Reshaping Nutrient Resorption Efficiency: Adaptive Strategies of Subtropical Slash Pine Plantations to Nitrogen and Phosphorus Additions
Abstract
1. Introduction
2. Materials and Methods
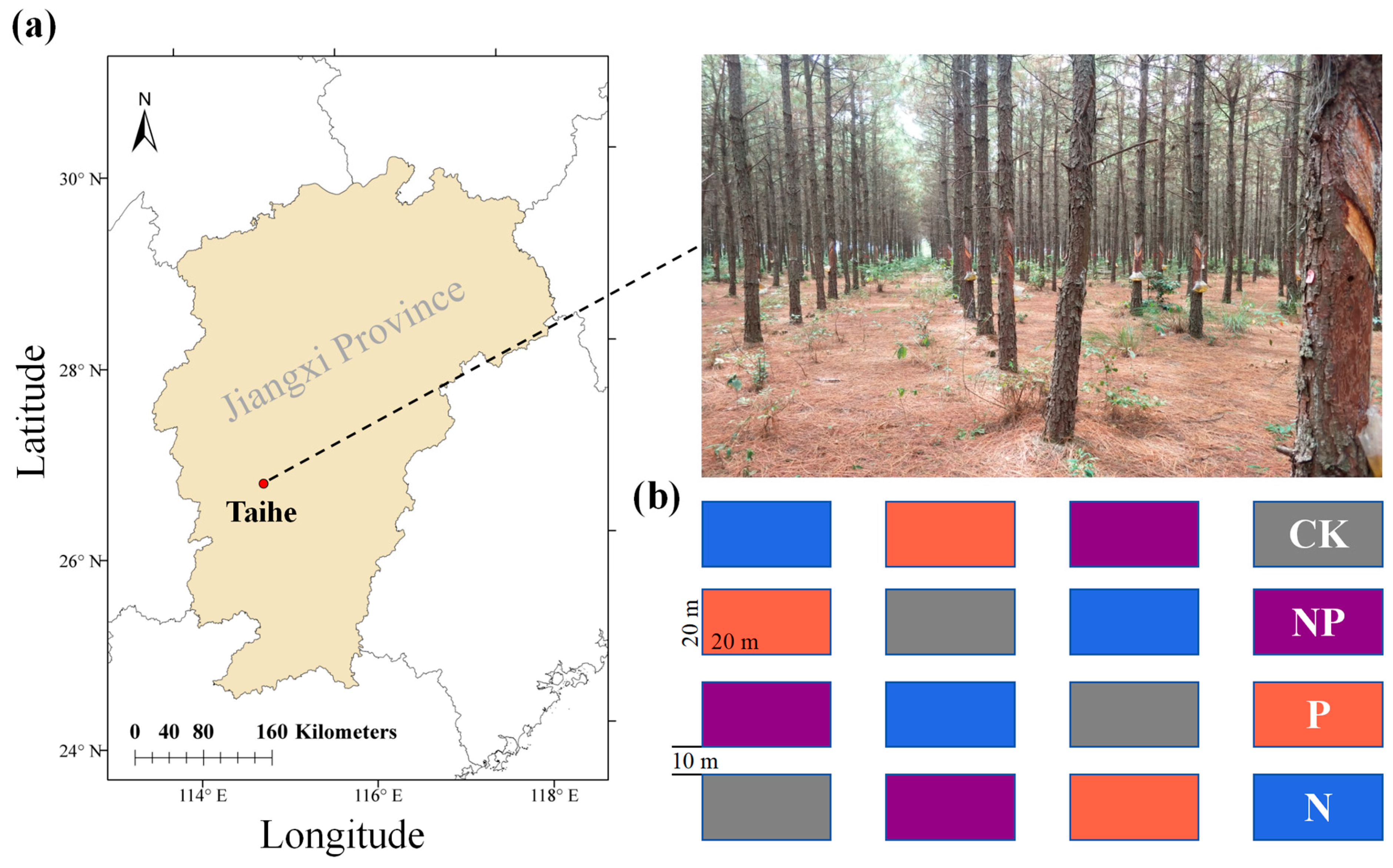
2.1. Study Site
2.2. Experimental Design Treatments
2.3. Sample Collection
2.4. Detection Methods
2.5. Parameter Calculations
2.6. Statistical Analyses
3. Results
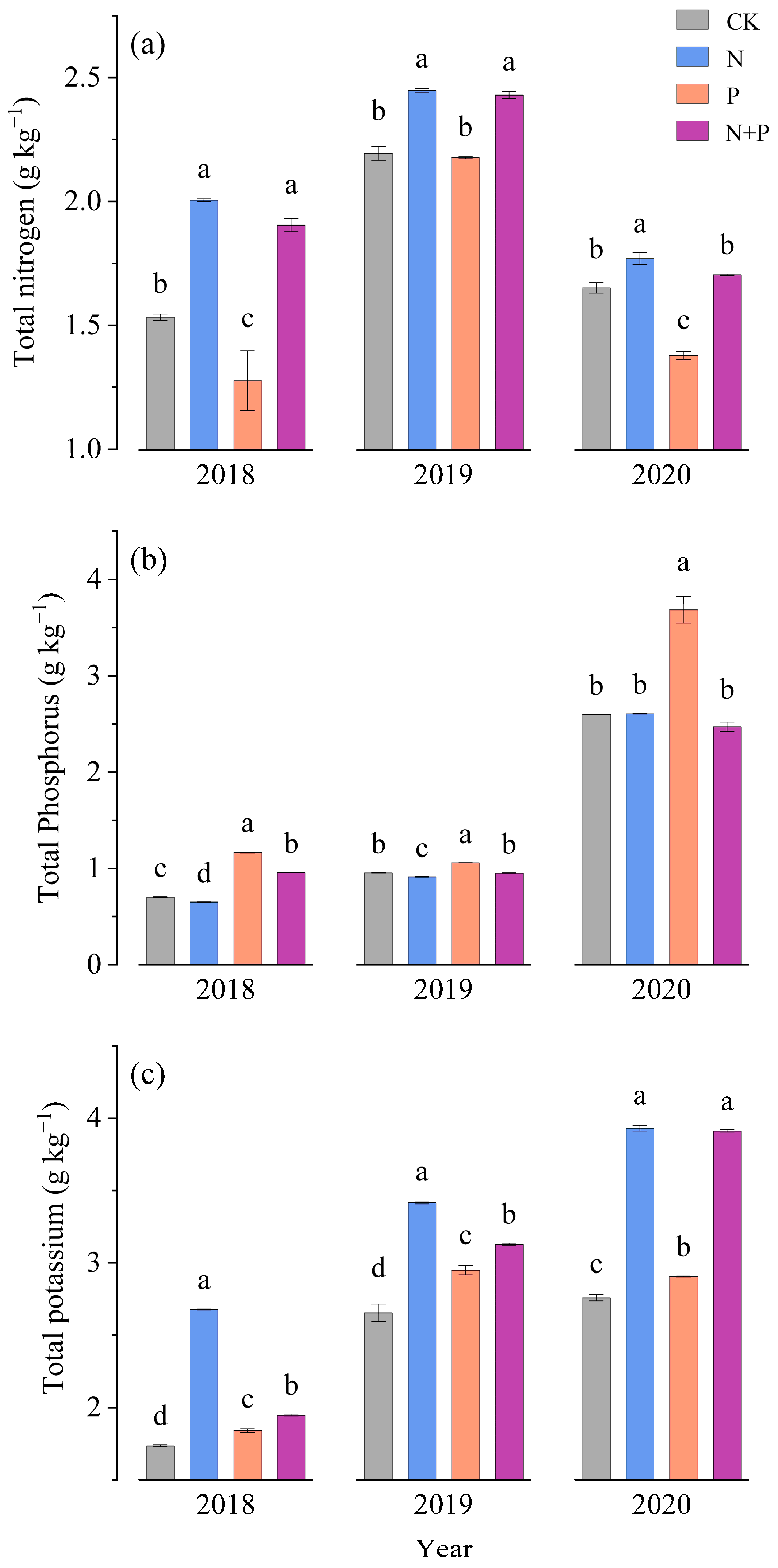
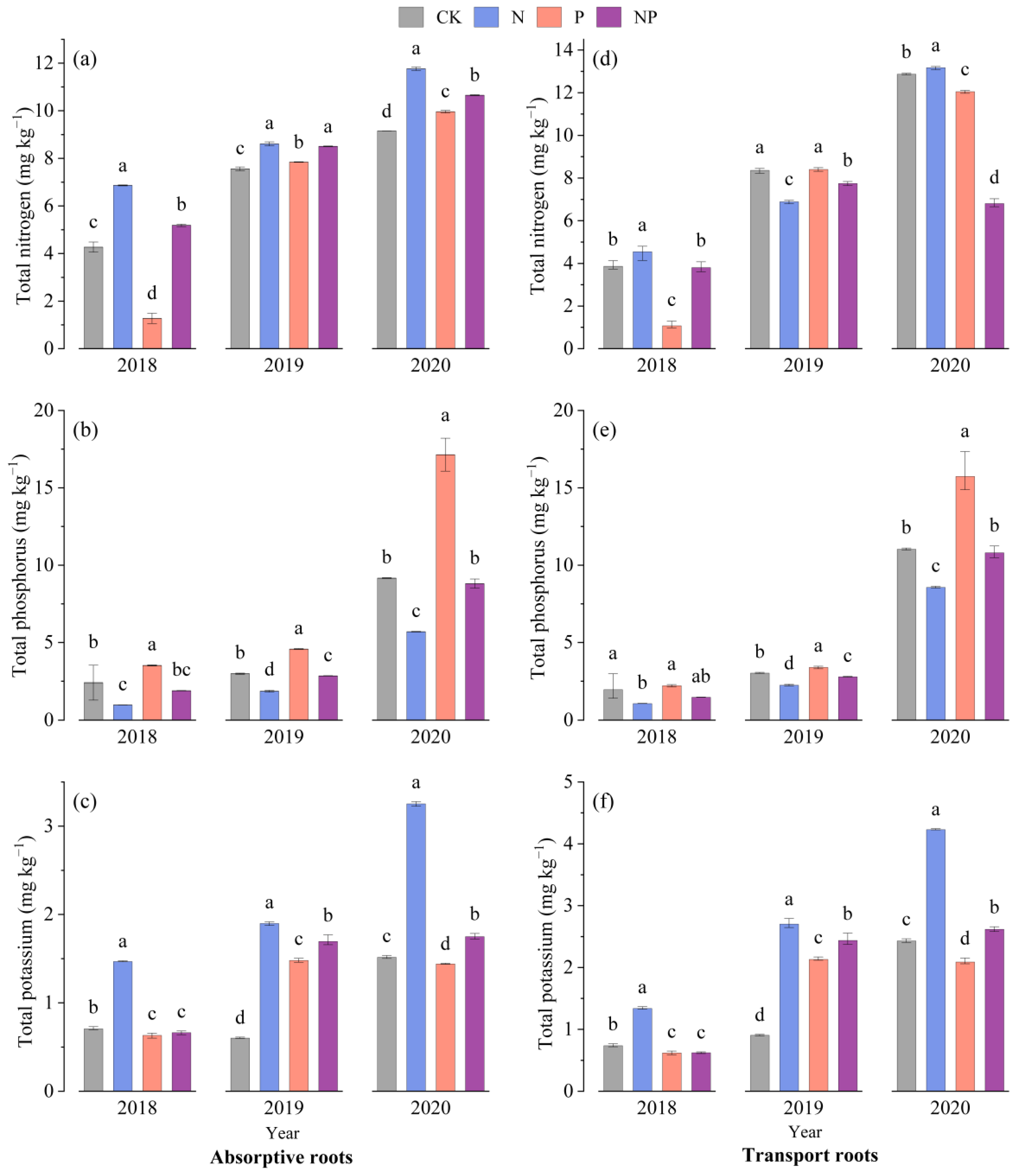
3.1. Dynamic Changes in Nutrient Content in Underground Part
3.2. Dynamic Changes in Nutrient Content in Aboveground Part
3.3. The Nutrient Transfer from Soil to Absorbing Root
3.4. The Nutrient Transfer from Absorbing Roots to Transportive Roots
3.5. The Nutrient Transfer from Transportive Roots to Twigs
3.6. The Nutrient Transfer from Twigs to Needles
3.7. The Nutrient Resorption Efficiency of Needles
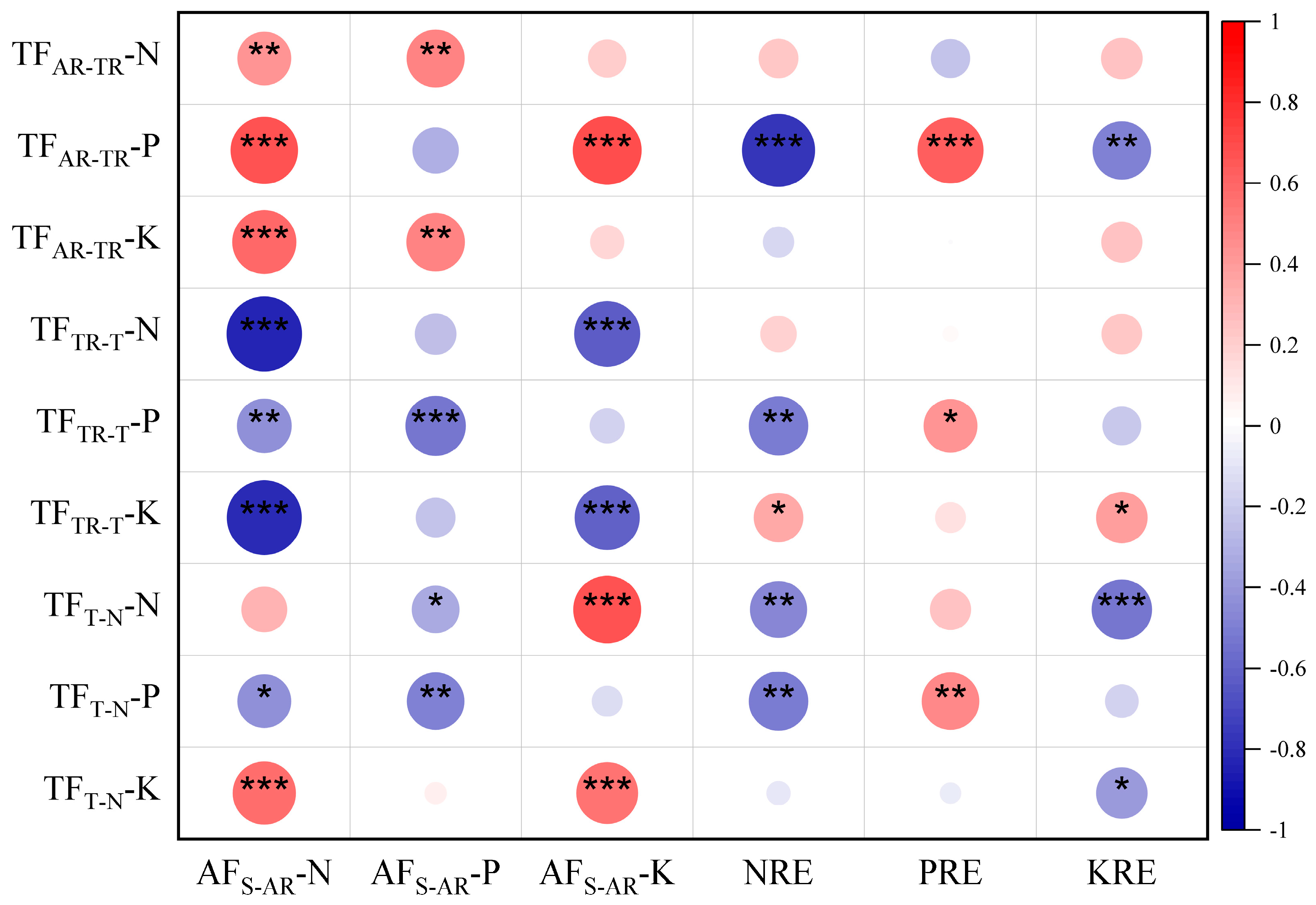
3.8. Relationship Between the Capacity of Nutrient Transport and Capture
3.9. Predominant Elements That Influence the Most Important Processes of Nutrient Movement
4. Discussion
4.1. Nutrient Dynamic Transport of Aboveground and Underground Parts
4.2. Interannual Variation in Plant Nutrient Capture and Resorption
4.3. The Potential Use of Transport Capacity as a Measure of the Trade-Off Between Capture and Absorption
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marklein, A.R.; Houlton, B.Z. Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol. 2012, 193, 696–704. [Google Scholar] [CrossRef]
- You, C.; Peng, C.; Xu, Z.; Liu, Y.; Zhang, L.; Yin, R.; Liu, L.; Li, H.; Wang, B.; Wang, L.; et al. Nitrogen addition mediates the response of foliar stoichiometry to phosphorus addition: A meta-analysis. Ecol. Process. 2021, 10, 58. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D.; Leyser, O. A plant’s diet, surviving in a variable nutrient environment. Science 2020, 368, eaba0196. [Google Scholar] [CrossRef]
- Elser, J.J.; Dobberfuhl, D.R.; MacKay, N.A.; Schampel, J.H. Organism Size, Life History, and N:P Stoichiometry: Toward a unified view of cellular and ecosystem processes. BioScience 1996, 46, 674–684. [Google Scholar] [CrossRef]
- McGroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C:N:P stoichiometry in forests worldwide: Implications of treeestrial redfield-type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]
- Guan, Z.-H.; Cao, Z.; Li, X.G.; Scholten, T.; Kühn, P.; Wang, L.; Yu, R.-P.; He, J.-S. Soil phosphorus availability mediates the effects of nitrogen addition on community- and species-level phosphorus-acquisition strategies in alpine grasslands. Sci. Total Environ. 2024, 906, 167630. [Google Scholar] [CrossRef]
- Ma, N.; Kou, L.; Li, S.; Dai, X.; Meng, S.; Jiang, L.; Xue, Y.; Zheng, J.; Fu, X.; Wang, H. Plant–soil feedback regulates the trade-off between phosphorus acquisition pathways in Pinus elliottii. Tree Physiol. 2023, 43, 1092–1103. [Google Scholar] [CrossRef]
- Yu, G.; Chen, J.; Yu, M.; Li, A.; Wang, Y.-P.; He, X.; Tang, X.; Liu, H.; Jiang, J.; Mo, J.; et al. Eighteen-year nitrogen addition does not increase plant phosphorus demand in a nitrogen-saturated tropical forest. J. Ecol. 2023, 111, 1545–1560. [Google Scholar] [CrossRef]
- Zhao, C.; Lin, Q.; Tian, D.; Ji, C.; Shen, H.; Fan, D.; Wang, X.; Fang, J. Nitrogen addition promotes conservative resource-use strategies via aggravating phosphorus limitation of evergreen trees in subtropical forest. Sci. Total Environ. 2023, 889, 164047. [Google Scholar] [CrossRef]
- Suonan, J.; Lu, X.; Li, X.; Hautier, Y.; Wang, C. Nitrogen addition strengthens the stabilizing effect of biodiversity on productivity by increasing plant trait diversity and species asynchrony in the artificial grassland communities. Front. Plant Sci. 2023, 14, 1301461. [Google Scholar] [CrossRef]
- Li, Q.; Yingxin, H.; Daowei, Z.; Shan, C. Mechanism of the trade-off between biological nitrogen fixation and phosphorus acquisition strategies of herbaceous legumes under nitrogen and phosphorus addition. Chin. J. Plant Ecol. 2021, 45, 286–297. [Google Scholar] [CrossRef]
- Kou, L.; Wang, H.; Gao, W.; Chen, W.; Yang, H.; Li, S. Nitrogen addition regulates tradeoff between root capture and foliar resorption of nitrogen and phosphorus in a subtropical pine plantation. Trees 2017, 31, 77–91. [Google Scholar] [CrossRef]
- See, C.R.; Yanai, R.D.; Fisk, M.C.; Vadeboncoeur, M.A.; Quintero, B.A.; Fahey, T.J. Soil nitrogen affects phosphorus recycling: Foliar resorption and plant–soil feedbacks in a northern hardwood forest. Ecology 2015, 96, 2488–2498. [Google Scholar] [CrossRef]
- Lambers, H.; Shane, M.W.; Cramer, M.D.; Pearse, S.J.; Veneklaas, E.J. Root Structure and Functioning for Efficient Acquisition of Phosphorus: Matching Morphological and Physiological Traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [CrossRef]
- Ren, H.; Gao, G.; Ma, Y.; Li, Z.; Wang, S.; Gu, J. Shift of root nitrogen-acquisition strategy with tree age is mediated by root functional traits along the collaboration gradient of the root economics space. Tree Physiol. 2023, 43, 1341–1353. [Google Scholar] [CrossRef]
- Wen, Z.; Li, H.; Shen, Q.; Tang, X.; Xiong, C.; Li, H.; Pang, J.; Ryan, M.H.; Lambers, H.; Shen, J. Tradeoffs among root morphology, exudation and mycorrhizal symbioses for phosphorus-acquisition strategies of 16 crop species. New Phytol. 2019, 223, 882–895. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Potassium: A neglected nutrient in global change. Glob. Ecol. Biogeogr. 2015, 24, 261–275. [Google Scholar] [CrossRef]
- Głąb, T.; Gondek, K. The influence of soil compaction and N fertilization on physico-chemical properties of Mollic Fluvisol soil under red clover/grass mixture. Geoderma 2014, 226–227, 204–212. [Google Scholar] [CrossRef]
- Wang, B.; Chen, J.; Huang, G.; Zhao, S.; Dong, F.; Zhang, Y.; He, W.; Wang, P.; Yan, Z. Growth and nutrient stoichiometry responses to N and P fertilization of 8-year old Masson pines (Pinus massoniana) in subtropical China. Plant Soil 2022, 477, 343–356. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. Negative effects of fertilization on plant nutrient resorption. Ecology 2015, 96, 373–380. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Guo, W.; Li, Y.; Wang, G.G.; Wu, T. Stoichiometric homeostasis, physiology, and growth responses of three tree species to nitrogen and phosphorus addition. Trees 2018, 32, 1377–1386. [Google Scholar] [CrossRef]
- Chen, F.-S.; Niklas, K.J.; Liu, Y.; Fang, X.-M.; Wan, S.-Z.; Wang, H. Nitrogen and phosphorus additions alter nutrient dynamics but not resorption efficiencies of Chinese fir leaves and twigs differing in age. Tree Physiol. 2015, 35, 1106–1117. [Google Scholar] [CrossRef]
- Hao, C.; Geshere Abdisa, G.; Wei, Z.; Xiaoyun, Z.; Mianhai, Z.; Qigui, M.; Tao, Z.; Jiangming, M. Nitrogen saturation in humid tropical forests after 6 years of nitrogen and phosphorus addition: Hypothesis testing. Funct. Ecol. 2015, 30, 305–313. [Google Scholar] [CrossRef]
- Fan, Y.; Zhong, X.; Lin, F.; Liu, C.; Yang, L.; Wang, M.; Chen, G.; Chen, Y.; Yang, Y. Responses of soil phosphorus fractions after nitrogen addition in a subtropical forest ecosystem: Insights from decreased Fe and Al oxides and increased plant roots. Geoderma 2019, 337, 246–255. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Zhu, B.; Homyak, P.M.; Chen, G.; Yao, X.; Wu, D.; Yang, Z.; Lyu, M.; Yang, Y. Long-term nitrogen deposition inhibits soil priming effects by enhancing phosphorus limitation in a subtropical forest. Glob. Change Biol. 2023, 29, 4081–4093. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, Z.; Liu, L.; Li, J.; Zhu, T.; Ma, J.; DeLuca, T.H.; Duan, M. Nitrogen-Fixing Plants Enhance Soil Phosphorus Availability by Promoting Transformations Among Phosphorus Fractions in a Subtropical Karst Forest. Forests 2025, 16, 360. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability Update on Microbial Phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef]
- Deng, Q.; Hui, D.; Sam Dennis; Reddy, K.C. Responses of terrestrial ecosystem phosphorus cycling to nitrogen addition: A meta-analysis. Glob. Ecol. Biogeogr. 2017, 26, 713–728. [Google Scholar] [CrossRef]
- Chen, Z.; Xiao, Y.; Dong, X.; Deng, Z.; Zhou, X.; Yan, G.; Zhang, J.; Han, S. Nitrogen addition promotes soil organic phosphorus accumulation through increasing microbial biomass phosphorus in a temperate forest. Plant Soil 2024, 1–16. [Google Scholar] [CrossRef]
- Ye, X.; Bu, W.; Hu, X.; Liu, B.; Liang, K.; Chen, F. Species divergence in seedling leaf traits and tree growth response to nitrogen and phosphorus additions in an evergreen broadleaved forest of subtropical China. J. For. Res. 2023, 34, 137–150. [Google Scholar] [CrossRef]
- Jia, T.; Yi, M.; Chen, F.; Lai, M.; Jin, C.; Nie, Z.; Zhou, L.; Xie, J.; Zhang, L. Response of C:N:P Stoichiometry to Phosphorus Addition and Homeostasis of Plant Tissues in a Subtropical Slash Pine Plantation. Forests 2023, 14, 1355. [Google Scholar] [CrossRef]
- Jia, T.; Fang, X.; Yuan, Y.; Fu, Y.; Yi, M.; Yuan, S.; Guo, S.; Lai, M.; Xie, J.; Zhang, L. Phosphorus addition alter the pine resin flow rate by regulating tree growth and non-structural carbohydrates in a subtropical slash pine plantation. Ind. Crops Prod. 2023, 199, 116782. [Google Scholar] [CrossRef]
- Ding, Z.; Tang, M.; Chen, X.; Yin, L.; Gui, H.; Zhu, B. Measuring rhizosphere effects of two tree species in a temperate forest: A comprehensive method comparison. Rhizosphere 2019, 10, 100153. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E. Methods of Soil Analysis. Part 3—Chemical Methods; ACSESS: Madison, WI, USA, 1996. [Google Scholar]
- Lu, R.K. Analysis Method of Soil Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000. [Google Scholar]
- Parkinson, J.A.; Allen, S.E. A wet oxidation procedure suitable for the determination of nitrogen and mineral nutrients in biological material. Commun. Soil Sci. Plan. 1975, 6, 1–11. [Google Scholar] [CrossRef]
- Wu, A.; Hu, X.; Wang, F.; Guo, C.; Wang, H.; Chen, F. Nitrogen deposition and phosphorus addition alter mobility of trace elements in subtropical forests in China. Sci. Total Environ. 2021, 781, 146778. [Google Scholar] [CrossRef]
- Ye, D.; Li, T.; Chen, G.; Zheng, Z.; Yu, H.; Zhang, X. Influence of swine manure on growth, P uptake and activities of acid phosphatase and phytase of Polygonum hydropiper. Chemosphere 2014, 105, 139–145. [Google Scholar] [CrossRef]
- Vergutz, L.; Manzoni, S.; Porporato, A.; Novais, R.F.; Jackson, R.B. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecol. Monogr. 2012, 82, 205–220. [Google Scholar] [CrossRef]
- Bargaz, A.; Lyamlouli, K.; Chtouki, M.; Zeroual, Y.; Dhiba, D. Soil Microbial Resources for Improving Fertilizers Efficiency in an Integrated Plant Nutrient Management System. Front. Microbiol. 2018, 9, 1606. [Google Scholar] [CrossRef]
- Wang, R.; Bicharanloo, B.; Hou, E.; Jiang, Y.; Dijkstra, F.A. Phosphorus Supply Increases Nitrogen Transformation Rates and Retention in Soil: A Global Meta-Analysis. Earth’s Future 2022, 10, e2021EF002479. [Google Scholar] [CrossRef]
- Song, L.; Liao, J.; Ma, F.; Wang, S.; Yan, Y.; Chen, C.; Zhou, Q.; Niu, S. Nitrogen additions increase soil microbial nitrate- rather than ammonium- immobilization. Biol. Fertil. Soils. 2025, 61, 1–16. [Google Scholar] [CrossRef]
- Magill, A.H.; Aber, J.D.; Hendricks, J.J.; Bowden, R.D.; Melillo, J.M.; Steudler, P.A. Biogeochemical response of forest ecosystems to simulated chronic nitrogen deposition. Ecol. Appl. 1997, 7, 402–415. [Google Scholar] [CrossRef]
- Aber, J.D.; Magill, A.; Boone, R.; Melillo, J.M.; Steudler, P. Plant and Soil Responses to Chronic Nitrogen Additions at the Harvard Forest, Massachusetts. Ecol. Appl. 1993, 3, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.E.; Poulton, P.R.; Fixen, P.E.; Curtin, D. Phosphorus: Its Efficient Use in Agriculture; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Song, Y.; Wan, G.-Y.; Wang, J.-X.; Zhang, Z.-S.; Xia, J.-Q.; Sun, L.-Q.; Lu, J.; Ma, C.-X.; Yu, L.-H.; Xiang, C.-B.; et al. Balanced nitrogen–iron sufficiency boosts grain yield and nitrogen use efficiency by promoting tillering. Mol. Plant 2023, 16, 1661–1677. [Google Scholar] [CrossRef]
- Fan, Y.; Lin, F.; Yang, L.; Zhong, X.; Wang, M.; Zhou, J.; Chen, Y.; Yang, Y. Decreased soil organic P fraction associated with ectomycorrhizal fungal activity to meet increased P demand under N application in a subtropical forest ecosystem. Biol. Fertil. Soils. 2018, 54, 149–161. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.-F.; Jin, M.-K.; Zhang, W.; Lambers, H.; Hui, D.; Liang, C.; Zhang, J.; Wu, D.; Sardans, J.; et al. Microbial controls over soil priming effects under chronic nitrogen and phosphorus additions in subtropical forests. ISME J. 2023, 17, 2160–2168. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, Y.; Li, A.; Wang, S.; Chen, J.; Mo, J.; Zheng, M. Root functional traits are important predictors for plant resource acquisition strategies in subtropical forests. Ecol. Appl. 2025, 35, e3082. [Google Scholar] [CrossRef]
- Yang, K.; Chen, G.; Xian, J.; Chang, H. Divergent adaptations of leaf functional traits to light intensity across common urban plant species in Lanzhou, northwestern China. Front. Plant Sci. 2023, 14, 1000647. [Google Scholar] [CrossRef]
- Xie, L.; Wang, L.; Pang, X.; Liu, Q.; Yin, C. Effects of soil water regime and nitrogen addition on ectomycorrhizal community structure of Picea asperata seedlings. J. Plant Nutr. Soil Sci. 2021, 184, 415–429. [Google Scholar] [CrossRef]
- Hedin, L.O. Global organization of terrestrial plant–nutrient interactions. Proc. Natl. Acad. Sci. USA 2004, 101, 10849–10850. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Yao, X.; Wen, Y.; Zhou, Z.; Lei, W.; Zhang, D.; Lin, H. Nitrogen-inducible GLK1 modulates phosphate starvation response via the PHR1-dependent pathway. New Phytol. 2022, 236, 1871–1887. [Google Scholar] [CrossRef] [PubMed]
- Lü, X.-T.; Reed, S.; Yu, Q.; He, N.-P.; Wang, Z.-W.; Han, X.-G. Convergent responses of nitrogen and phosphorus resorption to nitrogen inputs in a semiarid grassland. Glob. Change Biol. 2013, 19, 2775–2784. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.; Ma, C.; Lou, C.; Yuan, W.; Zhu, N.; Wang, G.G.; Wu, T. Leaf nitrogen and phosphorus stoichiometry of the halophytes across China. Front. Plant Sci. 2023, 14, 1276699. [Google Scholar] [CrossRef]
- DeMalach, N. Toward a mechanistic understanding of the effects of nitrogen and phosphorus additions on grassland diversity. Perspect. Plant Ecol. 2018, 32, 65–72. [Google Scholar] [CrossRef]
- Phoenix, G.K.; Booth, R.E.; Leake, J.R.; Read, D.J.; Grime, J.P.; Lee, J.A. Simulated pollutant nitrogen deposition increases P demand and enhances root-surface phosphatase activities of three plant functional types in a calcareous grassland. New Phytol. 2004, 161, 279–290. [Google Scholar] [CrossRef]
- Fang, X.; Yang, D.; Deng, L.; Zhang, Y.; Lin, Z.; Zhou, J.; Chen, Z.; Ma, X.; Guo, M.; Lu, Z.; et al. Phosphorus uptake, transport, and signaling in woody and model plants. For. Res. 2024, 4, e017. [Google Scholar] [CrossRef]
- Kou, L.; Chen, W.; Jiang, L.; Dai, X.; Fu, X.; Wang, H.; Li, S. Simulated nitrogen deposition affects stoichiometry of multiple elements in resource-acquiring plant organs in a seasonally dry subtropical forest. Sci. Total Environ. 2018, 624, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Xia, M.; Wei, X.; Chang, W.; Liu, Y.; Wang, Z. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol. 2008, 180, 673–683. [Google Scholar] [CrossRef]
- Gao, X.; Gao, Y.; Li, X.; Zhang, C.; Zeng, Q.; Yuan, X.; Chen, Y.; Yu, Y.; Fu, S. Eleven-year canopy nitrogen addition enhances the uptake of phosphorus by plants and accelerates its depletion in soil. Forests 2024, 15, 416. [Google Scholar] [CrossRef]
- Perring, M.P.; Hedin, L.O.; Levin, S.A.; McGroddy, M.; de Mazancourt, C. Increased plant growth from nitrogen addition should conserve phosphorus in terrestrial ecosystems. Proc. Natl. Acad. Sci. USA 2008, 105, 1971–1976. [Google Scholar] [CrossRef]


| Year | 2018 | 2019 | 2020 | |
|---|---|---|---|---|
| AFS-AR-N | CK | 2.79 ± 0.10 Cb | 3.45 ± 0.06 Bb | 5.55 ± 0.07 Ad |
| N | 3.42 ± 0.01 Ba | 3.52 ± 0.03 Bab | 6.65 ± 0.07 Ab | |
| P | 1.00 ± 0.00 Cc | 3.60 ± 0.01 Bab | 7.23 ± 0.07 Aa | |
| NP | 2.72 ± 0.02 Cb | 3.50 ± 0.02 Bab | 6.25 ± 0.00 Ac | |
| AFS-AR-P | CK | 3.43 ± 0.90 Aa | 3.13 ± 0.01 Ab | 3.53 ± 0.00 Ac |
| N | 1.51 ± 0.00 Cb | 2.05 ± 0.03 Bd | 2.19 ± 0.00 Ad | |
| P | 3.02 ± 0.01 Cab | 4.32 ± 0.01 Bab | 4.66 ± 0.01 Aa | |
| NP | 1.98 ± 0.01 Cab | 2.99 ± 0.01 Bc | 3.56 ± 0.01 Ab | |
| AFS-AR-K | CK | 0.41 ± 0.01 Bb | 0.23 ± 0.01 Cc | 0.55 ± 0.00 Ab |
| N | 0.55 ± 0.00 Ca | 0.56 ± 0.00 Bab | 0.83 ± 0.00 Aa | |
| P | 0.34 ± 0.01 Bc | 0.50 ± 0.01 Ab | 0.50 ± 0.00 Ac | |
| NP | 0.34 ± 0.01 Cc | 0.55 ± 0.01 Aab | 0.45 ± 0.01 Bd | |
| Year | 2018 | 2019 | 2020 | |
|---|---|---|---|---|
| TFAR-TR-N | CK | 0.90 ± 0.00 Ca | 1.11 ± 0.00 Ba | 1.41 ± 0.00 Aa |
| N | 0.66 ± 0.03 Cc | 0.80 ± 0.00 Bd | 1.12 ± 0.00 Ac | |
| P | 0.85 ± 0.00 Ca | 1.07 ± 0.01 Bb | 1.21 ± 0.01 Ab | |
| NP | 0.73 ± 0.03 Bb | 0.91 ± 0.01 Ac | 0.64 ± 0.01 Cd | |
| TFAR-TR-P | CK | 0.83 ± 0.02 Cb | 1.02 ± 0.00 Bb | 1.20 ± 0.00 Ac |
| N | 1.10 ± 0.00 Ca | 1.22 ± 0.00 Ba | 1.50 ± 0.00 Aa | |
| P | 0.64 ± 0.02 Cd | 0.74 ± 0.01 Bd | 0.92 ± 0.01 Ad | |
| NP | 0.78 ± 0.00 Cc | 0.98 ± 0.00 Bc | 1.23 ± 0.00 Ab | |
| TFAR-TR-K | CK | 1.04 ± 0.01 Ca | 1.50 ± 0.00 Ba | 1.60 ± 0.00 Aa |
| N | 0.91 ± 0.01 Cd | 1.42 ± 0.02 Ab | 1.30 ± 0.01 Bd | |
| P | 0.98 ± 0.00 Bb | 1.44 ± 0.01 Ab | 1.45 ± 0.02 Ac | |
| NP | 0.94 ± 0.01 Cc | 1.44 ± 0.00 Bb | 1.50 ± 0.00 Ab | |
| Year | 2018 | 2019 | 2020 | |
|---|---|---|---|---|
| TFTR-T-N | CK | 1.46 ± 0.06 Aa | 1.15 ± 0.01 Ba | 0.47 ± 0.00 Cb |
| N | 1.30 ± 0.01 Ab | 1.04 ± 0.04 Bb | 0.39 ± 0.03 Cc | |
| P | 1.36 ± 0.01 Ab | 1.18 ± 0.01 Ba | 0.46 ± 0.00 Cb | |
| NP | 1.50 ± 0.01 Aa | 1.15 ± 0.01 Ba | 1.12 ± 0.01 Ca | |
| TFTR-T-P | CK | 1.74 ± 0.03 Bc | 2.44 ± 0.02 Aa | 0.82 ± 0.01 Cb |
| N | 2.86 ± 0.06 Aa | 2.42 ± 0.27 Aa | 1.03 ± 0.01 Ba | |
| P | 0.74 ± 0.01 Bd | 1.64 ± 0.06 Ab | 0.70 ± 0.02 Bc | |
| NP | 2.01 ± 0.11 Ab | 2.19 ± 0.07 Aa | 1.05 ± 0.01 Ba | |
| TFTR-T-K | CK | 1.26 ± 0.06 Aa | 0.95 ± 0.01 Ba | 0.92 ± 0.01 Ba |
| N | 1.10 ± 0.01 Ab | 0.84 ± 0.04 Bb | 0.55 ± 0.00 Cb | |
| P | 1.16 ± 0.01 Ab | 0.98 ± 0.01 Ba | 0.56 ± 0.00 Cb | |
| NP | 1.30 ± 0.01 Aa | 0.95 ± 0.01 Ba | 0.92 ± 0.01 Ca | |
| Year | 2018 | 2019 | 2020 | |
|---|---|---|---|---|
| TFT-N-N | CK | 0.90 ± 0.00 Aab | 0.87 ± 0.00 Ba | 0.68 ± 0.00 Cb |
| N | 0.88 ± 0.00 Bbc | 0.86 ± 0.00 Bb | 2.75 ± 0.18 Aa | |
| P | 0.89 ± 0.00 Ab | 0.87 ± 0.00 Ba | 0.62 ± 0.00 Cb | |
| NP | 0.90 ± 0.00 Aa | 0.87 ± 0.00 Ba | 0.87 ± 0.00 Bb | |
| TFT-N-P | CK | 0.91 ± 0.00 Bc | 0.94 ± 0.00 Aa | 0.82 ± 0.00 Cb |
| N | 0.95 ± 0.00 Aa | 0.94 ± 0.01 Ba | 0.85 ± 0.00 Ba | |
| P | 0.80 ± 0.00 Bd | 0.91 ± 0.00 Ab | 0.79 ± 0.01 Bc | |
| NP | 0.92 ± 0.00 Ab | 0.93 ± 0.00 Aa | 0.86 ± 0.00 Ba | |
| TFT-N-K | CK | 0.88 ± 0.01 Bab | 0.84 ± 0.00 Ca | 0.96 ± 0.02 Aab |
| N | 0.86 ± 0.00 Bc | 0.82 ± 0.01 Bb | 1.40 ± 0.02 Aa | |
| P | 0.87 ± 0.00 Abc | 0.85 ± 0.00 Aa | 1.22 ± 0.26 Aab | |
| NP | 0.88 ± 0.00 Aa | 0.84 ± 0.00 Ba | 0.84 ± 0.00 Bb | |
| Year | 2018 | 2019 | 2020 | |
|---|---|---|---|---|
| NRE (%) | CK | 57.37 ± 1.22 Ab | 52.80 ± 0.26 Bb | 56.37 ± 0.13 Aa |
| N | 49.13 ± 0.27 Ad | 48.17 ± 0.38 Ac | 48.07 ± 1.11 Ac | |
| P | 61.27 ± 0.28 Aa | 56.77 ± 0.52 Ca | 58.53 ± 0.64 Ba | |
| NP | 56.67 ± 0.47 Ac | 53.07 ± 0.09 Bb | 52.90 ± 1.59 Bb | |
| PRE (%) | CK | 61.70 ± 0.87 Aa | 60.93 ± 0.30 Aab | 62.40 ± 0.06 Aa |
| N | 61.73 ± 0.28 Aa | 62.40 ± 0.67 Aa | 62.10 ± 0.42 Aa | |
| P | 52.27 ± 0.24 Ab | 51.20 ± 0.87 Ac | 51.17 ± 0.55 Ab | |
| NP | 61.07 ± 0.3 ABa | 60.27 ± 0.39 Bb | 61.67 ± 0.24 Aa | |
| KRE (%) | CK | 41.43 ± 0.79 Aa | 41.67 ± 0.54 Aab | 42.73 ± 0.50 Aa |
| N | 34.83 ± 0.28 Ac | 32.63 ± 0.47 Bc | 32.03 ± 0.30 Bd | |
| P | 41.40 ± 0.20 Aa | 40.83 ± 0.09 Ab | 37.47 ± 0.20 Bc | |
| NP | 36.7 ± 0.36 Cb | 42.4 ± 0.26 Aa | 40.30 ± 0.36 Bb | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Wu, A.; Jia, T.; Guo, S.; Yi, M.; Cheng, Z.; Lai, M.; Zhang, L. Reshaping Nutrient Resorption Efficiency: Adaptive Strategies of Subtropical Slash Pine Plantations to Nitrogen and Phosphorus Additions. Forests 2025, 16, 928. https://doi.org/10.3390/f16060928
Fu Y, Wu A, Jia T, Guo S, Yi M, Cheng Z, Lai M, Zhang L. Reshaping Nutrient Resorption Efficiency: Adaptive Strategies of Subtropical Slash Pine Plantations to Nitrogen and Phosphorus Additions. Forests. 2025; 16(6):928. https://doi.org/10.3390/f16060928
Chicago/Turabian StyleFu, Yuxin, Anqi Wu, Ting Jia, Shengmao Guo, Min Yi, Zishan Cheng, Meng Lai, and Lu Zhang. 2025. "Reshaping Nutrient Resorption Efficiency: Adaptive Strategies of Subtropical Slash Pine Plantations to Nitrogen and Phosphorus Additions" Forests 16, no. 6: 928. https://doi.org/10.3390/f16060928
APA StyleFu, Y., Wu, A., Jia, T., Guo, S., Yi, M., Cheng, Z., Lai, M., & Zhang, L. (2025). Reshaping Nutrient Resorption Efficiency: Adaptive Strategies of Subtropical Slash Pine Plantations to Nitrogen and Phosphorus Additions. Forests, 16(6), 928. https://doi.org/10.3390/f16060928






