A Contribution to the Knowledge of Polypores Occurring in City Parks: A Case Study of Five Parks in Wrocław (Lower Silesia, Poland)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.1.1. Site Characteristics–Wschodni Park
2.1.2. Site Characteristics–Zachodni Park
2.1.3. Site Characteristics–Grabiszyński Park
2.1.4. Site Characteristics–Południowy Park
2.1.5. Site Characteristics–Brochowski Park
2.2. Study Design and Sampling
2.3. Statistical Analyses
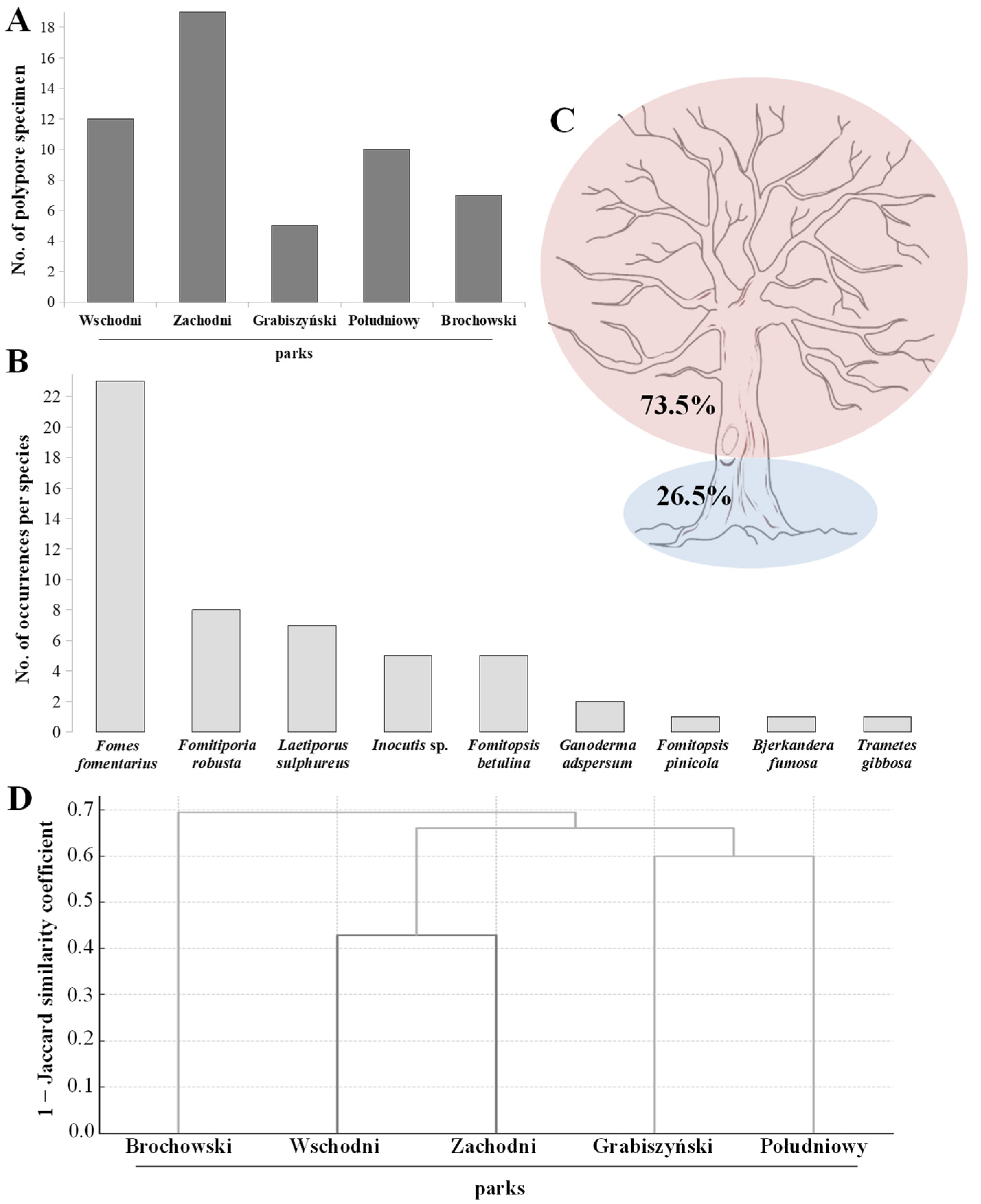
3. Results
3.1. Species Records–Wschodni Park
3.2. Species Records–Zachodni Park
3.3. Species Records–Grabiszyński Park
3.4. Species Records–Południowy Park
3.5. Species Records–Brochowski Park
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- He, X.; Yang, T.; Rinne-Garmston, K.T.; Ji, X.; Xu, Q.; Xu, Z.; Zheng, Y.; Zhao, Z.; Zhao, G.; Hu, Z. Wood-inhabiting fungal community characteristics responses to nutrient additions vary among tree taxonomic groups. J. Soils Sediments 2025, 25, 1497–1513. [Google Scholar] [CrossRef]
- Zjawiony, J.K. Biologically active compounds from Aphyllophorales (polypore) fungi. J. Nat. Prod. 2004, 67, 300–310. [Google Scholar] [CrossRef]
- Erkkilä, R.; Niemelä, T. Polypores in the parks and forests of the city of Helsinki. Karstenia 1986, 26, 1–40. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Y.F.; Nakasone, K.K.; Liu, S.L.; Huang, M.R.; He, S.H. Species diversity, taxonomy, multi-gene phylogeny, and divergence times of Meruliaceae (Polyporales, Basidiomycota). Mycology 2025, 1–42. [Google Scholar] [CrossRef]
- Wainhouse, M.; Boddy, L. Making hollow trees: Inoculating living trees with wood-decay fungi for the conservation of threatened taxa—A guide for conservationists. Glob. Ecol. Conserv. 2022, 33, e01967. [Google Scholar] [CrossRef]
- Runnel, K.; Miettinen, O.; Lõhmus, A. Polypore fungi as a flagship group to indicate changes in biodiversity—A test case from Estonia. IMA Fungus 2021, 12, 2. [Google Scholar] [CrossRef]
- Gdula, A.K.; Konwerski, S.; Olejniczak, I.; Rutkowski, T.; Skubała, P.; Zawieja, B.; Gwiazdowicz, D.J. The role of bracket fungi in creating alpha diversity of invertebrates in the Białowieża National Park, Poland. Ecol. Evol. 2021, 11, 6456–6470. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, J.; Ławrynowicz, M. Stan zbadania grzybów wielkoowocnikowych miast Polski. Wiadomości Bot. 1991, 35, 3–9. [Google Scholar]
- Bail, T. Zusammenstellung der Hymenomyceten in Schlesien und der Niederlausitz. Jahresber. Der Schlesischen Ges. Für Vaterl. Cult. 1860, 38, 88–109. [Google Scholar]
- Schröter, J. Zusammenstellung der im Breslauer botanischen Garten beobachteten Pilze. Jahresber. Der Schlesischen Ges. Für Vaterl. Cult. 1872, 50, 97–111. [Google Scholar]
- Schröter, J. Die Pilze Schlesiens. In Kryptogamen-Flora von Schlesien; Cohn, F., Ed.; J.U. Kern’s Verlag: Tübingen, Germany; p. 814.
- Dittrich, G. Pilzfunde im Breslauer Südpark. Jahresbericht der Schlesischen Gesellschaft für Vaterl. Culture 1931, 104, 129–139. [Google Scholar]
- Dittrich, G. Artkritische und standortkundliche Beiträge zur schlesischen Pilzflora. II. Berichte Der Dtsch. Bot. Ges. 1936, 54, 50–64. [Google Scholar]
- Dittrich, G. Artkritische und standortkundliche Beiträge zur schlesischen Pilzflora. III. Berichte Der Dtsch. Bot. Ges. 1937, 55, 67–73. [Google Scholar]
- Halama, M. Grzyby makroskopijne. In Leksykon Zieleni Wrocławia; Bińskowska, I., Szopińska, E., Eds.; Wydawnictwo Via Nova: Wrocław, Poland, 2013; pp. 818–819. [Google Scholar]
- Halama, M. Zagrożone, rzadkie i słabo poznane grzyby makroskopijne występujące na terenie miasta Wrocławia. Zesz. Nauk. Uniw. Przyr. We Wrocławiu 2016, 117, 37–48. [Google Scholar]
- Kopij, G. Breeding densities of woodpeckers (Picinae) in the inner and outer zones of a Central European city. Sylvia 2017, 53, 41–57. [Google Scholar]
- Bujoczek, L.; Bujoczek, M.; Zięba, S. How much, why and where? Deadwood in forest ecosystems: The case of Poland. Ecol. Indic. 2021, 121, 107027. [Google Scholar] [CrossRef]
- Spychała, K.; Kłosińska, K.; Salwińska, W.; Ogórek, R. Diversity of Soil-Borne Fungi Isolated from Places Frequently Visited by People in the City of Wrocław (Poland). Appl. Sci. 2024, 14, 2782. [Google Scholar] [CrossRef]
- Szopińska, E.; Popów-Nowicka, A. The Landscape and Environmental Values of Eastern Park in Wrocław. Archit. Kraj. 2009, 4, 70–73. (In Polish) [Google Scholar]
- Misztal, K. Breeding biology of the Wrocław population of Nuthatch Sitta europaea L. against the gradient of urbanization and in comparison to natural areas. Przegląd Przyr. 2008, XIX, 131–164. (In Polish) [Google Scholar]
- Grochowski, P. Breeding ecology of the blackbird Turdus Merula at the Grabiszyński park. Ptaki Śląska 2008, 17, 5–17. (In Polish) [Google Scholar]
- Bińkowska, I.; Szopińska, E. Leksykon Zieleni Wrocławia; Wydawnictwo Via Nova: Wrocław, Poland, 2013. [Google Scholar]
- Paciorkiewicz, P.; Chwałko, E.; Skała, C. Park Południowy. Wrocław I Okolice. Praktyczny Przewodnik; Pascal: Bielsko-Biała, Poland, 2005. [Google Scholar]
- Krop-Andrzejczuk, D. Spacer historyczno-przyrodniczy po parkach Brochowa i Bieńkowic. Maj Z Drzewami 2018, 2–15. Available online: http://miastodrzew.wroclaw.pl/wp-content/uploads/2018/06/Spacer-historyczno-przyrodniczy_Broch%C3%B3w_Bi%C5%84kowice_D.Krop-Andrzejczuk_v.2.pdf (accessed on 8 May 2025).
- Łakomy, P.; Kwaśna, H. Atlas Hub, 1st ed.; MULTICO: Warszawa, Poland, 2015. [Google Scholar]
- Szczepkowski, A. Grzyby nadrzewne w innym świetle—użytkowanie owocników. Stud. I Mater. CEPL W Rogowie 2012, 32, 171–189. [Google Scholar]
- Ryvarden, L.; Melo, I. Poroid fungi of Europe; Oslo Fungiflora: Oslo, Norway, 2014; Available online: https://fungi.myspecies.info/content/poroid-fungi-europe (accessed on 8 May 2025).
- Mirek, Z.; Piękoś-Mirkowa, H.; Zając, A.; Zając, M. Flowering plants and pteridophytes of Poland. A checklist. In Biodiversity of Poland; Mirek, Z., Ed.; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2002. [Google Scholar]
- Spellerberg, I.F.; Fedor, P.J. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the “Shannon-Wiener” Index. Glob. Ecol. Biogeogr. 2003, 12, 177–179. [Google Scholar] [CrossRef]
- Lakićević, M.; Srđević, B. Measuring Biodiversity in Forest Communities—A Role of Biodiversity Indices. Contemp. Agric. 2018, 67, 65–70. [Google Scholar] [CrossRef]
- Gáper, J.; Sliacka, I.; Hvolková, L. Diversity and ecology of polypores in urban vegetation of northern, central and southern Slovakia. Folia Oecologica 2014, 41, 17–23. [Google Scholar]
- Mamadashvili, G.; Brin, A.; Chumak, M.; Diedus, V.; Drössler, L.; Förster, B.; Georgiev, K.B.; Ghrejyan, T.; Hleb, R.; Kalashian, M.; et al. Drivers of wood-inhabiting fungal diversity in European and Oriental beech forests. Ecol. Evol. 2024, 14, e11660. [Google Scholar] [CrossRef]
- Sunhede, S. Wood and bark inhabiting fungi on Quercus in Northern Europe. In Proceedings of the International Conference on Mycology and Lichenology, Vilnius, Lithuania, 1993; p. 128. Available online: https://libris.kb.se/bib/10371170 (accessed on 8 May 2025).
- Wojewoda, W. Checklist of Polish Larger Basidiomycetes, 7th ed.; Mirek, Z., Ed.; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2003; ISBN 2013206534. [Google Scholar]
- Hagara, L.; Antonín, V.; Baier, J. Atlas Húb; Aventinum: Praha, Czech Republic, 2005. [Google Scholar]
- Tomšovský, M.; Kaeochulsri, S.; Kudláček, T.; Dálya, L.B. Ecological, morphological and phylogenetic survey of Fomes fomentarius and F. inzengae (Agaricomycetes, Polyporaceae) co-occurring in the same geographic area in Central Europe. Mycol. Prog. 2023, 22, 79. [Google Scholar] [CrossRef]
- Pirronitto, S.; Teng, F.; Verheyen, C.; Gaucet, V.; Henin, J.M.; Jourez, B.; Schmitz, S.; Chandelier, A. Characterization of Fomes fomentarius s.s. and F. inzengae in Belgian Beech Forests. Forests 2024, 15, 221. [Google Scholar] [CrossRef]
- Böhm, Š. Biodiverzita Dřevních hub v Urbanizovaném Prostředí České Republiky. Bachelor’s Thesis, Mendelova Univerzita v Brně, Faculty of Forestry and Wood Technology, Brno, Czech Republic, 2017. Available online: https://theses.cz/id/co0xwm/ (accessed on 10 December 2024).
- Kobza, M.; Ostrovský, R.; Adamčíková, K.; Pastirčáková, K. Stability of trees infected by wood decay fungi estimated by acoustic tomography: A field survey. Trees 2022, 36, 103–112. [Google Scholar] [CrossRef]
- Szczepkowski, A.; Referowska, E.; Lachowicz, H.; Bijak, S. Ability of Selected Basidiomycetous Fungi to Decay Black Locust Wood. Drewno. Pr. Nauk. Doniesienia. Komun. 2025, 68, 00049. [Google Scholar] [CrossRef]
- Sierota, Z.; Szczepkowski, A. Rozpoznawanie Chorób Infekcyjnych Drzew Leśnych; Butwiłowska, B., Ed.; Centrum Informacyjne Lasów Państwowych: Warszawa, Poland, 2014; ISBN 9788363895372.
- Schwarze, F.W.M.R.; Engels, J.; Mattheck, C. Fungal Strategies of Wood Decay in Trees; Springer Nature: Berlin/Heidelberg, Germany, 2000. [Google Scholar] [CrossRef]
- Sunhede, S.; Vasiliauskas, R. Ecology and decay pattern of Inocutis dryophila in Quercus robur. Karstenia 2003, 43, 45–53. [Google Scholar] [CrossRef][Green Version]
- Pleszczyńska, M.; Lemieszek, M.K.; Siwulski, M.; Wiater, A.; Rzeski, W.; Szczodrak, J. Fomitopsis betulina (formerly Piptoporus betulinus): The Iceman’s polypore fungus with modern biotechnological potential. World J. Microbiol. Biotechnol. 2017, 33, 83. [Google Scholar] [CrossRef] [PubMed]
- Han, M.L.; Chen, Y.Y.; Shen, L.L.; Song, J.; Vlasák, J.; Dai, Y.C.; Cui, B.K. Taxonomy and phylogeny of the brown-rot fungi: Fomitopsis and its related genera. Fungal Divers. 2016, 80, 343–373. [Google Scholar] [CrossRef]
- Giminder, A. Atlas grzybów. Jak Bezbłędnie Oznaczać 340 Gatunków Grzybów Europy Środkowej; Weltbild: Augsburg, Germany, 2011. [Google Scholar]
- Dubicki, A.; Dubicka, M. Klimat Wrocławia. In Informator O Stanie Środowiska Wrocławia; Smolnicki, K., Szykasiuk, M., Eds.; Dolnośląska Fundacja Ekorozwoju: Wrocław, Poland, 2002; pp. 9–25. [Google Scholar]
- Piętka, J.; Borowski, J. Beetles caught into sawdust substrate traps with Trametes gibbosa (Pers.: Fr.) Fr. mycelium in the Świętokrzyski National Park. Sylwan 2011, 155, 633–641. (In Polish) [Google Scholar]
- Loyd, A.L.; Linder, E.R.; Anger, N.A.; Richter, B.S.; Blanchette, R.A.; Smith, J.A. Pathogenicity of ganoderma species on landscape trees in the Southeastern United States. Plant Dis. 2018, 102, 1944–1949. [Google Scholar] [CrossRef] [PubMed]
- Türkkan, M.; Derviş, S.; Özgümüş, Ö.; Özer, G. Ganoderma butt rot of hazelnut (Corylus avellana) caused by Ganoderma adspersum in Türkiye. For. Pathol. 2024, 54, e12872. [Google Scholar] [CrossRef]
- Pagin-cláudio, F.; Gugliotta, A.D.M. Six new records of polypores (Agaricomycetes, Basidiomycota) from Southeast Brazil (Atlantic Forest) and perspectives in basic mycological research. Rodriguesia 2024, 76, e00672024. [Google Scholar] [CrossRef]
- Kaur, A.; Attri, S.; Kumar, A.; Mohana, P.; Singh, S.; Kaur, P.; Ram, E.; Dhingra, G.S.; Arora, S.; Singh, A.P. Valorization of Polypore Mushroom Phellinus fastuosus by Analyzing Antioxidative, Antiproliferative and Apoptosis Induction Potential. Waste Biomass Valorization 2023, 14, 2659–2672. [Google Scholar] [CrossRef]
- Gaurav, K.; Patil, V. Addition of Some Wood-Rotting Polypores from Satara District (M.S). J. Mycopathol. Res. 2023, 61, 235–237. [Google Scholar] [CrossRef]
- Patil, A.R.; Patil, Y.; Vedpathak, M. Diversity of Polypores (Basidiomycota: Aphyllophorales) from Kolhapur District (M.S.), India. Adv. Biores. 2025, S1, 36–39. [Google Scholar]
- Silenius, V. Forest Biodiversity Value and the Structural Characteristics of Forests and Polypore Species. Master’s Thesis, University of Jyväskylä, Jyväskylän yliopisto, Finland, 2024. [Google Scholar]
- Chemutai Sum, W.; Ebada, S.S.; Kellner, H.; Stadler, M. Comparative Study of Toxic Terpenoidal Aldehydes and Lactone Derivatives from the European Polypore Bondarzewia mesenterica. ACS Omega 2024, 9, 18668–18673. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, Y.; Yang, Z.; Liu, H.; Wu, F.; Dai, Y.; Yuan, Y. Polypore funga and species diversity in tropical forest ecosystems of Africa, America and Asia, and a comparison with temperate and boreal regions of the Northern Hemisphere. For. Ecosyst. 2024, 11, 100200. [Google Scholar] [CrossRef]
- Andjièrèyir, K.S.; Samson, N.; Sylvie, N.R.; Benovana, B.; Edouard, S.K.J.; Kounbo, D.; Elise, S. Taxonomic Study of Five Parasitic Polypores of the Hymenochaetaceae Family of TIN Vegetation in Western Burkina Faso. Am. J. Plant Sci. 2024, 15, 441–454. [Google Scholar] [CrossRef]
- Kujawa, A.; Gierczyk, B.; Kudławiec, B.; Stokłosa, N.; Bujakiewicz, A. Macromycetes of the palace park in poznań-radojewo (Wielkopolska region, Poland). Acta Mycol. 2020, 55, 1–19. [Google Scholar] [CrossRef]
- Wojewoda, W. Changes in macrofungal flora of Cracow (S. Poland). Veröff. Geobot. Inst. ETH Stift. Rubel Zürich 1991, 106, 150–161. [Google Scholar]
- Wołkowycki, M. Grzyby Poliporoidalne Białegostoku; Prezydent Miasta Białegostoku: Białystok, Poland, 2022; ISBN 9788395450495. [Google Scholar]
- Jim, C.Y.; Zhang, H. Species diversity and spatial differentiation of old-valuable trees in urban Hong Kong. Urban For. Urban Green. 2013, 12, 171–182. [Google Scholar] [CrossRef]

| Fungal spp. | Tree spp. or Type | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Acer pseudoplatanus | Betula pendula | Carpinus betulus | Fagus sylvatica | Quercus petraea | Quercus robur | Quercus rubra | Salix babylonica | U/I | |
| Inocutis sp. | 5 | ||||||||
| Fomitopsis betulina | 5 | ||||||||
| Fomes fomentarius | 2 | 7 | 1 | 1 | 3 | 1 | 1 | 7 | |
| Fomitopsis pinicola | 1 | ||||||||
| Fomitiporia robusta | 5 | 3 | |||||||
| Ganoderma adspersum | 1 | 1 | |||||||
| Laetiporus sulphureus | 4 | 1 | 1 | 1 | |||||
| Bjerkandera fumosa | 1 | ||||||||
| Trametes gibbosa | 1 | ||||||||
| Site No. | Latitude (N) | Longitude (E) | Fungal spp. | Age and Condition of Basidiocarps |
|---|---|---|---|---|
| 1 | 51°05′04.5″ | 17°04′34.4″ | Fomes fomentarius | young bcs |
| descr. | growing high on a damaged, mature “weeping” willow (Salix babylonica’ Babylon’) | |||
| 2 | 51°05′03.2″ | 17°04′37.1″ | Ganoderma adspersum | mostly old bcs (greenish from above due to algae growth), also single young fb |
| descr. | growing on a tree cut from the top, at the base of the trunk and at medium height (U/I sp.) | |||
| 3 | 51°05′02.9″ | 17°04′38.2″ | F. fomentarius | old bcs, turning light to very dark green from above due to algae growth |
| descr. | growing on a decaying trunk (outside and inside the trunk) (U/I sp.) | |||
| 4 | 51°05′03.9″ | 17°04′38.5″ | F. fomentarius | young single bcs, the edge of which was light green in color |
| descr. | growing on a damaged, broken tree (U/I sp.) | |||
| 5 | 51°05′03.9″ | 17°04′40.0″ | F. fomentarius | young bcs |
| descr. | growing on trunks (U/I tree sp.) | |||
| 6 | 51°05′04.0″ | 17°04′46.2″ | F. fomentarius | young bcs |
| descr. | growing on a very damaged, long-term pedunculate oak (Quercus robur) | |||
| 7 | 51°05′00.8″ | 17°04′53.6″ | F. fomentarius | middle-aged bcs |
| descr. | growing high on a tree cut from above (U/I tree sp.) | |||
| 8 | 51°05′04.9″ | 17°05′05.7″ | F. fomentarius | young bcs |
| descr. | growing high on a tree cut from above (U/I tree sp.) | |||
| 9 | 51°05′04.9″ | 17°05′03.6″ | Fomitiporia robusta | middle-aged single bcs |
| descr. | growing on a mature pedunculate oak, at medium height (Q. robur) | |||
| 10 | 51°05′11.3″ | 17°05′05.6″ | Trametes gibbosa | young bcs |
| descr. | numerous specimens growing on a young pedunculate oak (Q. robur) | |||
| 11 | 51°05′10.9″ | 17°05′05.6″ | Inocutis sp. | old bcs |
| descr. | growing high on a middle-aged pedunculate oak (Q. robur) | |||
| 12 | 51°05′09.4″ | 17°05′18.1″ | F. fomentarius | young single bcs |
| descr. | growing high on a mature pedunculate oak (Q. robur) | |||
| Site No. | Latitude (N) | Longitude (E) | Fungal spp. | Age and Condition of Basidiocarps |
|---|---|---|---|---|
| 1 | 51°07′48.7″ | 16°58′20.4″ | Fomitopsis betulina | middle-aged bcs |
| descr. | growing high on a Betula pendula cut from above | |||
| 2 | 51°07′50.9″ | 16°58′19.1″ | F. betulina | young bcs |
| descr. | growing high on a mature B. pendula | |||
| 3 | 51°07′51.2″ | 16°58′17.8″ | Ganoderma adspersum | old bcs |
| descr. | growing on a mature pedunculate oak, at medium height (Quercus robur) | |||
| 4 | 51°07′49.6″ | 16°58′24.3″ | Fomes fomentarius | young single bcs |
| descr. | growing high on a mature B. pendula | |||
| 5 | 51°07′44.2″ | 16°58′32.7″ | F. fomentarius | young bcs (top) and single older one (bottom) |
| descr. | numerous specimens growing on a middle-aged B. pendula | |||
| 6 | 51°07′43.9″ | 16°58′32.9″ | F. fomentarius | young bcs |
| descr. | numerous specimens growing on a middle-aged B. pendula | |||
| 7 | 51°07′44.3″ | 16°58′34.9″ | Laetiporus sulphureus | young bcs |
| descr. | growing at the base of the trunk of a mature Carpinus betulus | |||
| 8 | 51°07′43.9″ | 16°58′35.0″ | L. sulphureus | young single bcs |
| descr. | growing high on a mature C. betulus | |||
| 9 | 51°07′43.7″ | 16°58′37.3″ | Inocutis sp. | old bcs |
| descr. | growing on a mature Q. robur at medium height | |||
| 10 | 51°07′46.6″ | 16°58′40.9″ | L. sulphureus | young bcs |
| descr. | growing on a mature Quercus rubra at medium height | |||
| 11 | 51°07′46.0″ | 16°58′42.5″ | Inocutis sp. | old bcs |
| descr. | growing high on a mature Q. robur | |||
| 12 | 51°07′46.1″ | 16°58′42.8″ | L. sulphureus | young bcs |
| descr. | growing low on a mature Q. robur | |||
| 13 | 51°07′48.0″ | 16°58′37.1″ | F. fomentarius | damaged, young bcs |
| descr. | growing low and high on a tree cut from the top B. pendula | |||
| 14 | 51°07′47.1″ | 16°58′33.6″ | F. betulina | young single bcs |
| descr. | growing high on a mature B. pendula | |||
| 15 | 51°07′51.8″ | 16°58′25.9″ | Inocutis sp. | old bcs, taking on a greenish color over the entire surface due to algae growth |
| descr. | growing high on a mature Q. robur | |||
| 16 | 51°07′52.2″ | 16°58′25.9″ | Fomitiporia robusta | old bcs, turning green from above due to algae growth |
| descr. | growing at an average height on mature Q. robur | |||
| 17 | 51°07′52.4″ | 16°58′25.2″ | Inocutis sp. | old bcs |
| descr. | growing at an average height on mature Q. robur | |||
| 18 | 51°07′53.4″ | 16°58′08.3″ | F. fomentarius | young bcs, one taking on a greenish color over the entire surface due to algae growth |
| descr. | growing at an average to high height on a mature B. pendula | |||
| 19 | 51°07′52.5″ | 16°58′08.2″ | F. fomentarius | young bcs |
| descr. | growing at an average height on a mature B. pendula | |||
| Site No. | Latitude (N) | Longitude (E) | Fungal spp. | Age and Condition of Basidiocarps |
|---|---|---|---|---|
| 1 | 51°05′26.8″ | 16°58′49.5″ | Fomitopsis betulina | young bcs |
| descr. | growing on a mature Betula pendula, at medium height | |||
| 2 | 51°05′25.7″ | 16°58′47.8″ | F. betulina | young bcs |
| descr. | growing on a mature B. pendula cut from the top, at medium height | |||
| 3 | 51°05′29.3″ | 16°58′51.6″ | Laetiporus sulphureus | old bcs |
| descr. | growing at the base of the trunk of a mature Carpnius betulus | |||
| 4 | 51°05′28.7″ | 16°58′51.3″ | L. sulphureus | young bcs |
| descr. | growing at the base of the trunk of a mature C. betulus | |||
| 5 | 51°05′23.7″ | 16°58′56.8″ | Fomes fomentarius | young bcs |
| descr. | growing on a mature B. pendula, at medium height and a little below | |||
| Site No. | Latitude (N) | Longitude (E) | Fungal spp. | Age and Condition of Basidiocarps |
|---|---|---|---|---|
| 1 | 51°04′28.7″ | 17°00′44.7″ | Fomitopsis pinicola | old single bcs |
| descr. | growing at the base of the trunk of Quercus petraea | |||
| 2 | 51°04′26.1″ | 17°00′43.5″ | Laetiporus sulphureus | old single dried up bcs |
| descr. | growing high on Fagus sylvatica | |||
| 3 | 51°04′25.8″ | 17°00′41.7″ | Fomitiporia robusta | middle-aged single bcs |
| descr. | growing high on Quercus rubra | |||
| 4 | 51°04′26.2″ | 17°00′40.7″ | F. robusta | old single bcs, greenish from above due to algae growth |
| descr. | growing high on Q. rubra | |||
| 5 | 51°04′25.7″ | 17°00′40.3″ | F. robusta | old single bcs |
| descr. | growing high on Quercus robur | |||
| 6 | 51°04′24.7″ | 17°00′40.0″ | Fomes fomentarius | middle-aged single bcs |
| descr. | growing high on a tree cut from above (U/I tree sp.) | |||
| 7 | 51°04′36.0″ | 17°00′43.4″ | F. fomentarius | old bcs |
| descr. | growing on F. sylvatica, on medium height | |||
| 8 | 51°04′36.1″ | 17°00′40.5″ | F. fomentarius | middle-aged and old bcs |
| descr. | numerous specimens on Q. robur | |||
| 9 | 51°04′33.1″ | 17°00′36.7″ | F. fomentarius | young bcs |
| descr. | growing high on Q. rubra | |||
| 10 | 51°04′29.8″ | 17°00′38.9″ | F. robusta | old bcs, greenish from above due to algae growth |
| descr. | growing high on Q. rubra | |||
| Site No. | Latitude (N) | Longitude (E) | Fungal spp. | Age and Condition of Basidiocarps |
|---|---|---|---|---|
| 1 | 51°03′37.6″ | 17°04′22.0″ | Fomitoporia robusta | old bcs, turning green from above due to algae growth |
| descr. | growing high on a mature Quercus robur | |||
| 2 | 51°03′36.9″ | 17°04′21.9″ | Fomes fomentarius | young single bcs |
| descr. | growing high on a mature Acer pseudoplatanus | |||
| 3 | 51°03′36.2″ | 17°04′20.8″ | F. robusta | old bcs, turning green from above due to algae growth |
| descr. | growing at an average height on a mature Q. robur | |||
| 4 | 51°03′35.6″ | 17°04′15.4″ | F. fomentarius | young bcs |
| descr. | growing high on a mature A. pseudoplatanus | |||
| 5 | 51°03′37.5″ | 17°04′15.1″ | F. fomentarius | three young bcs and single old fb |
| descr. | growing high on a tree cut from above a mature Carpnius betulus | |||
| 6 | 51°03′40.6″ | 17°04′17.6″ | Bjerkandera fumosa | middle-aged bcs |
| descr. | growing from the bottom to a medium height on a damaged, mature A. pseudoplatanus | |||
| 7 | 51°03′44.8″ | 17°04′18.2″ | F. fomentarius | young bcs |
| descr. | growing on a mature tree cut from the top, at the base of the trunk (U/I tree sp.) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogórek, R.; Cal-Smok, M.; Suchodolski, J. A Contribution to the Knowledge of Polypores Occurring in City Parks: A Case Study of Five Parks in Wrocław (Lower Silesia, Poland). Forests 2025, 16, 908. https://doi.org/10.3390/f16060908
Ogórek R, Cal-Smok M, Suchodolski J. A Contribution to the Knowledge of Polypores Occurring in City Parks: A Case Study of Five Parks in Wrocław (Lower Silesia, Poland). Forests. 2025; 16(6):908. https://doi.org/10.3390/f16060908
Chicago/Turabian StyleOgórek, Rafał, Magdalena Cal-Smok, and Jakub Suchodolski. 2025. "A Contribution to the Knowledge of Polypores Occurring in City Parks: A Case Study of Five Parks in Wrocław (Lower Silesia, Poland)" Forests 16, no. 6: 908. https://doi.org/10.3390/f16060908
APA StyleOgórek, R., Cal-Smok, M., & Suchodolski, J. (2025). A Contribution to the Knowledge of Polypores Occurring in City Parks: A Case Study of Five Parks in Wrocław (Lower Silesia, Poland). Forests, 16(6), 908. https://doi.org/10.3390/f16060908








