Bite by Bite: How Ungulate Browsing Shapes North America’s Forest Future
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Review Approach
- What are the known effects and magnitude of ungulate browsing on forest regeneration across North America?
- Which preventive and control measures for ungulates have the greatest potential as management solutions for both wildlife and forestry managers?
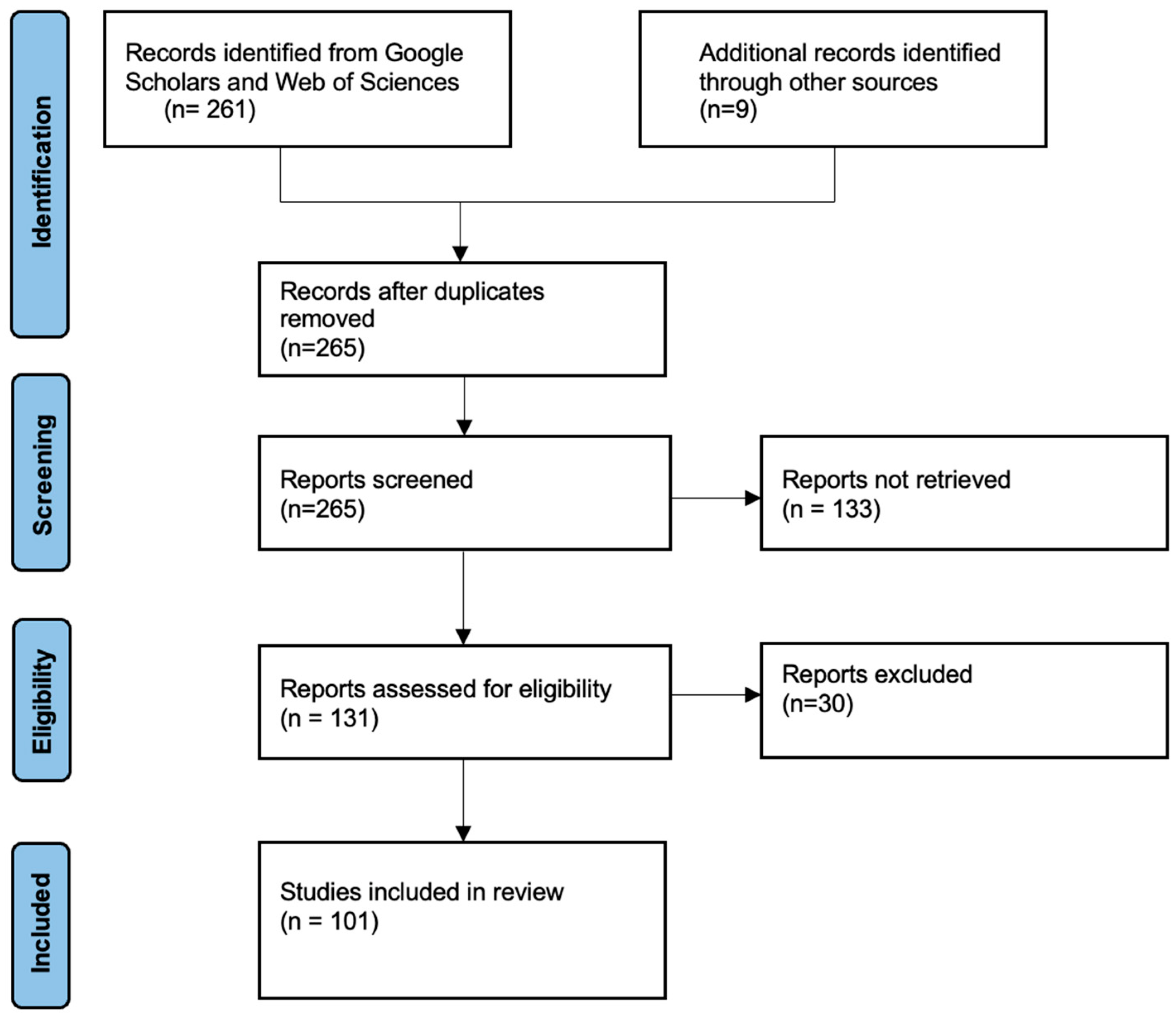
2.2. Search Strategy, Inclusion Criteria, and Screening Process
2.3. Analytical Framework
- Predator presence (yes or no);
- Dominant browsed species (broadleaves, coniferous, or mixed);
- Ungulate density per 1 km2 (low, medium, or high);
- Ungulate species (e.g., blacktail, elk, moose, or a combination of these species based on their presence).
3. Results
3.1. Overview of Ungulate Browsing Studies in North America
3.1.1. Canada
3.1.2. United States of America
3.2. Browsing Intensity Drivers
3.3. Relationship Between Predator Presence and Ungulate Density
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martin, P.S.; Klein, R.G. Prehistoric overkill: The global model. In Quaternary Extinctions: A Prehistoric Revolution; University of Arizona Press: Tucson, Arizona, 1984; pp. 354–403. [Google Scholar]
- Kay, C.E. Technical Commentary: Aboriginal Overkill and Native Burning: Implications for Modern Ecosystem Management. West. J. Appl. For. 1995, 10, 121–126. [Google Scholar] [CrossRef]
- Ambrose, S.E. Undaunted Courage: Meriwether Lewis, Thomas Jefferson, and the Opening of the American West; Simon & Schuster: New York, NY, USA, 1996. [Google Scholar]
- Angelstam, P.; Albulescu, A.C.; Andrianambinina, O.D.F.; Aszalós, R.; Borovichev, E.; Cardona, W.C.; Dobrynin, D.; Fedoriak, M.; Firm, D.; Hunter, M.L.; et al. Frontiers of protected areas versus forest exploitation: Assessing habitat network functionality in 16 case study regions globally. Ambio 2021, 50, 2286–2310. [Google Scholar] [CrossRef] [PubMed]
- Mantel, K. Wald und Forst in der Geschichte. Ein Lehr- und Handbuch; M. & H. Schaper: Hanover, Germany, 1990. [Google Scholar]
- Demeritt, D. Scientific forest conservation and the statistical picturing of nature’s limits in the progressive-era United States. Environ. Plan. D 2001, 19, 431–459. [Google Scholar] [CrossRef]
- Greeley, W.B. The Relation of Geography to Timber Supply. Econ. Geogr. 1925, 1, 1–14. [Google Scholar] [CrossRef]
- Søndergaard, S.A.; Fløjgaard, C.; Ejrnæs, R.; Svenning, J.C. Shifting baselines and the forgotten giants: Integrating megafauna into plant community ecology. Oikos 2025, 2025, e11134. [Google Scholar] [CrossRef]
- Laliberte, A.S.; Ripple, W.J. Range Contractions of North American Carnivores and Ungulates. BioScience 2004, 54, 123–138. [Google Scholar] [CrossRef]
- Malpeli, K.C.; Weiskopf, S.R.; Thompson, L.; Hardy, A.R. What are the effects of climate variability and change on ungulate life-histories, population dynamics, and migration in North America? A systematic map protocol. Environ. Evid. 2020, 9, 21. [Google Scholar] [CrossRef]
- Berger, J.; Stacey, P.B.; Bellis, L.; Johnson, M.P.; Johnson, M.P. A Mammalian Predator-Prey Imbalance: Grizzly Bear and Wolf Extinction Affect Avian Neotropical Migrants. Ecol. Appl. 2001, 11, 947–960. [Google Scholar]
- Kay, C. Are Ecosystems Structured from the Top-Down or Bottom-Up: A New Look at an Old Debate. Wildl. Socirty Bull. 1998, 26, 484–498. [Google Scholar]
- Ripple, W.J.; Estes, J.A.; Beschta, R.L.; Wilmers, C.C.; Ritchie, E.G.; Hebblewhite, M.; Berger, J.; Elmhagen, B.; Letnic, M.; Nelson, M.P.; et al. Status and ecological effects of the world’s largest carnivores. Science 2014, 343, 1241484. [Google Scholar] [CrossRef]
- Sporting Conservation Council. Strengthening America’s Hunting Heritage and Wildlife Conservation in the 21st Century: Challenges and Opportunities; Sporting Conservation Council: San Antonio, TX, USA, 2008. [Google Scholar]
- Krausman, P.R.; Bleich, V.C. Conservation and management of ungulates in North America. Int. J. Environ. Stud. 2013, 70, 372–382. [Google Scholar] [CrossRef]
- Mcshea, W.J. Ecology and management of white-tailed deer in a changing world. Ann. N. Y. Acad. Sci. 2012, 1249, 45–56. [Google Scholar] [CrossRef]
- Peterson, R.O.; Vucetich, J.A.; Page, R.E.; Chouinard, A. Temporal and Spatial Aspects of Predator-Prey Dynamics. Alces 2003, 39, 215–232. [Google Scholar]
- Barber-Meyer, S.M.; Mech, L.D.; White, P.J. Elk Calf Survival and Mortality Following Wolf Restoration to Yellowstone National Park. Wildl. Monogr. 2008, 169, 1–30. [Google Scholar] [CrossRef]
- Ripple, W.J.; Beschta, R.L. Large predators limit herbivore densities in northern forest ecosystems. Eur. J. Wildl. Res. 2012, 58, 733–742. [Google Scholar] [CrossRef]
- Leopold, A.; Sowls, L.K.; Spencer, D.L. Wiley Wildlife Society A Survey of Over-Populated Deer Ranges in the United States. J. Wildl. Manag. 1947, 11, 162–177. [Google Scholar] [CrossRef]
- Gordaliza, G.G.; García-Rovés, J.C.M.; López, R.; Aranda, I.; Gil, L.; Perea, R.; Rodríguez-Calcerrada, J. Herbivory legacy modifies leaf economic spectrum and drought tolerance in two tree species. Oecologia 2025, 207, 1–15. [Google Scholar] [CrossRef]
- Rooney, T.P.; Waller, D.M. Direct and indirect effects of white-tailed deer in forest ecosystems. For. Ecol. Manag. 2003, 181, 165–176. [Google Scholar] [CrossRef]
- Pascual-Rico, R.; Morales-Reyes, Z.; Aguilera-Alcalá, N.; Olszańska, A.; Sebastián-González, E.; Naidoo, R.; Moleón, M.; Lozano, J.; Botella, F.; von Wehrden, H.; et al. Usually hated, sometimes loved: A review of wild ungulates’ contributions to people. Sci. Total Environ. 2021, 801, 149652. [Google Scholar] [CrossRef]
- Côté, S.D.; Rooney, T.P.; Tremblay, J.-P.; Dussault, C.; Waller, D.M. Ecological Impacts of Deer Overabundance. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 113–147. [Google Scholar] [CrossRef]
- Russell, F.L.; Zippin, D.B.; Zippin, D.B.; Fowler, N.L. Effects of White-tailed Deer (Odocoileus virginianus) on Plants, Plant Populations and Communities: A Review. Am. Midl. Nat. 2001, 146, 1–26. [Google Scholar] [CrossRef]
- Boulanger, V.; Baltzinger, C.; Saïd, S.; Ballon, P.; Picard, J.F.; Dupouey, J.L. Decreasing deer browsing pressure influenced understory vegetation dynamics over 30 years. Ann. For. Sci. 2015, 72, 367–378. [Google Scholar] [CrossRef]
- Boulanger, V.; Baltzinger, C.; Saïd, S.; Ballon, P.; Picard, J.F.; Dupouey, J.L. Ranking temperate woody species along a gradient of browsing by deer. For. Ecol. Manag. 2009, 258, 1397–1406. [Google Scholar] [CrossRef]
- Czyżewski, S.; Svenning, J.C. Temperate forest plants are associated with heterogeneous semi-open canopy conditions shaped by large herbivores. Nat. Plants 2025, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Loreau, M.; Naeem, S.; Inchausti, P. Biodiversity & Ecosystem Functioning—Synthesis and Perspectives; OUP Oxford: Oxford, UK, 2002; Volume 12. [Google Scholar]
- Bauhus, J.; Puettmann, K.; Messier, C. Silviculture for old-growth attributes. For. Ecol. Manag. 2009, 258, 525–537. [Google Scholar] [CrossRef]
- Nicolescu, V.N. The Practice of Silviculture; Aldus: Brasov, Romania, 2018. [Google Scholar]
- Gill, R.M.A. A Review of Damage by Mammals in North Temperate Forests: 3. Impact on Trees and Forests. For. Int. J. For. Res. 1992, 65, 363–388. [Google Scholar] [CrossRef]
- Crystal-Ornelas, R.; Hudgins, E.J.; Cuthbert, R.N.; Haubrock, P.J.; Fantle-Lepczyk, J.; Angulo, E.; Kramer, A.M.; Ballesteros-Mejia, L.; Leroy, B.; Leung, B.; et al. Economic costs of biological invasions within north america. NeoBiota 2021, 67, 485–510. [Google Scholar] [CrossRef]
- Hardalau, D.; Codrean, C.; Iordache, D.; Fedorca, M.; Ionescu, O. The Expanding Thread of Ungulate Browsing—A Review of Forest Ecosystem Effects and Management Approaches in Europe. Forests 2024, 15, 1311. [Google Scholar] [CrossRef]
- Albulescu, A.C.; Manton, M.; Larion, D.; Angelstam, P. The Winding Road towards Sustainable Forest Management in Romania, 1989–2022: A Case Study of Post-Communist Social–Ecological Transition. Land 2022, 11, 1198. [Google Scholar] [CrossRef]
- Agresti, A. Tutorial on Modeling Ordered Categorical Response Data. Psychol. Bull. 1989, 105, 290–301. [Google Scholar] [CrossRef]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010. [Google Scholar]
- Hutcheson, G.D.; Moutinho, L.A.M. The SAGE Dictionary of Quantitative Management Research; SAGE Publications: Thousand Oaks, CA, USA, 2011; pp. 1–344. [Google Scholar]
- Waskom, M.L. Seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Bisong, E. Matplotlib and Seaborn. In Building Machine Learning and Deep Learning Models on Google Cloud Platform; Apress: Berkeley, CA, USA, 2019; pp. 151–165. [Google Scholar]
- Gommers, R.; Virtanen, P.; Haberland, M.; Burovski, E.; Reddy, T.; Weckesser, W.; Oliphant, T.E.; Cournapeau, D.; Nelson, A.; alexbrc; et al. scipy/scipy: SciPy 1.15.0. Zenodo 2025. Available online: https://zenodo.org/records/14630489 (accessed on 4 April 2025).
- Chouinard, A.; Filion, L. Detrimental effects of white-tailed deer browsing on balsam fir growth and recruitment in a second-growth stand on Anticosti Island, Québec. Ecoscience 2001, 8, 199–210. [Google Scholar] [CrossRef]
- Barrette, M.; Bélanger, L.; De Grandpré, L.; Ruel, J.C. Cumulative effects of chronic deer browsing and clear-cutting on regeneration processes in second-growth white spruce stands. For. Ecol. Manag. 2014, 329, 69–78. [Google Scholar] [CrossRef]
- Casabon, C.; Pothier, D. Browsing of tree regeneration by white-tailed deer in large clearcuts on Anticosti Island, Quebec. For. Ecol. Manag. 2007, 253, 112–119. [Google Scholar] [CrossRef]
- Dufresne, M.; Bradley, R.L.; Tremblay, J.P.; Poulin, M.; Pellerin, S. Clearcutting and deer browsing intensity interact in controlling nitrification rates in forest floor. Ecoscience 2009, 16, 361–368. [Google Scholar] [CrossRef]
- Potvin, F.; BeauprÉ, P.; Laprise, G. The eradication of balsam fir stands by white-tailed deer on Anticosti Island, Québec: A 150-year process. Ecoscience 2003, 10, 487–495. [Google Scholar] [CrossRef]
- Champagne, E.; Moore, B.D.; Côté, S.D.; Tremblay, J.P. Spatial correlations between browsing on balsam fir by white-tailed deer and the nutritional value of neighboring winter forage. Ecol. Evol. 2018, 8, 2812–2823. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, J.P.; Huot, J.; Potvin, F. Density-related effects of deer browsing on the regeneration dynamics of boreal forests. J. Appl. Ecol. 2007, 44, 552–562. [Google Scholar] [CrossRef]
- Thompson, I.D.; Mallik, A.U. Moose browsing and allopathic effects of Kalmia angustifolia on balsam fir regeneration in central Newfoundland. Can. J. For. Res. 1988, 19, 524–526. [Google Scholar] [CrossRef]
- Dumais, D.; Larouche, C.; Raymond, P.; Bédard, S.; Lambert, M.C. Survival and growth dynamics of red spruce seedlings planted under different forest cover densities and types. New For. 2019, 50, 573–592. [Google Scholar] [CrossRef]
- Vila, B.; Vourc’h, G.; Gillon, D.; Martin, J.L.; Guibal, F. Is escaping deer browse just a matter of time in Picea sitchensis? A chemical and dendroecological approach. Trees-Struct. Funct. 2002, 16, 488–496. [Google Scholar] [CrossRef]
- Vila, B.; Torre, F.; Guibal, F.; Martin, J.L. Growth change of young Picea sitchensis in response to deer browsing. For. Ecol. Manag. 2003, 180, 413–424. [Google Scholar] [CrossRef]
- Castleberry, S.B.; Ford, W.M.; Miller, K.V.; Smith, W.P. Influences of herbivory and canopy opening size on forest regeneration in a southern bottomland hardwood forest. For. Ecol. Manag. 2000, 131, 57–64. [Google Scholar] [CrossRef]
- Vila, B.; Torre, F.; Martin, J.L.; Guibal, F. Response of young Tsuga heterophylla to deer browsing: Developing tools to assess deer impact on forest dynamics. Trees-Struct. Funct. 2003, 17, 547–553. [Google Scholar] [CrossRef]
- Martin, J.L.; Stockton, S.A.; Allombert, S.; Gaston, A.J. Top-down and bottom-up consequences of unchecked ungulate browsing on plant and animal diversity in temperate forests: Lessons from a deer introduction. Biol. Invasions 2010, 12, 353–371. [Google Scholar] [CrossRef]
- Martin, J.L.; Baltzinger, C. Interaction among deer browsing, hunting, and tree regeneration. Can. J. For. Res. 2002, 32, 1254–1264. [Google Scholar] [CrossRef]
- Tarleton, P.; Lamb, E.G. Modification of plant communities by bison in Riding Mountain National Park. Écoscience 2021, 28, 67–80. [Google Scholar] [CrossRef]
- Bork, W.; Carlyle, C.N.; Cahill, J.F.; Haddow, R.E.; Hudson, R.J. Disentangling herbivore impacts on Populus tremuloides: A comparison of native ungulates and cattle in Canada’s Aspen Parkland. Oecologia 2013, 173, 895–904. [Google Scholar] [CrossRef]
- Komers, P.E.; Messier, F.; Gates, C.C. Group structure in wood bison: Nutritional and reproductive determinants. Can. J. Zool. 1993, 71, 1367–1371. [Google Scholar] [CrossRef]
- Redburn, M.J.; Strong, W.L.; Gates, C.C. Suitability of boreal mixedwood clearcuts as wood bison (Bison bison athabascae) foraging habitat in north-central Alberta, Canada. For. Ecol. Manag. 2008, 255, 2225–2235. [Google Scholar] [CrossRef]
- Jung, T.S. Winter diets of reintroduced bison (Bison bison) in northwestern Canada. Mamm. Res. 2015, 60, 385–391. [Google Scholar] [CrossRef]
- Jung, T.S.; Czetwertynski, S.M.; Schmiegelow, F.K.A. Boreal forest titans do not clash: Low overlap in winter habitat selection by moose (Alces americanus) and reintroduced bison (Bison bison). Eur. J. Wildl. Res. 2018, 64, 25. [Google Scholar] [CrossRef]
- Kittredge, B.; Ashton, P.M.S. Impact of Deer Browsing on Regeneration in Mixed Stands in Southern New England. North. J. Appl. For. 1995, 12, 115–120. [Google Scholar] [CrossRef]
- de la Cretaz, A.L.; Kelty, M.J. Development of tree regeneration in fern-dominated forest understories after reduction of deer browsing. Restor. Ecol. 2002, 10, 416–426. [Google Scholar] [CrossRef]
- DeGraaf, R.M.; Healy, W.M.; Brooks, R.T. Effects of thinning and deer browsing on breeding birds in New England oak woodlands. For. Ecol. Manag. 1991, 41, 179–191. [Google Scholar] [CrossRef]
- Stange, E.; Shea, K.L. Effects of deer browsing, fabric mats, and tree shelters on Quercus rubra seedlings. Restor. Ecol. 1998, 6, 29–34. [Google Scholar]
- Frelich, L.E.; Lorimer, C.G. Current and Predicted Long-term Effects of Deer Browsing in Hemlock Forests in Michigan, USA. Biol. Conserv. 1985, 34, 99–120. [Google Scholar] [CrossRef]
- Alverson, W.S.; Waller, D.M.; Solheim, S.L. Forests Too Deer: Edge Effects in Northern Wisconsin. Conserv. Biol. 1988, 2, 348–358. [Google Scholar] [CrossRef]
- Long, Z.T.; Carson, W.P.; Peterson, C.J. Can disturbance create refugia from herbivores: An example with Hemlock regeneration on treefall mounds. J. Torrey Bot. Soc. 1998, 125, 165–168. [Google Scholar] [CrossRef]
- Saunders, M.R.; Puettmann, K.J.; Bingham, D.C.; Weisberg, S. Use of Vegetational Characteristics and Browsing Patterns to Predict Deer Damage in Eastern White Pine (Pinus strobus) Plantations. North. J. Appl. For. 1999, 16, 96–102. [Google Scholar] [CrossRef]
- Anderson, C.; Chapman, K.A.; White, M.; Cornett, M. Effects of Browsing control on establishment and recruitment of eastern white pine (Pinus strobes L.) at Cathedral Grove, Lake Superior Highlands, Minnesota, USA. Nat. Areas J. 2002, 22, 202–210. [Google Scholar]
- Davidson, W.H. Deer Prefer Pine Seedlings Growing near Black Locust; USDA Forest Service Research Note; US Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Darby, PA, USA, 1970. [Google Scholar]
- Rooney, T.P.; Solheim, S.L.; Waller, D.M. Factors affecting the regeneration of northern white cedar in lowland forests of the Upper Great Lakes region, USA. For. Ecol. Manag. 2002, 163, 119–130. [Google Scholar] [CrossRef]
- Snyder, J.D.; Janke, R.A. Impact of Moose Browsing on Boreal-type Forests of Isle Royale National Park. Source Am. Midl. Nat. 1976, 95, 79–92. [Google Scholar] [CrossRef]
- Conover, M.R.; Kania, G.S. Annual variation in white-tailed deer damage in commercial nurseries. Ecosyst. Environ. 1995, 55, 213–217. [Google Scholar] [CrossRef]
- Michael, E. Impact of Deer Browsing on Regeneration of Balsam Fir in Canaan Valley, West Virginia. North. J. Appl. For. 1991, 9, 89–90. [Google Scholar] [CrossRef]
- Crouch, G. Postseason Hunting to Reduce Deer Damage to Douglas-Fir in Western Oregon; Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station: Portland, OR, USA, 1980. [Google Scholar]
- Grisez, T. Slash Helps Protect Seedlings From Deer Browsing. J. For. 1960, 58, 385–387. [Google Scholar]
- Ward, J.S.; Stephens, G.R. Protection of tree seedlings from deer browsing. In Proceedings of the 10th Central Hardwood Forest Conference, Morgantown, WV, USA, 5–8 March 1995; pp. 507–518. [Google Scholar]
- McInnes, P.F.; Naiman, R.J.; Pastor, J.; Cohen, Y. Effects of Moose Browsing on Vegetation and Litter of the Boreal Forest, Isle Royale, Michigan, USA. Ecology 1992, 73, 2059–2075. [Google Scholar] [CrossRef]
- Risenhoover, K.L. The influence of moose on the composition and structure of Isle Royale forests. Can. J. For. Res. 1987, 17, 357–364. [Google Scholar] [CrossRef]
- Andreozzi, A.; Pekins, P.J.; Langlais, M.L. Impact of moose browsing on forest regeneration in Northeast Vermont. Alces 2014, 50, 67–79. [Google Scholar]
- Pastor, J.; Dewey, B.; Naiman, R.J.; McInnes, P.F.; Cohen, Y. Moose Browsing and Soil Fertility in the Boreal Forests of Isle Royale National Park. Ecology 1993, 74, 467–480. [Google Scholar] [CrossRef]
- Angell, A.C.; Kielland, K. Establishment and growth of white spruce on a boreal forest floodplain: Interactions between microclimate and mammalian herbivory. For. Ecol. Manag. 2009, 258, 2475–2480. [Google Scholar] [CrossRef]
- Rossow, L.J.; Bryant, J.P.; Kielland, K. Effects of above-ground browsing by mammals on mycorrhizal infection in an early successional taiga ecosystem. Oecologia 1997, 110, 94–98. [Google Scholar] [CrossRef]
- Kielland, K.; Bryant, J.P. Moose herbivory in taiga: Effects on biogeochemistry and vegetation dynamics in primary succession. Oikos 1998, 82, 377–383. [Google Scholar] [CrossRef]
- Holmgren, M.; Groten, F.; Carracedo, M.R.; Vink, S.; Limpens, J. Rewilding Risks for Peatland Permafrost. Ecosystems 2023, 26, 1806–1818. [Google Scholar] [CrossRef]
- Ripple, W.J.; Larsen, E.J.; Renkin, R.A.; Smith, D.W. Trophic cascades among wolves, elk and aspen on Yellowstone National Park’s northern range. Biol. Conserv. 2001, 102, 227–234. [Google Scholar] [CrossRef]
- Ripple, W.J.; Beschta, R.L. Wolf reintroduction, predation risk, and cottonwood recovery in Yellowstone National Park. For. Ecol. Manag. 2003, 184, 299–313. [Google Scholar] [CrossRef]
- Middleton, A.D.; Kauffman, M.J.; Mcwhirter, D.E.; Cook, J.G.; Cook, R.C.; Nelson, A.A.; Jimenez, M.D.; Klaver, R.W. Animal migration amid shifting patterns of phenology and predation: Lessons from a Yellowstone elk herd. Ecology 2013, 94, 1245–1256. [Google Scholar] [CrossRef]
- Koontz, L.; Loomis, J.B. Economic Importance of Elk Hunting in Jackson Hole, Wyoming; US Geological Survey: Reston, VA, USA, 2005. [Google Scholar]
- Rudd, W.J.; Ward, A.L.; Irwin, L.L. Do Split Hunting Seasons Influence Elk Migrations from Yellowstone National Park? Source 1973, 11, 328–331. [Google Scholar]
- Andrus, R.A.; Hart, S.J.; Veblen, T.T. Forest recovery following synchronous outbreaks of spruce and western balsam bark beetle is slowed by ungulate browsing. Ecology 2020, 101, e02998. [Google Scholar] [CrossRef]
- Painter, L.E.; Ripple, W.J. Effects of bison on willow and cottonwood in northern Yellowstone National Park. For. Ecol. Manag. 2012, 264, 150–158. [Google Scholar] [CrossRef]
- Beschta, R.L.; Ripple, W.J.; Kauffman, J.B.; Painter, L.E. Bison limit ecosystem recovery in northern Yellowstone. Food Webs 2020, 23, e00142. [Google Scholar] [CrossRef]
- Painter, L.E.; Beschta, R.L.; Ripple, W.J. Bison alter the northern Yellowstone ecosystem by breaking aspen saplings. Ecol. Evol. 2023, 13, e10369. [Google Scholar] [CrossRef]
- Keigley, R.B. An increase in herbivory of cottonwood in Yellowstone National Park. Northwest Sci. 1997, 71, 127–136. [Google Scholar]
- Keigley, R.B. Architecture of Cottonwood as an Index of Browsing History in Yellowstone. Intermt. J. Sci. 1998, 11. [Google Scholar]
- Rose, J.R.; Cooper, D.J. The influence of floods and herbivory on cottonwood establishment and growth in Yellowstone National Park. Ecohydrology 2017, 10, e1768. [Google Scholar] [CrossRef]
- Zeigenfuss, L.C.; Schoenecker, K.A. Effects of Elk and Bison Herbivory on Narrowleaf Cottonwood. West. N. Am. Nat. 2021, 81, 97–112. [Google Scholar] [CrossRef]
- Kauffman, J.B.; Cummings, D.L.; Kauffman, C.; Beschta, R.L.; Brooks, J.; MacNeill, K.; Ripple, W.J. Bison influences on composition and diversity of riparian plant communities in Yellowstone National Park. Ecosphere 2023, 14, e4406. [Google Scholar] [CrossRef]
- McMillan, B.R.; Pfeiffer, K.A.; Kaufman, D.W. Vegetation Responses to an Animal-generated Disturbance (Bison Wallows) in Tallgrass Prairie. Am. Midl. Nat. 2011, 165, 60–73. [Google Scholar] [CrossRef]
- Coppedge, B.R.; Shaw, J.H. Effects of horning and rubbing behavior by bison (Bison bison) on woody vegetation in a tallgrass prairie landscape. Am. Midl. Nat. 1997, 138, 189–196. [Google Scholar] [CrossRef]
- Gill, R.M.A. A Review of Damage by Mammals in North Temperate Forests: 1. Deer. For. Int. J. For. Res. 1992, 65, 145–169. [Google Scholar] [CrossRef]
- Garrott, R.A.; White, P.J.; White, P.J.; White, C.A.V. Overabundance: An Issue for Conservation Biologists? Conserv. Biol. 1993, 7, 946–949. [Google Scholar] [CrossRef]
- Coulson, T. The Science of Overabundance: Deer Ecology and Population Management. Biodivers. Conserv. 1999, 8, 1719–1721. [Google Scholar] [CrossRef]
- McShea, W.J.; Underwood, H.B.; Underwood, H.B.; Rappole, J.H. The Science of Overabundance: Deer Ecology and Population Management. Wildl. Soc. Bull. 1997, 25, 578–580. [Google Scholar]
- Burbaitė, L.; Csányi, S. Roe deer population and harvest changes in Europe. Est. J. Ecol. 2009, 58, 169–180. [Google Scholar] [CrossRef]
- Bijl, H.; Csányi, S. Fallow Deer (Dama dama) Population and Harvest Changes in Europe since the Early 1980s. Sustainability 2022, 14, 12198. [Google Scholar] [CrossRef]
- Ionescu, A.; Hardalau, D.; Bakševičius, M.; Manton, M.; Popovici, D.-C.; Codrean, C.; Ionescu, G.; Iordache, D. Tracking population trends: Insights from deer hunting harvests in the Baltics, Central, and Eastern Europe. Cent. Eur. For. J. 2025, 71, 83–96. [Google Scholar] [CrossRef]
- Wisdom, M.J.; Nielson, R.M.; Rowland, M.M.; Proffitt, K.M. Modeling Landscape Use for Ungulates: Forgotten Tenets of Ecology, Management, and Inference. Front. Ecol. Evol. 2020, 8, 531719. [Google Scholar] [CrossRef]
- Flueck, W.T. Population regulation in large northern herbivores: Evolution, thermodynamics, and large predators. Z. Jagdwiss. 2018, 46, 139–166. [Google Scholar] [CrossRef]
- Didion, M.; Kupferschmid, A.D.; Bugmann, H. Long-term effects of ungulate browsing on forest composition and structure. For Ecol. Manag. 2009, 258, S44–S55. [Google Scholar] [CrossRef]
- White, M.A. Long-term effects of deer browsing: Composition, structure and productivity in a northeastern Minnesota old-growth forest. For. Ecol. Manag. 2012, 269, 222–228. [Google Scholar] [CrossRef]
- Hanberry, B.B.; Faison, E.K. Re-framing deer herbivory as a natural disturbance regime with ecological and socioeconomic outcomes in the eastern United States. Sci. Total Environ. 2023, 868, 161669. [Google Scholar] [CrossRef] [PubMed]
- Barnas, A.; Anholt, B.; Burton, A.C.; Carroll, K.; Côté, S.D.; Festa-Bianchet, M.; Fryxell, J.; St-Laurent, M.-H.; Fisher, J.T. The influence of habitat alteration on density of invading white-tailed deer should not be discounted. Glob. Change Biol. 2024, 30, e17498. [Google Scholar] [CrossRef]
- Vucetich, A.; Peterson, R.O.; Schaefer, C.L. The Effect of Prey and Predator Densities on Wolf Predation. Ecology 2002, 83, 3003. [Google Scholar] [CrossRef]
- Ausilio, G.; Sand, H.; Månsson, J.; Mathisen, K.M.; Wikenros, C. Ecological Effects of Wolves in Anthropogenic Landscapes: The Potential for Trophic Cascades Is Context-Dependent. Front. Ecol. Evol. 2021, 8, 577963. [Google Scholar] [CrossRef]
- Prugh, L.R.; Sivy, K.J.; Mahoney, P.J.; Ganz, T.R.; Ditmer, M.A.; van de Kerk, M.; Gilbert, S.L.; Montgomery, R.A. Designing studies of predation risk for improved inference in carnivore-ungulate systems. Biol. Conserv. 2019, 232, 194–207. [Google Scholar] [CrossRef]
- Donadio, E.; Buskirk, S.W. Linking predation risk, ungulate antipredator responses, and patterns of vegetation in the high Andes. J. Mammal. 2016, 97, 966–977. [Google Scholar] [CrossRef]
- Theuerkauf, J.; Rouys, S. Habitat selection by ungulates in relation to predation risk by wolves and humans in the Białowieża Forest, Poland. For. Ecol. Manag. 2008, 256, 1325–1332. [Google Scholar] [CrossRef]
- Jedrzejewski, W.; Jedrzejewska, B.; Okarma, H.; Schmidt, K.; Zub, K.; Musiani, M. Prey selection and predation by wolves in Białowieża Primeval Forest, Poland. J. Mammal. 2000, 81, 197–212. [Google Scholar] [CrossRef]
- Kuijper, D.P.J.; Schmidt, K.; Behnke, R.; Wikenros, C. Behavioural responses of ungulates to indirect cues of an ambush predator. Behaviour 2015, 152, 1019–1040. [Google Scholar]
- Kuijper, D.P.J.; Cromsigt, J.P.G.M.; Churski, M.; Adam, B.; Jedrzejewska, B.; Jedrzejewski, W. Do ungulates preferentially feed in forest gaps in European temperate forest? For. Ecol. Manag. 2009, 258, 1528–1535. [Google Scholar] [CrossRef]
- Hardalau, D.; Fedorca, M.; Popovici, D.-C.; Ionescu, G.; Fedorca, A.; Mirea, I.; Daniel, I.; Ionescu, O. Insights in Managing Ungulates Population and Forest Sustainability in Romania. Diversity 2025, 17, 194. [Google Scholar] [CrossRef]
- Atwood, T.C.; Gese, E.M.; Kunkel, K.E. Spatial Partitioning of Predation Risk in a Multiple Predator-Multiple Prey System. J. Wildl. Manag. 2009, 73, 876–884. [Google Scholar] [CrossRef]
- Van Beeck Calkoen, S.T.S.; Kreikenbohm, R.; Kuijper, D.P.J.; Heurich, M. Olfactory cues of large carnivores modify red deer behavior and browsing intensity. Behav. Ecol. 2021, 32, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Palazón, S. The Importance of Reintroducing Large Carnivores: The Brown Bear in the Pyrenees. Adv. Glob. Change Res. 2017, 62, 231–249. [Google Scholar]
- Tallian, A.; Ordiz, A.; Zimmermann, B.; Sand, H.; Wikenros, C.; Wabakken, P.; Bergqvist, G.; Kindberg, J. The return of large carnivores: Using hunter observation data to understand the role of predators on ungulate populations. Glob. Ecol. Conserv. 2021, 27, e01587. [Google Scholar] [CrossRef]
- Chapron, G.; Kaczensky, P.; Linnell, J.D.C.; Von Arx, M.; Huber, D.; Andrén, H.; López-Bao, J.V.; Adamec, M.; Álvares, F.; Anders, O.; et al. Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 2014, 346, 35–51. [Google Scholar] [CrossRef]
- Carpio, A.J.; Laguna, E.; Pascual-Rico, R.; Martínez-Jauregui, M.; Guerrero-Casado, J.; Vicente, J.; Soriguer, R.C.; Acevedo, P. The prohibition of recreational hunting of wild ungulates in Spanish National Parks: Challenges and opportunities. Sci. Total Environ. 2024, 926, 171363. [Google Scholar] [CrossRef]
- Licht, D.S.; Millspaugh, J.J.; Kunkel, K.E.; Kochanny, C.O.; Peterson, R.O. Using small populations of wolves for ecosystem restoration and stewardship. Bioscience 2010, 60, 147–153. [Google Scholar] [CrossRef]
- Valente, A.M.; Acevedo, P.; Figueiredo, A.M.; Fonseca, C.; Torres, R.T. Overabundant wild ungulate populations in Europe: Management with consideration of socio-ecological consequences. Mamm. Rev. 2020, 50, 353–366. [Google Scholar] [CrossRef]
- Hebblewhite, M.; White, C.A.; Nietvelt, C.G.; McKenzie, J.A.; Hurd, T.E.; Fryxell, J.M.; Bayley, S.E.; Paquet, P.C. Human activity mediates a trophic cascade caused by wolves. Ecology 2005, 86, 2135–2144. [Google Scholar] [CrossRef]
- Bond, W.J. Large parts of the world are brown or black: A different view on the ‘Green World’ hypothesis. J. Veg. Sci. 2005, 16, 261–266. [Google Scholar] [CrossRef]
- Kalábová, M.; Rinn, R.; Matejević, M.; Marković, V.; Kušta, T.; Löwe, R.; Lazaridou, D. Forests, wildlife, and economy: The role of hunting tourism in Czechia’s sustainable forest management. Front. For. Glob. Change 2025, 8, 1525311. [Google Scholar] [CrossRef]
- Kupferschmid, A.D.; Bütikofer, L.; Hothorn, T.; Schwyzer, A.; Brang, P. Ungulate species and abundance as well as environmental factors determine the probability of terminal shoot browsing on temperate forest trees. Forests 2020, 11, 764. [Google Scholar] [CrossRef]
- D’Aprile, D.; Vacchiano, G.; Vacchiano, G.; Meloni, F.; Garbarino, M.; Motta, R.; Ducoli, V.; Partel, P. Effects of Twenty Years of Ungulate Browsing on Forest Regeneration at Paneveggio Reserve, Italy. Forests 2020, 11, 612. [Google Scholar] [CrossRef]
- Dufresne, M.; Bradley, R.L.; Tremblay, J.P.; Côté, S.D. Evidence that soil depth and clay content control the post-disturbance regeneration of balsam fir and paper birch under heavy browsing from deer. Ecoscience 2011, 18, 363–368. [Google Scholar] [CrossRef]
- Velamazán, M.; Miguel, A.S.; Escribano, R.; Perea, R. Compatibility of regeneration silviculture and wild ungulates in a Mediterranean pine forest: Implications for tree recruitment and woody plant diversity. Ann. For. Sci. 2018, 75, 35. [Google Scholar] [CrossRef]
- Madalcho, A.B.; Gazda, A.; Wanic, T.; Szwagrzyk, J. Influence of Soil Quality on the Browsing Intensity of Ungulate Herbivores on Tree Species in European Forests. Forests 2024, 15, 708. [Google Scholar] [CrossRef]
- Nilsen, E.B.; Milner-Gulland, E.J.; Schofield, L.; Mysterud, A.; Stenseth, N.C.; Coulson, T. Wolf reintroduction to Scotland: Public attitudes and consequences for red deer management. Proc. R. Soc. B Biol. Sci. 2007, 274, 995–1002. [Google Scholar] [CrossRef]


| Class | Ungulate Size | Density Range (Animals/km2) |
|---|---|---|
| Low | Deer species | 0–15 |
| Moose or bison | 0–0.7 | |
| Medium | Deer species | >15–30 |
| Moose or bison | 0.7–1.25 | |
| High | Deer species | >30–50 |
| Moose or bison | >1.25 |
| Class | Browsing Rate (%) |
|---|---|
| Low | 0–30 |
| Medium | 31–70 |
| High | 71–100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hardalau, D.; Stefanescu, V.; Bakševičius, M.; Manton, M.; Ruffner, C.; Brazaitis, G.; Ionescu, G.; Ionescu, O. Bite by Bite: How Ungulate Browsing Shapes North America’s Forest Future. Forests 2025, 16, 1079. https://doi.org/10.3390/f16071079
Hardalau D, Stefanescu V, Bakševičius M, Manton M, Ruffner C, Brazaitis G, Ionescu G, Ionescu O. Bite by Bite: How Ungulate Browsing Shapes North America’s Forest Future. Forests. 2025; 16(7):1079. https://doi.org/10.3390/f16071079
Chicago/Turabian StyleHardalau, Darius, Vladut Stefanescu, Mindaugas Bakševičius, Michael Manton, Charles Ruffner, Gediminas Brazaitis, Georgeta Ionescu, and Ovidiu Ionescu. 2025. "Bite by Bite: How Ungulate Browsing Shapes North America’s Forest Future" Forests 16, no. 7: 1079. https://doi.org/10.3390/f16071079
APA StyleHardalau, D., Stefanescu, V., Bakševičius, M., Manton, M., Ruffner, C., Brazaitis, G., Ionescu, G., & Ionescu, O. (2025). Bite by Bite: How Ungulate Browsing Shapes North America’s Forest Future. Forests, 16(7), 1079. https://doi.org/10.3390/f16071079









