Estimating Carbon Acquisition in a Shade Cocoa Plantation in Southern Bahia, Brazil
Abstract
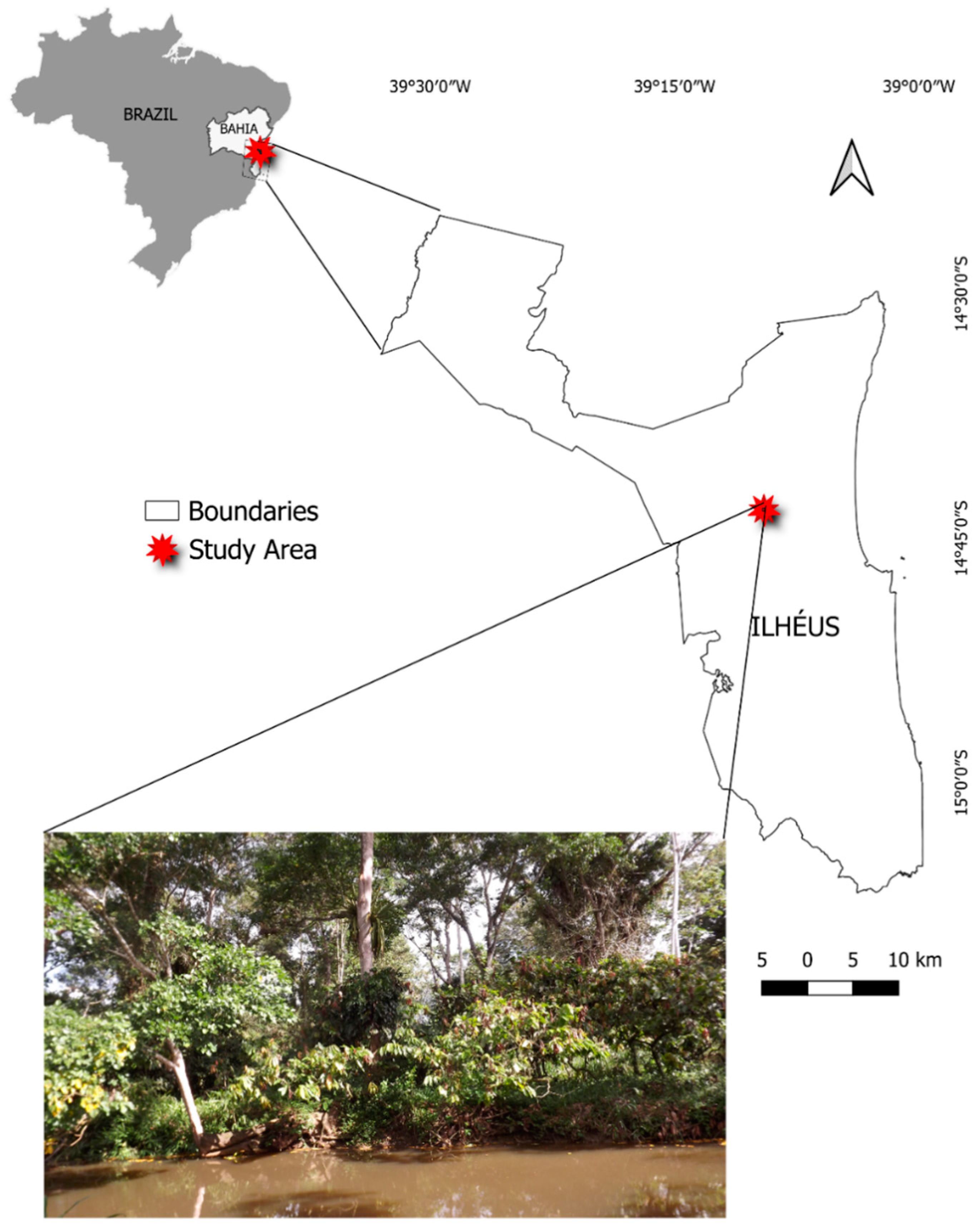
1. Introduction
2. Material and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lipper, L.; Thornton, P.; Campbell, B.M.; Baedeker, T.; Braimoh, A.; Bwalya, M.; Caron, P.; Cattaneo, A.; Garrity, D.; Henry, K.; et al. Climate-Smart Agriculture for Food Security. Nat. Clim. Chang. 2014, 4, 1068–1072. [Google Scholar] [CrossRef]
- Voora, V.; Bermúdez, S.; Larrea, C. Global Market Report: Cocoa; International Institute of Sustainable Development: Winnipeg, MB, Canada, 2019; p. 12. [Google Scholar]
- ICCO. Monthly Review of the Market August 2015; PLoS: London, UK, 2015; Available online: https://www.icco.org/statistics/#production (accessed on 25 March 2025).
- Somarriba, E.; Cerda, R.; Orozco, L.; Cifuentes, M.; Dávila, H.; Espin, T.; Mavisoy, H.; Ávila, G.; Alvarado, E.; Poveda, V.; et al. Carbon Stocks and Cocoa Yields in Agroforestry Systems of Central America. Agric. Ecosyst. Environ. 2013, 173, 46–57. [Google Scholar] [CrossRef]
- de Almeida-Rocha, J.M.; Peres, C.A.; Monsalvo, J.A.; Oliveira, L.D.C. Habitat determinants of golden-headed lion tamarin (Leontopithecus chrysomelas) occupancy of cacao agroforests: Gloomy conservation prospects for management intensification. Am. J. Primatol. 2020, 82, e23179. [Google Scholar] [CrossRef]
- McGinty, M.M. Native Forest Tree Conservation in Tropical Agroforests: Case Study of Cacao Farms in the Atlantic Forest of Southern Bahia, Brazil. Ph.D. Thesis, Columbia University, New York, NY, USA, 2012. [Google Scholar]
- Cassano, C.R.; Barlow, J.; Pardini, R. Large mammals in an agroforestry mosaic in the Brazilian Atlantic Forest. Biotropica 2012, 44, 818–825. [Google Scholar] [CrossRef]
- Heming, N.M.; Schroth, G.; Talora, D.C.; Faria, D. Cabruca Agroforestry Systems Reduce Vulnerability of Cacao Plantations to Climate Change in Southern Bahia. Agron. Sustain. Dev. 2022, 42, 48. [Google Scholar] [CrossRef]
- Abou Rajab, Y.; Leuschner, C.; Barus, H.; Tjoa, A.; Hertel, D. Cacao cultivation under diverse shade tree cover allows high carbon storage and sequestration without yield losses. PLoS ONE 2016, 11, e0149949. [Google Scholar] [CrossRef]
- Gama-Rodrigues, E.F.; Nair, P.K.R.; Nair, V.D.; Gama-Rodrigues, A.C.; Baligar, V.C.; Machado, R.C.R. Carbon Storage in Soil Size Fractions under Two Cacao Agroforestry Systems in Bahia, Brazil. Environ. Manag. 2010, 45, 274–283. [Google Scholar] [CrossRef]
- Rocha-Santos, L.; Faria, D.M.; Figueiredo, M.; Assad, E.; Estevam, C. Levantamento Dos Dados Da Flora Das Cabrucas e Estimativa de Estoque de Carbono Utilizando a Ferramenta GHG Protocol; 2021. Available online: https://arapyau.org.br/wp-content/uploads/2022/01/dados-da-flora-das-cabrucas-e-estimativa-de-estoque-de-carbono.pdf (accessed on 25 March 2025).
- Schroth, G.; Bede, L.C.; Paiva, A.O.; Cassano, C.R.; Amorim, A.M.; Faria, D.; Mariano-Neto, E.; Martini, A.M.Z.; Sambuichi, R.H.R.; Lôbo, R.N. Contribution of Agroforests to Landscape Carbon Storage. Mitig. Adapt. Strateg. Glob. Chang. 2015, 20, 1175–1190. [Google Scholar] [CrossRef]
- Pastore, K. Crédito de Carbono entra na “Receita” da Dengo Chocolates. 2023. Available online: https://neofeed.com.br/finde/credito-de-carbono-entra-na-receita-da-dengo-chocolates/ (accessed on 25 March 2025).
- Assunção, J.J.; Hansen, L.P.; Munson, T.; Scheinkman, J. Carbon Prices and Forest Preservation Over Space and Time in the Brazilian Amazon. SSRN Electron. J. 2023, 1, 1–48. [Google Scholar] [CrossRef]
- Kindermann, G.E.; Obersteiner, M.; Rametsteiner, E.; McCallum, I. Predicting the deforestation-trend under different carbon-prices. Carbon Balance Manag. 2006, 1, 1–17. [Google Scholar] [CrossRef]
- Sambuichi, R.H.R. Estrutura e Dinâmica Do Componente Arbóreo Em Área de Cabruca Na Região Cacaueira Do Sul Da Bahia, Brasil. Acta Bot. Bras. 2006, 20, 943–954. [Google Scholar] [CrossRef]
- Martini, A.M.Z.; Fiaschi, P.; Amorim, A.M.; Lima, J. A Hot-Point within a Hot-Spot: A High Diversity Site in in Brazil’ s Atlantic Forest. Biodivers. Conserv. 2007, 16, 3111–3128. [Google Scholar] [CrossRef]
- Thomas, W.W.; Carvalho, A.M.V.; Amorim, A.M.; Garrison, J.; Arbeláez, A.L. Plant Endemism in Two Forsts in Southern Bahia, Brazil. Biodivers. Conserv. 1998, 7, 311–322. [Google Scholar] [CrossRef]
- Mori, S.A.; Boom, B.M.; de Carvalino, A.M.; Santos, T.S. Ecological Importance of Myrtaceae in an Eastern Brazilian Wet Forest. Biotropica 1983, 15, 68–70. [Google Scholar] [CrossRef]
- Réjou-Méchain, M.; Tanguy, A.; Piponiot, C.; Chave, J.; Hérault, B. Biomass: An R Package for Estimating Above-Ground Biomass and Its Uncertainty in Tropical Forests. Methods Ecol. Evol. 2017, 8, 1163–1167. [Google Scholar] [CrossRef]
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.C.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved Allometric Models to Estimate the Aboveground Biomass of Tropical Trees. Glob. Change Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef]
- R Core Team, R. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Valladares, F.; Niinemets, Ü. Shade Tolerance, a Key Plant Feature of Complex Nature and Consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef]
- Swaine, M.D.; Whitmore, T.C. On the Definition of Ecological Species Groups in Tropical Rain Forests. Vegetatio 1988, 75, 81–86. [Google Scholar] [CrossRef]
- Maia, V.A.; Borges, A.; Santos, M.; de Aguiar-campos, N.; de Souza, C.R.; Coutinho, M.; de Oliveira, F.; Coelho, P.A.; Morel, J.D.; Silva, L.; et al. The Carbon Sink of Tropical Seasonal Forests in Southeastern Brazil Can Be under Threat. Sci. Adv. 2020, 6, eabd4548. [Google Scholar] [CrossRef]
- Vaast, P.; Somarriba, E. Trade-Offs between Crop Intensification and Ecosystem Services: The Role of Agroforestry in Cocoa Cultivation. Agrofor. Syst. 2014, 88, 947–956. [Google Scholar] [CrossRef]
- Vebrova, H.; Lojka, B.; Husband, T.P.; Zans, M.E.C.; van Damme, P.; Rollo, A.; Kalousova, M. Tree diversity in cacao agroforests in San Alejandro, Peruvian Amazon. Agrofor. Syst. 2014, 88, 1101–1115. [Google Scholar] [CrossRef]
- Guimarães, R.B.A.d.S.; da Silva, P.S.D.; Corrêa, M.M. Heterogeneidade na estrutura e diversidade de árvores de cabrucas no centro-sul do Estado da Bahia, Brasil. Hoehnea 2017, 44, 184–192. [Google Scholar] [CrossRef]
- Lobão, D.E.; Setenta, W.C.; Santos, E.S.; Curvelo, K.; Lobão, E.S.P.; Valle, R.R. Sistema cacau cabruca e a mata atlântica: Diversidade arbórea, conservação e potencial de produção. Agrotrópica 2011, 23, 115–124. [Google Scholar]
- Sambuichi, R.H.R.; Haridasan, M. Recovery of Species Richness and Conservation of Native Atlantic Forest Trees in the Cacao Plantations of Southern Bahia in Brazil. Biodivers. Conserv. 2007, 16, 3681–3701. [Google Scholar] [CrossRef]
- de Deyn, G.B.; Cornelissen, J.H.C.; Bardgett, R.D. Plant Functional Traits and Soil Carbon Sequestration in Contrasting Biomes. Ecol. Lett. 2008, 11, 516–531. [Google Scholar] [CrossRef]
- Shimamoto, C.Y.; Botosso, P.C.; Marques, M.C. How much carbon is sequestered during the restoration of tropical forests? Estimates from tree species in the Brazilian Atlantic forest. For. Ecol. Manag. 2014, 329, 1–9. [Google Scholar]
- Jantawong, K.; Elliott, S.; Wangpakapattanawong, P. Above-ground carbon sequestration during restoration of upland evergreen forest in northern Thailand. Open J. For. 2017, 7, 157–171. [Google Scholar] [CrossRef]
- Chiapetti, J.; Rocha, R.; Conceição, A.; Baiardi, A.; Szerman, D.; VanWey, L. Panorama da Cacauicultura no Território Litoral Sul da Bahia (2015–2019); Instituto Floresta Viva: Ilhéus, Brazil, 2020. [Google Scholar]
- Pinto, L.G.; Amaral, S.; Metzger, J.P.; Rosa, M.; Adorno, B.; Goncalves, G. Alarming Patterns of Mature Forest Loss in the Brazilian Atlantic Forest. Prepr. Res. Sq. 2024, 8, 256–264. [Google Scholar]
- Wang, N.; Quesada, B.; Xia, L.; Butterbach-Bahl, K.; Goodale, C.L.; Kiese, R. Effects of Climate Warming on Carbon Fluxes in Grasslands—A Global Meta-Analysis. Glob. Change Biol. 2019, 25, 1839–1851. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faria, D.; Mariano-Neto, E.; Sambuichi, R.H.R.; Rocha-Santos, L. Estimating Carbon Acquisition in a Shade Cocoa Plantation in Southern Bahia, Brazil. Forests 2025, 16, 929. https://doi.org/10.3390/f16060929
Faria D, Mariano-Neto E, Sambuichi RHR, Rocha-Santos L. Estimating Carbon Acquisition in a Shade Cocoa Plantation in Southern Bahia, Brazil. Forests. 2025; 16(6):929. https://doi.org/10.3390/f16060929
Chicago/Turabian StyleFaria, Deborah, Eduardo Mariano-Neto, Regina Helena Rosa Sambuichi, and Larissa Rocha-Santos. 2025. "Estimating Carbon Acquisition in a Shade Cocoa Plantation in Southern Bahia, Brazil" Forests 16, no. 6: 929. https://doi.org/10.3390/f16060929
APA StyleFaria, D., Mariano-Neto, E., Sambuichi, R. H. R., & Rocha-Santos, L. (2025). Estimating Carbon Acquisition in a Shade Cocoa Plantation in Southern Bahia, Brazil. Forests, 16(6), 929. https://doi.org/10.3390/f16060929






