Stem Heating Enhances Growth but Reduces Earlywood Lumen Size in Two Pine Species and a Ring-Porous Oak
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Tree Species
2.3. Climate Data
2.4. Growth and Phenology Monitoring
2.5. Stem Heating Experiment
2.6. Field Sampling and Dendrochronological Data
2.7. Changes in Concentrations of Non-Structural Carbohydrates (NSCs)
2.8. Wood Anatomy
2.9. Vaganov-Shashkin Process-Based Model of Radial Growth
2.10. Projecting Spring Phenology Variations in Response to Climate Scenarios
2.11. Statistical Analyses
3. Results
3.1. Leaf Phenology
3.2. Growth, Wood Anatomy, and Responses to Climate
3.3. Responses to Stem Heating: Growth, Sapwood NSCs, and Wood Anatomy
3.4. Models of Radial Growth
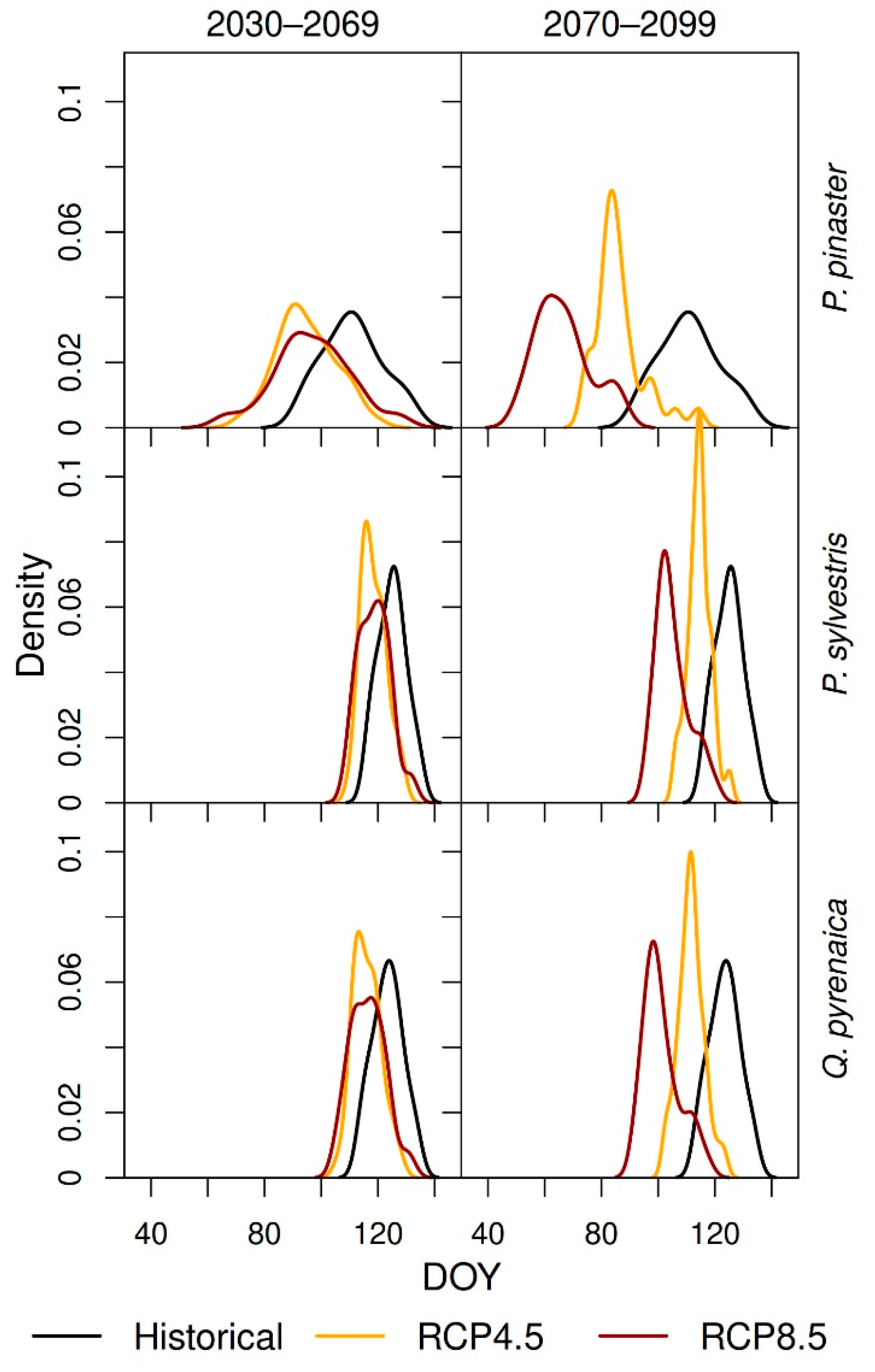
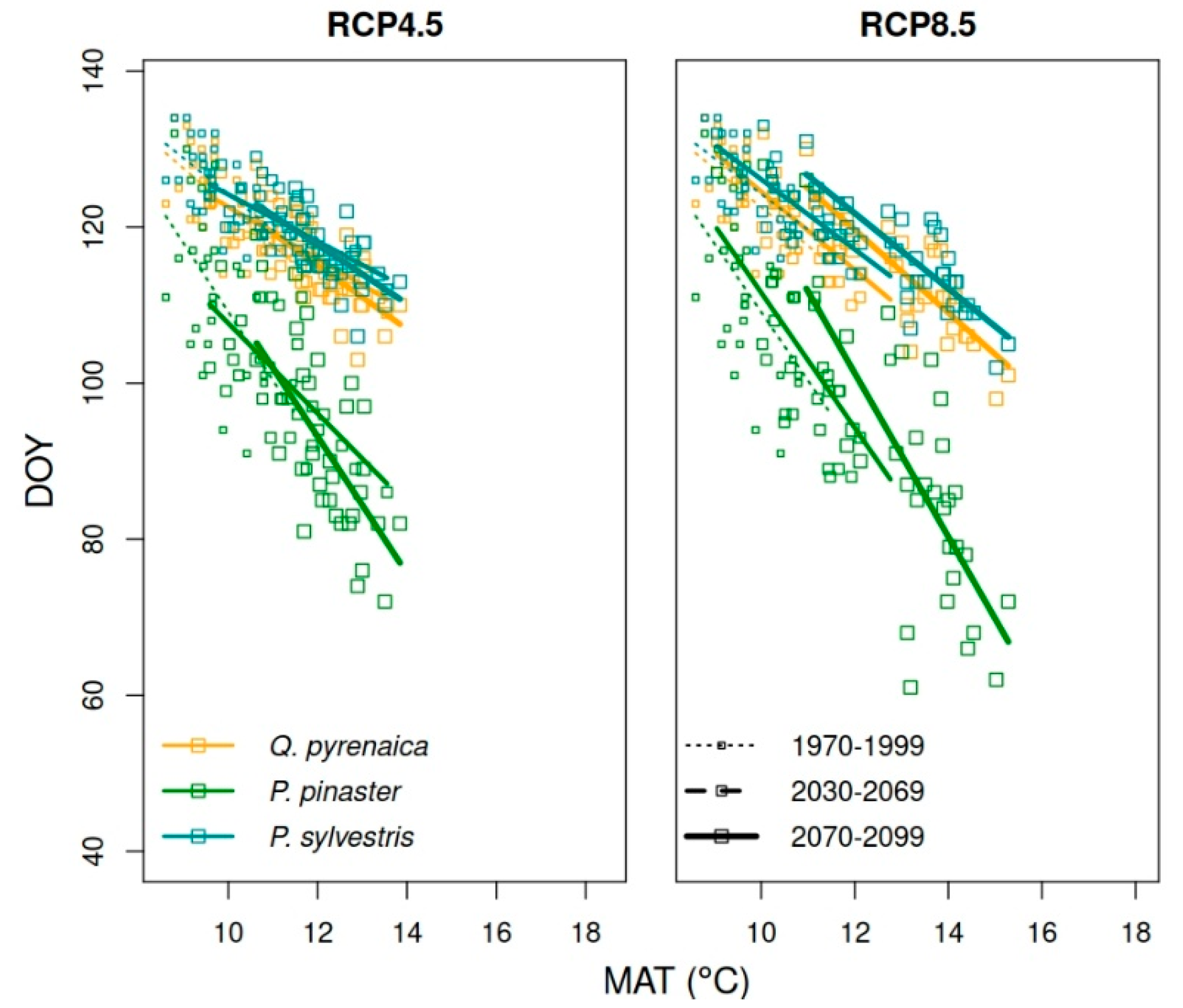
3.5. Forecasting Changes in Growth Phenology
4. Discussion
4.1. Species-Specific Responses to Stem Heating
4.2. Climatic Influences on Radial Growth
4.3. Responses of Earlywood Anatomy to Stem Heating: Hydraulic Adjustments
4.4. Implications for Spring Phenology and Tree Growth Under Warmer Climate Scenarios
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canadell, J.G.; Raupach, M.R. Managing forests for climate change mitigation. Science 2008, 320, 1456–1457. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.E.; Smiljanić, M.; Scharnweber, T.; Buras, A.; Cedro, A.; Cruz-García, R.; Drobyshev, I.; Janecka, K.; Jansons, Ā.; Kaczka, R.; et al. Tree growth influenced by warming winter climate and summer moisture availability in northern temperate forests. Glob. Change Biol. 2020, 26, 2505–2518. [Google Scholar] [CrossRef] [PubMed]
- Charrier, G.; Martin-StPaul, N.; Damesin, C.; Delpierre, N.; Hänninen, H.; Torres-Ruiz, J.M.; Davi, H. Interaction of drought and frost in tree ecophysiology: Rethinking the timing of risks. Ann. For. Sci. 2021, 78, 40. [Google Scholar] [CrossRef]
- Camarero, J.J.; Campelo, F.; Colangelo, M.; Valeriano, C.; Knorre, A.; Solé, G.; Rubio-Cuadrado, Á. Decoupled leaf-wood phenology in two pine species from contrasting climates: Longer growing seasons do not mean more radial growth. Agric. For. Meteorol. 2022, 327, 109223. [Google Scholar] [CrossRef]
- Dox, I.; Mariën, B.; Zuccarini, P.; Marchand, L.J.; Prislan, P.; Gričar, J.; Flores, O.; Gehrmann, F.; Fonti, P.; Lange, H.; et al. Wood growth phenology and its relationship with leaf phenology in deciduous forest trees of the temperate zone of Western Europe. Agric. For. Meteorol. 2022, 327, 109229. [Google Scholar] [CrossRef]
- Begum, S.; Nakaba, S.; Yamagishi, Y.; Oribe, Y.; Funada, R. Regulation of cambial activity in relation to environmental conditions: Understanding the role of temperature in wood formation of trees. Physiol. Plant. 2013, 147, 46–54. [Google Scholar] [CrossRef]
- Funada, R.; Yamagishi, Y.; Begum, S.; Kudo, K.; Nabeshima, E.; Nugroho, W.; Rahman, M.H.; Oribe, Y.; Nakaba, S. Xylogenesis in Trees: From Cambial Cell Division to Cell Death. In Secondary Xylem Biology; Kim, Y.S., Funada, R., Singh, A.P., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 25–43. [Google Scholar] [CrossRef]
- Begum, S.; Kudo, K.; Rahman, M.H.; Nakaba, S.; Yamagishi, Y.; Nabeshima, E.; Nugroho, W.D.; Oribe, Y.; Kitin, P.; Jin, H.-O.; et al. Climate change and the regulation of wood formation in trees by temperature. Trees Struct. Funct. 2018, 32, 3–15. [Google Scholar] [CrossRef]
- Oribe, Y.; Kubo, T. Effect of heat on cambial reactivation during winter dormancy in evergreen and deciduous conifers. Tree Physiol. 1997, 17, 81–87. [Google Scholar] [CrossRef]
- Oribe, Y.; Funada, R.; Shibagaki, M.; Kubo, T. Cambial reactivation in locally heated stems of the evergreen conifer Abies sachalinensis (Schmidt) Masters. Planta 2001, 212, 684–691. [Google Scholar] [CrossRef]
- Oribe, Y.; Funada, R.; Kubo, T. Relationships between cambial activity, cell differentiation and the localization of starch in storage tissues around the cambium in locally heated stems of Abies sachalinensis (Schmidt) Masters. Trees Struct. Funct. 2003, 17, 185–192. [Google Scholar] [CrossRef]
- Gričar, J.; Zupancic, M.; Čufar, K.; Oven, P. Regular cambial activity and xylem and phloem formation in locally heated and cooled stem portions of Norway spruce. Wood Sci. Technol. 2007, 41, 463–475. [Google Scholar] [CrossRef]
- Kudo, K.; Nabeshima, E.; Begum, S.; Yamagishi, Y.; Nakaba, S.; Oribe, Y.; Yasue, K.; Funada, R. The effects of localized heating and disbudding on cambial reactivation and formation of earlywood vessel in seedlings of the deciduous ring-porous hardwood, Quercus serrate. Ann. Bot. 2014, 113, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Kudo, K.; Yamagishi, Y.; Nakamura, Y.; Nakaba, S.; Begum, S.; Nugroho, W.D.; Arakawa, I.; Kitin, P.; Funada, R. Winter-spring temperature pattern is closely related to the onset of cambial reactivation in stems of the evergreen conifer Chamaecyparis pisifera. Sci. Rep. 2020, 10, 14341. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, A.; Mattana, S.; Emiliani, G.; Anichini, M.; Traversi, M.L.; Pavone, F.S.; Cicchi, R. Localized stem heating from the rest to growth phase induces latewood-like cell formation and slower stem radial growth in Norway spruce saplings. Tree Physiol. 2022, 42, 1149–1163. [Google Scholar] [CrossRef]
- Huang, J.-G.; Ma, Q.; Rossi, S.; Biondi, F.; Deslauriers, A.; Fonti, P.; Liang, E.; Mäkinen, H.; Oberhuber, W.; Rathgeber, C.B.K.; et al. Photoperiod and temperature as dominant environmental drivers triggering secondary growth resumption in Northern Hemisphere conifers. Proc. Natl. Acad. Sci. USA 2020, 117, 20645–20652. [Google Scholar] [CrossRef] [PubMed]
- Simard, S.; Giovannelli, A.; Treydte, K.; Traversi, M.L.; King, G.M.; Frank, D.; Fonti, P. Intra-annual dynamics of non-structural carbohydrates in the cambium of mature conifer trees reflects radial growth demands. Tree Physiol. 2013, 33, 913–923. [Google Scholar] [CrossRef]
- Yu, B.; Rossi, S.; Su, H.; Zhao, P.; Zhang, S.; Hu, B.; Li, X.; Chen, L.; Liang, H.; Huang, J.-G. Mismatch between primary and secondary growth and its consequences on wood formation in Qinghai spruce. Tree Physiol. 2023, 43, 1886–1902. [Google Scholar] [CrossRef]
- Stridbeck, P.; Björklund, J.; Fuentes, M.; Gunnarson, B.E.; Jönsson, A.M.; Linderholm, H.W.; Ljungqvist, F.C.; Olsson, C.; Rayner, D.; Rocha, E.; et al. Partly decoupled tree-ring width and leaf phenology response to 20th century temperature change in Sweden. Dendrochronologia 2022, 75, 125993. [Google Scholar] [CrossRef]
- Vaganov, E.A.; Hughes, M.K.; Shashkin, A.V. Growth Dynamics of Conifer Tree Rings; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Campelo, F.; Camarero, J.J. Temperature-photoperiod interactions improve simulations of early xylem phenology: Refining the Vaganov-Shashkin growth model. Dendrochronologia 2024, 85, 126215. [Google Scholar] [CrossRef]
- Tumajer, J.; Serra-Maluquer, X.; Gazol, A.; González de Andrés, E.; Colangelo, M.; Sangüesa-Barreda, G.; Olano, J.M.; Rozas, V.; García-Plazaola, J.I.; Fernández-Marín, B.; et al. Bimodal and unimodal radial growth of Mediterranean oaks along a coast-inland gradient. Agric. For. Meteorol. 2022, 327, 109234. [Google Scholar] [CrossRef]
- Campelo, F.; Ribas, M.; Gutiérrez, E. Plastic bimodal growth in a Mediterranean mixed-forest of Quercus ilex and Pinus halepensis. Dendrochronologia 2021, 67, 125836. [Google Scholar] [CrossRef]
- Valeriano, C.; Tumajer, T.; Gazol, A.; González de Andrés, E.; Sánchez-Salguero, R.; Colangelo, M.; Linares, J.C.; Valor, T.; Sangüesa-Barreda, G.; Camarero, J.J. Delineating vulnerability to drought using a process-based growth model in Pyrenean silver fir forests. For. Ecol. Manag. 2023, 541, 121069. [Google Scholar] [CrossRef]
- Aloni, R. Wood formation in deciduous hardwood trees. In Physiology of Trees; Raghavendra, A.S., Ed.; Wiley: New York, NY, USA, 1991; pp. 75–197. [Google Scholar]
- García-González, I.; Eckstein, D. Climatic signal of earlywood vessels of oak on a maritime site. Tree Physiol. 2003, 23, 497–504. [Google Scholar] [CrossRef]
- Fonti, P.; Solomonoff, N.; García-González, I. Earlywood vessels size of Castanea sativa records temperature before their formation. New Phytol. 2007, 173, 562–570. [Google Scholar] [CrossRef]
- Alla, A.Q.; Camarero, J.J. Contrasting responses of radial growth and wood anatomy to climate in a Mediterranean ring-porous oak: Implications for its future persistence or why the variance matters more than the mean. Eur. J. For. Res. 2012, 131, 1537–1550. [Google Scholar] [CrossRef]
- Penman, H.L. Natural evaporation from open water, bare soil and grass. Proc. R. Soc. Lond. Ser. A 1948, 194, 120–145. [Google Scholar]
- Willmott, C.J.; Rowe, C.M.; Mintz, Y. Climatology of the terrestrial seasonal water cycle. J. Climatol. 1985, 5, 589–606. [Google Scholar] [CrossRef]
- Zweifel, R.; Rigling, A.; Dobbertin, M. Species-specific stomatal response of trees to drought—A link to vegetation dynamics? J. Veg. Sci. 2009, 20, 442–454. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Tardif, J.C.; Conciatori, F. Attributing forest responses to global-change drivers: Limited evidence of a CO2-fertilization effect in Iberian pine growth. J. Biogeogr. 2015, 42, 2220–2233. [Google Scholar] [CrossRef]
- Mediavilla, S.; Escudero, A. Stomatal responses to drought at a Mediterranean site: A comparative study of co-occurring woody species differing in leaf longevity. Tree Physiol. 2003, 23, 987–996. [Google Scholar] [CrossRef]
- Fernández-de-Uña, L.; McDowell, N.G.; Cañellas, I.; Gea-Izquierdo, G. Disentangling the effect of competition, CO2 and climate on intrinsic water-use efficiency and tree growth. J. Ecol. 2016, 104, 678–690. [Google Scholar] [CrossRef]
- Rubio-Cuadrado, A.; Montes, F.; Pardos, P.; Camarero, J.J. Differences in hydrological niche and tree size explain growth resilience to drought in three Mediterranean oaks. Agric. For. Meteorol. 2024, 359, 110291. [Google Scholar] [CrossRef]
- Rossi, S.; Anfodillo, T.; Menardi, R. Trephor: A New Tool for Sampling Microcores from tree stems. IAWA J. 2006, 27, 89–97. [Google Scholar] [CrossRef]
- Holmes, R. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Campelo, F.; García-González, I.; Nabais, C. detrendeR—A Graphical User Interface to process and visualize tree-ring data using R. Dendrochronologia 2012, 30, 57–60. [Google Scholar] [CrossRef]
- Wigley, T.; Briffa, K.; Jones, P. On the average value of correlated time series, with applications in dendroclimatology and hydrometeorology. J. Clim. Appl. Meteorol. 1984, 23, 201–213. [Google Scholar] [CrossRef]
- Buysse, J.; Merckx, R. An improved colorimetric method to quantify sugar content of plant tissue. J. Exp. Bot. 1993, 44, 1627–1629. [Google Scholar] [CrossRef]
- Palacio, S.; Hernández, R.; Maestro-Martínez, M.; Camarero, J.J. Fast replenishment of initial carbon stores after defoliation by the pine processionary moth and its relationship to the regrowth ability of trees. Trees Struct. Funct. 2012, 26, 1627–1640. [Google Scholar] [CrossRef]
- Gärtner, H.; Nievergelt, D. The core-microtome, a new tool for surface preparation on cores and time series analysis of varying cell parameters. Dendrochronologia 2010, 28, 85–92. [Google Scholar] [CrossRef]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Dyachuk, P.; Arzac, A.; Peresunko, P.; Videnin, S.; Ilyin, V.; Assaulianov, R.; Babushkina, E.A.; Zhirnova, D.; Belokopytova, L.; Vaganov, E.A.; et al. AutoCellRow (ACR)—A new tool for the automatic quantification of cell radial files in conifer images. Dendrochronologia 2020, 60, 125687. [Google Scholar] [CrossRef]
- Scholz, A.; Klepsch, M.; Karimi, Z.; Jansen, S. How to quantify conduits in wood? Front. Plant Sci. 2013, 4, 56. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.M.; Calvin, K.V.; Smith, S.J.; Kyle, G.P.; Volke, A.; Patel, P.; Delgado-Arias, S.; Bond-Lamberty, B.; Wise, M.A.; Clarke, L.E.; et al. RCP4.5: A pathway for stabilization of radiative forcing by 2100. Clim. Change 2011, 109, 77–94. [Google Scholar] [CrossRef]
- Riahi, K.; Rao, S.; Krey, V.; Cho, C.; Chirkov, V.; Fischer, G.; Kindermann, G.; Nakicenovic, N.; Rafaj, P. RCP 8.5—A scenario of comparatively high greenhouse gas emissions. Clim. Change 2011, 109, 33–57. [Google Scholar] [CrossRef]
- Döscher, R.; Acosta, M.; Alessandri, A.; Anthoni, P.; Arneth, A.; Bergman, T.; Bernardello, R.; Boussetta, S.; Caron, L.-P.; Carver, G.; et al. The EC-Earth3 Earth System Model for the Climate Model Intercomparison Project 6. Geosci. Model Dev. 2022, 15, 2973–3020. [Google Scholar] [CrossRef]
- Eyring, V.; Bony, S.; Meehl, G.A.; Senior, C.A.; Stevens, B.; Stouffer, R.J.; Taylor, K.E. Overview of the coupled model intercomparison project phase 6 (CMIP6) experimental design and organization. Geosci. Model Dev. 2016, 9, 1937–1958. [Google Scholar] [CrossRef]
- Zang, C.; Biondi, F. Treeclim: An R package for the numerical calibration of proxy-climate relationships. Ecography 2015, 38, 431–436. [Google Scholar] [CrossRef]
- Bailey, L.D.; van de Pol, M. Climwin: An R Toolbox for Climate Window Analysis. PLoS ONE 2016, 11, e0167980. [Google Scholar] [CrossRef]
- van de Pol, M.; Bailey, L.D.; McLean, N.; Rijsdijk, L.; Lawson, C.R.; Brouwer, L. Identifying the Best Climatic Predictors in Ecology and Evolution. Methods Ecol. Evol. 2016, 7, 1246–1257. [Google Scholar] [CrossRef]
- Rubio-Cuadrado, Á.; Camarero, J.J.; Bosela, M. Applying climwin to dendrochronology: A breakthrough in the analyses of tree responses to environmental variability. Dendrochronologia 2022, 71, 125916. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2004. [Google Scholar]
- Campelo, F.; Rubio-Cuadrado, Á.; Montes, F.; Colangelo, M.; Valeriano, C.; Camarero, J.J. Growth phenology adjusts to seasonal changes in water availability in coexisting evergreen and deciduous Mediterranean oaks. For. Ecosyst. 2023, 10, 100134. [Google Scholar] [CrossRef]
- Campelo, F.; Vieira, J.; Nabais, C. Tree-ring growth and intra-annual density fluctuations of Pinus pinaster responses to climate: Does size matter? Trees Struct. Funct. 2013, 27, 763–772. [Google Scholar] [CrossRef]
- Alla, A.Q.; Camarero, J.J.; Palacio, S.; Montserrat-Martí, G. Revisiting the fate of buds: Size and position drive bud mortality and bursting in two coexisting Mediterranean Quercus species with contrasting leaf habit. Trees Struct. Funct. 2013, 27, 1375–1386. [Google Scholar] [CrossRef]
- Meier, A.R.; Saunders, M.R.; Michler, C.H. Epicormic buds in trees: A review of bud establishment, development and dormancy release. Tree Physiol. 2012, 32, 565–584. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Uña, L.; Aranda, I.; Rossi, S.; Fonti, P.; Cañellas, I.; Gea-Izquierdo, G. Divergent phenological and leaf gas exchange strategies of two competing tree species drive contrasting responses to drought at their altitudinal boundary. Tree Physiol. 2018, 38, 1152–1165. [Google Scholar] [CrossRef] [PubMed]
- Gazol, A.; Camarero, J.J.; Vicente-Serrano, S.M.; Sánchez-Salguero, R.; Gutiérrez, E.; de Luis, M.; Sangüesa-Barreda, G.; Novak, K.; Rozas, V.; Tíscar, P.A.; et al. Forest resilience to drought varies across biomes. Glob. Change Biol. 2018, 24, 2143–2158. [Google Scholar] [CrossRef]
- Fernández-de-Uña, L.; Rossi, S.; Aranda, I.; Fonti, P.; González-González, B.D.; Cañellas, I.; Gea-Izquierdo, G. Xylem and Leaf Functional Adjustments to Drought in Pinus sylvestris and Quercus pyrenaica at Their Elevational Boundary. Front. Plant Sci. 2017, 8, 1200. [Google Scholar] [CrossRef]
- Martín, J.A.; Esteban, L.G.; de Palacios, P.; Fernández, F.G. Variation in wood anatomical traits of Pinus sylvestris L. between Spanish regions of provenance. Trees Struct. Funct. 2010, 24, 1017–1028. [Google Scholar] [CrossRef]
- Gazol, A.; Oliva, J.; Valeriano, C.; Colangelo, M.; Camarero, J.J. Mixed Pine Forests in a Hotter and Drier World: The Great Resilience to Drought of Aleppo Pine Benefits It Over Other Coexisting Pine Species. Front. For. Glob. Change 2022, 5, 899425. [Google Scholar] [CrossRef]
- Schönbeck, L.; Gessler, A.; Hoch, G.; McDowell, N.G.; Rigling, A.; Schaub, M.; Li, M. Homeostatic levels of nonstructural carbohydrates after 13 yr of drought and irrigation in Pinus sylvestris. New Phytol. 2018, 219, 1314–1324. [Google Scholar] [CrossRef] [PubMed]
- Oberhuber, W.; Swidrak, I.; Pirkebner, D.; Gruber, A. Temporal dynamics of nonstructural carbohydrates and xylem growth in Pinus sylvestris exposed to drought. Can. J. For. Res. 2011, 41, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Matías, L.; Castro, J.; Villar-Salvador, P.; Quero, J.L.; Jump, A.S. Differential impact of hotter drought on seedling performance of five ecologically distinct pine species. Plant Ecol. 2017, 218, 201–212. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Griçar, J.; Seo, J.-W.; Rathgeber, C.B.; Anfodillo, T.; Morin, H.; Levanic, T.; Oven, P.; Jalkanen, R. Critical temperatures for xylogenesis in conifers of cold climates. Glob. Ecol. Biogeogr. 2008, 17, 696–707. [Google Scholar] [CrossRef]
- Camarero, J.J.; Olano, J.M.; Parras, A. Plastic bimodal xylogenesis in conifers from continental Mediterranean climates. New Phytol. 2010, 185, 471–480. [Google Scholar] [CrossRef]
- Hacke, U.G.; Sperry, J.S.; Pockman, W.T.; Davis, S.D.; McCulloh, K.A. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 2001, 126, 457–461. [Google Scholar] [CrossRef]
- Pittermann, J.; Sperry, J.S.; Wheeler, J.K.; Hacke, U.G.; Sikkema, E.H. Mechanical reinforcement of tracheids compromises the hydraulic efficiency of conifer xylem. Plant. Cell Environ. 2006, 29, 1618–1628. [Google Scholar] [CrossRef]
- Poyatos, R.; Aguadé, D.; Galiano, L.; Mencuccini, M.; Martínez-Vilalta, J. Drought-induced defoliation and long periods of near-zero gas exchange play a key role in accentuating metabolic decline of Scots pine. New Phytol. 2013, 200, 388–401. [Google Scholar] [CrossRef]
- Eilmann, B.; Zweifel, R.; Buchmann, N.; Graf Pannatier, E.; Rigling, A. Drought alters timing, quantity, and quality of wood formation in Scots pine. J. Exp. Bot. 2011, 62, 2763–2771. [Google Scholar] [CrossRef]
- Martin-Benito, D.; Beeckman, H.; Cañellas, I. Influence of drought on tree rings and tracheid features of Pinus nigra and Pinus sylvestris in a mesic Mediterranean forest. Eur. J. For. Res. 2013, 132, 33–45. [Google Scholar] [CrossRef]
- Corcuera, L.; Camarero, J.J.; Gil-Pelegrín, E. Effects of a severe drought on growth and wood-anatomical properties of Quercus faginea. IAWA J. 2004, 25, 185–204. [Google Scholar] [CrossRef]
- Corcuera, L.; Camarero, J.J.; Sisó, S.; Gil-Pelegrín, E. Radial-growth and wood-anatomical changes in overaged Quercus pyrenaica coppice stands: Functional responses in a new Mediterranean landscape. Trees Struct. Funct. 2006, 20, 91–98. [Google Scholar] [CrossRef]
- González-González, B.D.; Vázquez-Ruiz, R.A.; García-González, I. Effects of climate on earlywood vessel formation of Quercus robur and Q. pyrenaica at a site in the northwestern Iberian Peninsula. Can. J. For. Res. 2015, 45, 698–709. [Google Scholar] [CrossRef]
- Souto-Herrero, M.; Rozas, V.; García-González, I. Earlywood vessels and latewood width explain the role of climate on wood formation of Quercus pyrenaica Willd. across the Atlantic-Mediterranean boundary in NW Iberia. For. Ecol. Manag. 2018, 425, 126–137. [Google Scholar] [CrossRef]
- McCulloh, K.A.; Petitmermet, J.; Stefanski, A.; Rice, K.E.; Rich, R.L.; Montgomery, R.A.; Reich, P.B. Is it getting hot in here? Adjustment of hydraulic parameters in six boreal and temperate tree species after 5 years of warming. Glob. Change Biol. 2016, 22, 4124–4133. [Google Scholar] [CrossRef]
- Fu, Y.H.; Piao, S.; Op de Beeck, M.; Cong, N.; Zhao, H.; Zhang, Y.; Menzel, A.; Janssens, I.A. Recent spring phenology shifts in western Central Europe based on multiscale observations. Glob. Ecol. Biogeogr. 2014, 23, 1255–1263. [Google Scholar] [CrossRef]
- Chen, L.; Huang, J.; Ma, Q.; Hänninen, H.; Tremblay, F.; Bergeron, Y. Long-term changes in the impacts of global warming on leaf phenology of four temperate tree species. Glob. Change Biol. 2018, 25, 997–1004. [Google Scholar] [CrossRef]
- Körner, C.; Möhl, P.; Hiltbrunner, E. Four ways to define the growing season. Ecol. Lett. 2023, 26, 1277–1292. [Google Scholar] [CrossRef]



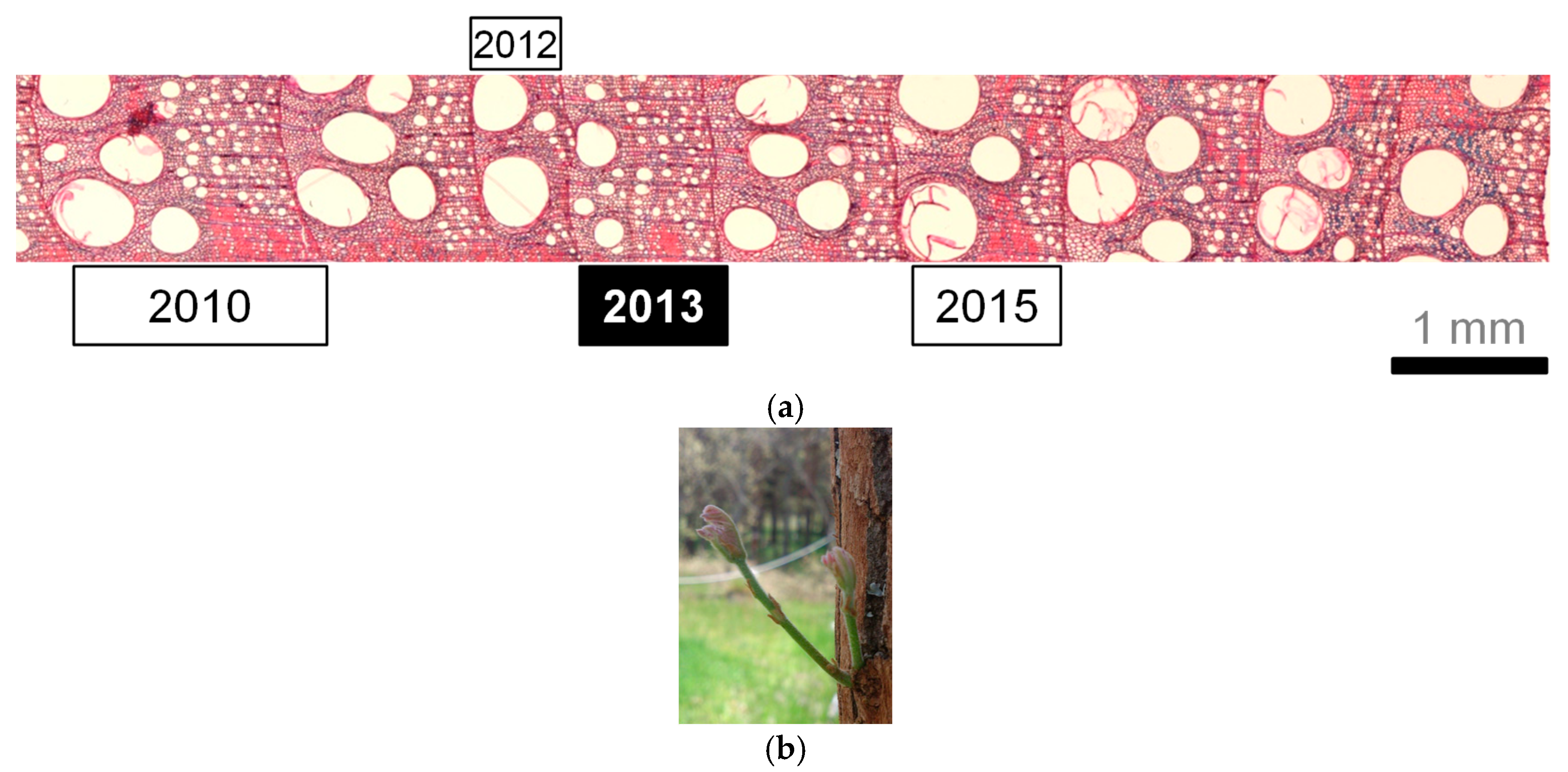


| Species | Heat Treatment | Dbh (cm) | Height (m) | Age at 1.3 m (Years) | Leaf Flushing, DOY (Date) | First Visible Leaves, DOY (Date) | Fully Unfolded Leaves, DOY (Date) | Onset Date of Xylem Formation, DOY (Date) |
|---|---|---|---|---|---|---|---|---|
| P. sylvestris | NH | 51.0 ± 1.1 a | 12.8 ± 0.7 a | 52 ± 1 a | 150 ± 2 a (29 May) | 158 ± 3 a (6 June) | 180 ± 4 a (28 June) | 128 ± 3 a (7 May) |
| H | 49.8 ± 0.9 a | 12.3 ± 0.9 a | 50 ± 1 a | 148 ± 3 a (27 May) | 156 ± 3 a (4 June) | 181 ± 4 a (29 June) | 125 ± 2 a (4 May) | |
| P. pinaster | NH | 57.2 ± 1.0 a | 12.1 ± 0.6 a | 50 ± 1 a | 148 ± 2 a (27 May) | 161 ± 2 a (9 June) | 180 ± 4 a (28 June) | 106 ± 4 b (15 April) |
| H | 58.0 ± 1.2 a | 11.9 ± 0.5 a | 53 ± 2 a | 145 ± 2 a (24 May) | 158 ± 3 a (6 June) | 176 ± 3 a (24 June) | 98 ± 4 a (7 April) | |
| Q. pyrenaica | NH | 25.0 ± 0.5 a | 11.8 ± 2.0 a | 48 ± 3 a | 140 ± 2 a (19 May) | 147 ± 3 a (26 May) | 154 ± 3 a (2 June) | 113 ± 2 b (22 April) |
| H | 29.6 ± 0.8 a | 11.4 ± 1.9 a | 52 ± 2 a | 138 ± 2 a (17 May) | 145 ± 3 a (24 May) | 152 ± 3 a (31 May) | 108 ± 2 a (17 April) |
| Species | No. Trees (No. Cores) | Mean Ring Width (mm) | AR1 | Mean Sensitivity | Rbar | EPS |
|---|---|---|---|---|---|---|
| Pinus pinaster | 20 (35) | 2.76 ± 1.18 | 0.68 ± 0.15 | 0.33 ± 0.05 | 0.62 | 0.98 |
| Pinus sylvestris | 20 (35) | 3.05 ± 1.12 | 0.62 ± 0.13 | 0.31 ± 0.07 | 0.51 | 0.96 |
| Quercus pyrenaica | 20 (35) | 1.92 ± 0.47 | 0.47 ± 0.16 | 0.30 ± 0.04 | 0.54 | 0.97 |
| Variable | Species | nov. | dec. | Jan. | Feb. | Mar. | Apr. | May | Jun. | Jul. | Aug. | Sep. | Oct. | Nov. | MJ | JJ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tmean | P. pinaster | 0.19 | 0.31 * | 0.22 | 0.52 *** | 0.08 | 0.14 | −0.24 | −0.36 * | −0.40 ** | −0.02 | 0.17 | 0.00 | −0.25 | −0.42 ** | −0.44 ** |
| P. sylvestris | 0.19 | 0.24 | 0.10 | 0.58 *** | 0.00 | 0.12 | −0.27 * | −0.34 * | −0.48 *** | −0.02 | 0.25 * | 0.06 | −0.35 ** | −0.45 *** | −0.46 *** | |
| Q. pyrenaica | 0.10 | 0.09 | 0.11 | 0.38 ** | −0.02 | 0.13 | −0.08 | −0.47 *** | −0.42 ** | 0.00 | 0.15 | −0.14 | −0.23 | −0.41 *** | −0.52 *** | |
| Pre | P. pinaster | 0.25 * | 0.31 * | 0.39 ** | 0.22 | −0.07 | 0.37 ** | 0.48 *** | 0.43 *** | 0.36 ** | −0.01 | −0.10 | −0.11 | −0.07 | 0.64 *** | 0.51 *** |
| P. sylvestris | 0.18 | 0.33 * | 0.39 ** | 0.14 | 0.01 | 0.30 * | 0.53 *** | 0.43 ** | 0.41 *** | −0.03 | −0.23 | −0.12 | −0.08 | 0.68 *** | 0.54 *** | |
| Q. pyrenaica | 0.25 * | 0.33 * | 0.32 | 0.13 | −0.03 | 0.32 * | 0.37 *** | 0.50 *** | 0.35 | −0.09 | −0.06 | 0.03 | 0.07 | 0.60 *** | 0.55 *** | |
| SWC | P. pinaster | 0.11 | 0.29 * | 0.41 ** | 0.41 *** | 0.33 ** | 0.48 *** | 0.68 *** | 0.74 *** | 0.80 *** | 0.68 *** | 0.54 *** | 0.30 * | 0.20 | 0.78 *** | 0.79 *** |
| P. sylvestris | 0.10 | 0.30 * | 0.41 *** | 0.39 ** | 0.34 ** | 0.46 *** | 0.69 *** | 0.74 *** | 0.84 *** | 0.69 *** | 0.47 *** | 0.24 | 0.15 | 0.80 *** | 0.81 *** | |
| Q. pyrenaica | 0.18 | 0.37 ** | 0.42 ** | 0.39 ** | 0.34 * | 0.46 *** | 0.59 *** | 0.70 *** | 0.77 *** | 0.60 *** | 0.49 *** | 0.37 *** | 0.34 * | 0.73 *** | 0.76 *** |
| Species | Variable | Climate Variable | ΔAICc | Windows Open | Windows Close | p-Value | Coefficient | R2 | pAICc |
|---|---|---|---|---|---|---|---|---|---|
| P. sylvestris | Earlywood width | Tmin | −11.64 | 11 | 63 | 0.000 | 0.088 | 0.247 | 0.035 |
| Tmax | −15.12 | 78 | 211 | 0.000 | −0.097 | 0.299 | 0.005 | ||
| Prec | −28.84 | 13 | 174 | 0.000 | 0.292 | 0.470 | <0.001 | ||
| Latewood width | Tmin | −13.27 | 16 | 77 | 0.000 | 0.119 | 0.272 | 0.023 | |
| Tmax | −20.68 | 177 | 207 | 0.000 | −0.084 | 0.374 | 0.002 | ||
| Prec | −20.09 | 141 | 246 | 0.000 | 0.317 | 0.366 | 0.004 | ||
| P. pinaster | Earlywood width | Tmin | −19.29 | 7 | 64 | 0.000 | 0.128 | 0.356 | <0.001 |
| Tmax | −18.48 | 144 | 211 | 0.000 | −0.091 | 0.345 | 0.003 | ||
| Prec | −41.02 | 275 (t − 1) | 180 | 0.000 | 0.521 | 0.587 | <0.001 | ||
| Latewood width | Tmin | −16.19 | 15 | 77 | 0.000 | 0.124 | 0.314 | 0.008 | |
| Tmax | −8.66 | 177 | 209 | 0.001 | −0.061 | 0.200 | 0.140 | ||
| Prec | −29.73 | 179 | 262 | 0.000 | 0.345 | 0.480 | <0.001 | ||
| Q. pyrenaica | Earlywood width | Tmin | −10.23 | 27 | 57 | 0.001 | 0.055 | 0.167 | 0.105 |
| Tmax | −7.3 | 108 | 160 | 0.003 | −0.049 | 0.640 | 0.394 | ||
| Prec | −6.65 | 187 | 229 | 0.004 | 0.376 | 0.618 | 0.681 | ||
| Latewood width | Tmin | −8.57 | 105 | 266 | 0.001 | 0.055 | 0.724 | 0.160 | |
| Tmax | −9.12 | 162 | 205 | 0.001 | −0.049 | 0.175 | 0.097 | ||
| Prec | −26.34 | 140 | 179 | 0.000 | 0.148 | 0.384 | 0.001 |
| Historical | RCP 4.5 | RCP 8.5 | |||
|---|---|---|---|---|---|
| 1970–1999 | 2030–2069 | 2070–2099 | 2030–2069 | 2070–2099 | |
| Q. pyrenaica | 4.95 | 3.33 | 4.09 | 5.02 | 5.32 |
| P. pinaster | 8.78 | 5.78 | 8.78 | 8.67 | 10.49 |
| P. sylvestris | 4.58 | 3.01 | 3.77 | 4.49 | 4.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camarero, J.J.; Campelo, F.; Revilla de Lucas, J.; Colangelo, M.; Rubio-Cuadrado, Á. Stem Heating Enhances Growth but Reduces Earlywood Lumen Size in Two Pine Species and a Ring-Porous Oak. Forests 2025, 16, 1080. https://doi.org/10.3390/f16071080
Camarero JJ, Campelo F, Revilla de Lucas J, Colangelo M, Rubio-Cuadrado Á. Stem Heating Enhances Growth but Reduces Earlywood Lumen Size in Two Pine Species and a Ring-Porous Oak. Forests. 2025; 16(7):1080. https://doi.org/10.3390/f16071080
Chicago/Turabian StyleCamarero, J. Julio, Filipe Campelo, Jesús Revilla de Lucas, Michele Colangelo, and Álvaro Rubio-Cuadrado. 2025. "Stem Heating Enhances Growth but Reduces Earlywood Lumen Size in Two Pine Species and a Ring-Porous Oak" Forests 16, no. 7: 1080. https://doi.org/10.3390/f16071080
APA StyleCamarero, J. J., Campelo, F., Revilla de Lucas, J., Colangelo, M., & Rubio-Cuadrado, Á. (2025). Stem Heating Enhances Growth but Reduces Earlywood Lumen Size in Two Pine Species and a Ring-Porous Oak. Forests, 16(7), 1080. https://doi.org/10.3390/f16071080









