Tree and Liana Growth in Three Neotropical Dry Forests: Coherent Patterns and Individualistic Responses to Climate Variability
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Field Sampling
2.2. Climate Data and Indices
2.3. Processing Wood Samples and Ring-Width Measurements
2.4. Statistical Analyses
3. Results
3.1. Examining Tree-Ring Boundaries in Species from the Colombian TDF
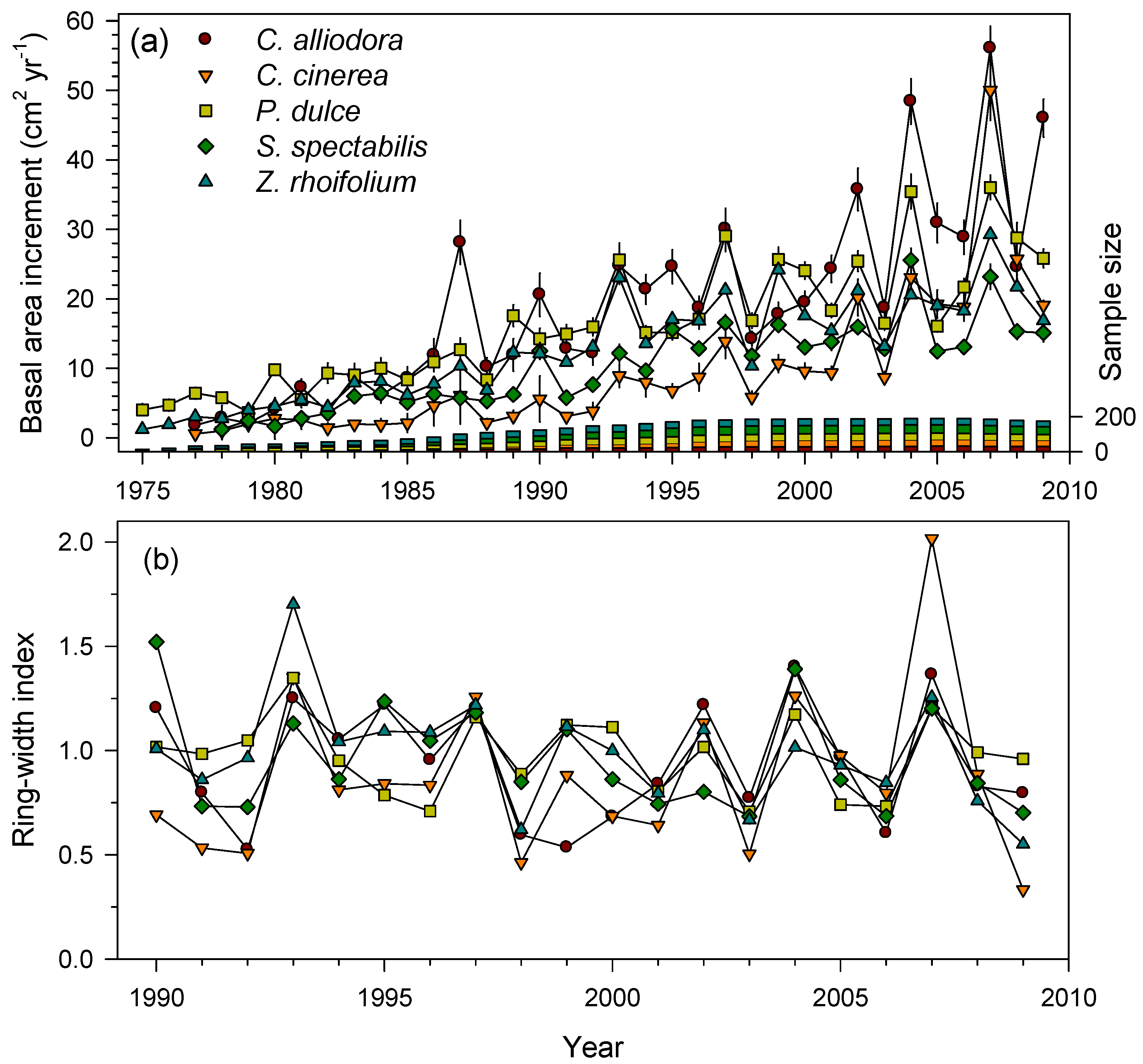
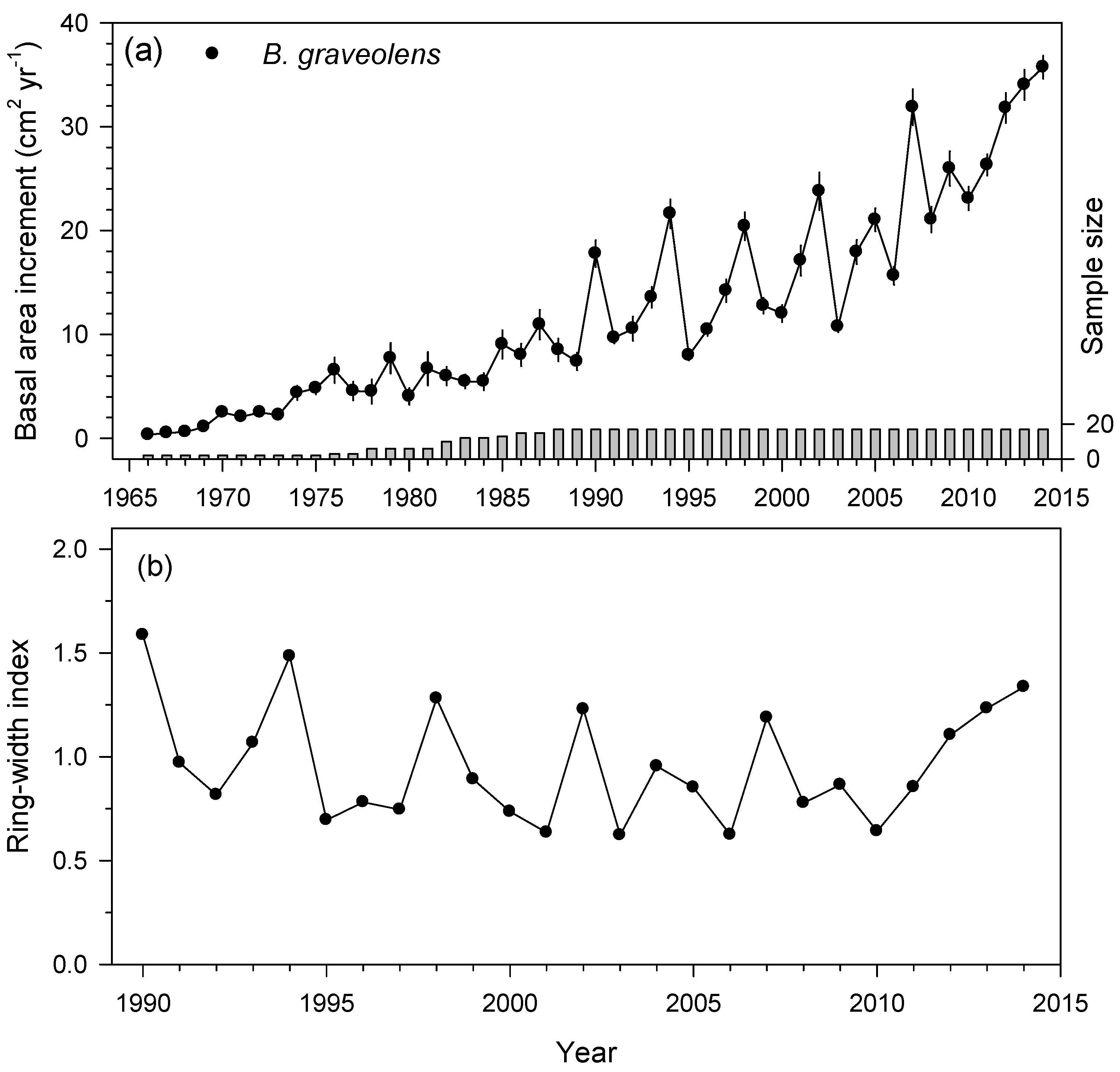
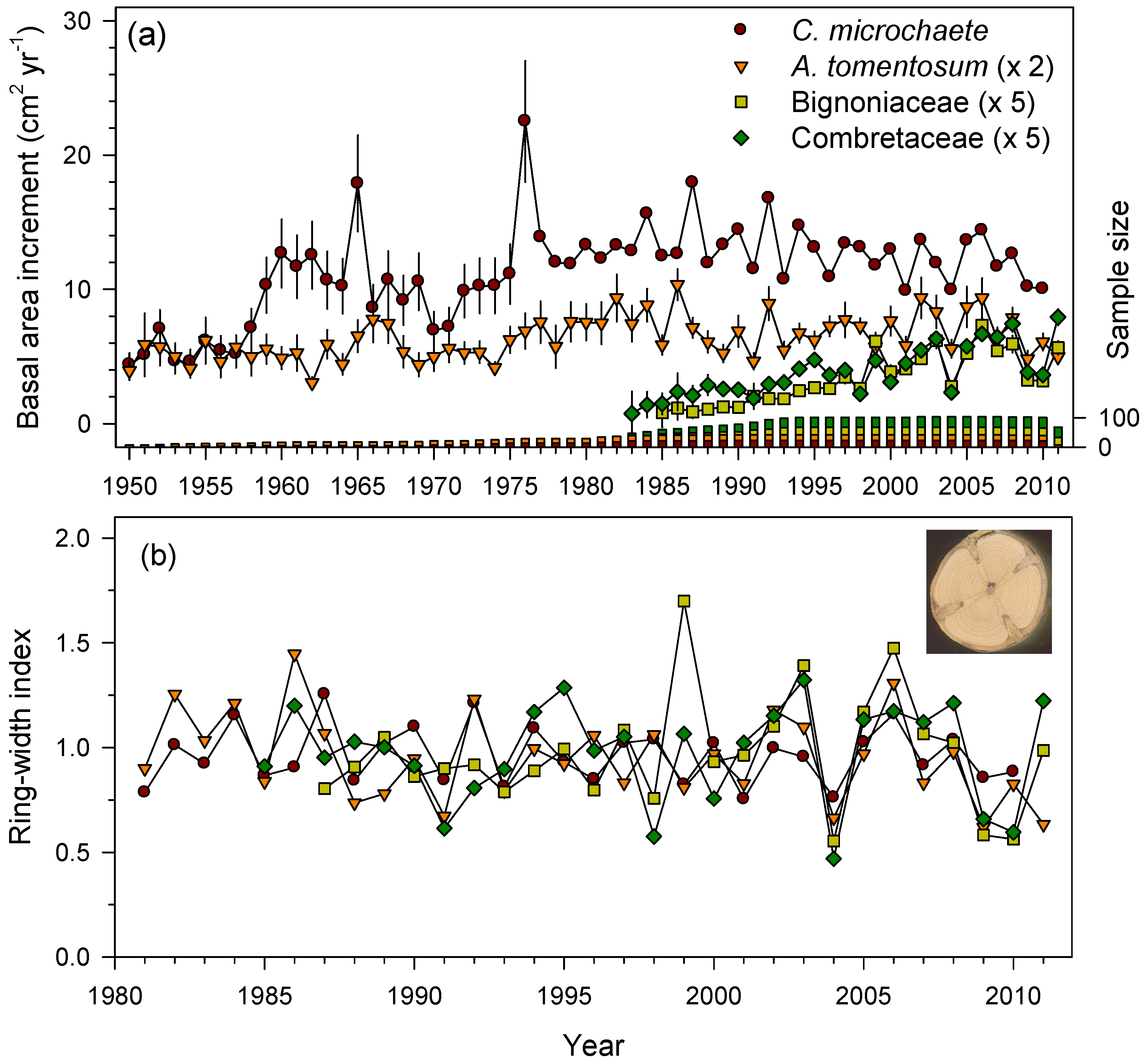
3.2. Ring-Width Data and Growth Patterns in the Three TDFs
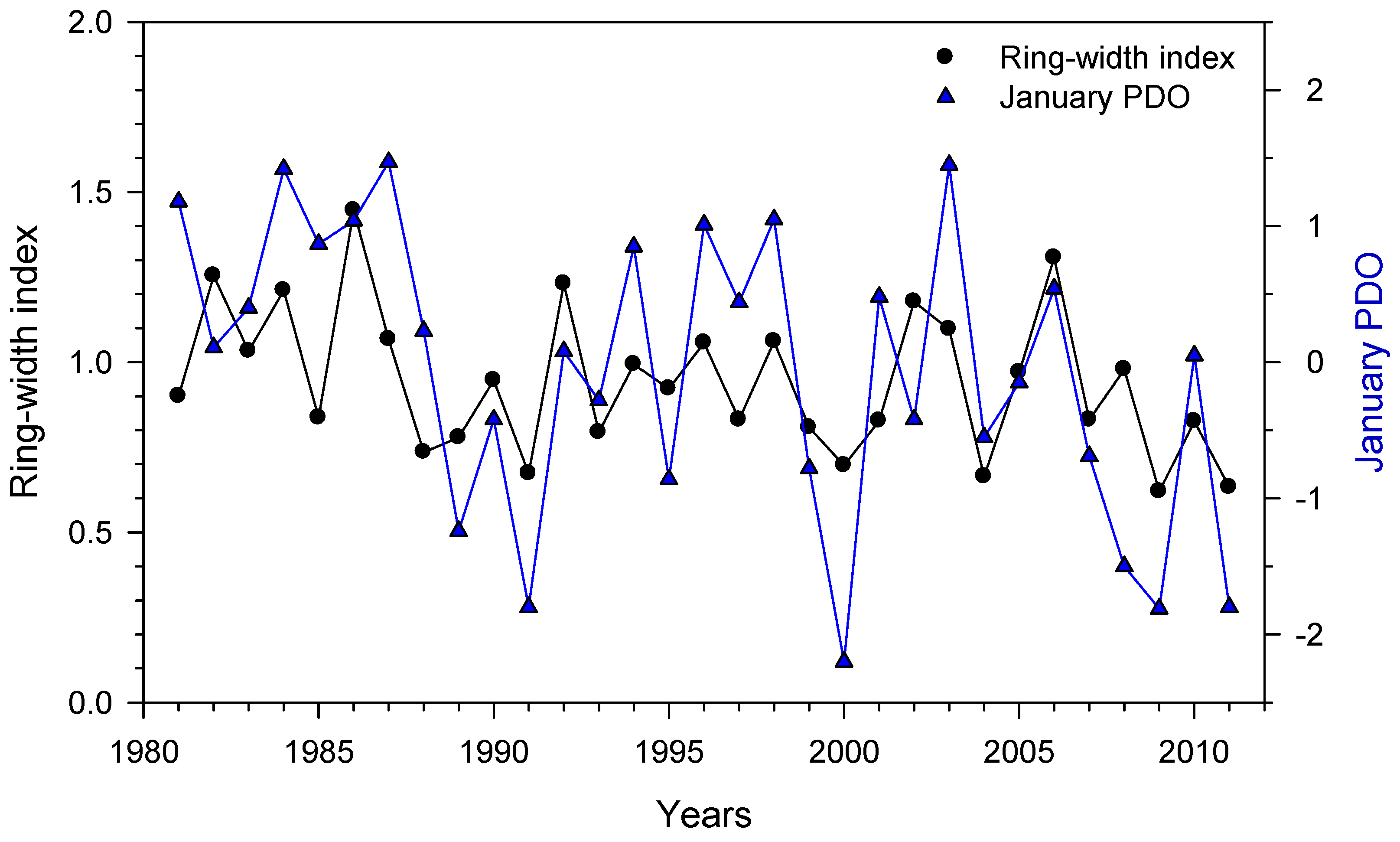
3.3. Relationships Between Growth Indices and Climate
3.4. Relationships Between Growth and Teleconnection Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibson, L.; Lee, T.M.; Koh, L.P.; Brook, B.W.; Gardner, T.A.; Barlow, J.; Peres, C.A.; Bradshaw, C.J.A.; Laurance, W.F.; Lovejoy, T.E.; et al. Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 2011, 478, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.H.; Hérault, B.; Bonal, D.; Stahl, C.; Anderson, L.O.; Baker, T.R.; Becker, G.S.; Beeckman, H.; Boanerges Souza, D.; Botosso, P.C.; et al. Climate seasonality limits carbon assimilation and storage in tropical forests. Biogeosciences 2016, 13, 2537–2562. [Google Scholar] [CrossRef]
- Rozendaal, D.M.; Zuidema, P.A. Dendroecology in the tropics: A review. Trees Struct. Funct. 2011, 25, 3–16. [Google Scholar] [CrossRef]
- Zuidema, P.A.; Brienen, R.J.W.; Schöngart, J. Tropical forest warming: Looking backwards for more insights. Trends Ecol. Evol. 2012, 27, 193–194. [Google Scholar] [CrossRef]
- Brienen, R.J.W.; Schöngart, J.; Zuidema, P.A. Tree Rings in the Tropics: Insights into the Ecology and Climate Sensitivity of Tropical Trees. In Tropical Tree Physiology; Goldstein, G., Santiago, L., Eds.; Springer: Cham, Switzerland, 2016; pp. 439–461. [Google Scholar] [CrossRef]
- Schöngart, J.; Bräuning, A.; Barbosa, A.C.M.C.; Lisi, C.S.; de Oliveira, J.M. Dendroecological studies in the neotropics: History, status and future challenges. In Dendroecology: Tree-Ring Analyses Applied to Ecological Studies; Amoroso, M.M., Daniels, L.D., Baker, P.J., Camarero, J.J., Eds.; Springer: Cham, Switzerland, 2017; pp. 35–73. [Google Scholar]
- Zuidema, P.A.; Babst, F.; Groenendijk, P.; Trouet, V.; Abiyu, A.; Acuña-Soto, R.; Adenesky-Filho, E.; Alfaro-Sánchez, R.; Aragão, J.R.V.; Assis-Pereira, G.; et al. Tropical tree growth driven by dry-season climate variability. Nat. Geosci. 2022, 15, 269–276. [Google Scholar] [CrossRef]
- Worbes, M. One hundred years of tree-ring research in the tropics –a brief history and an outlook to future challenges. Dendrochronologia 2002, 20, 217–231. [Google Scholar] [CrossRef]
- Quesada-Román, A.; Ballesteros-Cánovas, J.A.; George, S.; Stoffel, M. Tropical and subtropical dendrochronology: Approaches, applications, and prospects. Ecol. Indic. 2022, 144, 109506. [Google Scholar] [CrossRef]
- Bullock, S.H.; Mooney, H.A.; Medina, E. Seasonally Dry Tropical Forest; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Pennington, R.T.; Prado, D.E.; Pendry, C.A. Neotropical seasonally dry forest and Quaternary vegetation changes. J. Biogeogr. 2000, 27, 261–273. [Google Scholar] [CrossRef]
- Worbes, M. How to measure growth dynamics in tropical trees a review. IAWA J. 1995, 16, 337–351. [Google Scholar] [CrossRef]
- Borchert, R. Climatic periodicity, phenology, and cambium activity in tropical dry forest trees. IAWA J. 1999, 20, 239–247. [Google Scholar] [CrossRef]
- Borchert, R. Soil and stem water storage determine phenology and distribution of tropical dry forest trees. Ecology 1994, 75, 1437–1449. [Google Scholar] [CrossRef]
- Herrera-Ramirez, D.; Andreu-Hayles, L.; del Valle, J.I.; Santos, G.M.; Gonzalez, P.L.M. Nonannual tree rings in a climate-sensitive Prioria copaifera chronology in the Atrato River, Colombia. Ecol. Evol. 2017, 7, 6334–6345. [Google Scholar] [CrossRef] [PubMed]
- Speer, J.H. Fundamentals of Tree Ring Research; University of Arizona Press: Tucson, AZ, USA, 2010. [Google Scholar]
- Stokes, M.A.; Smiley, T.L. An Introduction to Tree-Ring Dating; University of Arizona Press: Tucson, AZ, USA, 2022. [Google Scholar]
- Brienen, R.J.W.; Lebrija-Trejos, E.; Van Breugel, M.; Pérez-García, E.A.; Bongers, F.; Meave, J.A.; Martínez-Ramos, M. The Potential of Tree Rings for the Study of Forest Succession in Southern Mexico. Biotropica 2009, 41, 186–195. [Google Scholar] [CrossRef]
- López, L.; Villalba, R.; Peña-Claros, M. Determining the annual periodicity of growth rings in seven tree species of a tropical moist forest in Santa Cruz, Bolivia. For. Syst. 2012, 21, 508–514. [Google Scholar] [CrossRef]
- López, L.; Villalba, R. Reliable estimates of radial growth for eight tropical species based on wood anatomical patterns. J. Trop. For. Sci. 2016, 28, 139–152. [Google Scholar]
- Fichtler, E.; Worbes, M. Wood anatomical variables in tropical trees and their relation to site conditions and individual tree morphology. IAWA J. 2012, 33, 119–140. [Google Scholar] [CrossRef]
- Tarelkin, Y.; Delvaux, C.; de Ridder, M.; el Berkani, T.; de Cannière, C.; Beeckman, H. Growth-ring distinctness and boundary anatomy variability in tropical trees. IAWA J. 2016, 37, 275–294. [Google Scholar] [CrossRef]
- Volland-Voigt, F.; Bräuning, A.; Ganzhi, O.; Peters, T.; Maza, H. Radial stem variations of Tabebuia chrysantha (Bignoniaceae) in different tropical forest ecosystems of southern Ecuador. Trees Struct. Funct. 2010, 25, 39–48. [Google Scholar] [CrossRef]
- Mendivelso, H.A.; Camarero, J.J.; Gutiérrez, E.; Zuidema, P.A. Time-dependent effects of climate and drought on tree growth in a Neotropical dry forest: Short-term tolerance vs. long-term sensitivity. Agric. For. Meteorol. 2014, 188, 13–23. [Google Scholar] [CrossRef]
- Mendivelso, H.A.; Camarero, J.J.; Gutiérrez, E.; Castaño-Naranjo, A. Climate influences on leaf phenology, xylogenesis and radial stem changes at hourly to monthly scales in two tropical dry forests. Agric. For. Meteorol. 2016, 216, 20–36. [Google Scholar] [CrossRef]
- García-Cervigón, A.I.; Camarero, J.J.; Cueva, E.; Espinosa, C.I.; Escudero, A. Climate seasonality and tree growth strategies in a tropical dry forest. J. Veg. Sci. 2020, 31, 266–280. [Google Scholar] [CrossRef]
- García-Cervigón, A.I.; Natalia Mercado, L.; Mendivelso, H.A.; Toledo, M.; Camarero, J.J. Adjusting xylem anatomy and growth to inter-annual climate variability in two Fabaceae species (Centrolobium microchaete, Cenostigma pluviosum) from Bolivian dry tropical forests. Dendrochronologia 2021, 67, 125840. [Google Scholar] [CrossRef]
- Brienen, R.J.W.; Zuidema, P.A. Relating tree growth to rainfall in Bolivian rain forests: A test for six species using tree ring analysis. Oecologia 2005, 146, 1–12. [Google Scholar] [CrossRef] [PubMed]
- López, L.; Villalba, R. Climate influences on the radial growth of Centrolobium microchaete, a valuable timber species from the tropical dry forests in Bolivia. Biotropica 2011, 43, 41–49. [Google Scholar] [CrossRef]
- Mendivelso, H.A.; Camarero, J.J.; Royo Obregón, O.; Gutiérrez, E.; Toledo, M. Differential growth responses to water balance of coexisting deciduous tree species are linked to wood density in a Bolivian tropical dry forest. PLoS ONE 2013, 8, e73855. [Google Scholar] [CrossRef]
- López, L.; Rodríguez-Catón, M.; Villalba, R. Convergence in growth responses of tropical trees to climate driven by water stress. Ecography 2019, 42, 1899–1912. [Google Scholar] [CrossRef]
- Fritz das Neves Brandes, A.; Campos Rizzieri, Y.; Tamaio, N.; Pace, M.R.; Barros, C.F. A global review on wood growth rings in lianas. Dendrochronologia 2022, 71, 125920. [Google Scholar] [CrossRef]
- Schnitzer, S.A.; Carson, W.P. Lianas suppress tree regeneration and diversity in treefall gaps. Ecol. Lett. 2010, 13, 849–857. [Google Scholar] [CrossRef]
- Brandes, A.F.N.; Lisi, C.S.; Barros, C.F. Dendrochronology of lianas of the Leguminosae family from the Atlantic Forest, Brazil. Trees Struct. Funct. 2011, 25, 133–144. [Google Scholar] [CrossRef]
- Espinosa, C.I.; Camarero, J.J.; Gusmán, A.A. Site-dependent growth responses to climate in two major tree species from tropical dry forests of southwest Ecuador. Dendrochronologia 2018, 52, 1119. [Google Scholar] [CrossRef]
- Killeen, T.J.; Jardim, A.; Mamani, F.; Rojas, N. Diversity, composition and structure of a tropical semideciduous forest in the Chiquitanía region of Santa Cruz, Bolivia. J. Trop. Ecol. 1998, 14, 803–827. [Google Scholar] [CrossRef]
- Alvira, D.; Putz, F.E.; Fredericksen, T.S. Liana loads and post-logging liana densities after liana cutting in a lowland forest in Bolivia. For. Ecol. Manag. 2004, 190, 73–86. [Google Scholar] [CrossRef]
- Villegas, Z.; Peña-Claros, M.; Mostacedo, B.; Alarcón, A.; Licona, J.C.; Leaño, C.; Pariona, W.; Choque, U. Silvicultural treatments enhance growth rates of future crop trees in a tropical dry forest. For. Ecol. Manag. 2009, 258, 971–977. [Google Scholar] [CrossRef]
- Harris, I.; Osborn, T.J.; Jones, P.; Lister, D. Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Sci. Data 2020, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, R.; Mabresa, A.; Luckman, B.; Evans, M.; Masiokas, M.; Ektved, T.M. “El Niño” events recorded in dry-forest species of the lowlands of northwest Peru. Dendrochronologia 2005, 22, 181–186. [Google Scholar] [CrossRef]
- Pucha-Cofrep, D.; Peters, T.; Bräuning, A. Wet season precipitation during the past century reconstructed from tree-rings of a tropical dry forest in Southern Ecuador. Glob. Planet. Change 2015, 133, 65–78. [Google Scholar] [CrossRef]
- Ropelewski, C.F.; Jones, P.D. An extension of the Tahiti–Darwin Southern Oscillation Index. Mon. Weather. Rev. 1987, 115, 2161–2163. [Google Scholar] [CrossRef]
- Huang, B.; Thorne, P.W.; Banzon, V.F.; Boyer, T.; Chepurin, G.; Lawrimore, J.H.; Menne, M.J.; Smith, T.M.; Vose, R.S.; Zhang, H.-M. Extended Reconstructed Sea Surface Temperature version 5 (ERSSTv5), Upgrades, validations, and intercomparisons. J. Clim. 2017, 30, 8179–8205. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Blackburn Press: Caldwell, ID, USA, 2001. [Google Scholar]
- Schulman, E. Dendroclimatic Change in Semiarid America; University of Arizona Press: Tucson, AZ, USA, 1956; 142p. [Google Scholar]
- Maxwell, R.S.; Larsson, L.-A. Measuring tree-ring widths using the CooRecorder software application. Dendrochronologia 2021, 67, 125841. [Google Scholar] [CrossRef]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 1983, 43, 69–78. [Google Scholar]
- Biondi, F.; Qeadan, F. A theory-driven approach to tree-ring standardization: Defining the biological trend from expected basal area increment. Tree-Ring Res. 2008, 64, 81–96. [Google Scholar] [CrossRef]
- Briffa, K.R.; Jones, P.D. Basic chronology statistics and assessment. In Methods of Dendrochronology: Applications in the Environmental Sciences; Cook, E.R., Kairiukstis, L.A., Eds.; Kluwer: Dordrecht, The Netherlands, 1990; pp. 439–461. Available online: https://link.springer.com/book/10.1007/978-94-015-7879-0 (accessed on 9 January 2025).
- Bunn, A.G. Statistical and visual crossdating in R using the dplR library. Dendrochronologia 2010, 28, 251–258. [Google Scholar] [CrossRef]
- Bunn, A.G.; Korpela, M.; Biondi, F.; Campelo, F.; Mérian, P.; Qeadan, F.; Zang, C. dplR: Dendrochronology Program Library in R. R package, Version 1.7.8. 2025. Available online: https://cran.r-project.org/web/packages/dplR/index.html (accessed on 21 February 2025).
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.r-project.org/ (accessed on 21 February 2025).
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.7-0. 2025. Available online: https://github.com/vegandevs/vegan (accessed on 9 January 2025).
- Zang, C.; Biondi, F. Treeclim: An R package for the numerical calibration of proxy-climate relationships. Ecography 2015, 38, 431–436. [Google Scholar] [CrossRef]
- Roig, F.A. Dendrocronología en los bosques del neotrópico: Revisión y prospección futura. In Dendrocronología en América Latina; Roig, F.A., Ed.; EDIUNC: Mendoza, Argentina, 2000; pp. 307–357. [Google Scholar]
- Roig, F.A.; Jiménez-Osornio, J.J.; Villanueva-Díaz, J.; Luckman, B.; Thiessen, H.; Medina, A.; Noellemeyr, E.J. Anatomy of growth rings the Yucatán Peninsula. Dendrochronologia 2005, 22, 187–193. [Google Scholar] [CrossRef]
- Callado, C.H.; Roig, F.A.; Tomazello-Filho, M.; Barros, C.F. Cambial growth periodicity studies of South American woody species—A review. IAWA J. 2013, 34, 213–230. [Google Scholar] [CrossRef]
- Mariaux, A. Les cernes dans le bois tropicaux africains, nature et périodicité. Rev. Bois Forêt Trop. 1967, 114, 23–37. [Google Scholar]
- Fichtler, E.; Clark, D.A.; Worbes, M. Age and long-term growth of trees in an old-growth tropical rain forest, based on analyses of tree rings and 14C. Biotropica 2003, 35, 306–317. [Google Scholar]
- Dünisch, O.; Ribeiro Montóia, V.; Bauch, J. Dendroecological investigations on Swietenia macrophylla King and Cedrela odorata L. (Meliaceae) in the central Amazon. Trees Struct. Funct. 2003, 17, 244–250. [Google Scholar] [CrossRef]
- Eamus, D. Ecophysiological traits of deciduous and evergreen woody species in the seasonally dry tropics. Trends Ecol. Evol. 1999, 14, 11–16. [Google Scholar] [CrossRef]
- Markesteijn, L.; Poorter, L.; Paz, H.; Sack, L.; Bongers, F. Ecological differentiation in xylem cavitation resistance is associated with stem and leaf structural traits. Plant Cell Environ. 2011, 34, 137–148. [Google Scholar] [CrossRef]
- Pineda-García, F.; Paz, H.; Meinzer, F.C. Drought resistance in early and late secondary successional species from a tropical dry forest: The interplay between xylem resistance to embolism, sapwood water storage and leaf shedding. Plant Cell Environ. 2013, 36, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, R.K.; Tripathi, A.; Raghubanshi, A.S.; Singh, J.S. Functional traits indicate a continuum of tree drought strategies across a soil water availability gradient in a tropical dry forest. For. Ecol. Manag. 2021, 482, 118740. [Google Scholar] [CrossRef]
- Briceño-J, A.M.; Rangel-Ch, O.J.; Bogino, S.M. Tree ring study of Cordia alliodora (Ruiz & Pav.) in Colombia. Colomb. For. 2016, 19, 219–232. [Google Scholar] [CrossRef][Green Version]
- Volland, F.; Pucha, D.; Bräuning, A. Hydro-climatic variability in southern Ecuador reflected by tree-ring oxygen isotopes. Erdkunde 2016, 70, 69–82. [Google Scholar] [CrossRef]
- García-Cervigón, A.I.; Camarero, J.J.; Espinosa, C.I. Intra-annual stem increment patterns and climatic responses in five tree species from an Ecuadorian tropical dry forest. Trees Struct. Funct. 2017, 1057–1067. [Google Scholar] [CrossRef]
- Spann, S.; Volland, F.; Pucha, D.; Peters, T.; Cueva, E.; Bräuning, A. Climate variability, tree increment patterns and ENSO-related carbon sequestration reduction of the tropical dry forest species Loxopterygium huasango of Southern Ecuador. Trees Struct. Funct. 2016, 30, 1245–1258. [Google Scholar] [CrossRef]
- Paredes-Villanueva, K.; Sánchez-Salguero, R.; Manzanedo, R.D.; Sopepi, R.Q. Growth rate and climatic response of Machaerium scleroxylon in a dry tropical forest in southeastern Santa Cruz, Bolivia. Tree-Ring Res. 2013, 69, 63–79. [Google Scholar] [CrossRef]
- Paredes-Villanueva, K.; López, L.; Brookhouse, M.; Navarro-Cerrillo, R.M. Rainfall and temperature variability in Bolivia derived from the tree-ring width of Amburana cearensis (Fr. Allem.) AC Smith. Dendrochronologia 2015, 35, 80–86. [Google Scholar] [CrossRef]
- López, L.; Villalba, R.; Stahle, D. High-fidelity representation of climate variations by Amburana cearensis tree-ring chronologies across a tropical forest transition in South America. Dendrochronologia 2022, 72, 125932. [Google Scholar] [CrossRef]
- Godoy-Veiga, M.; Cintra, B.B.L.; Stríkis, N.M.; Cruz, F.W.; Grohmann, C.H.; Santos, M.S.; Regev, L.; Boaretto, E.; Ceccantini, G.; Locosselli, G.M. The value of climate responses of individual trees to detect areas of climate-change refugia, a tree-ring study in the Brazilian seasonally dry tropical forests. For. Ecol. Manag. 2021, 488, 118971. [Google Scholar] [CrossRef]
- Angyalossy, V.; Angeles, G.; Pace, M.R.; Lima, A.C.; Dias-Leme, C.L.; Lohmann, L.G.; Madero-Vega, C. An overview of the anatomy, development and evolution of the vascular system of lianas. Plant Ecol. Div. 2012, 5, 167–182. [Google Scholar] [CrossRef]
- Carlquist, S. Observations on functional wood histology of vines and lianas: Vessel dimorphism, tracheids, vasicentric tracheids, narrow vessels, and parenchyma. Aliso 1985, 11, 139–157. [Google Scholar] [CrossRef]
- Enquist, B.J.; Leffler, A.J. Long-term tree ring chronologies from sympatric tropical dry-forest trees: Individualistic responses to climatic variation. J. Trop. Ecol. 2001, 17, 41–60. [Google Scholar] [CrossRef]
- Aceituno, P. On the functioning of the Southern Oscillation in the South American sector. Part I: Surface climate. Mon. Wea. Rev. 1988, 116, 505–524. [Google Scholar] [CrossRef]
- Grimm, M.; Barros, V.; Doyle, M. Climate Variability in Southern South America Associated with El Niño and La Niña Events. J. Clim. 2000, 13, 35–58. [Google Scholar] [CrossRef]
- Seiler, C.; Hutjes, R.W.A.; Kabat, P. Climate Variability and Trends in Bolivia. J. Clim. 2013, 52, 130–146. [Google Scholar] [CrossRef]
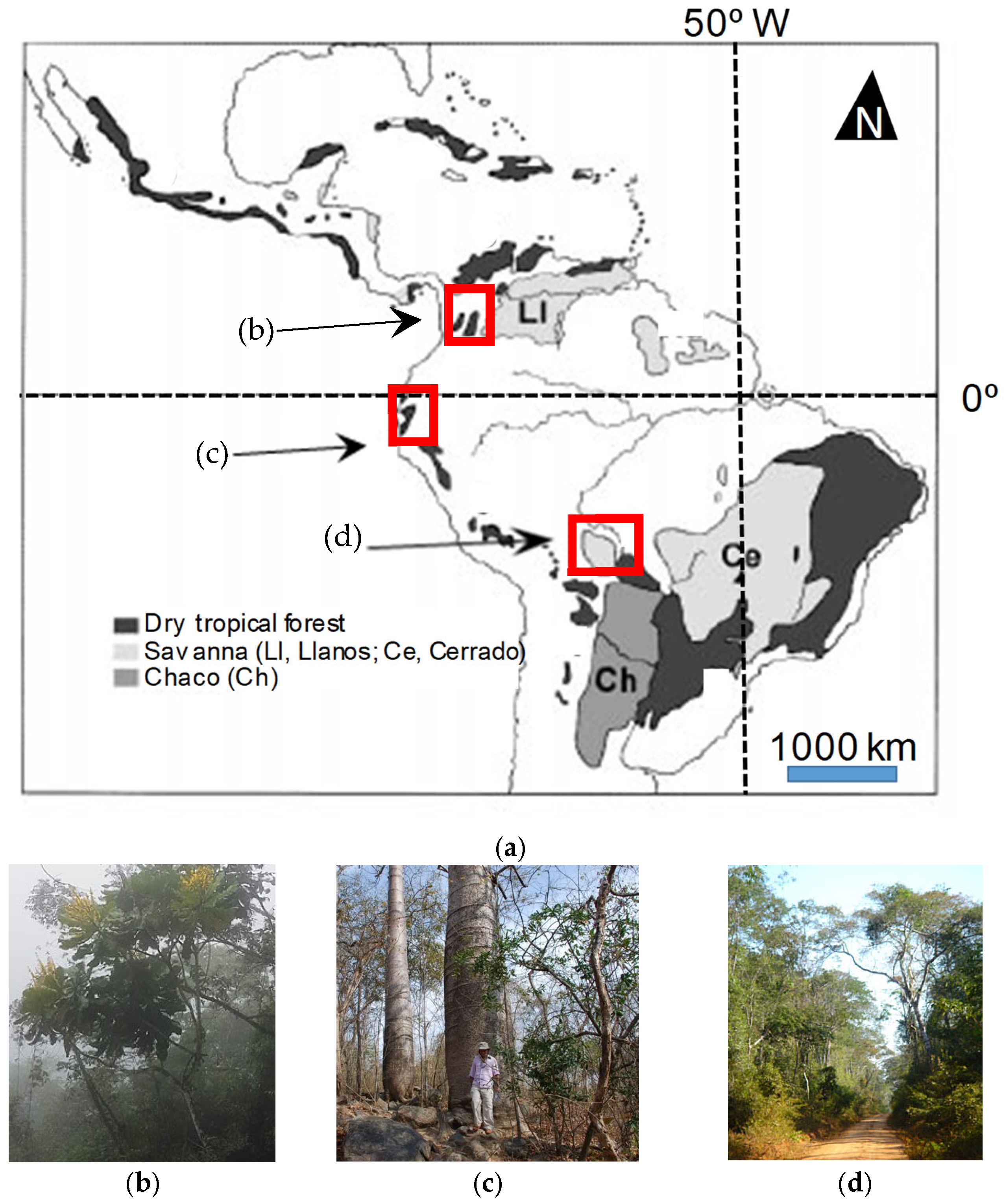



| Variable | Characteristics | ||
|---|---|---|---|
| Site, country | Tuluá, Colombia | Arenillas, Ecuador | INPA, Bolivia |
| Latitude | 4.33° N | 3.52° S | 16.12° S |
| Longitude | 76.17° W | 80.13° W | 61.72° W |
| Elevation (m a.s.l.) | 1220 | 21 | 380 |
| Annual temperature (°C) | 23.6 | 25.9 | 24.3 |
| Annual precipitation (mm) | 1193 | 661 | 1160 |
| No. tree species (individuals) | 19 (151) | 1 (11) | 2 (38) |
| No. liana species (individuals) | — | — | 2 (30) |
| Species | Tree-Ring Detail | Distinctiveness | Wood Structure | |
|---|---|---|---|---|
| In | Out | |||
| Amyris pinnata | 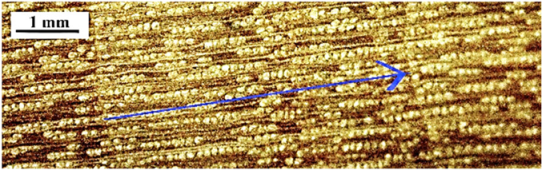 | − − | + − | 2 |
| Beilschmiedia sp. | 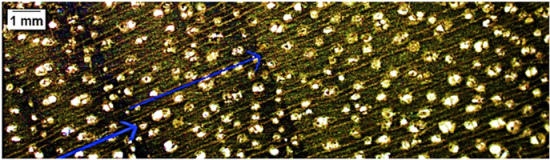 | − − | + | 1, 2 |
| Ceiba pentandra | 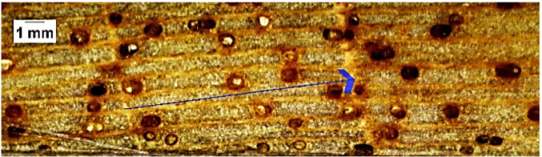 | + − | + − | 2 |
| Citharexylum kunthianum |  | + | + | 1, 2, 4 |
| Cordia alliodora | 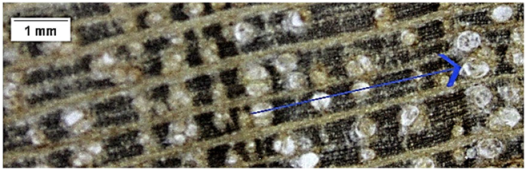 | + | + | 2 |
| Croton gossypiifolius |  | − − | + − | 1, 2 |
| Cupania cinerea | 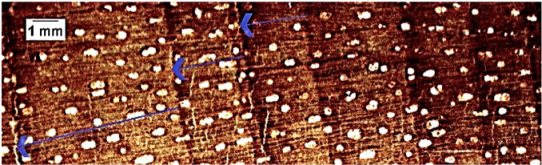 | + | + | 2 |
| Eugenia sp. | 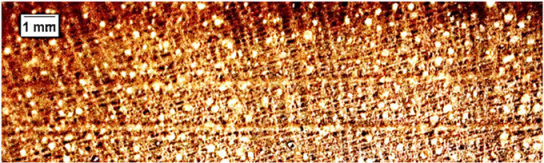 | − | − | 2 |
| Genipa americana |  | − | − | 2 |
| Guarea guidonia | 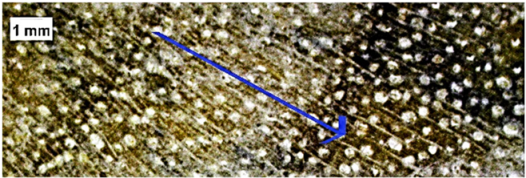 | − | − | 1, 2 |
| Hymenaea courbaril | 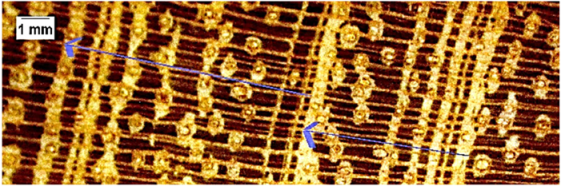 | + | + | 3 |
| Machaerium capote |  | − | + − | 3 |
| Pithecellobium dulce | 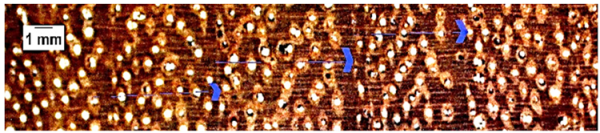 | + | + | 1, 2 |
| Rapanea guianensis |  | − | − | – |
| Sapindus saponaria | 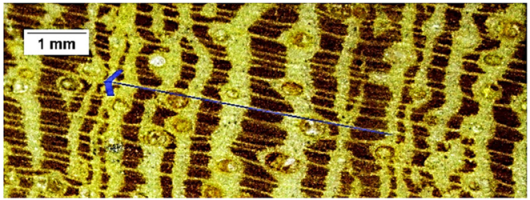 | + | + − | 3 |
| Senna spectabilis | 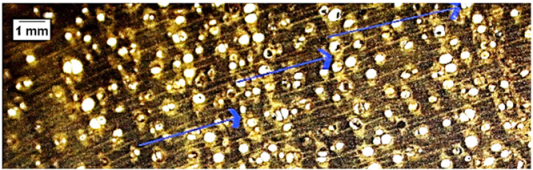 | + | + − | 2, 4 |
| Zanthoxylum monophyllum |  | + − | + − | 2 |
| Zanthoxylum rhoifolium |  | + − | + | 1, 2 |
| Zanthoxylum verrucosum | 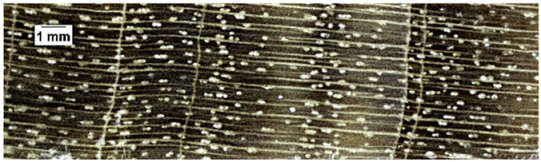 | + − | + | 1, 2 |
| Site, Country | Species (Sample Type) | Timespan | No Series | Ring Width (mm) | AR1 | MSx | Corr | Rbar |
|---|---|---|---|---|---|---|---|---|
| J.M. Céspedes-El Vínculo, Colombia | C. alliodora (CO) | 1975–2009 | 32 | 4.80 ± 1.36 b | 0.21 ± 0.14 a | 0.63 ± 0.15 a | 0.43 | 0.24 |
| C. cinerea (CO) | 1976–2009 | 38 | 3.43 ± 1.23 a | 0.20 ± 0.12 a | 0.65 ± 0.10 a | 0.53 | 0.33 | |
| P. dulce (CO) | 1971–2009 | 36 | 3.57 ± 1.04 a | 0.25 ± 0.18 a | 0.48 ± 0.09 a | 0.42 | 0.22 | |
| S. spectabilis (CO) | 1977–2009 | 48 | 3.12 ± 0.75 a | 0.23 ± 0.15 a | 0.51 ± 0.13 a | 0.42 | 0.19 | |
| Z. rhoifolium (CO) | 1970–2009 | 38 | 3.27 ± 1.12 a | 0.24 ± 0.16 a | 0.50 ± 0.09 a | 0.38 | 0.20 | |
| Arenillas, Ecuador | B. graveolens (CO) | 1965–2014 | 15 | 2.89 ± 0.66 | 0.31 ± 0.19 | 0.52 ± 0.09 | 0.37 | 0.23 |
| INPA, Bolivia | C. microchaete (CS) | 1941–2010 | 26 | 1.72 ± 0.44 b | 0.34 ± 0.18 a | 0.37 ± 0.07 a | 0.31 | 0.29 |
| A. tomentosum (CS) | 1936–2011 | 38 | 0.96 ± 0.20 a | 0.37 ± 0.19 a | 0.42 ± 0.06 a | 0.30 | 0.22 | |
| Bignoniaceae # (CS) | 1984–2011 | 26 | 0.84 ± 0.21 a | 0.45 ± 0.13 a | 0.47 ± 0.11 a | 0.45 | 0.28 | |
| Combretaceae # (CS) | 1982–2011 | 34 | 0.90 ± 0.24 a | 0.44 ± 0.17 a | 0.53 ± 0.11 b | 0.48 | 0.21 |
| C. alliodora | C. cinerea | P. dulce | S. spectabilis | Z. rhoifolium | |
|---|---|---|---|---|---|
| C. alliodora | 0.001 | 0.119 | 0.001 | 0.008 | |
| C. cinerea | 0.711 | 0.015 | 0.016 | 0.001 | |
| P. dulce | 0.360 | 0.534 | 0.049 | 0.004 | |
| S. spectabilis | 0.687 | 0.529 | 0.445 | 0.010 | |
| Z. rhoifolium | 0.573 | 0.712 | 0.617 | 0.559 |
| C. microchaete | A. tomentosum | Bignoniaceae | Combretaceae | |
|---|---|---|---|---|
| C. microchaete | 0.000 | 0.454 | 0.275 | |
| A. tomentosum | 0.716 | 0.079 | 0.045 | |
| Bignoniaceae | 0.160 | 0.365 | 0.000 | |
| Combretaceae | 0.232 | 0.413 | 0.687 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camarero, J.J.; Valeriano, C. Tree and Liana Growth in Three Neotropical Dry Forests: Coherent Patterns and Individualistic Responses to Climate Variability. Forests 2025, 16, 542. https://doi.org/10.3390/f16030542
Camarero JJ, Valeriano C. Tree and Liana Growth in Three Neotropical Dry Forests: Coherent Patterns and Individualistic Responses to Climate Variability. Forests. 2025; 16(3):542. https://doi.org/10.3390/f16030542
Chicago/Turabian StyleCamarero, J. Julio, and Cristina Valeriano. 2025. "Tree and Liana Growth in Three Neotropical Dry Forests: Coherent Patterns and Individualistic Responses to Climate Variability" Forests 16, no. 3: 542. https://doi.org/10.3390/f16030542
APA StyleCamarero, J. J., & Valeriano, C. (2025). Tree and Liana Growth in Three Neotropical Dry Forests: Coherent Patterns and Individualistic Responses to Climate Variability. Forests, 16(3), 542. https://doi.org/10.3390/f16030542







