Plants 2024, 13, 789. [Google Scholar] [CrossRef] [PubMed]
- Arismendi, W.A.; Ortiz-Ardila, A.E.; Delgado, C.V.; Lugo, L.; Sequeda-Castañeda, L.G.; Celis-Zambrano, C.A. Modified Tannins and Their Application in Wastewater Treatment. Water Sci. Technol. 2018, 78, 1115–1128. [Google Scholar] [CrossRef]
- Romero, R.; Contreras, D.; Segura, C.; Schwederski, B.; Kaim, W. Hydroxyl Radical Production by a Heterogeneous Fenton Reaction Supported in Insoluble Tannin from Bark of Pinus Radiata. Environ. Sci. Pollut. Res. 2017, 24, 6135–6142. [Google Scholar] [CrossRef]
- Palma, G.; Freer, J.; Baeza, J. Removal of Metal Ions by Modified Pinus Radiata Bark and Tannins from Water Solutions. Water Res. 2003, 37, 4974–4980. [Google Scholar] [CrossRef]
- TANAC S.A. Available online: www.tanac.com.br (accessed on 29 June 2025).
- SILVATEAM. Available online: www.silvateam.com (accessed on 29 June 2025).
- Pizzi, A. Tannins: Prospectives and Actual Industrial Applications. Biomolecules 2019, 9, 344. [Google Scholar] [CrossRef]
- Costa, N.A.; Pereira, J.; Ferra, J.; Cruz, P.; Martins, J.; Magalhães, F.D.; Mendes, A.; Carvalho, L.H. Scavengers for Achieving Zero Formaldehyde Emission of Wood-Based Panels. Wood Sci. Technol. 2013, 47, 1261–1272. [Google Scholar] [CrossRef]
- Santos, J.; Pereira, J.; Paiva, N.; Ferra, J.; Magalhães, F.D.; Martins, J.M.; de Carvalho, L.H. Water Resistance Evaluation of a MFU Resins with Different Molar Ratio Catalyzed with Citric Acid. Int. J. Adhes. Adhes. 2022, 117, 103020. [Google Scholar] [CrossRef]
- Fernandes, R.A.; Ferreira, N.; Lopes, S.; Santos, J.; Bento Pereira, N.; Ferreira, N.O.; Nunes, L.; Martins, J.M.; Carvalho, L.H. Development of an Innovative Lightweight Composite Material with Thermal Insulation Properties Based on Cardoon and Polyurethane. Polymers 2024, 16, 137. [Google Scholar] [CrossRef]
- Arias, A.; González-Rodríguez, S.; Vetroni Barros, M.; Salvador, R.; de Francisco, A.C.; Moro Piekarski, C.; Moreira, M.T. Recent Developments in Bio-Based Adhesives from Renewable Natural Resources. J. Clean. Prod. 2021, 314, 127892. [Google Scholar] [CrossRef]
- Pizzi, A. Pine Tannin Adhesives for Particleboard. Holz Als Roh-Und Werkst. 1982, 40, 293–301. [Google Scholar] [CrossRef]
- von Leyser, E.; Pizzi, A. The Formulation and Commercialization of Glulam Pine Tannin Adhesives in Chile. Holz Als Roh Werkst. 1990, 48, 25–29. [Google Scholar] [CrossRef]
- Pizzi, A.; von Leyser, E.R.; Valenzuela, J.; Clark, J.G. The Chemistry and Development of Pine Tannin Adhesives for Exterior Particleboard. Holzforschung 1993, 47, 168–174. [Google Scholar] [CrossRef]
- Pizzi, A.; Valenzuela, J.; Westermeyer, C. Non-Emulsifiable, Water-Based, Mixed Diisocyanate Adhesive Systems for Exterior Plywood Part II. Theory Application and Industrial Results. Holzforschung 1993, 47, 68–71. [Google Scholar] [CrossRef]
- Pizzi, A.; Valenzuela, J.; Westermeyer, C. Low Formaldehyde Emission, Fast Pressing, Pine and Pecan Tannin Adhesives for Exterior Particleboard. Holz Als Roh Werkst. 1994, 52, 311–315. [Google Scholar] [CrossRef]
- Bocalandro, C.; Sanhueza, V.; Gómez-Caravaca, A.M.; González-Álvarez, J.; Fernández, K.; Roeckel, M.; Rodríguez-Estrada, M.T. Comparison of the Composition of Pinus Radiata Bark Extracts Obtained at Bench- and Pilot-Scales. Ind. Crops Prod. 2012, 38, 21–26. [Google Scholar] [CrossRef]
- Navarrete, P.; Mansouri, H.R.; Pizzi, A.; Tapin-Lingua, S.; Benjelloun-Mlayah, B.; Pasch, H.; Rigolet, S. Wood Panel Adhesives from Low Molecular Mass Lignin and Tannin without Synthetic Resins. J. Adhes. Sci. Technol. 2010, 24, 1597–1610. [Google Scholar] [CrossRef]
- Mansouri, H.R.; Navarrete, P.; Pizzi, A.; Tapin-Lingua, S.; Benjelloun-Mlayah, B.; Pasch, H.; Rigolet, S. Synthetic-Resin-Free Wood Panel Adhesives from Mixed Low Molecular Mass Lignin and Tannin. Eur. J. Wood Wood Prod. 2011, 69, 221–229. [Google Scholar] [CrossRef]
- Iždinský, J.; Vidholdová, Z.; Reinprecht, L. Particleboards from Recycled Wood. Forests 2020, 11, 1166. [Google Scholar] [CrossRef]
- Gonçalves, S.; Curling, S.F.; Ormondroyd, G.; Paiva, N.T.; Martins, J.; Magalhães, F.D.; Carvalho, L.H. Volatile Organic Compound (VOC) Emissions from No-Added-Formaldehyde Lignosulphonate/PMDI Particleboards and Their Effect on Indoor Air Quality. Wood Mater. Sci. Eng. 2024, 1–9. [Google Scholar] [CrossRef]
- Finsa: Fibrapan Bio. Available online: https://www.finsa.com/en/fp/fibrapan-fibrapan_bio/fibrapan/fibrapan-bio (accessed on 30 June 2025).
- El Guerrouj, B.; Taibi, M.; Elbouzidi, A.; Bouhassoun, S.; Loukili, E.H.; Moubchir, T.; Haddou, M.; Hammouti, Y.; Khoulati, A.; Addi, M.; et al. The Effect of Altitude on the Chemical Composition, Antioxidant and Antimicrobial Activities of Eucalyptus Globulus Labill. Essential Oils. Trop. J. Nat. Prod. Res. 2023, 7, 5279–5285. [Google Scholar] [CrossRef]
- Čmiková, N.; Galovičová, L.; Schwarzová, M.; Vukic, M.D.; Vukovic, N.L.; Kowalczewski, P.Ł.; Bakay, L.; Kluz, M.I.; Puchalski, C.; Kačániová, M. Chemical Composition and Biological Activities of Eucalyptus Globulus Essential Oil. Plants 2023, 12, 1076. [Google Scholar] [CrossRef] [PubMed]
- Hernández, F.; Cordano, G.; Medina, J.; Rivera, E. Pino Radiata y Eucaliptus Citriodora: Fuente de Materias Primas Para La Industria Cosmética. Rev. Col. Químico Farm. 1979, 35, 30–36. [Google Scholar]
- Sisubalan, N.; Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Composition, Bioactivities, Safety Concerns, and Impact of Essential Oil on Pets’ and Animals’ Health. Preprints 2023, 1–25. [Google Scholar] [CrossRef]
- Chariguamán Coello, A.; Montero Mora, M.C.; Velastegui Freire, C.A.; Guerrero Naranjo, L.E. Evaluación de los Compuestos Bioactivos y Aplicaciones Terapéuticas del Aceite Esencial de Eucalyptus Globulus. Sapiens Int. Multidiscip. J. 2025, 2, 1–11. [Google Scholar] [CrossRef]
- González-Burgos, E.; Liaudanskas, M.; Viškelis, J.; Žvikas, V.; Janulis, V.; Gómez-Serranillos, M.P. Antioxidant Activity, Neuroprotective Properties and Bioactive Constituents Analysis of Varying Polarity Extracts from Eucalyptus Globulus Leaves. J. Food Drug Anal. 2018, 26, 1293–1302. [Google Scholar] [CrossRef]
- Nadjib, B.M.; Amine, F.M.; Abdelkrim, K.; Fairouz, S.; Maamar, M. Liquid and Vapour Phase Antibacterial Activity of Eucalyptus Globulus Essential Oil = Susceptibility of Selected Respiratory Tract Pathogens. Am. J. Infect. Dis. 2014, 10, 105–117. [Google Scholar] [CrossRef]
- Macedo-Ramírez, Y.; Mejía-Delgado, E. Antifungal Efficacy of Eucalyptus Globulus Ethanolic Extract on In Vitro Candida Albicans. Rev. Méd. Trujillo 2019, 14, 79–91. [Google Scholar] [CrossRef]
- Shiekh, R.A.E.; Atwa, A.M.; Elgindy, A.M.; Mustafa, A.M.; Senna, M.M.; Alkabbani, M.A.; Ibrahim, K.M. Therapeutic Applications of Eucalyptus Essential Oils. Inflammopharmacology 2025, 33, 163–182. [Google Scholar] [CrossRef]
- Müller-Heupt, L.K.; Vierengel, N.; Groß, J.; Opatz, T.; Deschner, J.; von Loewenich, F.D. Antimicrobial Activity of Eucalyptus Globulus, Azadirachta Indica, Glycyrrhiza Glabra, Rheum Palmatum Extracts and Rhein against Porphyromonas Gingivalis. Antibiotics 2022, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, S.A.; Pelaez-Prestel, H.F.; Ras-Carmona, A.; Mozas-Gutierrez, J.; Reyes-Manzanas, R.; Reche, P.A. Eucalyptus Essential Oil Inhibits Cell Infection by SARS-CoV-2 Spike Pseudotyped Lentivirus. Biomedicines 2024, 12, 1885. [Google Scholar] [CrossRef]
- Mieres-Castro, D.; Ahmar, S.; Shabbir, R.; Mora-Poblete, F. Antiviral Activities of Eucalyptus Essential Oils: Their Effectiveness as Therapeutic Targets against Human Viruses. Pharmaceuticals 2021, 14, 1210. [Google Scholar] [CrossRef]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, Medicinal and Toxicological Significance of Eucalyptus Leaf Essential Oil: A Review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef]
- Lin, W.; Jianbo, S.; Wanzhong, L.; Yanna, L.; Weiwei, S.; Gang, W.; Chunzhen, Z. Protective Effect of Eucalyptus Oil on Pulmonary Destruction and Inflammation in Chronic Obstructive Pulmonary Disease (COPD) in Rats. J. Med. Plants Res. 2017, 11, 129–136. [Google Scholar] [CrossRef]
- Baptista, E.B.; Zimmermann-Franco, D.C.; Lataliza, A.A.B.; Raposo, N.R.B. Chemical Composition and Antifungal Activity of Essential Oil from Eucalyptus Smithii against Dermatophytes. Rev. Soc. Bras. Med. Trop. 2015, 48, 746–752. [Google Scholar] [CrossRef]
- González-Guiñez, R.; Silva-Aguayo, G.; Urbina-Parra, A.; Gerding-González, M. Aceite Esencial de Eucalyptus globulus Labill Y Eucalyptus nitens H. Deane & Maiden (MYRTACEAE) Para El Control de Sitophilus zeamais Motschulsky. Chil. J. Agric. Anim. Sci. 2016, 32, 204–216. [Google Scholar]
- Pheko-Ofitlhile, T.; Phiri, N.; Serame, E.; Lepodise, L.M. Chemical Profiling and Spectroscopic Studies of Leaf Volatile Oil of Eucalyptus globulus from Botswana Isolated by Conventional Hydrodistillation and Ultrasound Assisted Hydrodistillation. Nat. Prod. Res. 2024, 1–8. [Google Scholar] [CrossRef]
- Hasni, S.; Riguene, H.; Mendiola, J.A.; Ibáñez, E.; Montero, L.; Domínguez-Rodríguez, G.; Ghazghazi, H.; Rigane, G.; Salem, R. Ben Optimization of a Pressurized Extraction Process Based on a Ternary Solvent System for the Recovery of Neuroprotective Compounds from Eucalyptus Marginata Leaves. Int. J. Mol. Sci. 2025, 26, 94. [Google Scholar] [CrossRef]
- Xu, G.; Guo, J.; Sun, C. Eucalyptol Ameliorates Early Brain Injury after Subarachnoid Haemorrhage via Antioxidant and Anti-Inflammatory Effects in a Rat Model. Pharm. Biol. 2021, 59, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Kurniawati, A.; Al Anas, M.M. The Effect of a Candidate Feed Additive Derived from the Essential Oils of Pinus merkusii (Jungh & de vriese) and Melaleuca leucadendra (l.) on the Kinetics of Gas Production and Methane Emitted during In-Vitro Ruminal Fermentation. In Proceedings of the BIO Web of Conferences, Yogyakarta, Indonesia, 23 August 2021; EDP Sciences: Les Ulis, France, 2021; Volume 33, p. 04009. [Google Scholar]
- Nikolić, B.M.; Ballian, D.; Mitić, Z.S. Diversity of Needle Terpenes Among Pinus Taxa. Forests 2025, 16, 623. [Google Scholar] [CrossRef]
- Mirković, S.; Tadić, V.; Milenković, M.T.; Ušjak, D.; Racić, G.; Bojović, D.; Žugić, A. Antimicrobial Activities of Essential Oils of Different Pinus Species from Bosnia and Herzegovina. Pharmaceutics 2024, 16, 1331. [Google Scholar] [CrossRef] [PubMed]
- Ancuceanu, R.; Anghel, A.I.; Hovaneț, M.V.; Ciobanu, A.M.; Lascu, B.E.; Dinu, M. Antioxidant Activity of Essential Oils from Pinaceae Species. Antioxidants 2024, 13, 286. [Google Scholar] [CrossRef] [PubMed]
- Semerdjieva, I.; Cantrell, C.L.; Zheljazkov, V.D.; Radoukova, T.; Koleva-Valkova, L.H.; Astatkie, T.; Kačániová, M.; Borisova, D. Chemical Profile, Antioxidant and Antimicrobial Activity of Pinus Heldreichii Christ. Distributed in Bulgaria. Heliyon 2024, 10, e22967. [Google Scholar] [CrossRef]
- Morris, H.; Jansen, S. Bark: Its Anatomy, Function and Diversity. Int. Dendrol. Soc. 2017, 51–61. [Google Scholar]
- FAOSTAT—Forestry Production and Trade. Available online: https://www.fao.org/faostat/en/#data/FO (accessed on 11 November 2024).
- Neiva, D.M.; Ek, M.; Sels, B.F.; Samec, J.S.M. Toward Sustainable Upgrading of Bark. Chem. Catal. 2024, 4, 101022. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Oliveira, E.L.G.; Freire, C.S.R.; Couto, R.M.; Simões, P.C.; Neto, C.P.; Silvestre, A.J.D.; Silva, C.M. Supercritical Fluid Extraction of Eucalyptus Globulus Bark—A Promising Approach for Triterpenoid Production. Int. J. Mol. Sci. 2012, 13, 7648–7662. [Google Scholar] [CrossRef]
- Neiva, D.M.; Araújo, S.; Gominho, J.; de Cássia Carneiro, A.; Pereira, H. Potential of Eucalyptus Globulus Industrial Bark as a Biorefinery Feedstock: Chemical and Fuel Characterization. Ind. Crops Prod. 2018, 123, 262–270. [Google Scholar] [CrossRef]
- Fuentealba, C.; Segovia, C.; Pradena-Miquel, M.; César, A.G. Efficient Bio-Based Insulation Panels Produced from Eucalyptus Bark Waste. Forests 2024, 15, 1628. [Google Scholar] [CrossRef]
- Feng, S.; Cheng, S.; Yuan, Z.; Leitch, M.; Xu, C. Valorization of Bark for Chemicals and Materials: A Review. Renew. Sustain. Energy Rev. 2013, 26, 560–578. [Google Scholar] [CrossRef]
- Xiao, M.Z.; Chen, W.J.; Cao, X.F.; Chen, Y.Y.; Zhao, B.C.; Jiang, Z.H.; Yuan, T.Q.; Sun, R.C. Unmasking the Heterogeneity of Carbohydrates in Heartwood, Sapwood, and Bark of Eucalyptus. Carboh. Polym. 2020, 238, 116212. [Google Scholar] [CrossRef]
- Fuentealba, C.; Segovia, C.; Vibert, C.; Brosse, N. Eucalyptus Globulus Bark as Valuable Raw Material to the Development of Bio-Based Material. In Springer Proceedings in Materials; Springer: Berlin/Heidelberg, Germany, 2024; Volume 43, pp. 76–87. [Google Scholar]
- Quilhó, T.; Pereira, H. Within and Between-Tree Variation of Bark Content and Wood Density of Eucalyptus Globulus in Commercial Plantations. IAWA J. 2001, 22, 255–265. [Google Scholar] [CrossRef]
- Mustapha, L.S.; Obayomi, O.V.; Yahya, M.D.; Lau, S.Y.; Obayomi, K.S. Exploring the Synergistic Effects of Calcium Chloride Modification on Stem Bark Eucalyptus Biochar for Cr(VI) and Pb(II) Ions Removal: Kinetics, Isotherm, Thermodynamic and Optimization Studies. Bioresour. Technol. Rep. 2024, 25, 101699. [Google Scholar] [CrossRef]
- Laloon, K.; Junsiri, C.; Sanchumpu, P.; Ansuree, P. Factors Affecting the Biomass Pellet Using Industrial Eucalyptus Bark Residue. Biomass Conver. Biorefin. 2024, 14, 10101–10113. [Google Scholar] [CrossRef]
- Rodrigues, T.; Torres, C.A.V.; Marques, S.; Gírio, F.; Freitas, F.; Reis, M.A.M. Polyhydroxyalkanoate Production from Eucalyptus Bark’s Enzymatic Hydrolysate. Materials 2024, 17, 1773. [Google Scholar] [CrossRef]
- Tamayo-Peña, J.A.; Tovar, L.P.; Pacheco, L.C.A.; Gonçalves, A.R.; Franco, T.T. Eucalyptus Grandis Forestry Residue Valorization: Distinct and Integrated Pretreatment Methods for Enhanced Xylooligosaccharide Production. Bioenergy Res. 2024, 17, 1503–1521. [Google Scholar] [CrossRef]
- Casas-Ledón, Y.; Daza Salgado, K.; Cea, J.; Arteaga-Pérez, L.E.; Fuentealba, C. Life Cycle Assessment of Innovative Insulation Panels Based on Eucalyptus Bark Fibers. J. Clean. Prod. 2020, 249, 119356. [Google Scholar] [CrossRef]
- Bakatovich, A.; Bakatovich, N.; Silva, A.; Gaspar, F. Thermal Insulation Materials Based on Eucalyptus Bark Fibres. Constr. Build. Mater. 2024, 449, 138559. [Google Scholar] [CrossRef]
- Fuentealba, C.; Berg, A.; Salazar, J.; Vega, J.; Michanickl, A. Un Proceso de Obtención de Un Material Fibroso a Partir de Corteza Útil Para Fabricar Materiales Aislantes. CL2016003408A1, 2 June 2017. Available online: https://www.patentguru.com/CL2016003408A1 (accessed on 23 April 2025).
- Asdrubali, F.; D’Alessandro, F.; Schiavoni, S. A Review of Unconventional Sustainable Building Insulation Materials. Sustain. Mater. Technol. 2015, 4, 1–17. [Google Scholar] [CrossRef]
- Korjenic, A.; Petránek, V.; Zach, J.; Hroudová, J. Development and Performance Evaluation of Natural Thermal-Insulation Materials Composed of Renewable Resources. Energy Build. 2011, 43, 2518–2523. [Google Scholar] [CrossRef]
- Ardente, F.; Beccali, M.; Cellura, M.; Mistretta, M. Building Energy Performance: A LCA Case Study of Kenaf-Fibres Insulation Board. Energy Build. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- AISLACOR: Aislante Sustentable. Available online: https://aislacor.cl/ (accessed on 26 June 2025).
- Chemetova, C.; Mota, D.; Fabião, A.; Gomimho, J.; Ribeiro, H. Valorization of Eucalyptus Globulus Bark as a Growing-Media Component for Potted Plants. In Proceedings of the 15th International Conference on Environmental Science and Technology (CEST) 2017, Rhodes, Greece, 31 August–2 September 2017; pp. 1–5. [Google Scholar]
- Chemetova, C.; Fabião, A.; Gominho, J.; Ribeiro, H. Range Analysis of Eucalyptus Globulus Bark Low-Temperature Hydrothermal Treatment to Produce a New Component for Growing Media Industry. Waste Manag. 2018, 79, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chemetova, C.; Mota, D.; Fabião, A.; Gominho, J.; Ribeiro, H. Low-Temperature Hydrothermally Treated Eucalyptus Globulus Bark: From By-Product to Horticultural Fiber-Based Growing Media Viability. J. Clean. Prod. 2021, 319, 128805. [Google Scholar] [CrossRef]
- Gizaw, D.G.; Periyasamy, S.; Baylie, H.; Tassew Redda, Z.; Asaithambi, P.; Jayakumar, M.; Baskar, G.; Pugazhendhi, A. Advances in Solid Biofuels Production through Torrefaction: Potential Biomass, Types of Torrefaction and Reactors, Influencing Process Parameters and Future Opportunities—A Review. Process Saf. Environ. Prot. 2024, 186, 1307–1319. [Google Scholar] [CrossRef]
- Blackwood Technology. Available online: https://www.blackwood-technology.com/ (accessed on 25 June 2025).
- Arteaga-Pérez, L.E.; Segura, C.; Espinoza, D.; Radovic, L.R.; Jiménez, R. Torrefaction of Pinus radiata and Eucalyptus globulus: A Combined Experimental and Modeling Approach to Process Synthesis. Energy Sustain. Dev. 2015, 29, 13–23. [Google Scholar] [CrossRef]
- Arteaga-Pérez, L.E.; Grandón, H.; Flores, M.; Segura, C.; Kelley, S.S. Steam Torrefaction of Eucalyptus globulus for Producing Black Pellets: A Pilot-Scale Experience. Bioresour. Technol. 2017, 238, 194–204. [Google Scholar] [CrossRef]
- Pérez-Jeldres, R.; Cornejo, P.; Flores, M.; Gordon, A.; García, X. A Modeling Approach to Co-Firing Biomass/Coal Blends in Pulverized Coal Utility Boilers: Synergistic Effects and Emissions Profiles. Energy 2017, 120, 663–674. [Google Scholar] [CrossRef]
- Ensyn Technology. Available online: https://www.ensyn.com/ (accessed on 25 June 2025).
- BTG Bioliquids. Available online: https://www.btg-bioliquids.com/plants/ (accessed on 25 June 2025).
- Vandana, T.U.; Tripathy, B.K.; Mishra, R.K.; Sharma, A.; Mohanty, K. A Review on Waste Biomass-Derived Biochar: Production, Characterisation, and Advanced Analytical Techniques for Pollutants Assessment in Water and Wastewater. Process Saf. Environ. Prot. 2025, 201, 107505. [Google Scholar] [CrossRef]
- Wilkomirsky, I.; Moreno, E.; Berg, A. Bio-Oil Production from Biomass by Flash Pyrolysis in a Three-Stage Fluidized Bed Reactors System. J. Mat. Sci. Chem. Eng. 2014, 2, 6–10. [Google Scholar] [CrossRef]
- Gómez Cápiro, O.; Aravena Riquelme, K.A.; Jiménez, R.; Arteaga-Pérez, L.E. Carbothermic Reduction of Carbon Aerogel-Supported Fe during the Catalytic Decomposition of Toluene. Catal. Today 2021, 372, 82–88. [Google Scholar] [CrossRef]
- Gómez-Cápiro, O.; Matschuk, K.; Schulzke, T.; Concepción, R.J.; Arteaga-Pérez, L.E. Carbon Aerogel-Supported Iron for Gasification Gas Cleaning: Tars Decomposition. Catalysts 2022, 12, 391. [Google Scholar] [CrossRef]
- Domínguez, J.I.; Blanco Machín, E.; Travieso Pedroso, D.; Wagemman Herrera, E.; Cuevas Barraza, C. Advanced Thermoconversion Technology for Municipal Solid Waste Energetic Valorization. Renew. Energy 2024, 228, 120604. [Google Scholar] [CrossRef]
- Rodríguez, A.G.; Rodrigues, M.; Sotomayor, O. Towards a Sustainable Bioeconomy in Latin America and the Caribbean: Elements for a Regional Vision. Nat. Resour. Dev. Ser. 2019, 191, 1–49. [Google Scholar]
- Tan, E.C.D.; Lamers, P. Circular Bioeconomy Concepts—A Perspective. Front. Sustain. 2021, 2, 701509. [Google Scholar] [CrossRef]
- European Commission. Communication on Innovating for Sustainable Growth: A Bioeconomy for Europe; European Commission: Brussels, Belgium, 2012. [Google Scholar]
- Biomass Research and Development Board. The Bioeconomy Initiative: Implementation Framework; Biomass Research and Development Board: Washington, DC, USA, 2018.
- Gobierno de Chile. Contribución Determinada a Nivel Nacional (NDC) de Chile; Gobierno de Chile: Santiago, Chile, 2020. [Google Scholar]
- Ministerio del Medio Ambiente (MMA). Hoja de Ruta Para Un Chile Circular al 2040; Ministerio del Medio Ambiente (MMA): Santiago, Chile, 2021; pp. 1–67. [Google Scholar]
- Gobierno de Chile. Estrategia Nacional de Residuos Orgánicos Chile 2040; Gobierno de Chile: Santiago, Chile, 2020. [Google Scholar]
- Ministerio del Medio Ambiente (MMA). Ley 20920. Ley 20920. Establece Marco para la Gestión de Residuos, la Responsabilidad Extendida del Productor y Fomento al Reciclaje; Ministerio del Medio Ambiente (MMA): Santiago, Chile, 2016; pp. 17–20. [Google Scholar]
- Papageorgiou, S.; Massai, C. Chile’s Forests: A Pillar for Inclusive and Sustainable Development-Country Forest Note; World Bank: Washington, DC, USA, 2020; pp. 1–106. [Google Scholar]
- Espinosa Gutiérrez, G.; Casas Ledón, Y. Life Cycle Thinking: An Essential Framework for Eco-Industrial Development. In Eco-Industrial Development as an Industrial Strategy from a German-Chilean Research Partnership; Braun, A., Espinoza, G., Tröger, D., Hirth, T., Eds.; Springer Nature: Cham, Switzerland, 2025; pp. 69–92. [Google Scholar]
- Casas-Ledón, Y.; Flores, M.; Jiménez, R.; Ronsse, F.; Dewulf, J.; Arteaga-Pérez, L.E. On the Environmental and Economic Issues Associated with the Forestry Residues-to-Heat and Electricity Route in Chile: Sawdust Gasification as a Case Study. Energy 2019, 170, 763–776. [Google Scholar] [CrossRef]
- Weldu, Y.W. Life Cycle Human Health and Ecosystem Quality Implication of Biomass-Based Strategies to Climate Change Mitigation. Renew. Energy 2017, 108, 11–18. [Google Scholar] [CrossRef]
- Cleary, J.; Caspersen, J.P. Comparing the Life Cycle Impacts of Using Harvest Residue as Feedstock for Small- and Large-Scale Bioenergy Systems (Part I). Energy 2015, 88, 917–926. [Google Scholar] [CrossRef]
- Röder, M.; Whittaker, C.; Thornley, P. How Certain Are Greenhouse Gas Reductions from Bioenergy? Life Cycle Assessment and Uncertainty Analysis of Wood Pellet-to-Electricity Supply Chains from Forest Residues. Biomass Bioenergy 2015, 79, 50–63. [Google Scholar] [CrossRef]
- Arteaga-Pérez, L.E.; Vega, M.; Rodríguez, L.C.; Flores, M.; Zaror, C.A.; Casas Ledón, Y. Life-Cycle Assessment of Coal-Biomass Based Electricity in Chile: Focus on Using Raw vs Torrefied Wood. Energy Sustain. Dev. 2015, 29, 81–90. [Google Scholar] [CrossRef]
- Murphy, F.; Sosa, A.; McDonnell, K.; Devlin, G. Life Cycle Assessment of Biomass-to-Energy Systems in Ireland Modelled with Biomass Supply Chain Optimisation Based on Greenhouse Gas Emission Reduction. Energy 2016, 109, 1040–1055. [Google Scholar] [CrossRef]
- Ricciardi, P.; Belloni, E.; Cotana, F. Innovative Panels with Recycled Materials: Thermal and Acoustic Performance and Life Cycle Assessment. Appl. Energy 2014, 134, 150–162. [Google Scholar] [CrossRef]
- Buratti, C.; Belloni, E.; Lascaro, E.; Merli, F.; Ricciardi, P. Rice Husk Panels for Building Applications: Thermal, Acoustic and Environmental Characterization and Comparison with Other Innovative Recycled Waste Materials. Constr. Build. Mater. 2018, 171, 338–349. [Google Scholar] [CrossRef]
- Schiavoni, S.; D’Alessandro, F.; Bianchi, F.; Asdrubali, F. Insulation Materials for the Building Sector: A Review and Comparative Analysis. Renew. Sustain. Energy Rev. 2016, 62, 988–1011. [Google Scholar] [CrossRef]
- Atilgan, B.; Azapagic, A. Life Cycle Environmental Impacts of Electricity from Fossil Fuels in Turkey. J. Clean. Prod. 2015, 106, 555–564. [Google Scholar] [CrossRef]
- Cherubini, F.; Bird, N.D.; Cowie, A.; Jungmeier, G.; Schlamadinger, B.; Woess-Gallasch, S. Energy- and Greenhouse Gas-Based LCA of Biofuel and Bioenergy Systems: Key Issues, Ranges and Recommendations. Resour. Conserv. Recycl. 2009, 53, 434–447. [Google Scholar] [CrossRef]
- Huang, Y.F.; Syu, F.S.; Chiueh, P.-T.; Lo, S.L. Life Cycle Assessment of Biochar Cofiring with Coal. Bioresour. Technol. 2013, 131, 166–171. [Google Scholar] [CrossRef]
- Barrio, A.; Francisco, F.B.; Leoncini, A.; Wietschel, L.; Thorenz, A. Life Cycle Sustainability Assessment of a Novel Bio-Based Multilayer Panel for Construction Applications. Resources 2021, 10, 98. [Google Scholar] [CrossRef]
- Le, D.L.; Salomone, R.; Nguyen, Q.T. Circular Bio-Based Building Materials: A Literature Review of Case Studies and Sustainability Assessment Methods. Build. Environ. 2023, 244, 110774. [Google Scholar] [CrossRef]
- Braun, A.C.; Troeger, D.; Garcia, R.; Aguayo, M.; Barra, R.; Vogt, J. Assessing the Impact of Plantation Forestry on Plant Biodiversity: A Comparison of Sites in Central Chile and Chilean Patagonia. Glob. Ecol. Conserv. 2017, 10, 159–172. [Google Scholar] [CrossRef]
- Echeverria, C.; Coomes, D.; Salas, J.; Rey-Benayas, J.M.; Lara, A.; Newton, A. Rapid Deforestation and Fragmentation of Chilean Temperate Forests. Biol. Conserv. 2006, 130, 481–494. [Google Scholar] [CrossRef]
- Banfield, C.C.; Braun, A.C.; Barra, R.; Castillo, A.; Vogt, J. Erosion Proxies in an Exotic Tree Plantation Question the Appropriate Land Use in Central Chile. CATENA 2018, 161, 77–84. [Google Scholar] [CrossRef]
- Díaz, M.E.; Figueroa, R.; Alonso, M.L.S.; Vidal-Abarca, M.R. Exploring the Complex Relations between Water Resources and Social Indicators: The Biobío Basin (Chile). Ecosyst. Serv. 2018, 31, 84–92. [Google Scholar] [CrossRef]
- Cerda, R.; Gallardo-Cobos, R.; Sánchez-Zamora, P. An Analysis of the Impact of Forest Policy on Rural Areas of Chile. Forests 2020, 11, 1105. [Google Scholar] [CrossRef]
- Berasaluce, M.; Díaz-Siefer, P.; Rodríguez-Díaz, P.; Mena-Carrasco, M.; Ibarra, J.T.; Celis-Diez, J.L.; Mondaca, P. Social-Environmental Conflicts in Chile: Is There Any Potential for an Ecological Constitution? Sustainability 2021, 13, 12701. [Google Scholar] [CrossRef]
- Carte, L.; Hofflinger, Á.; Polk, M.H. Expanding Exotic Forest Plantations and Declining Rural Populations in La Araucanía, Chile. Land 2021, 10, 283. [Google Scholar] [CrossRef]
- Carranza, D.M.; Varas-Belemmi, K.; De Veer, D.; Iglesias-Müller, C.; Coral-Santacruz, D.; Méndez, F.A.; Torres-Lagos, E.; Squeo, F.A.; Gaymer, C.F. Socio-Environmental Conflicts: An Underestimated Threat to Biodiversity Conservation in Chile. Environ. Sci. Policy 2020, 110, 46–59. [Google Scholar] [CrossRef]
- Reyes, R.; Nelson, H. A Tale of Two Forests: Why Forests and Forest Conflicts Are Both Growing in Chile. Int. For. Rev. 2014, 16, 379–388. [Google Scholar] [CrossRef]
- Obtén La Certificación FSC. Available online: https://cl.fsc.org/cl-es/obten-la-certificacion (accessed on 8 July 2025).
- Tricallotis, M.; Gunningham, N.; Kanowski, P. The Impacts of Forest Certification for Chilean Forestry Businesses. For. Policy Econ. 2018, 92, 82–91. [Google Scholar] [CrossRef]
- Barañano, L.; Unamunzaga, O.; Garbisu, N.; Briers, S.; Orfanidou, T.; Schmid, B.; de Arano, I.M.; Araujo, A.; Garbisu, C. Assessment of the Development of Forest-Based Bioeconomy in European Regions. Sustainability 2022, 14, 4747. [Google Scholar] [CrossRef]
- Emmanuel Miassi, Y.; Fabrice Dossa, K. Circular Economy Initiatives for Forest-Based Bioeconomy: Harnessing the Potential of Non-Wood Biomaterials. Waste Manag. Bull. 2024, 2, 270–278. [Google Scholar] [CrossRef]



| Wood Products | Consumption (m3 swb) | Wood Products Flow (m3 swb) | ||
|---|---|---|---|---|
| Primary Industry | Domestic Market | Foreign Market | ||
| Export Logs | 62,524 | 62,524 | ||
| Chips Logs | 4,269,813 | Wood Chips (a) | 5,379,515 (b) | 2,775,772 |
| Sawlogs | 13,491,275 | Sawnwood (a) | 4,863,916 | 2,022,935 |
| Pulplogs | 17,730,716 | Wood pulp | 898,837 ton | 4,430,600 ton |
| Logs for Panels and Veneers | 4,144,743 | Panels and Veneers | 1,604,956 | 1,295,644 |
| Logs for Posts and Poles | 249,276 | Post and Poles | 191,709 | 49,446 |
| Total Industrial Roundwood Consumption | 39,948,347 | |||
| Plant Species | Main Compound | Biological Effect | Action Mechanism | Ref. |
|---|---|---|---|---|
| E. globulus | 1,8-cineol (eucaliptol) | Antimicrobial (Gram-positive bacteria and fungi) | Inhibition of Gram-positive bacteria and fungi growth by altering cell membrane permeability | [87] |
| Antioxidant (presence of flavonoids and tannins) | Neutralization of free radicals due to the presence of flavonoids and tannins | [88] | ||
| Antimicrobial (Staphylococcus aureus) | Inhibition of bacterial cell wall synthesis | [89] | ||
| Antifungal (Candida albicans) | Alteration of fungal cell membrane integrity | [90] | ||
| Antimicrobial (Escherichia coli and Staphylococcus aureus) | Disruption of bacterial cell membrane | [91] | ||
| Antimicrobial (periodontal pathogens) | Inhibition of bacterial virulence factors | [92] | ||
| Antiviral (molecular modeling) | Potential inhibition of SARS-CoV-2 by blocking viral proteins | [93] | ||
| Antiviral | Inhibition of viral replication | [94] | ||
| Multiple (antimicrobial, antioxidant, anti-inflammatory) | Various mechanisms including cell membrane alteration | [95] | ||
| 1,8-cineol (eucaliptol), α-pineno | Antibacterial | Disruption of bacterial cell membrane | [83] | |
| α-terpineol, γ-terpineno | Anti-inflammatory | Inhibition of pro-inflammatory cytokine production | [96] | |
| Globulol and epiglobulol | Antifungal (dermatophytes) | Inhibition of ergosterol synthesis | [97] | |
| E. nitens | 1,8-cineol (eucaliptol) (59.85%), α-pineno (18.36%) | Insecticide (fumigant and repellent against Sitophilus zeamais) | Neurotoxicity in insects | [98] |
| Extract Type | Main Compounds | Biological Effect | Action Mechanism | Ref. |
|---|---|---|---|---|
| Wax | Methyl 4-ketohex-5-enoate; 1-naphthanol; dioctyl adipate; eicosanebiotic acid dimethyl ester | Toxicity evaluation for cosmetic application | Non-toxic up to 2% concentration | [49] |
| EO | α-pinene, β-pinene, δ-3-carene, β-caryophyllene, limonene/β-phellandrene, and germacrene D | Multiple effects: antiviral, antibacterial, antifungal, herbicidal | Biological activity dependent on dominant terpene components | [103] |
| EO | 1,8-cineole (63.1%), p-cymene (7.7%), α-pinene (7.3%), and α-limonene (6.9%) | Antimicrobial against S. aureus and E. coli | Disruption of bacterial cell membrane integrity | [104] |
| Hydrolate | Phenolic compounds, organic acids, and water-soluble terpenes | Antimicrobial against E. coli, S. aureus, and C. albicans | Inhibition of microbial growth through multiple mechanisms | [105] |
| Oleoresin | Volatile fraction (essential oil), solid fraction (rosin) containing abietic and pimaric acids | Anti-inflammatory and antimicrobial | Inhibition of inflammatory mediators; disruption of microbial membranes | [106] |
| Material | Density (kg/m3) | Thermal Conductivity (W/mK) | Reference |
|---|---|---|---|
| Eucalyptus bark | 97.8 ± 6.5 | 0.0379 ± 0.00052 | [115] |
| Eucalyptus bark | 80–300 | 0.064–0.077 | [112] |
| Eucalyptus bark | 140–160 | 0.042 | [122] |
| Kenaf | 30–180 | 0.034–0.043 | [124] |
| Jute | 26.1 | 0.0458 | |
| Flax | 32.1 | 0.0429 | [125] |
| Hemp | 79.6 | 0.0475 | |
| Hemp | 40.2 | 0.0393 | |
| Rock wool | 40–200 | 0.0330–0.040 | [124] |
| EPS * | 15–35 | 0.0310–0.0380 | |
| Polyurethane | 24 | 0.0240 | [126] |
| Bioeconomy Routes | Raw Materials | kgCO2eq/kWh | References |
| Bioenergy Pathway | |||
| Combustion | Forest residues (FRs) | 0.063 | [154] |
| Pellets from FR | 0.615 | [156] | |
| Pellets from SRa | 0.731 | [156] | |
| Co-firing CHP | Coal and raw pellets | 2.32 | [157] |
| Co-firing CHP | Coal and torrefied pellets | 2.58 | [157] |
| Co-firing CHP | Peat and biomass | 0.72 | [158] |
| Gasification–ICE | FR a | 0.049 | [154] |
| Gasification–ICE | Wood chip | 0.038 | [155] |
| Gasification–ICE | SR b | 0.17–0.20 | [153] |
| Bio-Based Materials | Embodied Energy MJ/f.ub c | kgCO2eq/f.ub | References |
| Expanded polyurethane | 125 | 5.1 | [159] |
| Expanded polystyrene | 130 | 5.0 | [159] |
| Glass wool | 229 | 9.8 | [159] |
| Rice husk | 45 | 1.9 | [160] |
| Kenaf fibers | 42.3 | 1.1 | [161] |
| Eucalyptus bark fibers | 16–72 | 1.4–5.9 | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentealba, C.; Ferrer, V.; Arteaga-Perez, L.E.; Santos, J.; Delgado, N.; Casas-Ledón, Y.; Bravo-Arrepol, G.; Pereira, M.; Andrade, A.; Escobar-Avello, D.; et al. Actualized Scope of Forestry Biomass Valorization in Chile: Fostering the Bioeconomy. Forests 2025, 16, 1208. https://doi.org/10.3390/f16081208
Fuentealba C, Ferrer V, Arteaga-Perez LE, Santos J, Delgado N, Casas-Ledón Y, Bravo-Arrepol G, Pereira M, Andrade A, Escobar-Avello D, et al. Actualized Scope of Forestry Biomass Valorization in Chile: Fostering the Bioeconomy. Forests. 2025; 16(8):1208. https://doi.org/10.3390/f16081208
Chicago/Turabian StyleFuentealba, Cecilia, Victor Ferrer, Luis E. Arteaga-Perez, Jorge Santos, Nacarid Delgado, Yannay Casas-Ledón, Gastón Bravo-Arrepol, Miguel Pereira, Andrea Andrade, Danilo Escobar-Avello, and et al. 2025. "Actualized Scope of Forestry Biomass Valorization in Chile: Fostering the Bioeconomy" Forests 16, no. 8: 1208. https://doi.org/10.3390/f16081208
APA StyleFuentealba, C., Ferrer, V., Arteaga-Perez, L. E., Santos, J., Delgado, N., Casas-Ledón, Y., Bravo-Arrepol, G., Pereira, M., Andrade, A., Escobar-Avello, D., & Cabrera-Barjas, G. (2025). Actualized Scope of Forestry Biomass Valorization in Chile: Fostering the Bioeconomy. Forests, 16(8), 1208. https://doi.org/10.3390/f16081208










