Fire Impact on Diversity and Forest Structure of Castanea sativa Mill. Stands in Managed and Oldfield Areas of Tenerife (Canary Islands, Spain)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling Design
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Transect | pH | OM% | P | Ca | Mg | K | Na | EC |
| ppm | meq/100 g | |||||||
| P1CA | 6.15 | 6.32 | 23.30 | 7.33 | 4.91 | 0.91 | 0.95 | 0.141 |
| P2CA | 5.81 | 5.45 | 30.41 | 4.76 | 3.22 | 0.66 | 0.88 | 0.137 |
| P3CA | 6.26 | 6.05 | 38.71 | 13.44 | 8.42 | 1.33 | 1.20 | 0.167 |
| P1CE | 5.25 | 5.25 | 52.94 | 6.13 | 1.29 | 1.15 | 0.65 | 0.273 |
| P2CE | 6.31 | 4.68 | 36.93 | 8.98 | 4.98 | 3.69 | 0.92 | 0.137 |
| P3CE | 5.09 | 5.75 | 58.27 | 3.31 | 0.80 | 1.47 | 0.61 | 0.229 |
| P1QA | 6.63 | 6.18 | 27.45 | 12.98 | 8.59 | 0.93 | 0.90 | 0.142 |
| P2QA | 6.52 | 5.88 | 27.45 | 12.94 | 7.33 | 0.78 | 0.85 | 0.152 |
| P3QA | 6.28 | 5.37 | 50.57 | 9.25 | 6.67 | 0.55 | 0.84 | 0.113 |
| P1QE | 5.95 | 4.60 | 26.26 | 5.13 | 1.51 | 2.25 | 0.69 | 0.174 |
| P2QE | 5.45 | 4.82 | 103.33 | 4.42 | 1.76 | 1.16 | 0.64 | 0.147 |
| P3QE | 6.04 | 5.29 | 35.75 | 10.75 | 6.03 | 2.14 | 0.82 | 0.143 |
Appendix B
| Species | Code | Origin | Endemism |
| Aichryson laxum (Haw.) Bramwell | Aila | NS | * |
| Anethum foeniculum L. | Fovu | ISN | |
| Andryala sp. | Ansp | ||
| Asplenium adiantum-nigrum L. | Asad | NS | |
| Bidens pilosa L. | Bipi | ISN | |
| Brachypodium distachyon (L.) P. Beauv. | Brdi | NP | |
| Brassicaceae | Brsp | ||
| Brachypodium sylvaticum (Huds.) P. Beauv. | Brsy | NP | |
| Castanea sativa Mill. | Casa | ISI | |
| Allium sp. | Allium | ||
| Apium sp. | Apium | ||
| Asphodelus sp. | Asphodelus | ||
| Fumaria sp. | Fumaria | ||
| Geranium sp. | Geranium | ||
| Gladiolus sp. | Gladiolus | ||
| Hypochaeris sp. | Hypochaeris | ||
| Spergula sp. | Spergula | ||
| Chenopodiastrum murale (L.) S. Fuentes, Uotila & Borsch | Chmu | IP | |
| Chenopodium album L. | Chal | NP | |
| Cistus symphytifolius Lam. | Cisy | NS | * |
| Cistus monspeliensis L. | Cymo | NP | |
| Cyperus sp. | Cysp | ||
| Davallia canariensis (L.) Sm. | Daca | NS | |
| Daphne gnidium L. | Dagn | NS | |
| Digitalis canariensis L. | Isca | NS | * |
| Echium plantagineum L. | Ecpl | NP | |
| Erica canariensis Rivas-Mart., M. Osorio & Wildpret | Erar | NS | |
| Erigeron bonariensis L. | Cobo | ISN | |
| Erigeron sumatrensis Retz. | Cosu | ISN | |
| Erigeron sp. | Cosp | ||
| Fabaceae | Fasp | ||
| Galium aparine L. | Gaap | NP | |
| Galium scabrum L. | Gasc | NP | |
| Galactites tomentosus Moench | Gato | NP | |
| Geranium reuteri Aedo & Muñoz Garm. | Gere | NS | * |
| Hypericum grandifolium Choisy | Hygr | NS | |
| Ilex canariensis Poir | Ilca | NS | |
| Laurus novocanariensis Rivas-Mart., Lousa, Fern. Prieto, E. Días, J.C. Costa & C. Aguiar | Lano | NS | * |
| Lathyrus tingitanus L. | Lati | IP | |
| Lathyrus sp. | Lathyrus | ||
| Lupinus sp. | Lusp | ||
| Lysimachia arvensis (L.) U. Manns & Anderb. | Anar | NP | |
| Micromeria sp. | Micromeria | NS | * |
| Morella faya (Aiton) Wilbur | Myfa | NS | |
| Myosotis sp. | Myosotis | ||
| Ornithopus compressus L. | Orco | NP | |
| Origanum vulgare L. | Orvi | NP | |
| Oxalis pes-caprae L. | Oxpe | ISI | |
| Poaceae | Gram | ||
| Polygonum aviculare L. | Poav | NP | |
| Pteridium aquilinum (L.) Kuhn in Von der Decken | Ptaq | NP | |
| Ranunculus cortusifolius Willd. | Raco | NS | |
| Rumex sp. | Rumex | ||
| Rubus ulmifolius Schott | Ruul | NP | |
| Silene vulgaris (Moench) Garcke | Siin | NP | |
| Smilax aspera L. | Smas | NS | |
| Solanum villosum Mill. subsp. miniatum (Bernh. ex Willd.) Edmonds | Soal | NP | |
| Solanum nigrum L. | Soni | NP | |
| Solanum tuberosum L. | Sotu | ISN | |
| Sonchus oleraceus L. | Sool | NP | |
| Spartium junceum L. | Spju | ISI | |
| Stellaria media (L.) Vill. | Stme | IP | |
| Torilis arvensis (Huds.) Link | Toar | NP | |
| Tropaeolum majus L. | Trma | ISI | |
| Urtica stachyoides Webb & Berthel. | Urst | NS | * |
| Vicia disperma DC. | Vidi | NP | |
| Viburnum rugosum Pers. | Viru | NS | * |
| Vicia sp. | Vicia |
References
- Pyne, S.J. Fire: A Brief History; NewSouth Publishing: Sydney, Australia, 2019. [Google Scholar]
- Pickett, S.T.A.; White, P.S. The Ecology of Natural Disturbance and Patch Dynamics; Academic Press: New York, NY, USA, 1985. [Google Scholar]
- Rowe, N.P.; Jones, T.P. Devonian charcoal. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2000, 164, 347–354. [Google Scholar] [CrossRef]
- Song, Y.; Xu, C.; Li, X.; Oppong, F. Lightning-Induced Wildfires: An Overview. Fire 2024, 7, 79. [Google Scholar] [CrossRef]
- Pausas, J.; Keeley, J.E. Wildfires as an ecosystem service. Front. Ecol. Environ. 2019, 17, 289–295. [Google Scholar] [CrossRef]
- Arévalo, J.R.; Álvarez, P.; Narvaez, N.; Walker, K. The effects of fire in the regeneration of a Quercus douglasii stand in Quail Ridge Reserve, Berryessa Valley (California). J. For. Res. 2009, 14, 81–87. [Google Scholar] [CrossRef]
- Arno, S.F.; Fiedler, C.E. Mimicking Nature’s: Restoring Fire-Prone Forests in the West; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Serra Ràfols, E. Las datas de Tenerife (Libros de I a IV de datos originales); Fontes Rerum Canarium. Colección de textos y documentos para la Historia de Canarias. XII; Consejo Superior de Investigaciones Científicas; Instituto de Estudios Canarios en la Universidad de La Laguna: La Laguna, Spain, 1978; p. 423. [Google Scholar]
- Martín, V. Los Paisajes Agrarios. In Gran Atlas Temático de Canarias; Morales, G., Pérez, R., Eds.; Editorial Insular Canaria: Santa Cruz de Tenerife, Spain, 2000; pp. 87–106. [Google Scholar]
- Hernández, A.; Hernández, J.Z. Veinte años de desarrollo rural endógeno en Canarias: Evolución y retos pendientes. In La Sociología en Canarias (1999–2019); Facultad de Ciencias Sociales y de la Comunicación ULL y Colegio Oficial de Ciencias Políticas y Sociología de Canarias: Santa Cruz de Tenerife, Spain, 2020; pp. 31–50. [Google Scholar]
- Conedera, M.; Lucini, L.; Valese, E.; Ascoli, D.; Pezzatti, G.B. Fire resistance and vegetative recruitment ability of different deciduous tree species after low-to-moderate-intensity surface fires in southern Switzerland. In VI International Conference on Forest Fire Research; ADAI/CEIF University of Coimbra: Coimbra, Portugal, 2010; p. 12. [Google Scholar]
- Saulino, L.; Rita, A.; Stinca, A.; Liuzzi, G.; Silvestro, R.; Rossi, S.; Saracino, A. Wildfire promotes the invasion of Robinia pseudoacacia in the unmanaged Mediterranean Castanea sativa coppice forests. Front. For. Glob. Change 2023, 6, 1177551. [Google Scholar] [CrossRef]
- Del Arco, M.J.; Pérez, P.L.; Rodríguez, O.; Salas, M.; Wildpret, W. Atlas Cartográfico de los Pinares Canarios II: Tenerife; Viceconsejería de Medio Ambiente y Conservación de la Naturaleza, Gobierno de Canarias: Santa Cruz de Tenerife, Spain, 1992. [Google Scholar]
- Arévalo, J.R.; Bernardos, M.; González-Montelongo, C.; Grillo, F. Prescribed Burning Effect on the Richness, Diversity and Forest Structure of an Endemic Reforested Pinus canariensis Stand (Canary Islands). Fire 2023, 6, 150. [Google Scholar] [CrossRef]
- Del Arco, M.; González-González, R.; Garzón-Machado, V.; Pizarro-Hernández, B. Climate change impacts on vegetation in the Canary Islands: A review. Land 2019, 8, 5. [Google Scholar] [CrossRef]
- Fernández-Palacios, J.M.; Arévalo, J.R. Regeneration strategies of tree species in the laurel forest of Tenerife. Plant Ecol. 1998, 137, 21–29. [Google Scholar] [CrossRef]
- Fernández-Caldas, E.; Tejedor, M.; Quantin, P. Los Suelos Volcánicos de Canarias; Servicio de Publicaciones de La Universidad de La Laguna: La Laguna, Spain, 1985. [Google Scholar]
- Ríos-Mesa, D.; Pereira-Lorenzo, S.; González-Díaz, A.J.; González-Díaz, E. Hernández-González, J.Z.; González-Díaz, E.; Galán Saúco, V. The status of Chestnut cultivation and utilization in the Canary Islands. Adv. Hortic. Sci. 2011, 25, 90–98. [Google Scholar] [CrossRef]
- Pereira, S.; Ríos, D.; González, A.J.; Ramos, A.M. Los castañeros de Canarias. In Caracterización Morfológica y Molecular de las Variedades de Tenerife y La Palma; CCBAT–CAP: Canary Islands, Spain, 2007; ISBN 84-87340-80-6. [Google Scholar]
- Ríos-Mesa, D. La Castaña. Cabildo Insular de Tenerife, Área de Aguas, Agricultura, Ganadería y Pesca, Servicio Técnico de Agricultura y Desarrollo Rural. In Cocinando con Castañas de Tenerife; Cabildo Insular de Tenerife: Santa Cruz de Tenerife, Spain, 2004; pp. 11–39. [Google Scholar]
- Plan Forestal de Canarias. ANUNCIO de 7 de julio de 1999. In Proceedings of the por el que se hace público el Acuerdo Adoptado por el Gobierno de la Comunidad Autónoma de Canarias en la sesión de 25 de mayo de 1999, de Aprobación del Plan Forestal de Canarias. BOC n.º 117, Las Palmas de Gran Canaria, Spain, 31 August 1999; pp. 13092–13093. [Google Scholar]
- Real Decreto 1628/2011, de 14 de noviembre, por el que se regula el listado y catálogo español de especies exóticas invasoras. In BOE n.º 298, de 12 de Diciembre de 2011; Referencia: BOE-A-2011-19398; Ministerio de Medio Ambiente, y Medio Rural y Marino: Madrid, Spain, 2011.
- Real Decreto 630/2013, de 2 de agosto, por el que se regula el Catálogo español de especies exóticas invasoras. In BOE n.º 185, de 3 de Agosto de 2013; Referencia: BOE-A-2013-8565; Ministerio de Medio Ambiente, y Medio Rural y Marino: Madrid, Spain, 2011.
- Lemmon, P.E. A new instrument for measuring forest overstory density. J For. 1957, 55, 667–668. [Google Scholar]
- Arévalo, J.R.; Fernández-Palacios, J.M. Treefall gap characteristics and regeneration in the laurel forest of Tenerife. J. Veg. Sci. 2009, 9, 297–306. [Google Scholar] [CrossRef]
- Banco de Datos de Biodiversidad de Canarias. Gobierno de Canarias. Available online: https://www.biodiversidadcanarias.es/biota (accessed on 5 April 2025).
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemist, 15th ed.; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- Anon. Official Methods of Analysis; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 1986; Volume I. [Google Scholar]
- Anderson, M.; Gorley, R.N.; Clarke, R.K. Permanova+ for Primer: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Smith, B.; Wilson, J.B. A consumer’s guide to evenness indices. Oikos 1996, 76, 70–82. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 5 April 2025).
- McCune, B.; Grace, J.B.; Urban, D.L. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002; Volume 28. [Google Scholar]
- De Cáceres, M.; Legendre, P.; Moretti, M. Improving indicator species analysis by combining groups of sites. Oikos 2010, 119, 1674–1684. [Google Scholar] [CrossRef]
- ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 5.1); Microcomputer Power: Ithaca, NY, USA, 2018. [Google Scholar]
- Hill, M.O.; Gauch, H.J. Detrended Correspondence Analysis: An improved ordination technique. Vegetatio 1980, 42, 47–58. [Google Scholar] [CrossRef]
- Valbuena-Carabaña, M.; López de Heredia, U.; Fuentes-Utrilla, P.; González-Doncel, I.; Gil, L. Historical and recent changes in the Spanish forests: A socio-economic process. Rev. Biol. Trop. 2010, 58, 1127–1146. [Google Scholar] [CrossRef]
- Conedera, M.; Krebs, P.; Tinner, W.; Pradella, M.; Torriani, D. The cultivation of Castanea sativa (Mill.) in Europe, from its origin to its diffusion on a continental scale. Veg. Hist. Archaeobotany 2004, 13, 161–179. [Google Scholar] [CrossRef]
- Fernandes, P.M.; Davies, G.M.; Ascoli, D.; Fernández, C.; Moreira, F.; Rigolot, E.; Vega, J.A.; Molina, D. Prescribed burning in southern Europe: Developing fire management in a dynamic landscape. Front. Ecol. Environ. 2013, 11 (Suppl. S1), e4–e14. [Google Scholar] [CrossRef]
- Grime, J.P. Competitive exclusion in herbaceous vegetation. Nature 1973, 242, 344–347. [Google Scholar] [CrossRef]
- Pausas, J.G.; Ribeiro, E.; Vallejo, R. Post-fire regeneration variability of Pinus halepensis in the eastern Iberian Peninsula. For. Ecol. Manag. 2004, 203, 251–259. [Google Scholar] [CrossRef]
- Perring, M.P.; Standish, R.J.; Hobbs, R.J. Incorporating novelty and novel ecosystems into restoration planning and practice in the 21st century. Ecol. Process. 2015, 4, 4. [Google Scholar] [CrossRef]
- Blondel, J. The Mediterranean Region: Biological Diversity in Space and Time; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Santana, J.; Reino, L.; Stoate, C.; Moreira, F.; Ribeiro, P.F.; Beja, P. Combined effects of landscape composition and heterogeneity on farmland avian diversity. Ecol. Evol. 2017, 7, 1212–1223. [Google Scholar] [CrossRef] [PubMed]
- Plieninger, T.; Hui, C.; Gaertner, M.; Huntsinger, L. The impact of land abandonment on species richness and abundance in the Mediterranean Basin: A meta-analysis. PLoS ONE 2014, 9, e98355. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Smith, K.; López-Vertedor, G.; Zhang, L.; Martínez-Fernández, M. Global functional trait responses of graminoids to fire regimes: A meta-analysis. Fire 2023, 6, 329. [Google Scholar]
- Dupouey, J.L.; Dambrine, E.; Laffite, J.D.; Moares, C. Irreversible impact of past land use on forest soils and biodiversity. Ecology 2002, 83, 2978–2984. [Google Scholar] [CrossRef]
- Kobziar, L.N.; Hiers, J.K.; Belcher, C.M.; Bond, W.J.; Enquist, C.A.; Loudermilk, E.L.; O’Brien, J.J.; Pausas, J.G.; Prichard, S.J.; Smith, A.M.S.; et al. Principles of Fire Ecology. Fire Ecol. 2024, 20, 39. [Google Scholar] [CrossRef]
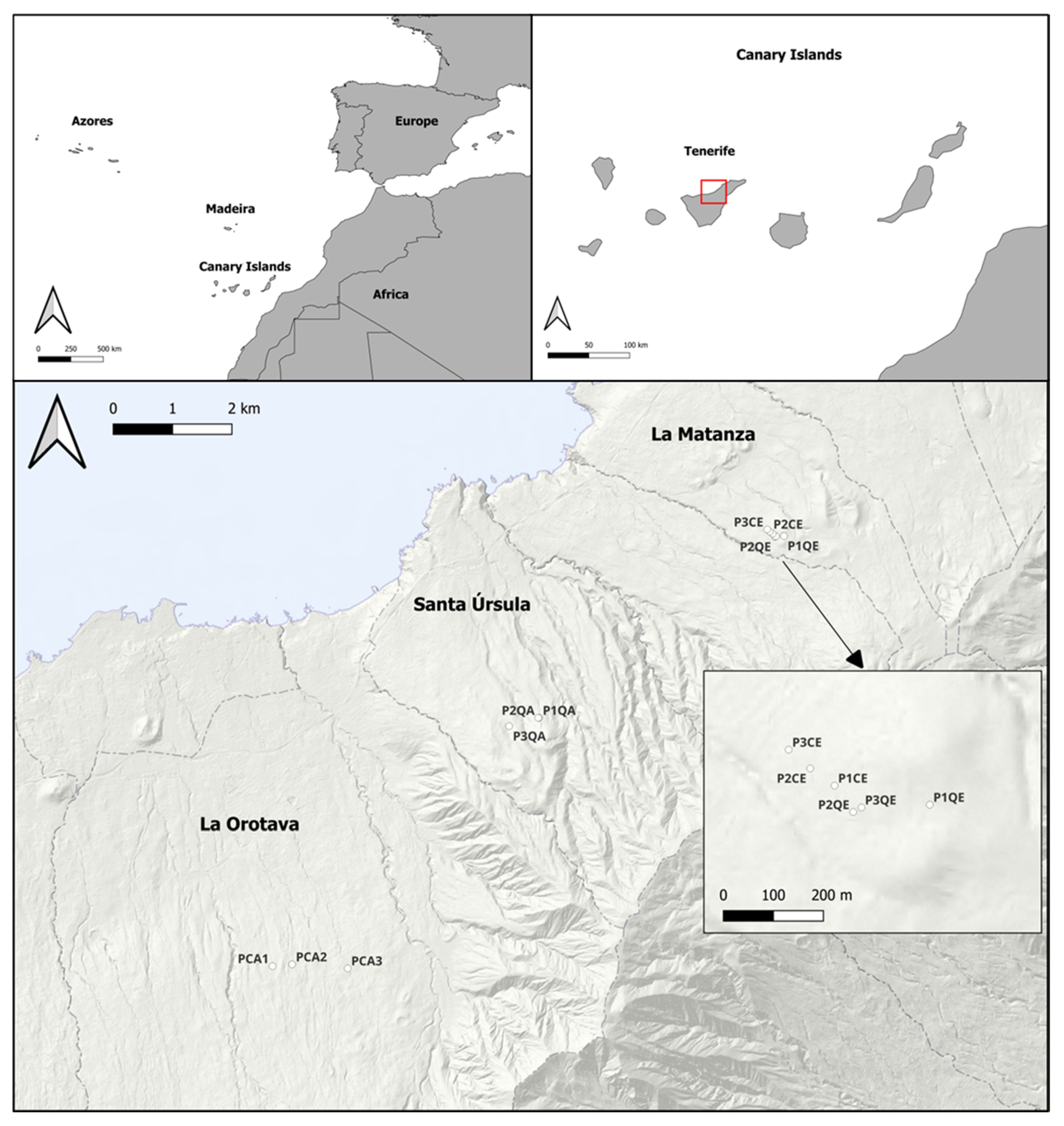
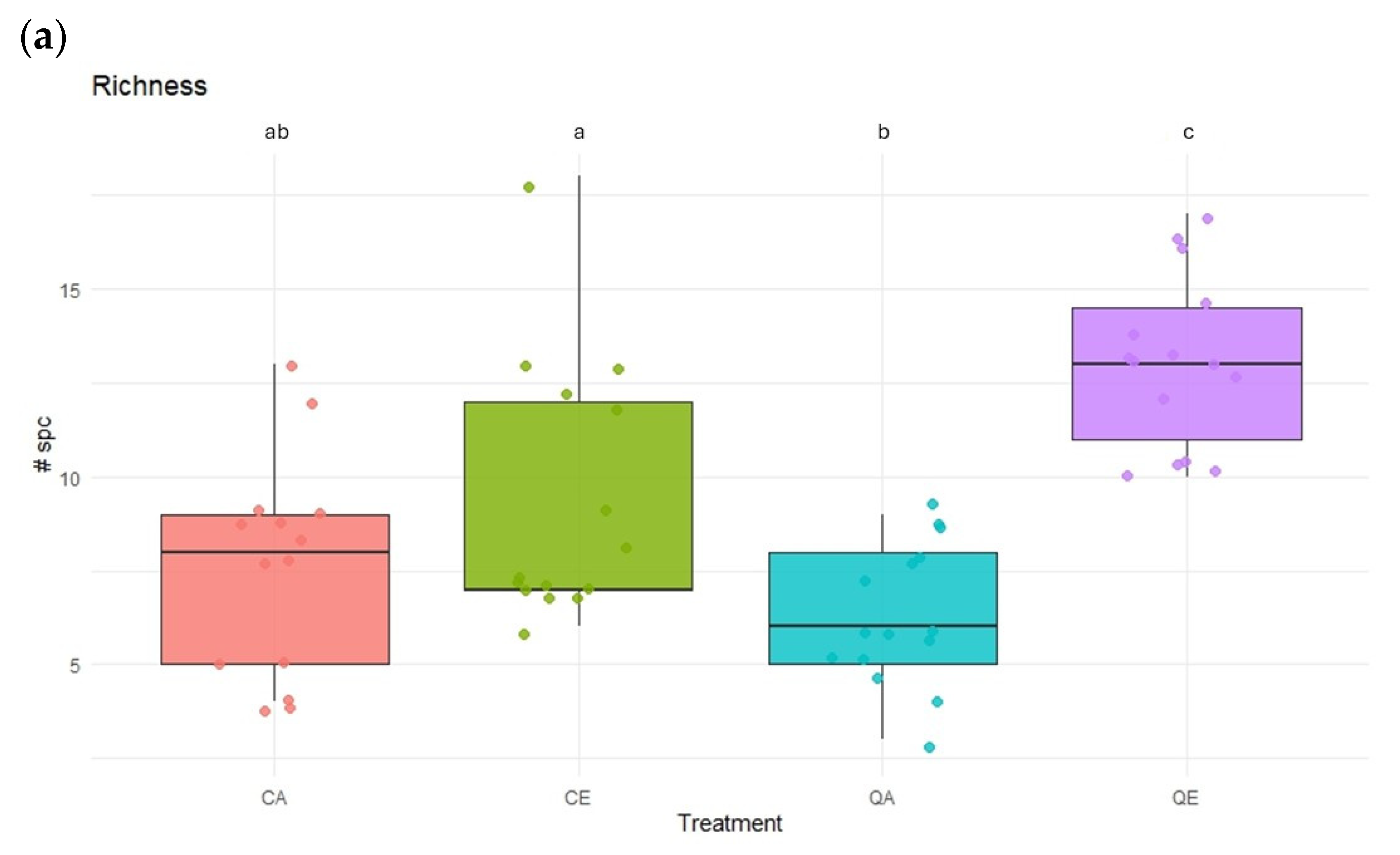

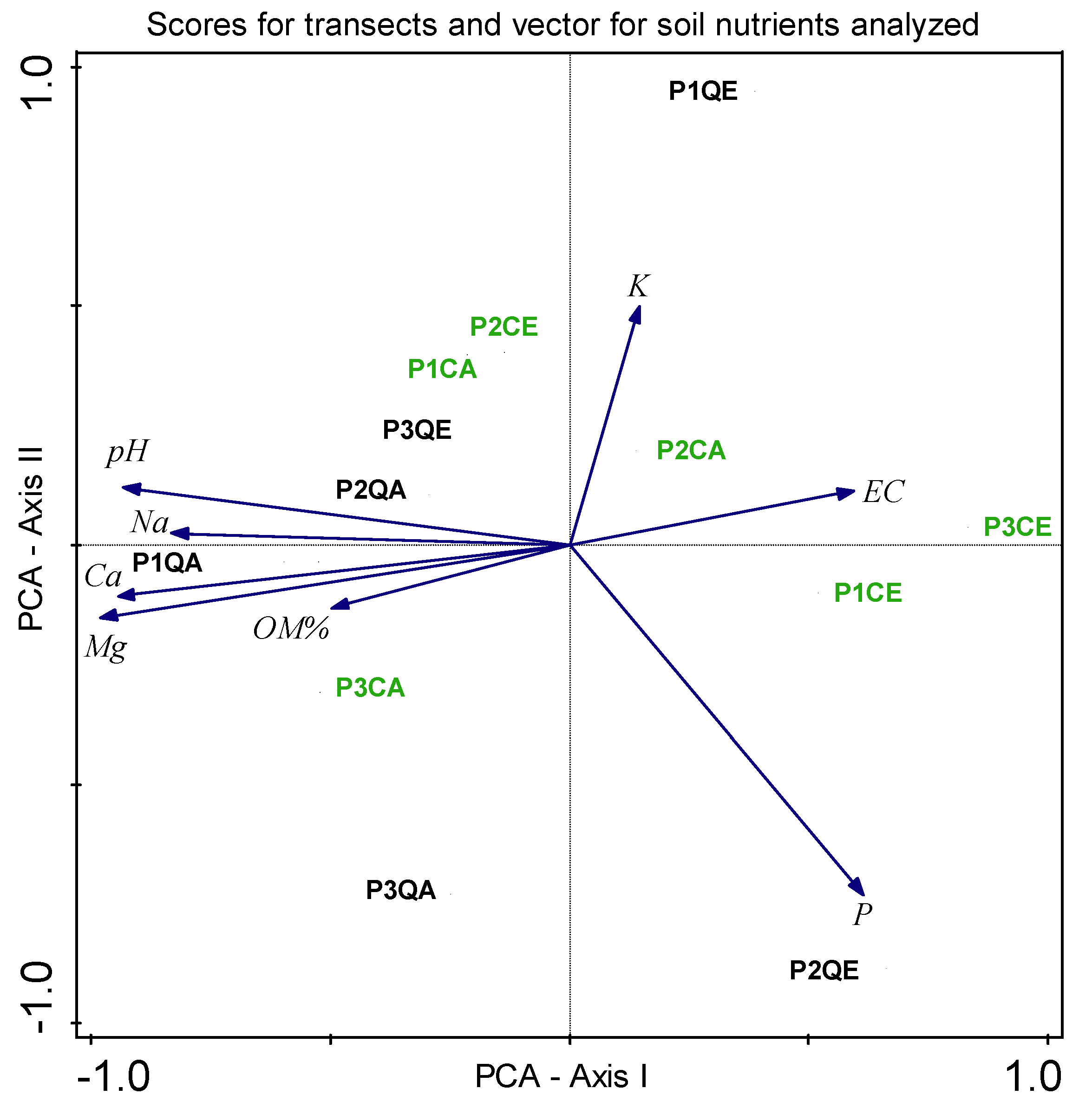
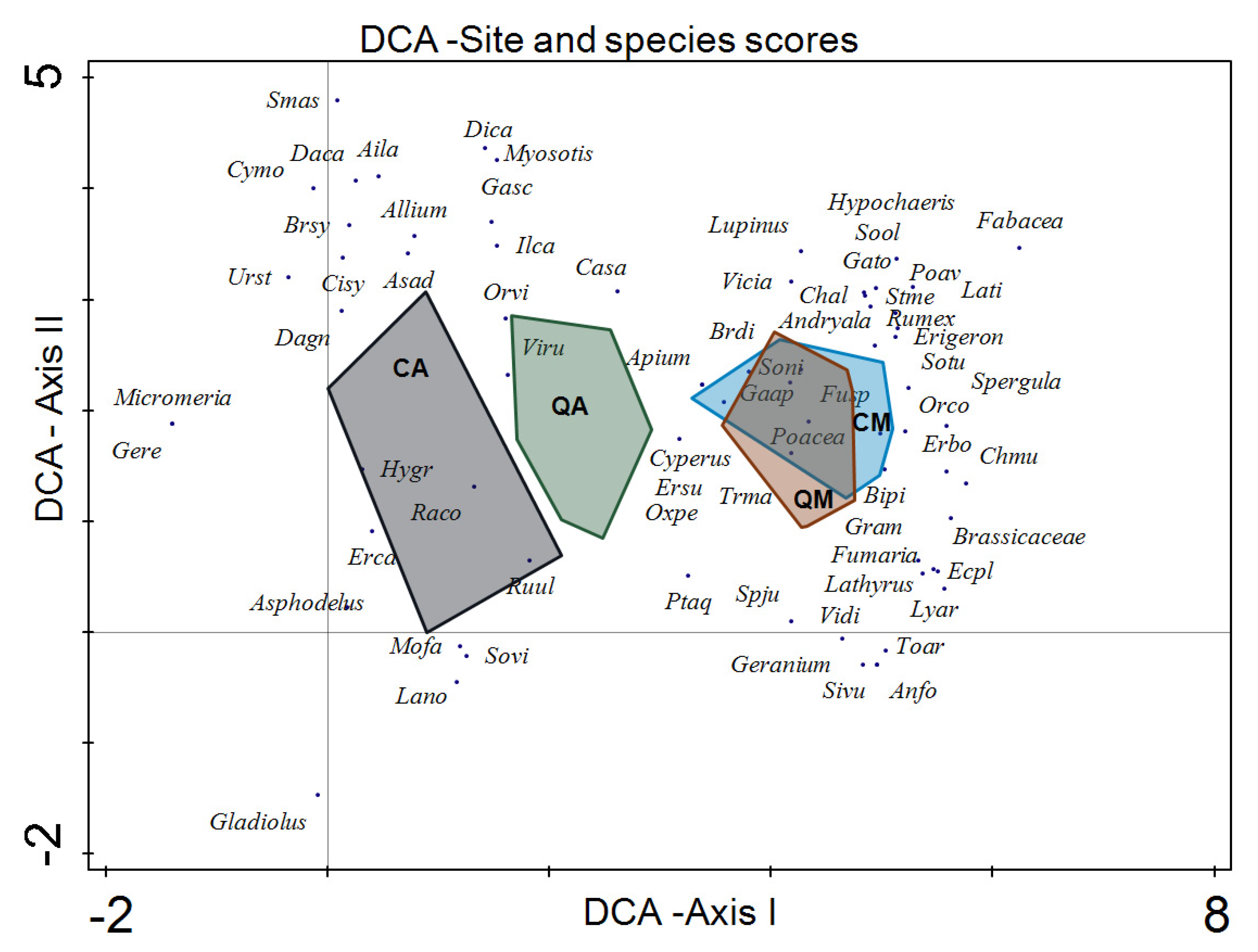
| Transect | Category | Management | Coordinates | Slope (°Sex) | Aspect (Degrees) | Altitude (m a.s.l.) | Canopy Cover (%) |
|---|---|---|---|---|---|---|---|
| P1CA | Control | Oldfield | 28°21′55.5″ N; 16°31′26.0″ W | 2 | 247 | 890 | 80 |
| P1QA | Burned | Oldfield | 28°24′13.1″ N; 16°28′42.9″ W | 10 | 238 | 986 | 71.8 |
| P2CA | Control | Oldfield | 28°21′56.6″ N; 16°31′13.9″ W | 3 | 60 | 899 | 85 |
| P2QA | Burned | Oldfield | 28°24′13.1″ N; 16°28′43.5″ W | 10 | 216 | 974 | 76.8 |
| P3CA | Control | Oldfield | 28°21′54.8″ N; 16°30′39.5″ W | 27 | 280 | 961 | 65 |
| P3QA | Burned | Oldfield | 28°24′08.3″ N; 16°29′01.4″ W | 0 | 13 | 909 | 55 |
| P1CE | Control | Managed | 28°25′55.6″ N; 16°26′19.7″ W | 2 | 35 | 905 | 70 |
| P1QE | Burned | Managed | 28°25′54.4″ N; 16°26′12.6″ W | 2 | 342 | 967 | 23 |
| P2CE | Control | Managed | 28°25′56.7″ N; 16°26′21.5″ W | 2 | 290 | 886 | 94 |
| P2QE | Burned | Managed | 28°25′53.9″ N; 16°26′18.3″ W | 1 | 180 | 922 | 62.8 |
| P3CE | Control | Managed | 28°25′57.9″ N; 16°26′23.1″ W | 3 | 192 | 870 | 82 |
| P3QE | Burned | Managed | 28°25′54.2″ N; 16°26′17.7″ W | 1 | 183 | 928 | 65.6 |
| Castanea sativa | Erica arborea | Ilex canariensis | Morella faya | Sonchus canariensis | Viburnum tinus | Total | Rich * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B.A. | Dens. | B.A. | Dens. | B.A. | Dens. | B.A. | Dens. | B.A. | Dens. | B.A. | Dens. | B.A. | Dens. | ||
| P1CA | 16.29 | 160 | 115.81 | 1160 | - | - | - | - | - | - | - | - | 218.96 | 1320 | 2 |
| P1QA | 121.30 | 360 | 25.45 | 280 | - | - | - | - | - | - | 0.08 | 80 | 266.95 | 720 | 3 |
| P2CA | 88.67 | 280 | 580.88 | 2040 | - | - | - | - | - | - | - | - | 1123.45 | 2320 | 2 |
| P2QA | 1862.65 | 1600 | 2.64 | 160 | - | - | - | - | - | - | 0.08 | 40 | 2030.78 | 1800 | 3 |
| P3CA | 275.25 | 840 | 1231.11 | 3440 | 1.39 | 80 | - | - | 0.08 | 40 | - | - | 2823.36 | 4400 | 4 |
| P3QA | 322.71 | 280 | - | - | - | - | 0.62 | 40 | - | - | - | - | 351.51 | 340 | 2 |
| P1CE | 95.11 | 120 | - | - | - | - | - | - | - | - | - | - | 95.11 | 120 | 1 |
| P1QE | 399.27 | 640 | - | - | - | - | - | - | - | - | - | - | 399.27 | 640 | 1 |
| P2CE | 248.95 | 160 | - | - | - | - | - | - | - | - | - | - | 248.95 | 160 | 1 |
| P2QE | 535.86 | 560 | - | - | - | - | - | - | - | - | - | - | 535.86 | 560 | 1 |
| P3CE | 448.88 | 200 | - | - | - | - | - | - | - | - | - | - | 448.88 | 200 | 1 |
| P3QE | 606.83 | 760 | - | - | - | - | - | - | - | - | - | - | 606.83 | 760 | 1 |
| Castanea sativa | Erica arborea | Ilex canariensis | Morella faya | Sonchus canariensis | Viburnum rugosum | Total | |
|---|---|---|---|---|---|---|---|
| P1CA | 336 | 552 | - | - | - | - | 888 |
| P1QA | 248 | 8 | - | - | - | 120 | 376 |
| P2CA | 296 | 472 | - | - | - | - | 768 |
| P2QA | 8 | - | - | - | - | - | 8 |
| P3CA | 384 | 1408 | - | - | - | - | 1792 |
| P3QA | 1104 | - | - | 96 | - | - | 1200 |
| P1CE | - | - | - | - | - | - | - |
| P1QE | 1440 | - | - | - | - | - | 1440 |
| P2CE | - | - | - | - | - | - | - |
| P2QE | 584 | - | - | - | - | - | 584 |
| P3CE | - | - | - | - | - | - | - |
| P3QE | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Montelongo, C.; Hernández, J.Z.; Ríos, D.; Velázquez-Barrera, M.E.; Arévalo, J.R. Fire Impact on Diversity and Forest Structure of Castanea sativa Mill. Stands in Managed and Oldfield Areas of Tenerife (Canary Islands, Spain). Forests 2025, 16, 1062. https://doi.org/10.3390/f16071062
González-Montelongo C, Hernández JZ, Ríos D, Velázquez-Barrera ME, Arévalo JR. Fire Impact on Diversity and Forest Structure of Castanea sativa Mill. Stands in Managed and Oldfield Areas of Tenerife (Canary Islands, Spain). Forests. 2025; 16(7):1062. https://doi.org/10.3390/f16071062
Chicago/Turabian StyleGonzález-Montelongo, Cristina, José Zoilo Hernández, Domingo Ríos, María Encarnación Velázquez-Barrera, and José Ramón Arévalo. 2025. "Fire Impact on Diversity and Forest Structure of Castanea sativa Mill. Stands in Managed and Oldfield Areas of Tenerife (Canary Islands, Spain)" Forests 16, no. 7: 1062. https://doi.org/10.3390/f16071062
APA StyleGonzález-Montelongo, C., Hernández, J. Z., Ríos, D., Velázquez-Barrera, M. E., & Arévalo, J. R. (2025). Fire Impact on Diversity and Forest Structure of Castanea sativa Mill. Stands in Managed and Oldfield Areas of Tenerife (Canary Islands, Spain). Forests, 16(7), 1062. https://doi.org/10.3390/f16071062









