1. Introduction
Oak trees are one of the broad-leaved deciduous species of most significant economic and ecological value within the forest ecosystems that are widely distributed across South Korea, where these species collectively cover over 25% of South Korea’s forested area. In South Korea, five oak species predominantly found across the country include
Quercus acutissima,
Q. aliena,
Q. mongolica,
Q. serrata, and
Q. variabilis [
1].
Oak wilt mortality was first detected in
Q. mongolica in Gyeonggi Province, South Korea, in 2004 and has subsequently spread throughout the country [
2,
3], inflicting considerable damage on forest ecosystems. Although the number of affected trees has gradually decreased due to the continuous monitoring, prevention, and control strategies for the disease, the number of affected trees surged in 2006, reaching a peak of 331,000 damaged trees in 2011. Since 2017, the number of damaged trees has stabilized at around 150,000 to 170,000 annually (
http://www.forest.go.kr (accessed on 1 March 2022)).
The pathogen,
Dryadomyces quercus-mongolicae, is linked to this oak wilt mortality, facilitated by mass infestations of the wood-boring ambrosia beetle vector,
Platypus koryoensis. This wood-boring ambrosia beetle transmits the fungal inoculum into oak trees that are stressed or weakened by various factors during its boring activity, facilitating systemic infection and wilting [
4,
5,
6,
7]. The wood-boring ambrosia beetle,
P. koryoensis, was reported to be distributed domestically as early as the 1930s. Given the fact that the distribution of
P. koryoensis has been documented in Russia and Korea, it is suggested that the wood-boring ambrosia beetle is a native species in these regions [
8].
It is widely accepted that entomopathogenic fungi play a role in regulating the density of host insects within natural ecosystems. These fungi exhibit high host specificity, targeting only the intended pest insects and thereby posing no known toxicity to the environment, livestock, or humans [
9]. Due to the development of resistance to chemical insecticides in pests and the challenges posed by hard-to-control pests, many studies have focused on biological or environmentally friendly control methods. The representative entomopathogenic fungi being studied include
Beauveria bassiana,
B. brongniartii,
Hirsutella thompsonii,
Isaria fumosorosea,
Lecanicillium muscarium,
Metarhizium anisopliae, and Nomuraea, which are commercially available [
9,
10]. In addition,
Purpureocillium lilacinum has been increasingly recognized beyond its traditional nematophagous role—namely, for its entomopathogenic properties. In this regard, it was shown that
P. lilacinum significantly reduced egg hatching and juvenile survival of
M. incognita in vitro and decreased root galling in eggplants, while also enhancing plant growth and photosynthetic pigment levels [
11].
In cases where entomopathogenic fungi have been utilized for environmentally friendly pest control in agriculture, an isolate of
I. fumosorosea FG340 is a representative example of their application in controlling thrips that affect cucumbers [
12]. In addition, the efficacy of entomopathogenic fungi as an environmentally friendly control strategy against cherry scale insects, a pest of cherry trees, has been evaluated using
B. bassiana ARP14 [
13].
In this study, the Tenebrio molitor baiting system was employed to isolate entomopathogenic fungi from the soil in South Korea, and those that were successfully recovered were subjected to identification based on morphology and DNA sequence comparisons. In addition, three strains, B. bassiana GHA, B. bassiana KACC 43988, and M. anisopliae KACC 40969, were obtained from KACC and utilized in this study. Different fungal strains were assessed to select those suitable for use as control agents against P. koryoensis, the vector of oak wilt disease, and to provide foundational data. This involved evaluating their stability under various environmental conditions, as well as analyzing their insecticidal efficacy and median lethal time (LT50) against the insect vector.
2. Materials and Methods
2.1. Sampling of the Insect Vector
The insect vector,
Platypus koryoensis, was targeted in the Suri-Mt. area located in Gunpo City [37.401388, 126.894722] and the forested area of Jung-dong in Hwaseong City [37.211944, 127.148611], where significant infestation was observed in
Quercus mongolica trees showing numerous entry holes by the insect vector and weakened vitality due to previous-year damage. Collection was conducted using mass trapping systems and multi-funnel traps (Lindgren multiple-funnel traps) [
14], and only vigorous individuals, confirmed within three days post-capture, were used for subsequent experiments.
2.2. Isolates of Entomopathogenic Fungi
The entomopathogenic fungus used in the experiment was isolated from soil collected in a Quercus dentata stand within the Mulhyanggi Arboretum, Osan City, Gyeonggi Province [37.165000, 127.056388]. Isolation was performed using the Tenebrio molitor baiting system, in which larvae of the mealworm beetle (Tenebrio molitor) were employed to selectively attract and recover fungal pathogens from the soil.
The collected soil was sieved through a 2 mm mesh, and approximately 100 mL of the sieved soil was placed in a container, into which five T. molitor larvae were introduced. The containers were incubated at approximately 25 °C for 14 days, during which the soil was mixed once daily, and the larvae were monitored for signs of fungal infection. Infected larvae were surface-sterilized by immersion in 3% sodium hypochlorite solution for 3 min, followed by two rinses in sterile distilled water, each for 3 min. The larvae were then placed on 90 mm Petri dishes lined with filter paper moistened with sterile distilled water and incubated at 25 °C for more than 7 days to induce sporulation. Fungal isolates were purified on potato dextrose agar (PDA, Difco, Detroit, MI, USA) and subcultured on Sabouraud dextrose agar (SDA, Difco, Detroit, MI, USA) for use in the experiment, which served as a conducive medium for the growth of pathogenic fungi due to its lower pH and high dextrose content.
The fungal isolates were further obtained from the Korea Agricultural Culture Collection (KACC), with one isolate of Beauveria bassiana and one isolate of Metarhizium anisopliae. Additionally, one isolate of B. bassiana GHA was obtained from the Department of Biological Resources at Andong National University and used in this study.
Although four isolates were initially considered—two isolates of B. bassiana (GHA and KACC 43988), one M. anisopliae (KACC 40969), and one Purpureocillium lilacinum (GG18031) recovered from soil—only three were eventually used in the insect bioassays. B. bassiana KACC 43988 was excluded due to poor sporulation and low viability during pre-testing. This selection ensured consistency and reliability in subsequent bioefficacy evaluations.
2.3. Identification of Entomopathogenic Fungi
2.3.1. Morphology
SDA medium was dispensed at 15 mL per 90 mm Petri dish, followed by streak inoculation to observe fungal colony growth. Morphological identification was conducted using the slide culture technique, and observations were made under a phase-contrast microscope [
15].
Morphological characteristics of the fungal colonies were assessed by inoculating 100 µL of a conidial suspension (5 × 107 conidia/mL) onto solid media, followed by incubation in the dark at 25 °C for 15 days. For slide culture, fungal mycelia grown on the medium were cut into 5 mm discs (Ø 5 mm) along with the medium and placed onto slide glasses, covered with cover slips, and incubated in the dark at 25 °C for 4 days. After incubation, hyphae and conidia were observed using a phase-contrast microscope at 400× magnification.
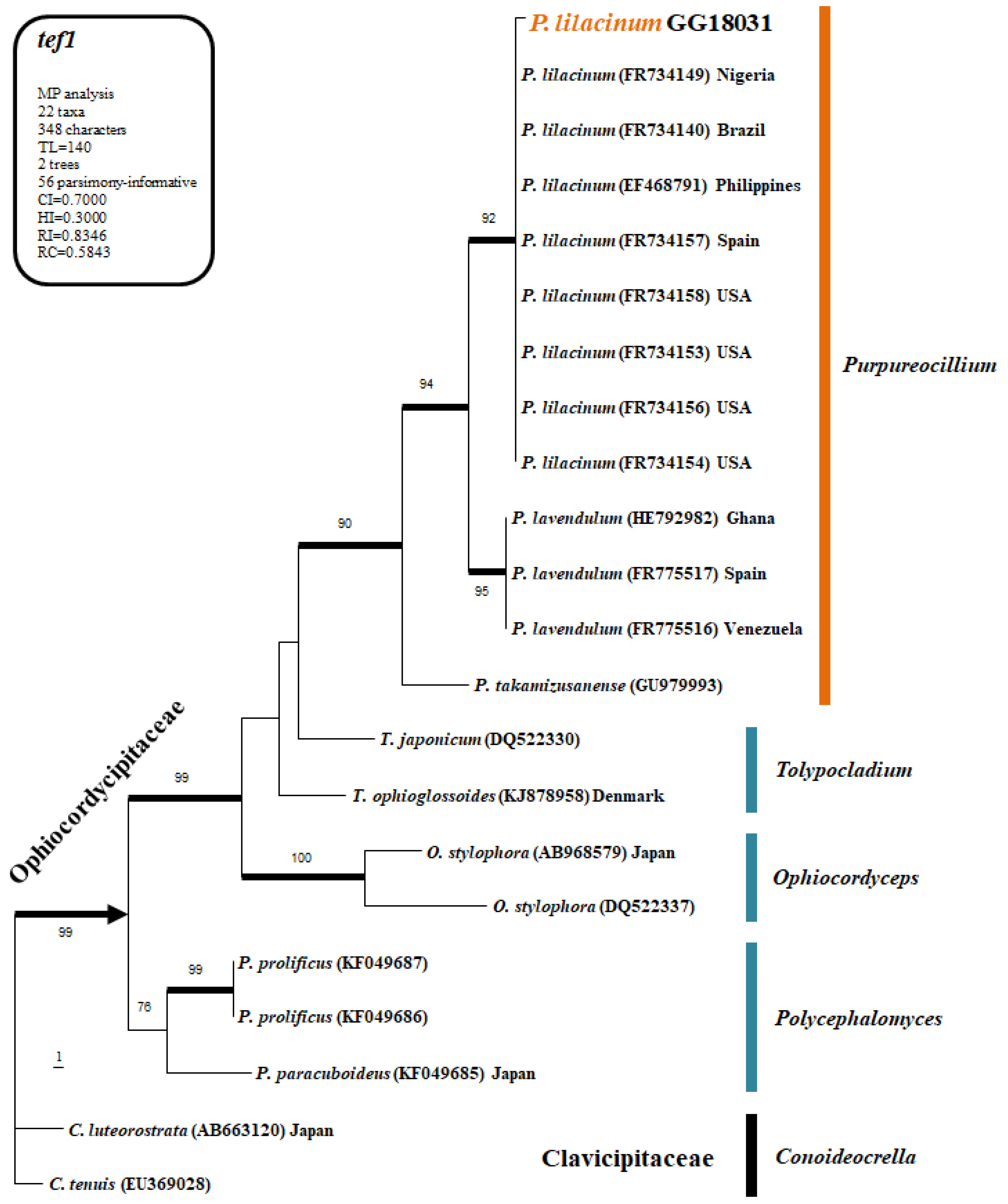
2.3.2. Sequence Comparisons
The fungal isolate obtained from soil was subjected to genomic DNA extraction, and sequence analysis was performed using the ITS1, ITS4, and tef1 primers. The PCR products were sent to Bionic Inc. (Seoul, Republic of Korea) for sequencing. The obtained sequences were aligned using ClustalX v.1.81 and edited and curated with PHYDIT v. 3.2. Phylogenetic analysis was conducted using PAUP v. 4.0 b10 for Maximum Parsimony (MP) bootstrap analysis and MrBayes v. 3.1.2 for Bayesian Inference (BI). For the heuristic search to generate the most parsimonious tree, optimization was performed with 1000 random addition sequence replications and tree bisection-reconnection (TBR) branch swapping. Only trees with bootstrap values of 70% or higher were used in the final phylogenetic tree.
2.4. Evaluation of Control Efficacy
The four entomopathogenic fungal isolates were cultured on SDA and PDA media at 25 °C for 10–14 days to induce sporulation. The conidia that developed on the surface were collected inside a clean bench using 0.02% Tween 20. The number of conidia was counted using a hemacytometer, and a conidial suspension (1.0 × 108 conidia/mL) was prepared for subsequent use.
A total of 100 µL of conidial suspension (1 × 108 conidia/mL) was dispensed into each sterilized 25 mL glass vial, amounting to approximately 1 × 107 conidia per vial. The suspension was evenly distributed and air-dried for 24 h at room temperature to allow the liquid to evaporate, leaving only the conidia inside. Five P. koryoensis beetles were then introduced into each vial and sealed with a loosely fitting cap to prevent escape. For the control group, an identical volume of 0.02% Tween-20 solution (without spores) was applied and similarly dried to ensure consistent experimental conditions. The vials were incubated at 25 °C, 40% humidity, and in the dark, with 5 beetles per vial and 3 replicates per treatment.
The P. koryoensis beetles introduced into the vials air-dried at room temperature were examined under a compound microscope at 8 h intervals to determine their survival or death. Individuals showing no movement were considered to have died from infection. The LT50 (lethal time for 50% mortality) for each entomopathogenic fungus was measured. In subsequent observations, the presence of fungal infection in the beetles was confirmed using a microscope.
2.5. Evaluation of the Heat, UV Stability, and Cold Tolerance for Germination of Entomopathogenic Fungal Spore
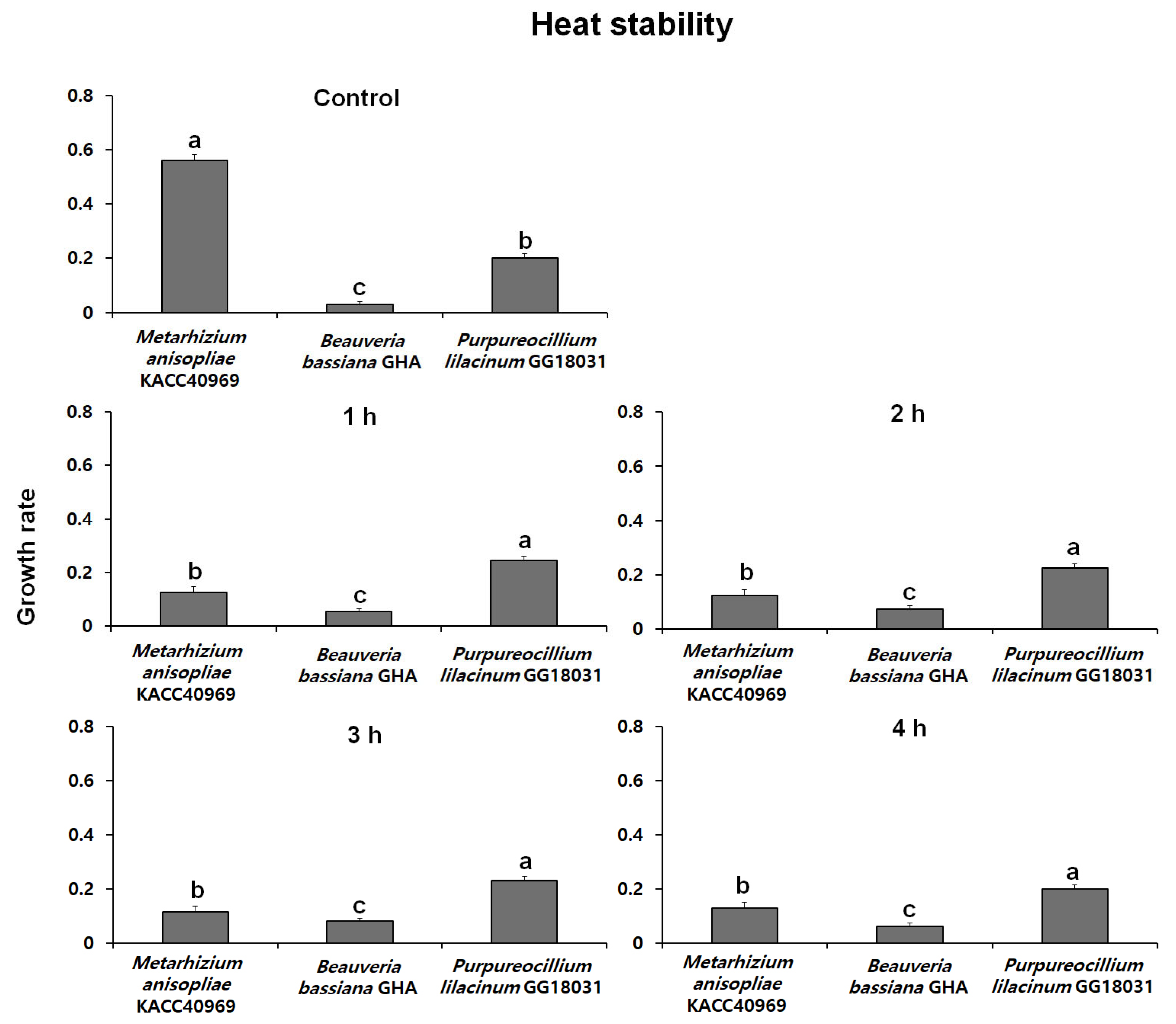
2.5.1. Evaluation of Heat Stability
Fungal conidia were induced to form by incubating on SDA medium at 25 °C in the dark. After conidia were collected, a conidial suspension (100 µL, 1 × 108 conidia/mL with 0.02% Tween-20) was prepared and placed in sterilized 1.5 mL microtubes. The suspension was then subjected to treatment in a water bath at 45 °C for 1, 2, 3, and 4 h. The control group was treated with the same conidial suspension at 25 °C.
The conidial suspension treated at high temperatures was inoculated with 10 µL into a 96-well plate, with each well containing 200 µL of PDB medium. The plate was incubated at 25 °C in the dark for 48 h. Fungal mycelial growth was then assessed at a wavelength of 650 nm using the VersaMaxPLUS ROM v1.17. The heat stability was evaluated with 5 replicate experiments, and the stability index was expressed as a percentage by measuring the absorbance of the treatment groups relative to the control group.
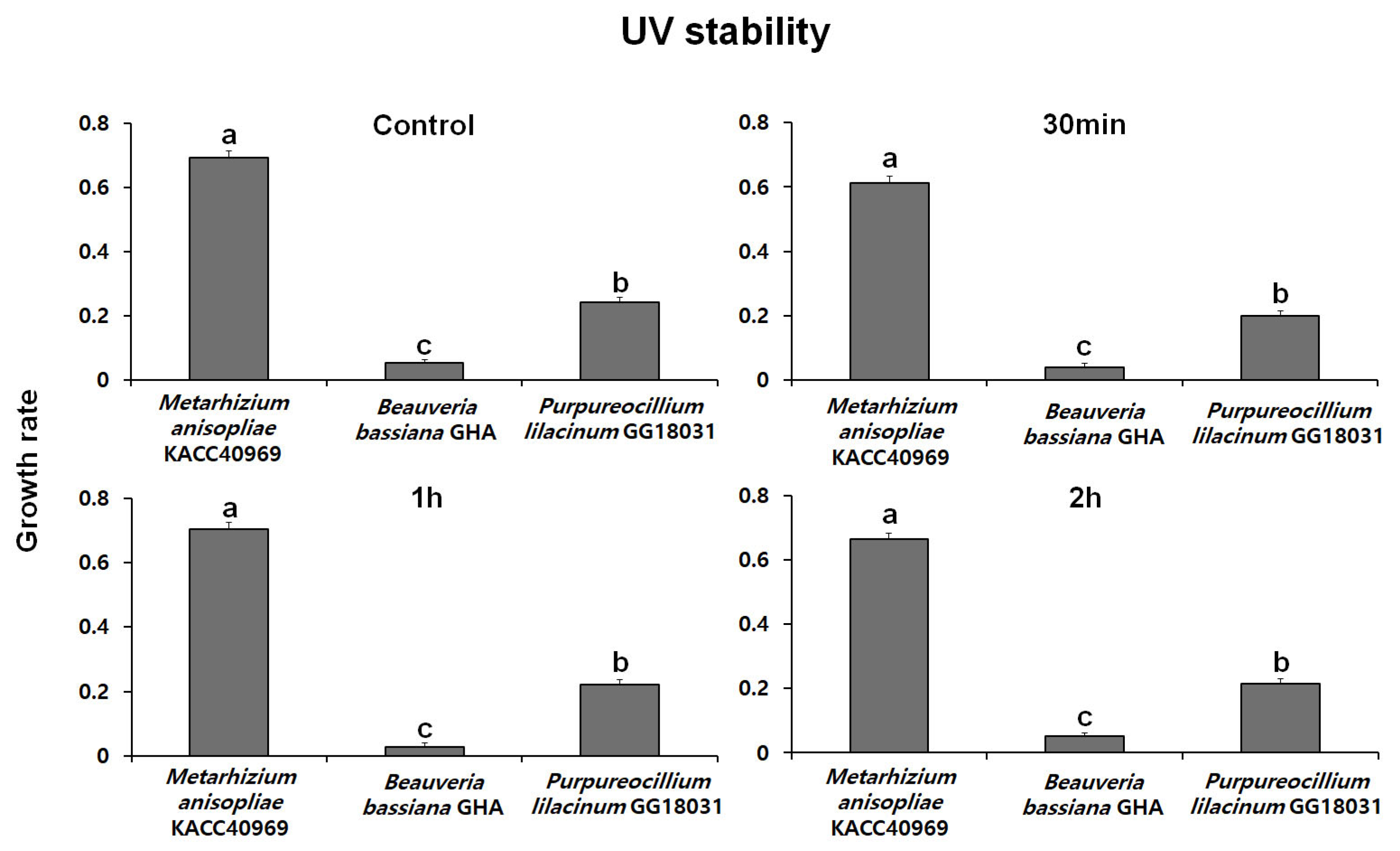
2.5.2. Evaluation of UV Stability
Fungal conidia were induced to form by incubating on SDA medium at 25 °C in the dark. After conidia were collected, a conidial suspension (100 µL, 1 × 108 conidia/mL with 0.02% Tween-20) was prepared and placed in a 96-well plate. The suspension was then exposed to UV treatment for 30 min, 1 h, and 2 h using a UV lamp. After UV treatment, 10 µL of the conidial suspension was inoculated into a 96-well plate containing 200 µL of PDB medium
The conidial suspension was incubated at 25 °C in the dark for 48 h. Fungal mycelial growth was then assessed at a wave-length of 650 nm using SoftMax Pro [Molecular Devices, VersaMaxPLUS ROM v1.17]. The UV stability was evaluated with 5 replicate experiments, and the stability index was expressed by measuring the absorbance of the treatment groups relative to the control group.
2.5.3. Evaluation of Cold Germination Ability
Fungal conidia were induced to form by incubating on SDA medium at 25 °C in the dark. After conidia were collected, a conidial suspension (1 × 107 conidia/mL with 0.02% Tween-20) was prepared. A 50 µL aliquot of the suspension was then inoculated onto PDA medium using the streak plate method.
The treated media were incubated in the dark for 15 days at 4 °C and 15 °C, respectively. Conidial germination was observed at 1, 2, 3, 6, 10, and 15 days using a phase-contrast microscope (LEICA DM5500B, Wetzlar, Germany) at magnifications of 200×, 400×, or 600×. A total of 100 conidia were randomly observed three times for each time point. The control group was treated at 25 °C with the same conidial suspension. The experiment was performed with 5 replicates.
For the cold germination assay, a lower concentration of 1 × 107 conidia/mL was intentionally used to facilitate clearer microscopic observations by avoiding spore clumping or overgrowth. This concentration was sufficient to accurately monitor individual germination events over time. The higher concentration (1 × 108 conidia/mL) used in other assays was optimized for uniform coating and stronger bioassay efficacy.
4. Discussion
In this study, the potential of three entomopathogenic fungal strains,
Metarhizium anisopliae KACC40969,
Beauveria bassiana GHA, and
Purpureocillium lilacinum GG18031, that can be used as a bio-control agent was evaluated against the insect vector,
Platypus koryoensis, responsible for transmitting the fungus associated with oak wilt disease in South Korea [
8,
17].
Analysis of insecticidal efficacy and LT50 revealed that all three fungal strains significantly reduced the survival rate of the insect vectors, with treated groups surviving for 6 days post-infection compared to 9 days in the untreated control. Among the strains tested, M. anisopliae KACC40969 showed the most rapid effect, with the shortest LT50 of 58.7 h. These results suggest that eco-friendly control methods can reduce reliance on chemical pesticides and serve as effective biological control strategies. In conclusion, this study demonstrates that entomopathogenic fungi represent a promising alternative for managing P. koryoensis. Furthermore, these findings may provide a valuable foundation for the development of sustainable forest management strategies in the future.
Thermal stability at 45 °C was evaluated over time using spores produced from each fungal strain. The growth rates of B. bassiana GHA and P. lilacinum GG18031 remained unchanged compared to the control regardless of exposure duration. In contrast, M. anisopliae KACC40969 exhibited a markedly reduced growth rate following high-temperature treatment, indicating a high sensitivity to heat stress. In addition, analysis of spore germination under ultraviolet (UV) exposure showed no significant differences compared to the control in all strains. However, M. anisopliae KACC40969 exhibited a relatively higher germination rate compared to the other entomopathogenic fungal strains. Spore germination was completely inhibited at 4 °C for all tested strains, indicating a temperature threshold between 4 °C and 15 °C. At 15 °C, all strains reached 100% germination, although at a slower rate than at 25 °C. These findings highlight that while low temperatures delay the germination process, they do not compromise the viability of the fungal spores. This suggests that the selected strains are capable of functioning effectively across a moderate temperature range relevant to temperate forest environments.
While previous studies on the efficacy of entomopathogenic fungi have largely focused on crops and ornamental plants [
9,
10,
12], this study provides meaningful insights by focusing on a forest pathosystem. Specifically, we assessed the experimental efficacy and practical applicability of a biological control strategy targeting the insect vector [
8] responsible for transmitting pathogenic fungi that cause severe damage to oak species—one of the dominant broadleaf tree groups in Korea. These findings contribute to expanding the scope of biological control research into forest ecosystems and highlight the potential of environmentally friendly management strategies for major forest pests and diseases, which might include the use of biological control agents, aiming to minimize non-target toxicity, preserve soil microbial integrity, and reduce disruptions to food webs and broader ecosystem processes [
18,
19].
While this study provides important insights under controlled conditions, the observed mortality in the control group points to the need for further refinement in bioassay methodology, such as improving ventilation or reducing stress-related factors in confined test environments. This limitation has also been consistently mentioned in previous research [
20,
21]. Future field trials should also consider these environmental stresses, alongside temperature, humidity, and UV exposure, which can influence fungal efficacy. Moreover, broader soil sampling and formulation improvements should be considered for subsequent research to enhance isolate diversity and environmental resilience [
22].