The Habitats of European Oak (Quercus) in Poland and General Oak Wood Color Issues
Abstract
1. Introduction
2. Materials and Methods
- Collaborative scope definition: The review framework was defined jointly by forestry researchers and veneer manufacturers. This ensured alignment between scientific investigation and industry needs, such as improving raw material classification, minimizing waste due to color mismatch, and enhancing surface appearance in high-end veneer products.
- Literature screening and selection: Sources were screened to exclude duplicates, non-peer-reviewed material, and studies not relevant to forest habitat, oak species characteristics, or wood color. Special attention was given to studies focused on Polish and Central European forest conditions, which reflect the primary sourcing regions for many veneer producers.
- Categorization into thematic areas: The selected literature was grouped into five primary themes, developed with direct input from industry experts:
- Forest habitat types and site conditions;
- Species-specific traits of Quercus robur and Quercus petraea;
- Abiotic factors (e.g., soil, climate, light exposure);
- Biotic influences (e.g., fungi, pathogens, insects);
- Industrial and technological processes (e.g., thermal modification, drying, storage methods).
- Integration of forest management data: National datasets from the Polish Forest Data Bank, State Forests, and Forest Management Plans were incorporated to contextualize habitat variation, forest typology, and timber availability. These ecological data were cross-referenced with veneer production practices to identify key factors influencing raw material consistency.
- Industry validation and application: Findings were reviewed internally and double checked in veneer industry environment (Balti Spoon and Lubelski Fornir) to evaluate real-world implications for material sorting, color grading, and production efficiency. This input helped refine the interpretation of ecological data in relation to challenges encountered during procurement and processing, including seasonal variability, storage practices, and end-user expectations for surface appearance.
3. Results and Discussion
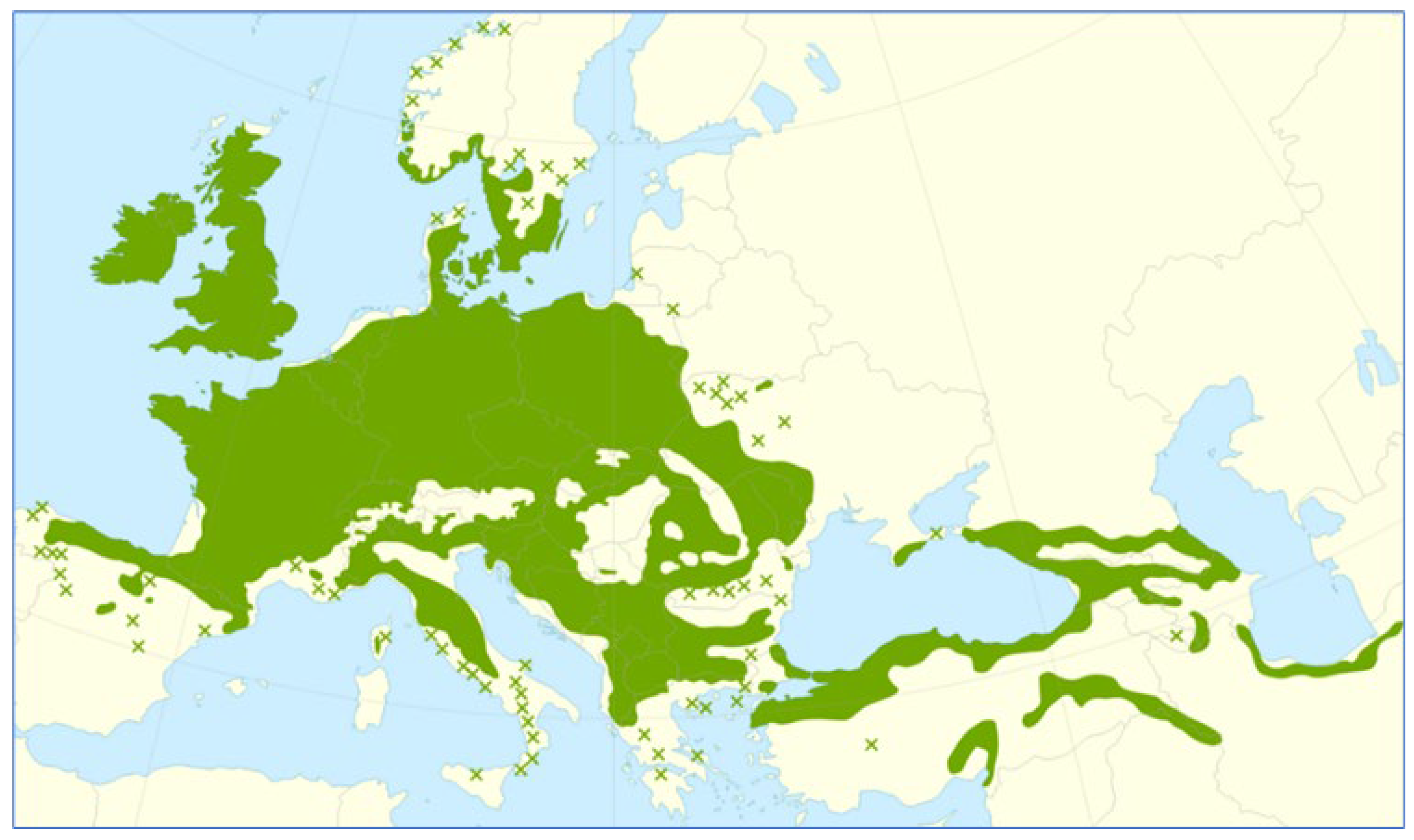
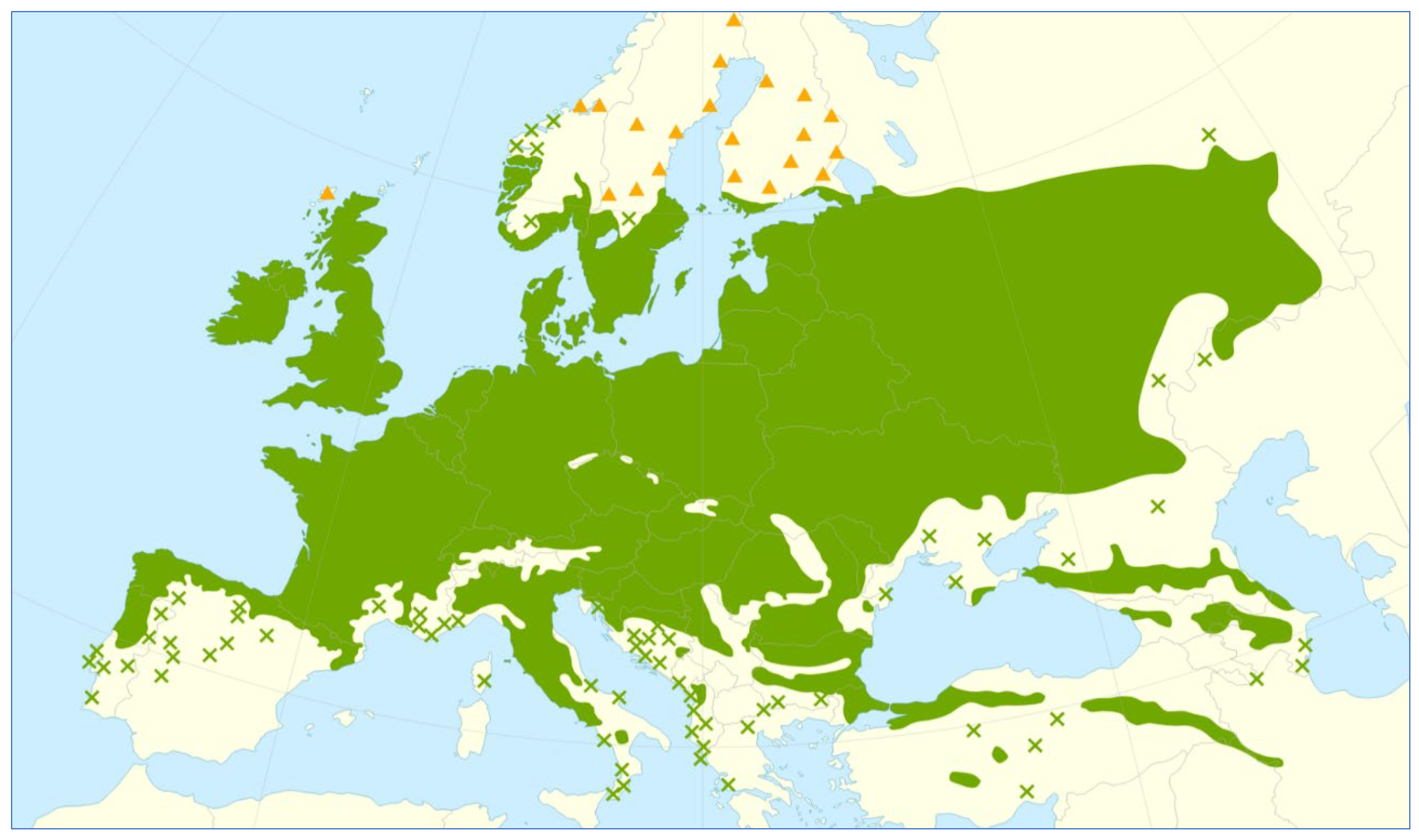
3.1. Oak Wood Habitats in Poland
- -
- Quercus spp. including Q. alba L and other species;
- -
- Quercus spp. including Q. rubra L. and other species;
- -
- Quercus spp., especially Q. mongolica Fisch. ex Turcz. var. Grosseserrata Rehd. and Wils.
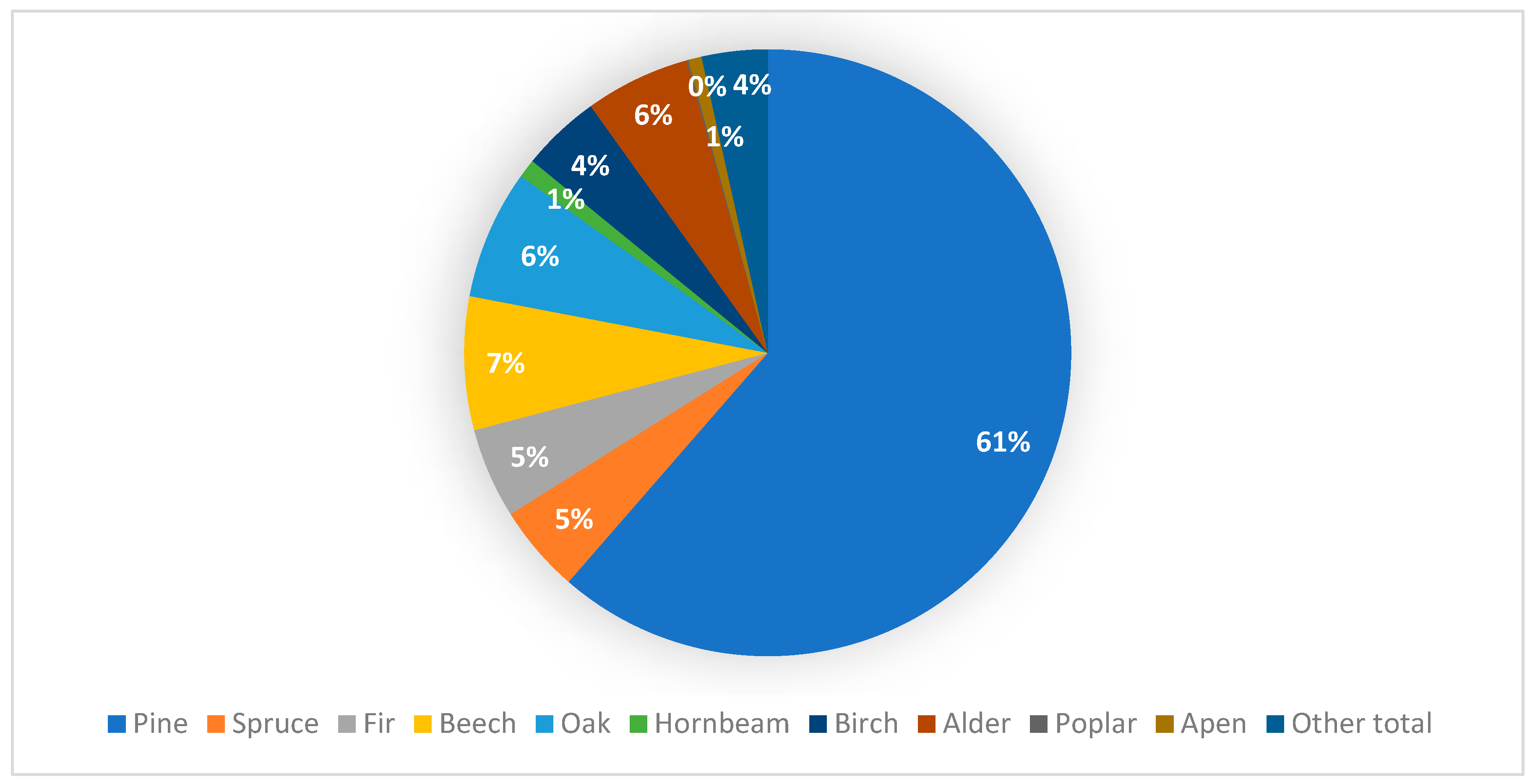
3.2. Forest Management and Changes in Oak Wood
3.3. Changes in Wood Coloration
| Group of Factors | Name | Impact |
|---|---|---|
| Fungi | Aureobasidium pullulans |
|
| Dothichiza pithyophila | ||
| Aspergillus Niger | ||
| Penicillium spp. | ||
| Trichoderma spp. | ||
| Bacteria | Brenneria goodwinii Gibbsiella quercinecans, |
|
| Insects | Coleoptera (beetle) |
|
| Blattodea (cockroaches and termites) |
| |
| Hymenoptera (ants, bees, wasps) |
| |
| Diptera, Ephemeroptera, Lepidoptera |
|
3.4. Production-Driven Factors Determining the Change of Wood Color
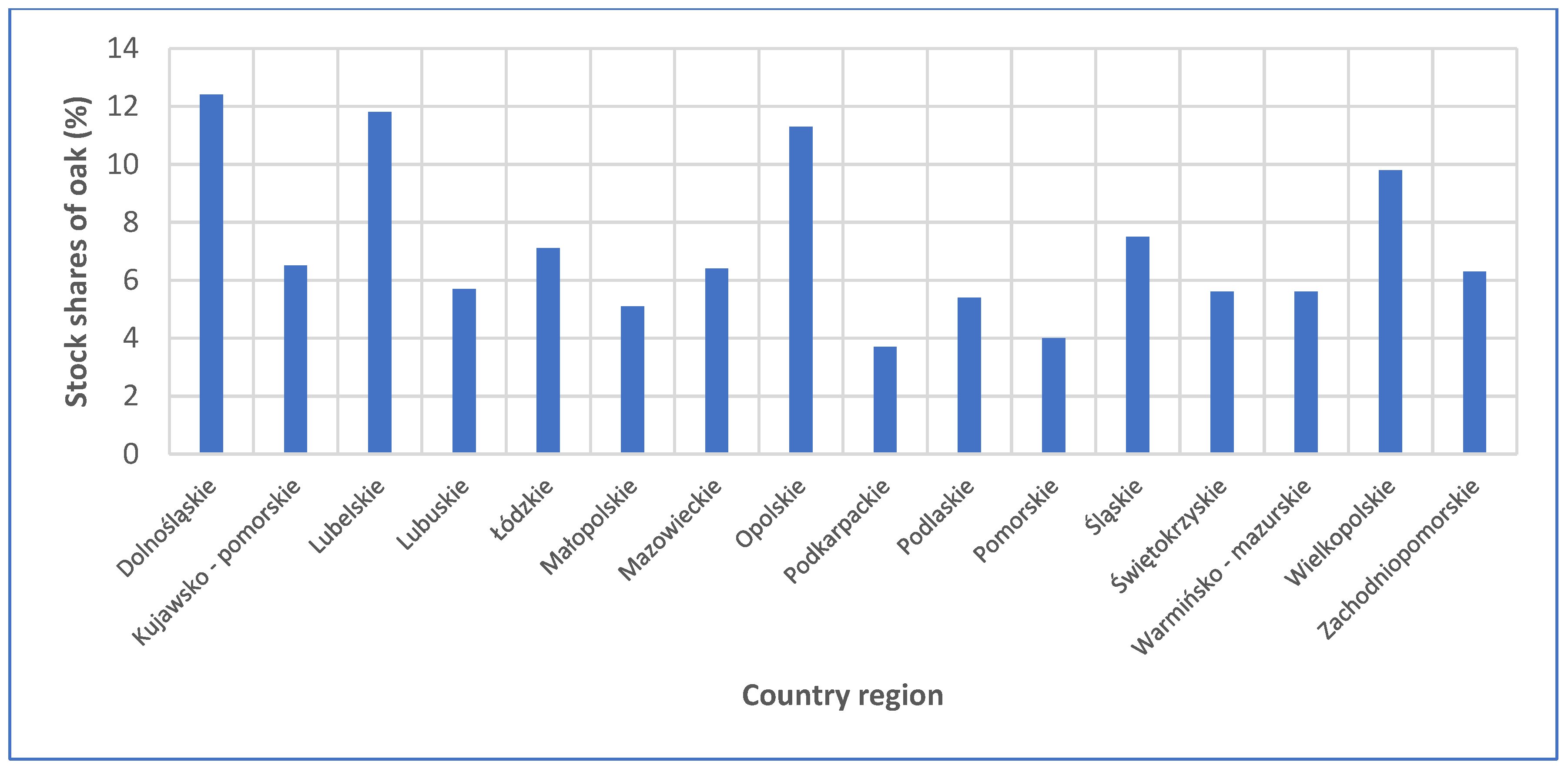
3.5. Correlation of Habitat with Wood Color
4. Conclusions
- Clarify habitat–color relationships using colorimetric and environmental data;
- Examine species-specific responses to drying and modification methods;
- Explore how genetics and site conditions jointly shape wood color;
- Validate findings in real-world veneer and furniture production.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eaton, E.G.S.D.J.; Caudullo, G.; Oliveira, S.; De Rigo, D. Quercus robur and Quercus petraea in Europe: Distribution, Habitat, Usage and Threats. European Atlas of Forest Tree Species; Publications Office of the EU: Luxembourg, 2016; pp. 160–163. [Google Scholar]
- EN 13556:2004; Round and Sawn Timber–Nomenclature of Timbers Used in Europe. CEN Brussels: Bruxelles, Brussels, 2004.
- PN-74/D-95011; Drewno okleinowe. Polski Komitet Normalizacji, Miar i Jakości w dniu26.10.1974. Polski Komitet Normalizacyjny: Warszawa, Poland, 1974.
- PN−EN 1316−2013; Drewno okrągłe liściaste: Klasyfikacja jakościowa. Cz. 1: Dąb i buk. Polski Komitet Normalizacyjny: Warszawa, Poland, 2013.
- Ouden, J.D.; Jansen, P.A.; Smit, R. Jays, mice and oaks: Predation and dispersal of Quercus robur and Q. petraea in North-western Europe. Seed fate: Predation, dispersal and seedling establishment. CABI Digit. Libr. 2005, 223–239. [Google Scholar] [CrossRef]
- Bobiec, A.; Reif, A.; Öllerer, K. Seeing the oakscape beyond the forest: A landscape approach to the oak regeneration in Europe. Landscape Ecol. 2018, 33, 513–528. [Google Scholar] [CrossRef]
- Berthold, D.; Meinlschmidt, P.; Ritter, N. Hardwood processing in Germany–Challenges and opportunities for the wood based panel industry. In Proceedings of the 6th International Scientific Conference on Hardwood Processing; Natural Resources Institute Finland: Helsinki, Finland, 2017; pp. 97–108. [Google Scholar]
- Cassens, D.L. Factors determining the suitability of trees and logs for the face veneer industry. In Proceedings of the 14th Central Hardwood Forest Conference, Wooster, OH, USA, 16–19 March 2004; Yaussy, D.A., Hix, D.M., Long, R.P., Goebel, P.C., Eds.; US Department of Agriculture, Forest Service, Northeastern Research Station: Newtown Square, PA, USA, 2004; pp. 130–139. [Google Scholar]
- Cao, S.; Cheng, S.; Cai, J. Research progress and prospects of wood high-temperature heat treatment technology. BioResources 2022, 17, 3702. [Google Scholar] [CrossRef]
- Reh, R.; Kristak, L.; Kral, P.; Pipiska, T.; Jopek, M. Perspectives on Using Alder, Larch, and Birch Wood Species to Maintain the Increasing Particleboard Production Flow. Polymers 2024, 16, 1532. [Google Scholar] [CrossRef] [PubMed]
- Salca, E.A. Black Alder (Alnus glutinosa L.)—A Resource for Value-Added Products in Furniture Industry Under European Screening. Curr. For. Rep. 2019, 5, 41–54. [Google Scholar] [CrossRef]
- Yazaki, Y. Wood colors and their coloring matters: A review. Nat. Prod. Commun. 2015, 10, 505–512. [Google Scholar] [CrossRef]
- Kamperidou, V.; Aidinidis, E.; Barboutis, I. Impact of Structural Defects on the Surface Quality of Hardwood Species Sliced Veneers. Appl. Sci. 2020, 10, 6265. [Google Scholar] [CrossRef]
- Scalbert, A.; Monties, B.; Dupouey, J.L.; Becker, M. Polyphenols Extractibles du Bois de Chene: Variabilite Interspecifique, Interindividuelle et Effet de la Duraminisation [Quercus robur, Quercus Petraea, Quercus Rubra]; Groupe Polyphenols Bulletin de Liaison no. 13.: Montpellier, France, 1986. [Google Scholar]
- Janin, G.; Mazet, J.F.; Flot, J.L.; Hofmann, P. Couleur et qualité du bois de chêne de tranchage: Chêne sessile, Chêne pédonculé et Chêne rouge. Rev. For. Française 1990, 42, 134–139. [Google Scholar] [CrossRef]
- Klumpers, J.; Janin, G.; Becker, M.; Lévy, G. The influences of age, extractive content and soil water on wood color in oak: The possible genetic determination of wood color. Ann. Des Sci. For. 1993, 50 (Suppl. S1), 403s–409s. [Google Scholar] [CrossRef]
- Zahri, S.; Belloncle, C.; Charrier, F.; Pardon, P.; Quideau, S.; Charrier, B. UV light impact on ellagitannins and wood surface colour of European oak (Quercus petraea and Quercus robur). Appl. Surf. Sci. 2007, 253, 4985–4989. [Google Scholar] [CrossRef]
- Available online: https://www.webofscience.com/WOS (accessed on 12 January 2025).
- Available online: https://scholar.google.com/ (accessed on 12 January 2025).
- Available online: https://www.scopus.com/ (accessed on 12 January 2025).
- Konatowska, M.; Młynarczyk, A.; Rutkowski, P.; Kujawa, K. Impact of Site Conditions on Quercus robur and Quercus petraea Growth and Distribution Under Global Climate Change. Remote Sens. 2024, 16, 4094. [Google Scholar] [CrossRef]
- Szymanski, S. Siedlisko jako podstawa planowania hodowlanego. Sylwan 1985, 129, 10–11. [Google Scholar]
- Kleinschmit, J.R.G.; Bacilieri, R.; Kremer, A.; Roloff, O. Comparison of Morphological and Genetic Traits of Pedunculate Oak (Q. robur L.) and Sessile Oak (Q. petraea (MATT.) Liebl.). Silvae Genet. 1995, 44, 5–6. [Google Scholar]
- Kowalkowski, A. Rola gleboznawstwa i geologii w typologicznej analizie lasu. Sylwan 1999, 143, 95–117. [Google Scholar]
- Andrzejczyk, T.; Sewerniak., P. Gleby i siedliska drzewostanów nasiennych dębu szypułkowego (Quercus robur) i dębu bezszypułkowego (Q. petraea) w Polsce. Sylwan 2016, 160, 674–683. [Google Scholar] [CrossRef]
- Ducousso, A.; Bordacs, S. EUFORGEN Technical Guidelines for Genetic Conservation and Use for Pedunculate and Sessile Oaks (Quercus Robur and Q. Petraea); International Plant Genetic Resources Institute: Rome, Italy, 2004. [Google Scholar]
- Caudullo, G.; Welk, E.; San-Miguel-Ayanz, J. Chorological maps for the main European woody species. Data Brief 2017, 12, 662–666. [Google Scholar] [CrossRef]
- Paschalis-Jakubowicz, P.; Kulik, P.; Lachowicz, I.H. Potential volume of the highest quality timber in Poland. Sylwan 2015, 159, 188–200. [Google Scholar] [CrossRef]
- Mirski, R.; Malinowski, Z.; Wieruszewski, M. Quality and value analysis of oak wood in the submission sale of valuable wood in the Regional Directorate of the State Forests in Katowice. Sylwan 2020, 164, 467–473. [Google Scholar] [CrossRef]
- Malinowski, Z.; Lis, W.; Wieruszewski, M. Submisje jako kierunek dystrybucji cennego surowca drzewnego. Sylwan 2016, 160, 531–538. Available online: http://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-3c19bc05-e440-47a4-85ef-3220eccfaed9 (accessed on 12 January 2025).
- Vieitez, A.M.; Corredoira, E.; Martínez, M.T.; San-José, M.C.; Sánchez, C.; Valladares, S.; Ballester, A. Application of biotechnological tools to Quercus improvement. Eur. J. For. Res. 2011, 131, 519–539. [Google Scholar] [CrossRef]
- Rozkrut, D. Rocznik Statystyczny Leśnictwa 2024. Główny Urząd Statystyczny. (Statistical Yearbook of Forestry 2024); Central Statistical Office Urząd Statystyczny w Białymstoku: Warszawa, Białystok, 2024. [Google Scholar]
- Państwowe, L. Instrukcja Urządzania Lasu—Część I. Dostęp 2 Listopad 2024. Available online: https://www.lasy.gov.pl/pl/publikacje/copy_of_gospodarka-lesna/urzadzanie/iul/instrukcja-urzadzenia-lasu-2024/instrukcja-urzadzania-lasu-czesc-i.pdf/view (accessed on 7 January 2025).
- Bank Danych o Lasach. (Forest Data Bank). Available online: https://www.bdl.lasy.gov.pl/portal/tworzenie-zestawienia-rup (accessed on 7 January 2025).
- Wieruszewski, M.; Mydlarz, K. The Influence of Habitat Conditions on the Properties of Pinewood. Forests 2021, 12, 1311. [Google Scholar] [CrossRef]
- Lasota, J.; Zwydak, M.; Zwydak, M.; Wanic, T.; Brożek, S. Soil diversity of mixed coniferous forest communities. Soil Sci. Annu. 2011, 62, 54–72. [Google Scholar]
- Matuszkiewicz, J.M. Zespoły Leśne Polski; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2001. [Google Scholar]
- Wyniki Monitoringu Siedlisk z lat 2016-2018—Monitoring Gatunków i Siedlisk Przyrodniczych. Available online: https://siedliska.gios.gov.pl/wyniki-monitoringu-menu/2015-2018/szczegolowe-wyniki-dla-siedlisk-przyrodniczych (accessed on 7 January 2025).
- Regionalna Dyrekcja Ochrony Środowiska we Wrocławiu. Plan Zadań Ochronnych dla obszaru Natura 2000 Dąbrowy Janikowskie PLH020089—Regionalna Dyrekcja Ochrony Środowiska we Wrocławiu—Portal Gov.pl. Prezentacja Wyników Inwentaryzacji Przyrodniczej z zakresu Ekspertyzy Fitosocjologicznej. Available online: https://www.gov.pl/web/rdos-wroclaw/plan-zadan-ochronnych-dla-obszaru-natura-2000-dabrowy-janikowskie-plh020089 (accessed on 7 January 2025).
- Przewodniki Metodyczne: Siedliska Przyrodnicze—Monitoring Gatunków i Siedlisk Przyrodniczych. Biblioteka Monitoringu Środowiska Warszawa 2012. Available online: https://siedliska.gios.gov.pl/publikacje-menu/przewodniki-metodyczne/dla-siedlisk-przyrodniczych. (accessed on 7 January 2025).
- Oszako, T. Causes of oak stand decline. Sylwan 2007, 151, 62–72. [Google Scholar] [CrossRef]
- Kuźminski, R.; Łakomy, P.; Mazur, A. Zamieranie dębów—historia, Przyczyny i Objawy. Zarządzanie Ochroną Przyrody w Lasach 2007, [01]. Available online: http://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-b444f684-f398-4d01-902d-f9761b49efc5 (accessed on 7 January 2025).
- After the Oaks—Dieback of oak Trees on the Krotoszyn Plateau. Available online: https://www.drewno.pl/artykuly/122,po-debach-zamieranie-debow-na-plycie-krotoszynskiej.html (accessed on 7 January 2025).
- Państwowe, L. Instrukcja Urządzania Lasu—część II. Dostęp 11 Styczeń 2025. Available online: https://www.lasy.gov.pl/pl/publikacje/copy_of_gospodarka-lesna/urzadzanie/iul/instrukcja-urzadzenia-lasu-2024/instrukcja-urzadzania-lasu-czesc-ii.pdf/view (accessed on 7 January 2025).
- Marciniak, P.; Radomska, N. Submisja jako forma sprzedaży drewna cennego na terenie RDLP w Gdańsku. Zarządzanie Ochr. Przyr. W Lasach 2022, XIV, 107–124. [Google Scholar] [CrossRef]
- Zastocki, D.; Dobosz, L.; Moskalik, T.; Sadowski, J. Submission sale of valuable wood on the example of the Krosno Regional Directorate of the State Forests. Sylwan 2012, 156, 483–493. [Google Scholar] [CrossRef]
- Zastocki, D.; Oktaba, J.; Lachowicz, H. Changes in the Market of Precious Wood: A Case Study of Submission System in Poland. Forests 2021, 12, 421. [Google Scholar] [CrossRef]
- Directive 2009/147/EC of the European Parliament and of the Council of 30 November 2009 on the Conservation of Wild Birds. Available online: http://data.europa.eu/eli/dir/2009/147/oj (accessed on 7 January 2025).
- Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora. Available online: http://data.europa.eu/eli/dir/1992/43/oj (accessed on 7 January 2025).
- The Natura 2000 Protected Areas Network. Available online: https://www.eea.europa.eu/themes/biodiversity/natura-2000/the-natura-2000-protected-areas-network (accessed on 7 January 2025).
- Wyniki Monitoringu Siedlisk z lat 2006-2008—Monitoring Gatunków i Siedlisk Przyrodniczych. Available online: https://siedliska.gios.gov.pl/wyniki-monitoringu-menu/2006-2008/szczegolowe-wyniki-dla-siedlisk-przyrodniczych#:~:text=W%20latach%202006-2008%20monitoringiem%20obj%C4%99to%2020%20typ%C3%B3w%20siedlisk,oko%C5%82o%20170%20specjalnych%20obszarach%20ochrony%20siedlisk%20Natura%202000 (accessed on 7 January 2025).
- Wyniki Monitoringu Siedlisk z roku 2021—Monitoring Gatunków i Siedlisk Przyrodniczych. Available online: https://siedliska.gios.gov.pl/wyniki-monitoringu-menu/2020-2021/wyniki-monitoringu-siedlisk. (accessed on 7 January 2025).
- EU Biodiversity Strategy for 2030. Available online: https://www.eea.europa.eu/policy-documents/eu-biodiversity-strategy-for-2030-1 (accessed on 7 January 2025).
- Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. EU Biodiversity Strategy 2030 Bringing Nature Back into our Lives 2020. Available online: https://eur-lex.europa.eu/legal-content/PL/TXT/?uri=celex%3A52020DC0380 (accessed on 7 January 2025).
- Kropat, M.; Hubbe, M.A.; Laleicke, F. Natural, Accelerated, and Simulated Weathering of Wood: A Review. BioResources 2020, 15, 9998–10062. [Google Scholar] [CrossRef]
- Barcík, Š.; Gašparík, M.; Razumov, E.Y. Effect of temperature on the color changes of wood during thermal modification. Cellul. Chem. Technol 2015, 49, 789–798. [Google Scholar] [CrossRef]
- Barcík, Š.; Gašparík, M.; Razumov, E.Y. Effect of thermal modification on the colour changes of oak wood. Wood Res. 2015, 60, 385–396. [Google Scholar]
- Rozanska, A.N.N.A.; Sokolek, A.; Barski, A. Influence of traditional wood surface modification methods on changes in aesthetic and resistance properties. Ann. Wars. Univ. Life Sci. SGGW. For. Wood Technol. 2021, 113, 74–88. [Google Scholar] [CrossRef]
- Jirouš-Rajković, V.; Miklečić, J. Enhancing Weathering Resistance of Wood—A Review. Polymers 2021, 13, 1980. [Google Scholar] [CrossRef]
- George, B.; Suttie, E.; Merlin, A.; Deglise, X. Photodegradation and photostabilisation of wood—The state of the art. Polym. Degrad. Stab. 2005, 88, 268–274. [Google Scholar] [CrossRef]
- Bailleres, H.; Nepveu, G.; Charrier, B. Quantitative assessment of total phenol contents of European oak (Quercus petraea and Quercus robur) by diffuse reflectance NIR spectroscopy on solid wood surfaces. Holzforschung 2008, 62, 679–687. [Google Scholar] [CrossRef]
- Tomak, E.D.; Ermeydan, M.A.; Can, A.; Aydın, M.A. A comparative study on photodegradation of twenty-three wood species after UV irradiation. Radiat. Phys. Chem. 2024, 223, 111986. [Google Scholar] [CrossRef]
- Valášková, V.; Šnajdr, J.; Bittner, B.; Cajthaml, T.; Merhautová, V.; Hofrichter, M.; Baldrian, P. Production of lignocellulose-degrading enzymes and degradation of leaf litter by saprotrophic basidiomycetes isolated from a Quercus petraea forest. Soil Biol. Biochem. 2007, 39, 2651–2660. [Google Scholar] [CrossRef]
- Simpson, W.T. Drying Wood: A Review—Part II. Dry. Technol. 1983, 2, 353–368. [Google Scholar] [CrossRef]
- Kržišnik, D.; Lesar, B.; Thaler, N.; Humar, M. Influence of Natural and Artificial Weathering on the Colour Change of Different Wood and Wood-Based Materials. Forests 2018, 9, 488. [Google Scholar] [CrossRef]
- Lyubenova, A.; Baranowska, M.; Menkis, A.; Davydenko, K.; Nowakowska, J.; Borowik, P.; Oszako, T. Prospects for Oak Cultivation in Europe Under Changing Environmental Conditions and Increasing Pressure from Harmful Organisms. Forests 2024, 15, 2164. [Google Scholar] [CrossRef]
- Cohan, F.M. Tracking bacterial responses to global warming with an ecotype-based systematics. Clin. Microbiol. Infect. 2009, 15, 54–59. [Google Scholar] [CrossRef]
- Mieszkin, S.; Richet, P.; Bach, C.; Lambrot, C.; Augusto, L.; Buée, M.; Uroz, S. Oak decaying wood harbors taxonomically and functionally different bacterial communities in sapwood and heartwood. Soil Biol. Biochem. 2021, 155, 108160. [Google Scholar] [CrossRef]
- Shigo, A.L. Successions of Microorganisms and Patterns of Discoloration and Decay after Wounding in Red Oak and White Oak. Phytopathology 1972, 62, 256–259. [Google Scholar] [CrossRef]
- Kielak, A.M.; Barreto, C.C.; Kowalchuk, G.A.; Van Veen, J.A.; Kuramae, E.E. The ecology of Acidobacteria: Moving beyond genes and genomes. Front. Microbiol. 2016, 7, 744. [Google Scholar] [CrossRef]
- Rinta-Kanto, J.M.; Sinkko, H.; Rajala, T.; Al-Soud, W.A.; Sørensen, S.J.; Tamminen, M.V.; Timonen, S. Natural decay process affects the abundance and community structure of Bacteria and Archaea in Picea abies logs. FEMS Microbiol. Ecol. 2016, 92, fiw087. [Google Scholar] [CrossRef] [PubMed]
- Prewitt, L.; Kang, Y.; Kakumanu, M.L.; Williams, M. Fungal and Bacterial Community Succession Differs for Three Wood Types during Decay in a Forest Soil. Microb. Ecol. 2014, 68, 212–221. [Google Scholar] [CrossRef]
- Moll, J.; Kellner, H.; Leonhardt, S.; Stengel, E.; Dahl, A.B.; Assler, C.; Buscot, F.; Hofrichter, M.; Hoppe, B. Bacteria inhabiting deadwood of 13 tree species are heterogeneously distributed between sapwood and heartwood. Environ. Microbiol. 2018, 20, 3744–3756. [Google Scholar] [CrossRef]
- Hoppe, B.; Krüger, D.; Kahl, T.; Arnstadt, T.; Buscot, F.; Bauhus, J.; Wubet, T. A pyrosequencing insight into sprawling bacterial diversity and community dynamics in decaying deadwood logs of Fagus sylvatica and Picea abies. Sci. Rep. 2015, 5, 9456. [Google Scholar] [CrossRef]
- Nicolitch, O.; Colin, Y.; Turpault, M.P.; Fauchery, L.; Uroz, S. Tree roots select specific bacterial communities in the subsurface critical zone. Soil Biol. Biochem. 2017, 115, 109–123. [Google Scholar] [CrossRef]
- Onet, A.; Grenni, P.; Onet, C.; Stoian, V.; Crisan, V. Forest Soil Microbiomes: A Review of Key Research from 2003 to 2023. Forests 2025, 16, 148. [Google Scholar] [CrossRef]
- Lladó, S.; Žifčáková, L.; Větrovský, T.; Eichlerová, I.; Baldrian, P. Functional screening of abundant bacteria from acidic forest soil indicates the metabolic potential of Acidobacteria subdivision 1 for polysaccharide decomposition. Biol. Fertil. Soils 2016, 52, 251–260. [Google Scholar] [CrossRef]
- Nicolitch, O.; Colin, Y.; Turpault, M.P.; Uroz, S. Soil type determines the distribution of nutrient mobilizing bacterial communities in the rhizosphere of beech trees. Soil Biol. Biochem. 2016, 103, 429–445. [Google Scholar] [CrossRef]
- Hervé, V.; Ketter, E.; Pierrat, J.C.; Gelhaye, E.; Frey-Klett, P. Impact of Phanerochaete chrysosporium on the Functional Diversity of Bacterial Communities Associated with Decaying Wood. PLoS ONE 2016, 11, e0147100. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.; Woodward, S. Temporal changes in functional diversity of culturable populations in Sitka spruce stumps. For. Pathol. 2007, 37, 217–235. [Google Scholar] [CrossRef]
- Vala skova, V.; De Boer, W.; Gunnewiek, P.J.K.; Pospisek, M.; Baldrian, P. Phylogenetic composition and properties of bacteria coexisting with the fungus Hypholoma fasciculare in decaying wood. ISME J. 2009, 3, 1218. [Google Scholar] [CrossRef]
- Vorob’ev, A.V.; de Boer, W.; Folman, L.B.; Bodelier, P.L.; Doronina, N.V.; Suzina, N.E.; Trotsenko, Y.A.; Dedysh, S.N. Methylovirgula ligni gen. nov., sp. nov., an obligately acidophilic, facultatively methylotrophic bacterium with a highly divergent mxaF gene. Int. J. Syst. Evol. Microbiol. 2009, 59, 2538–2545. [Google Scholar] [CrossRef]
- Pournou, A. Wood Deterioration by Insects. In Biodeterioration of Wooden Cultural Heritage: Organisms and Decay Mechanisms in Aquatic and Terrestrial Ecosystems; Anastasia Pournou, A., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 425–526. [Google Scholar] [CrossRef]
- Vidholdova, Z.; Slabejová, G.; Kaloč, J. Influence of wood pre-weathering on selected surface properties of the system wood–coating film. Acta Fac. Xylologiae Zvolen Res. Publica Slovaca 2017, 59, 67–77. [Google Scholar] [CrossRef]
- Tarocinski, E. Protection of valuable leafy trees’ wood assortments in forest and wood stores. Sylwan 1961, 105920, 57–66. [Google Scholar]
- Maleszewski, S. Utilization of raw material reserves for veener production. Sylwan 1958, 102, 75–85. Available online: http://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-1e82085a-9054-4a9f-9679-eded27490c57 (accessed on 12 January 2025).
- Bukara, B.; Milić, G. Development of discolouration during conventional drying of oak timber. Wood Technol. Prod. Des. 2024, 13, 187. [Google Scholar]
- Sandoval-Torres, S.; Jomaa, W.; Marc, F.; Puiggali, J.-R. Colour alteration and chemistry changes in oak wood (Quercus pedunculata Ehrh) during plain vacuum drying. Wood Sci. Technol. 2010, 46, 177–191. [Google Scholar] [CrossRef]
- Charrier, B.; Haluk, J.P.; Metche, I.M. Characterization of European Oakwood Constituents Acting in the Brown Discolouration during Kiln Drying. Holzforschung 1995, 49, 168–172. [Google Scholar] [CrossRef]
- Barański, J.; Konopka, A.; Vilkovska, T.; Klement, I.; Vilkovsky, P. Deformation and Surface Color Changes of Beech and Oak Wood Lamellas Resulting from the Drying Process. BioResources 2020, 15, 8965–8980. [Google Scholar] [CrossRef]
- Tolvaj, L. Optical Properties of Wood: Measurement Methods and Result Evaluations; Springer Nature: Berlin/Heidelberg, Germany, 2023; Volume 45. [Google Scholar]
- Halalisan, F.; Romero, C.; Popa, B.; Landin, G.A.; Talpa, N.; Abrudan, I.V. Global assessment of FSC forest management certification auditing through analysis of accreditation reports. Land Use Policy 2023, 131, 106724. [Google Scholar] [CrossRef]
- Mosedale, J.R.; Charrier, B.; Janin, G. Genetic control of wood colour, density and heartwood ellagitannin concentration in European oak (Quercus petraea and Q. robur). For. Int. J. For. Res. 1996, 69, 111–124. [Google Scholar] [CrossRef]
- Luostarinen, K. Effects of Environmental and Internal Factors of Trees and Timber Treatment on Colour of Dried Birch (Betula pendula) Wood; Dissertationes Florestales; Faculty of Forestry, University of Joensuu: Joensuu, Finland, 2006; p. 19. [Google Scholar] [CrossRef]
- Brunner, C.C.; Shaw, G.B.; Butler, D.A.; Funck, J.W. Using color in machine vision systems for wood processing. Wood Fiber Sci. 1990, 22, 413–428. Available online: https://wfs.swst.org/index.php/wfs/article/view/1536/1536 (accessed on 7 January 2025).
- Hălălișan, A.-F.; Dinulică, F.; Gurean, D.M.; Codrean, C.; Neykov, N.; Antov, P.; Bardarov, N. Wood Colour Variations of Quercus Species in Romania. Forests 2023, 14, 230. [Google Scholar] [CrossRef]
- Vieira, H.C.; da Silva, E.L.; dos Santos, J.X.; de Muñiz, G.I.B.; Morrone, S.R.; Nisgoski, S. Wood colorimetry of native species of Myrtaceae from a Araucaria Forest. Floresta 2019, 49, 353. [Google Scholar] [CrossRef]
- Boardman, B.E.; Senft, J.F.; McCabe, G.P.; Ladisch, C.M. Colorimetric analysis in grading black walnut veneer. Wood Fiber Sci. 1992, 24, 99–107. [Google Scholar]
- Musat, E.C.; Salca, E.A.; Dinulică, F.; Ciobanu, V.; Dumitrascu, A.E. Evaluation of Color Variability of Oak Veneers for Sorting. Bioresources 2016, 11, 573–584. [Google Scholar] [CrossRef]
- Defoirdt, N.; Wuijtens, I.; De Boever, L.; Coppens, H.; Van den Bulcke, J.; Van Acker, J. A colour assessment methodology for oak wood. Ann. For. Sci. 2012, 69, 939–946. [Google Scholar] [CrossRef][Green Version]

| Abiotic | Physical |
|
| Chemical |
| |
| Mechanical |
| |
| Natural | Physical |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolarek, E.; Kowalska, J.; Pałubicki, B.; Wieruszewski, M. The Habitats of European Oak (Quercus) in Poland and General Oak Wood Color Issues. Forests 2025, 16, 1063. https://doi.org/10.3390/f16071063
Smolarek E, Kowalska J, Pałubicki B, Wieruszewski M. The Habitats of European Oak (Quercus) in Poland and General Oak Wood Color Issues. Forests. 2025; 16(7):1063. https://doi.org/10.3390/f16071063
Chicago/Turabian StyleSmolarek, Edmund, Jolanta Kowalska, Bartosz Pałubicki, and Marek Wieruszewski. 2025. "The Habitats of European Oak (Quercus) in Poland and General Oak Wood Color Issues" Forests 16, no. 7: 1063. https://doi.org/10.3390/f16071063
APA StyleSmolarek, E., Kowalska, J., Pałubicki, B., & Wieruszewski, M. (2025). The Habitats of European Oak (Quercus) in Poland and General Oak Wood Color Issues. Forests, 16(7), 1063. https://doi.org/10.3390/f16071063







