Integrative Analysis of Transcriptome and Metabolome Reveals Light Quality-Mediated Regulation of Adventitious Shoot Proliferation in Chinese Fir
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Determination of Morphological Indices
2.3. Determination of Physiological-Biochemical Indices
2.4. RNA Extraction, cDNA Library Construction, RNA Sequencing, and Data Analysis
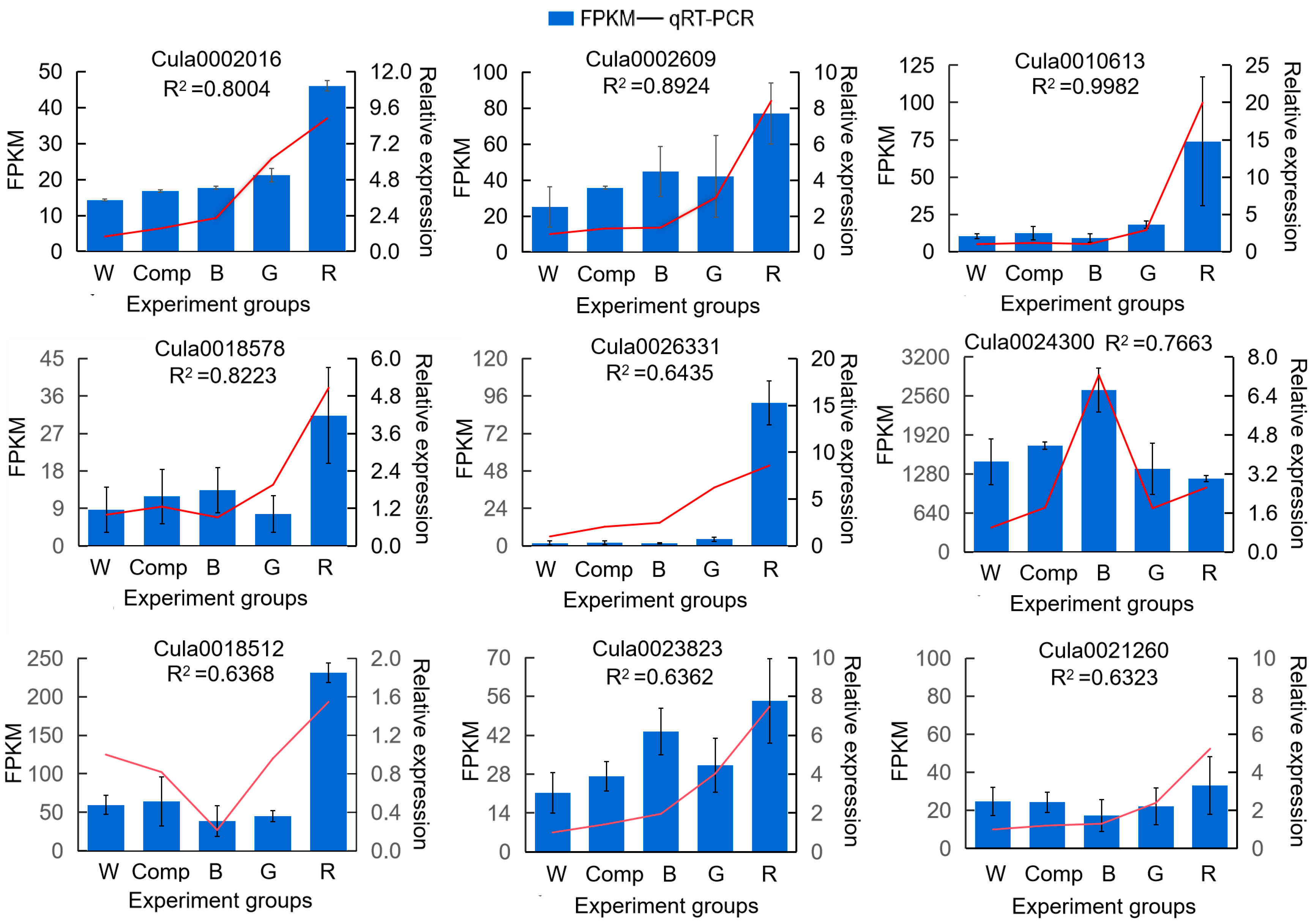
2.5. Quantitative Real-Time PCR (qRT–PCR)
2.6. Untargeted Metabonomic Analysis
3. Result
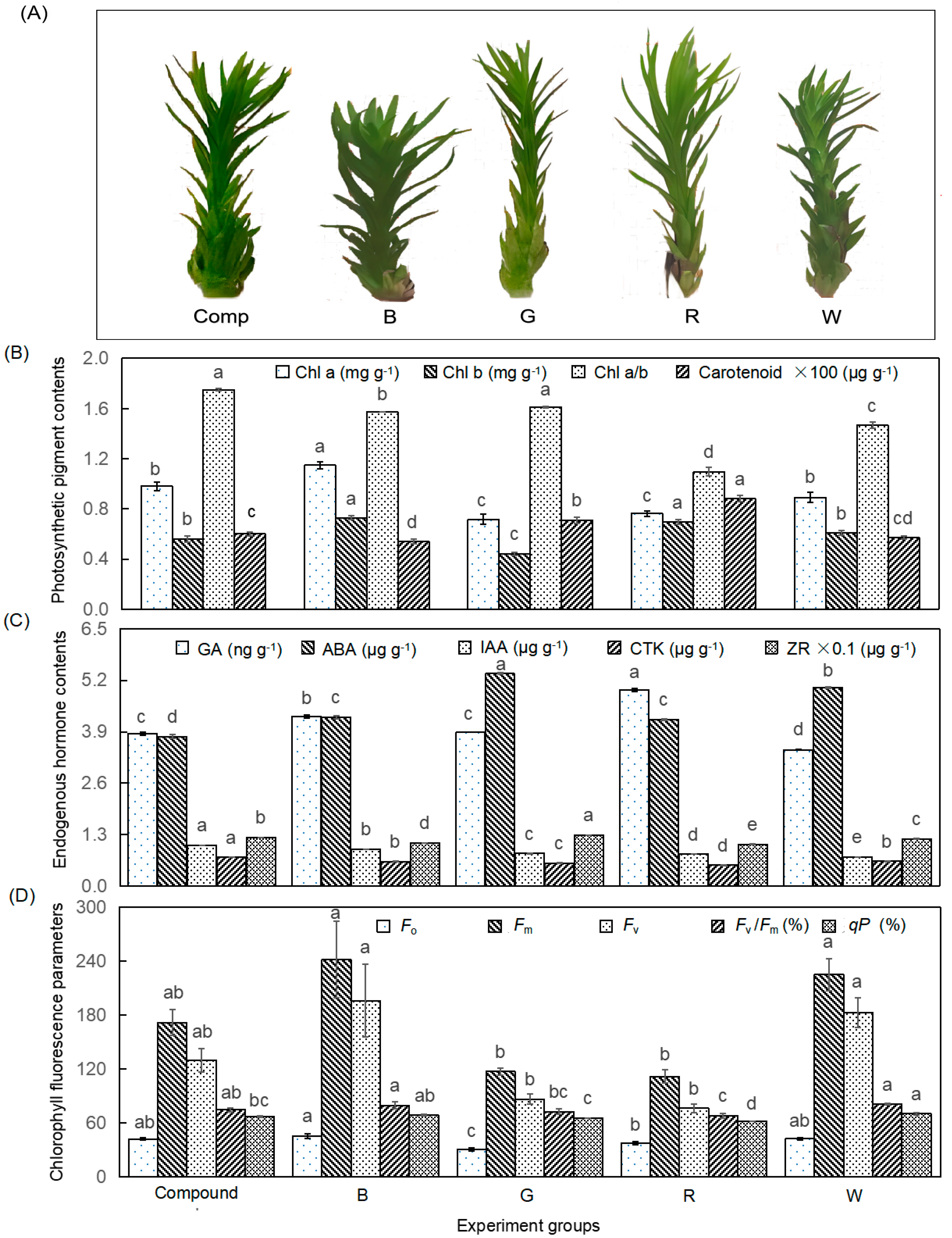
3.1. Light Quality Regulation on Morphology, Physiology and Biochemistry
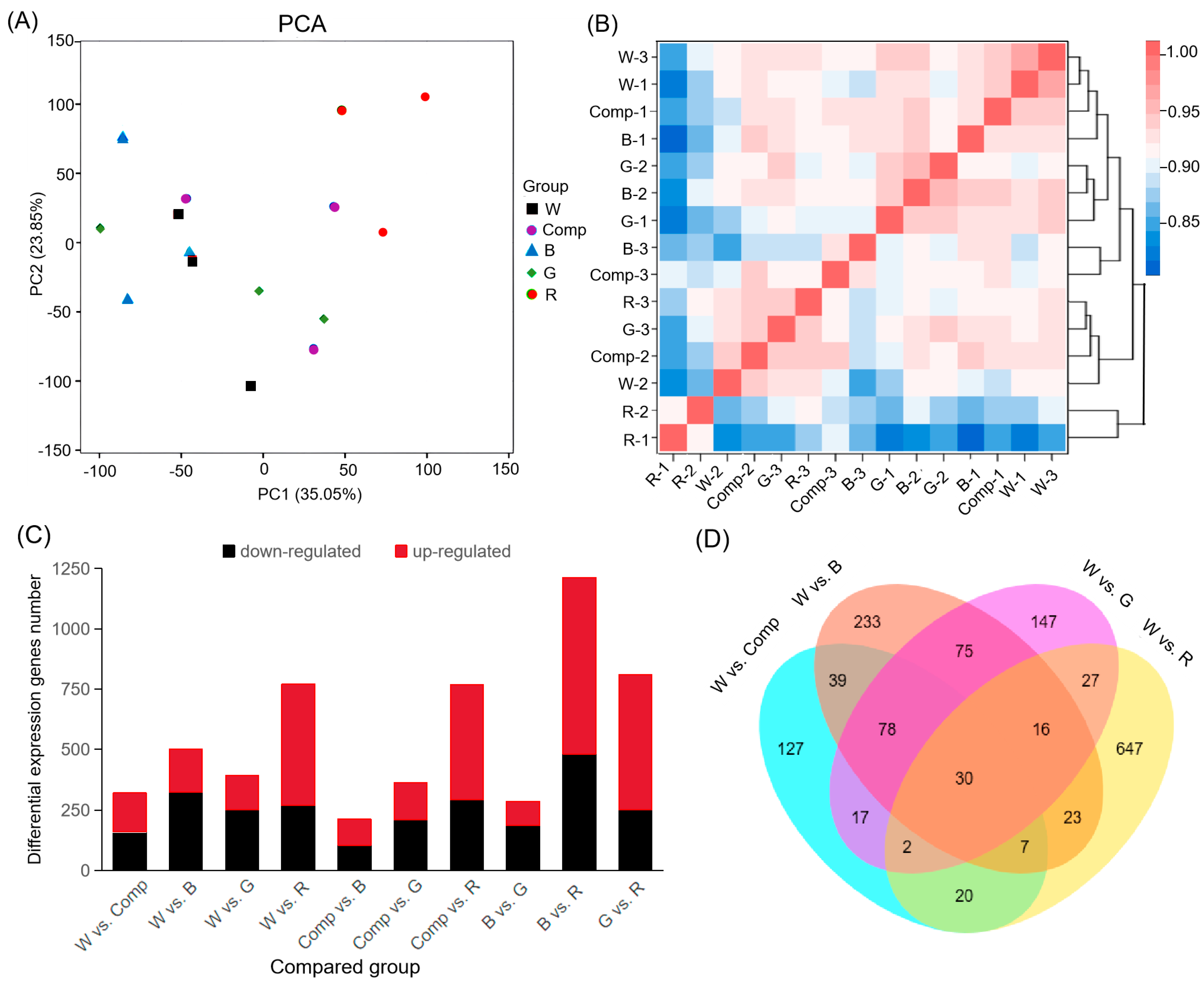
3.2. Transcriptome Sequencing Results and Alignment with the Reference Genome
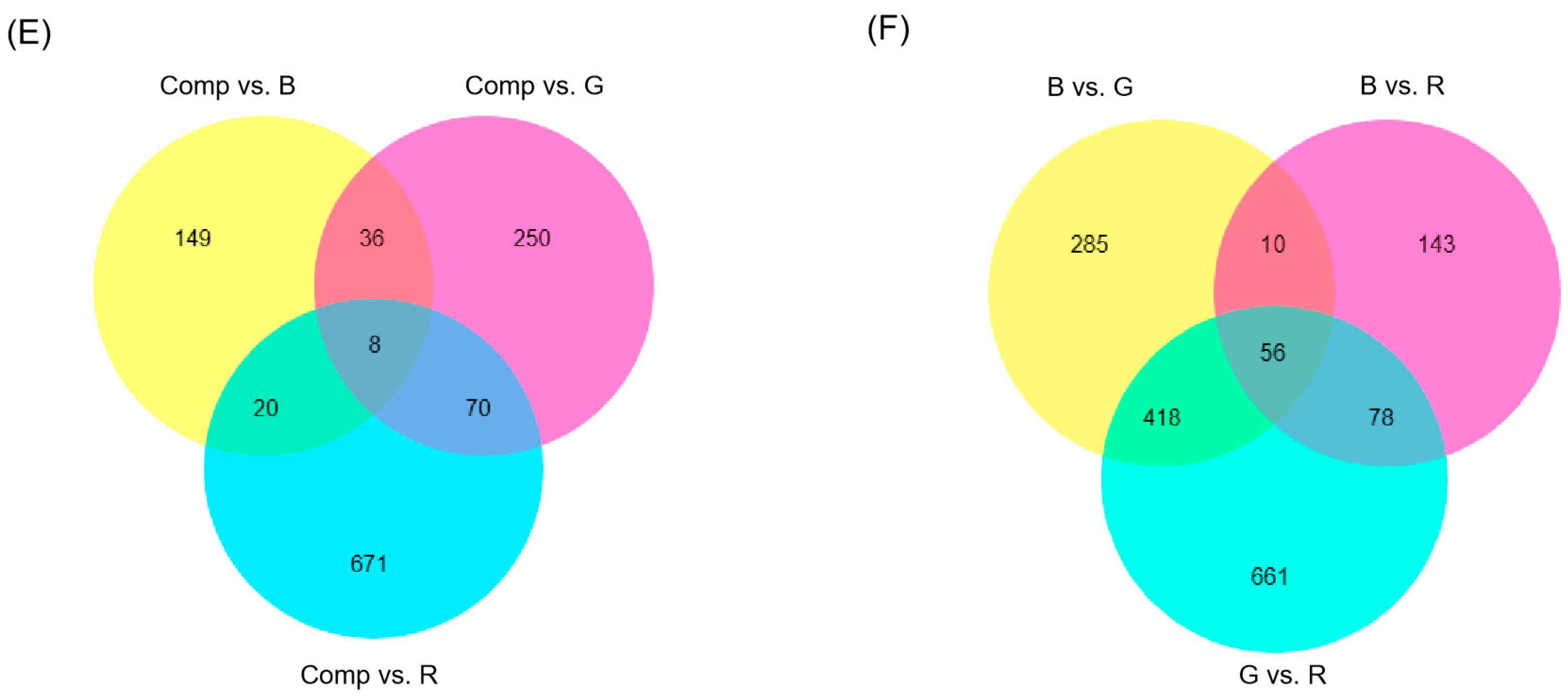
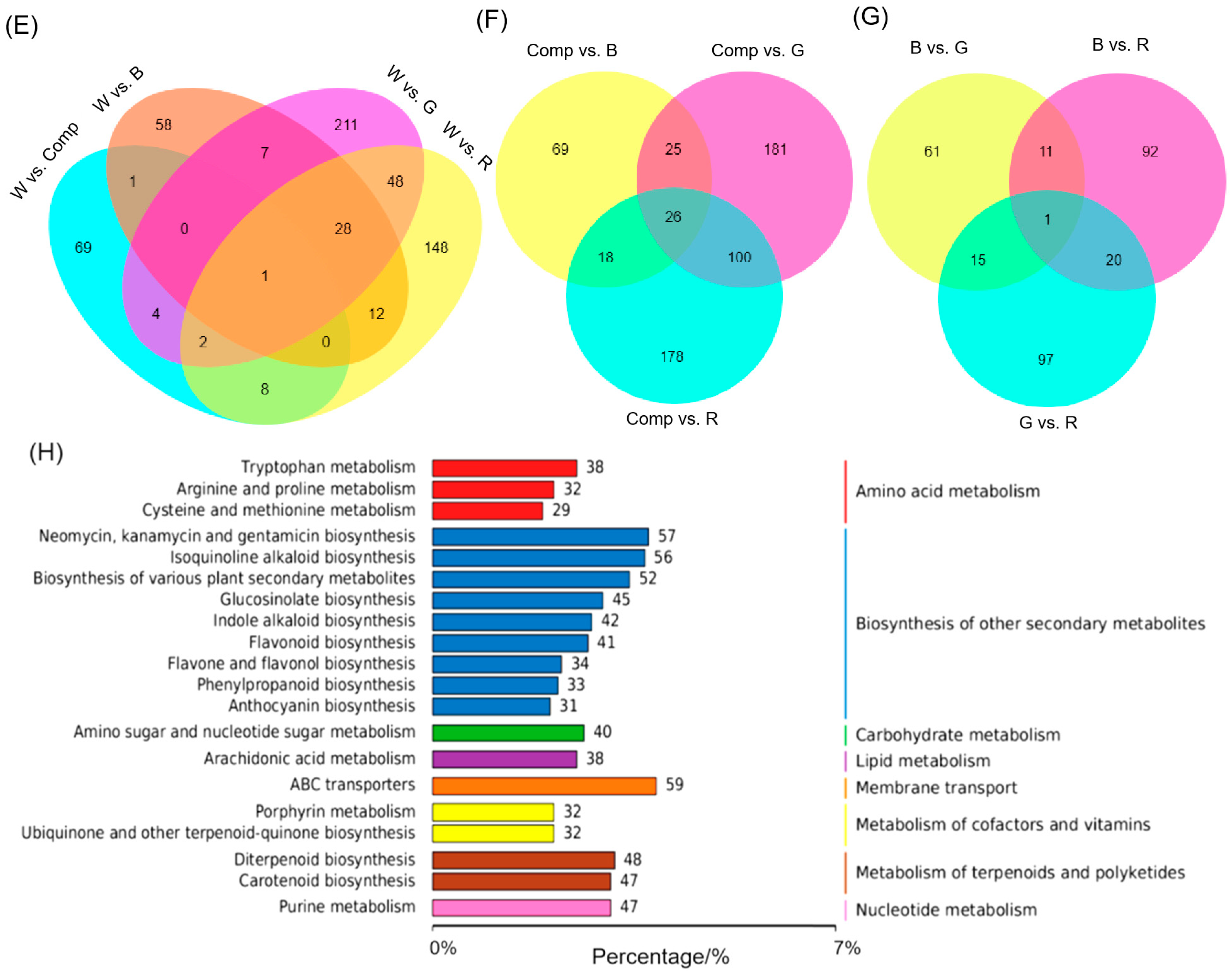
3.3. Transcriptome Analysis of Adventitious Shoots Under Different Light Quality
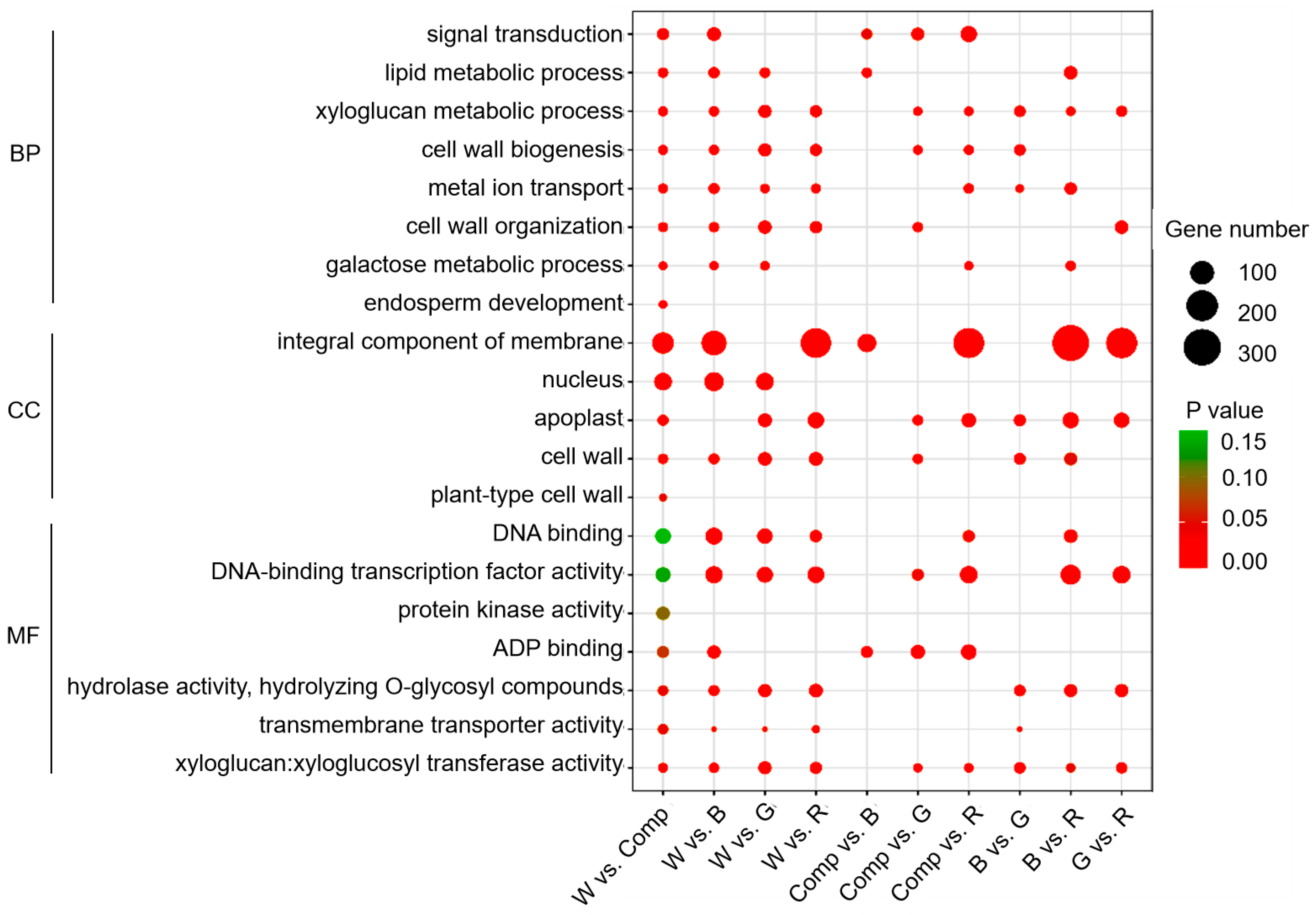
3.4. GO and KEGG Enrichment Analyses
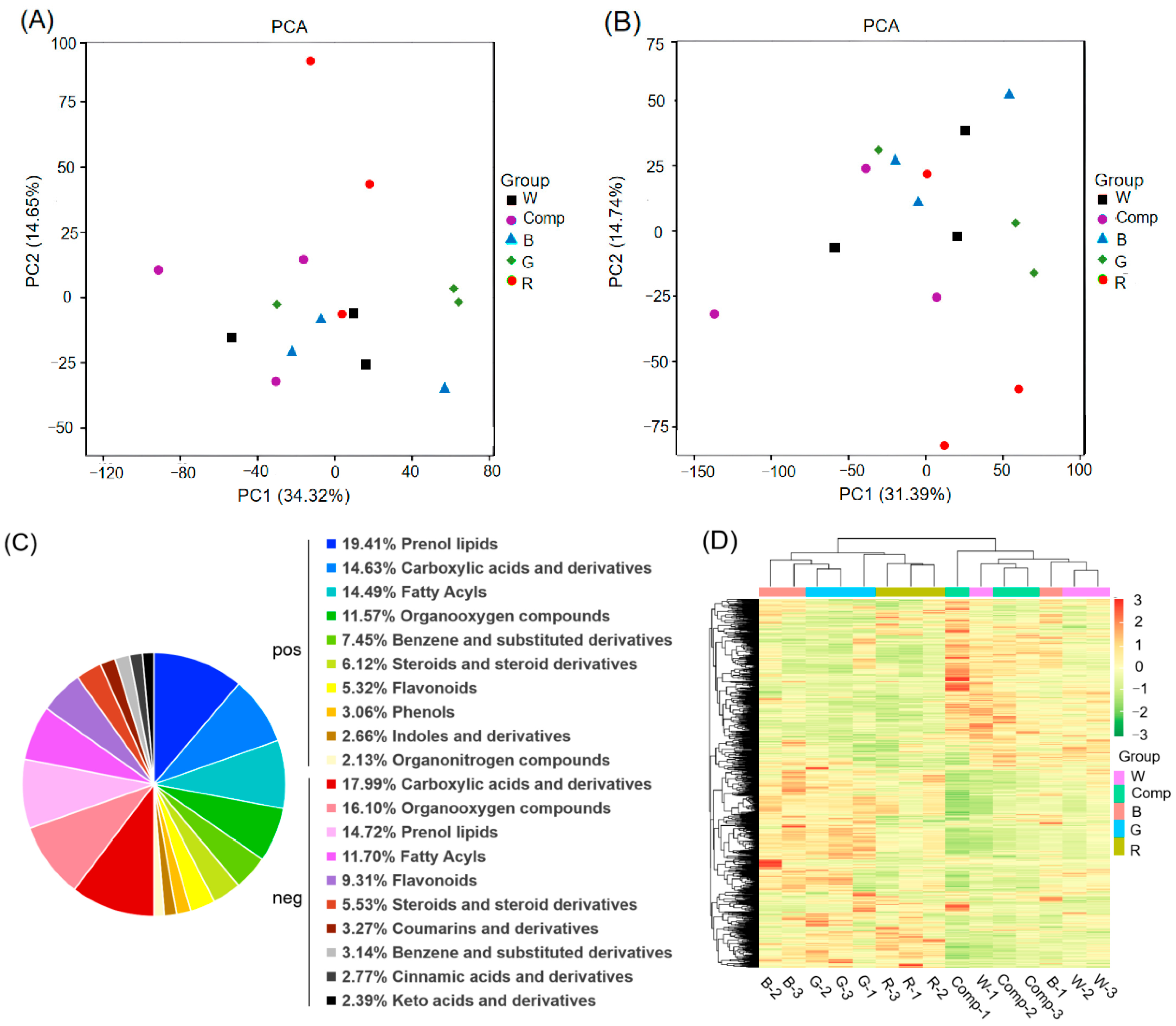
3.5. Metabolite Analysis of Adventitious Shoots Under Different Light Quality
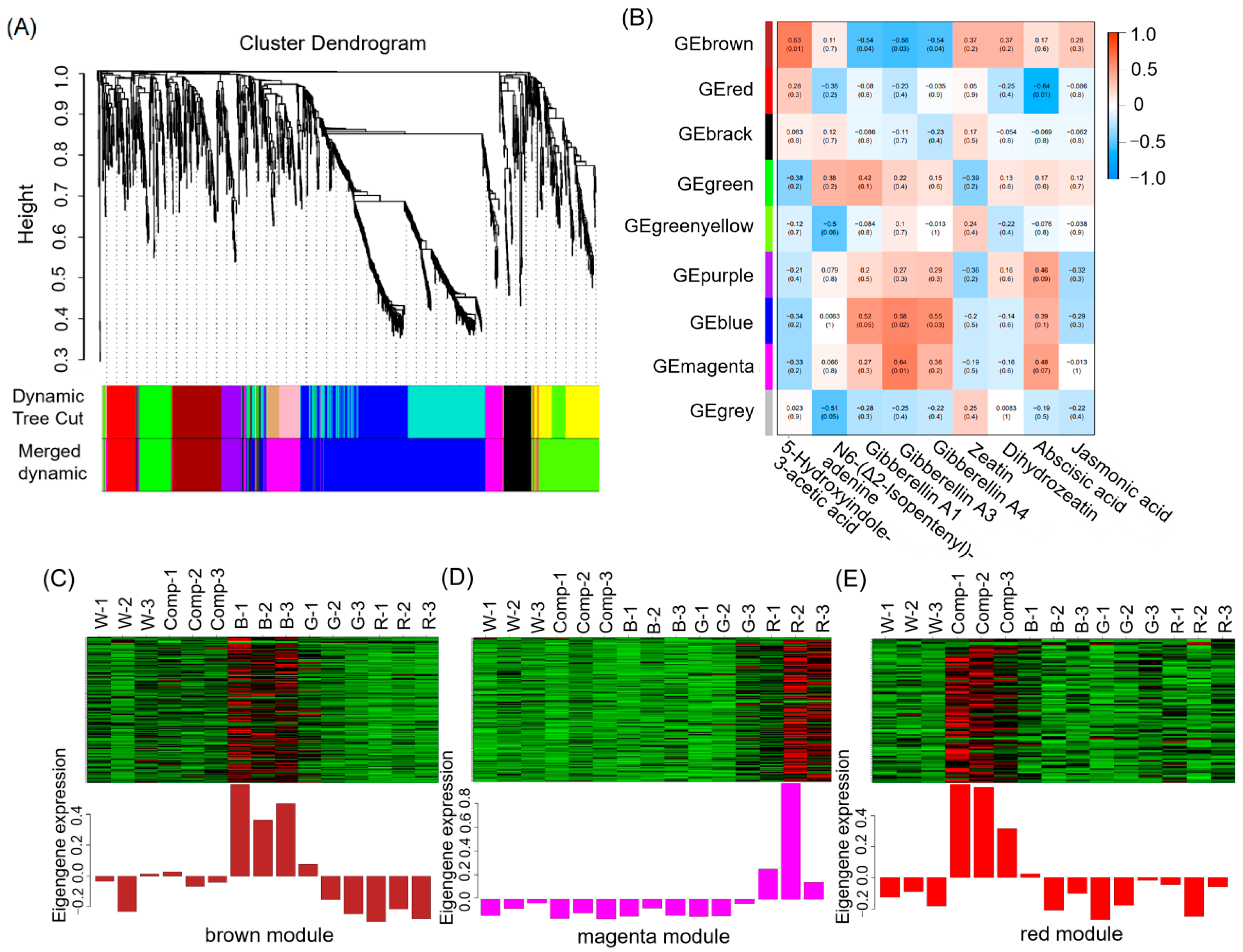
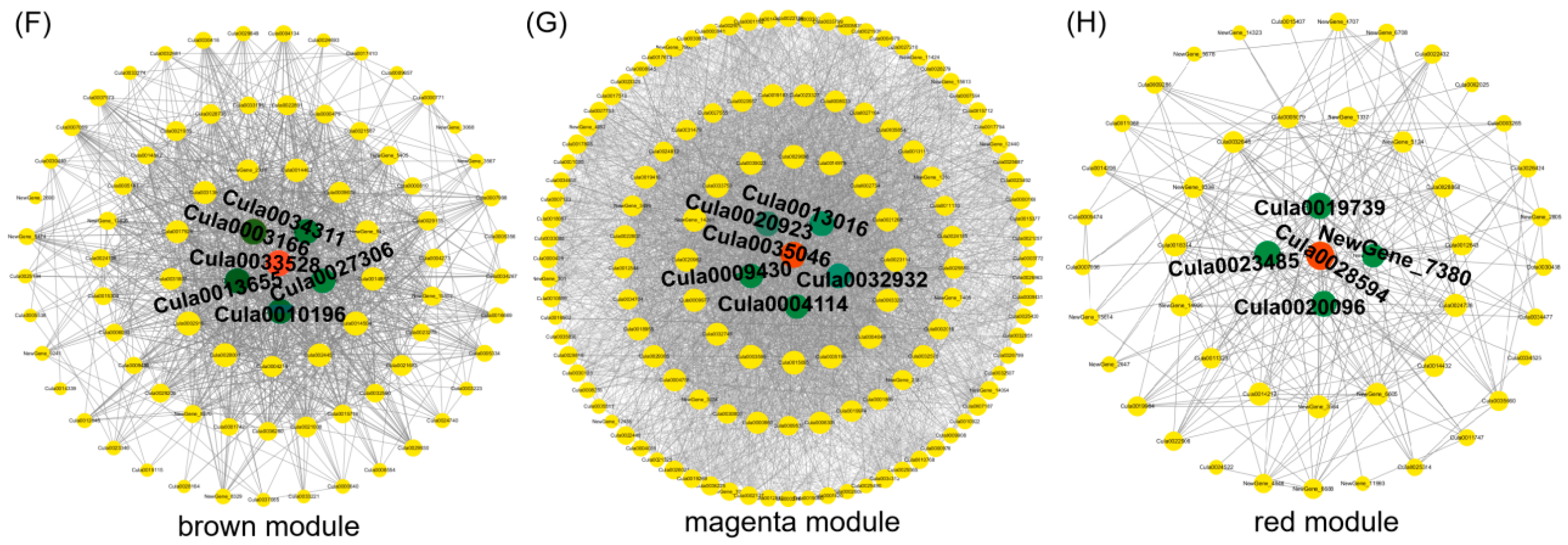
3.6. Co-Expression Network Analysis of Weighted Genes
3.7. Variation of Gene Expression and Metabolite Accumulation in Key Pathways Regulated by Light Quality
4. Discussion
4.1. Light Quality Regulation on Morphology, Physiology, and Biochemistry
4.2. Light Quality Regulation on DEGs and DMs
4.3. Key Genes and Networks for Light Quality Regulating Proliferation of Chinese Fir Seedlings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.Z.; Fan, X.C.; Song, M.F.; Yao, S.T.; Liu, T.; Ding, W.S.; Liu, L.; Zhang, M.; Zhan, W.; Yan, L.; et al. Cryptochrome 1b represses gibberellin signaling to enhance lodging resistance in maize. Plant Physiol. 2024, 194, 902–917. [Google Scholar] [CrossRef] [PubMed]
- Riikonen, J. Applications of different light spectra in growing forest tree seedlings. Forests 2021, 12, 1194. [Google Scholar] [CrossRef]
- Tang, Z.Q.; Yu, J.H.; Xie, J.M.; Lyu, J.; Feng, Z.; Dawuda, M.M.; Liao, W.; Wu, Y.; Hu, L. Physiological and growth response of pepper (Capsicum annuum L.) seedlings to supplementary red/blue light revealed through transcriptomic analysis. Agronomy 2019, 9, 139. [Google Scholar] [CrossRef]
- Shen, Y.; Fan, K.; Wang, Y.; Wang, H.; Ding, S.; Song, D.; Shen, J.; Li, H.; Song, Y.; Han, X.; et al. Red and blue light affect the formation of adventitious roots of tea cuttings (Camellia sinensis) by regulating hormone synthesis and signal transduction pathways of mature leaves. Front. Plant Sci. 2022, 13, 943662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Jiang, L.Y.; Li, Y.L.; Chen, Q.; Ye, Y.T.; Zhang, Y.; Luo, Y.; Sun, B.; Wang, X.; Tang, H. Effect of red and blue light on anthocyanin accumulation and differential gene expression in strawberry (Fragaria × ananassa). Molecules 2018, 23, 820. [Google Scholar] [CrossRef]
- Wang, Y.T.; Jiang, Z.H.; Li, W.X.; Yang, X.L.; Li, C.M.; Cai, D.X.; Pan, Y.; Su, W.; Chen, R. Supplementary low far-red light promotes proliferation and photosynthetic capacity of blueberry in vitro plantlets. Int. J. Mol. Sci. 2024, 25, 688. [Google Scholar] [CrossRef] [PubMed]
- Li, X.B.; Yang, K.G.; Ma, J.; Wu, S.D.; Xu, E.Y.; Liu, Y.Y.; Wang, Y.; Luo, A.; Fan, Y. Transcriptome analysis reveals different red and blue light duration promote growth and main medicinal ingredients of Dendrobium nobile Lindl. Ind. Crops Prod. 2023, 204, 117347. [Google Scholar] [CrossRef]
- Cai, X.B.; Huq, E. Shining light on plant growth: Recent insights into phytochrome-interacting factors. J. Exp. Bot. 2025, 76, 646–663. [Google Scholar] [CrossRef] [PubMed]
- Nie, R.X.; Wei, X.L.; Jin, N.Q.; Su, S.C.; Chen, X.Y. Response of photosynthetic pigments, gas exchange and chlorophyll fluorescence parameters to light quality in Phoebe bournei seedlings. Plant Growth Regul. 2024, 103, 675–687. [Google Scholar] [CrossRef]
- Zhang, J.L. China Forest Resources Report (2014–2018); China Forestry Publishing House: Beijing, China, 2019; pp. 10–50. [Google Scholar]
- Tang, Y.; Li, L.Y.; Xu, S.S.; Zhong, M.H.; Zheng, X.Y.; Ye, Y.Q. Effect of different light qualities on rooting of tissue cultured Cunninghamia lanceolata seedlings and its mechanism. Acta Bot. Boreali-Occident. Sin. 2022, 42, 609–618. [Google Scholar]
- Zhou, B.Y.; Sun, L.J.; Wu, P.F.; Li, M.; Ma, X.Q. Research progress in cultivation of large-diameter timber tree of Chinese fir. World For. Res. 2024, 37, 54–58. [Google Scholar]
- Zhou, J.Y. The Research of Rapid Propagation Technology Based on Light Emitting Diode of the Chinese Fir. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2013. [Google Scholar]
- Huang, J.; Wu, P.F.; Cheng, H.; Ma, X.Q. Callus subculture and regeneration of Cunninghamia lanceolata. J. Environ. 2018, 38, 20–26. [Google Scholar]
- Li, X.Y.; Zeng, B.S. The proliferative efficiency and the photoprotective response of Aquilaria crassna Pierre. Plantlets under different light quality. Mol. Plant Breed. 2021, 19, 291–298. [Google Scholar]
- Zhou, J.Y.; Peng, Z.Q.; Xu, S.S.; Ding, G.C.; Lin, S.Z. Effect of light quality on the growth of tissue culture chlorophyll and chlorophyll fluorescence in Cunninghamia lanceolata. Chin. J. Trop. Crops 2016, 37, 1759–1765. [Google Scholar]
- Xu, Y.; Yang, M.; Cheng, F.; Liu, S.; Liang, Y. Effects of LED photoperiods and light qualities on in vitro growth and chlorophyll fluorescence of Cunninghamia lanceolata. BMC Plant Biol. 2020, 20, 269. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.X.; Ye, Y.Q.; Xu, S.S.; Lin, K.M. Optimization of the light environment for the propagation of tissue-cultured Cunninghamia lanceolata seedlings. J. Environ. 2023, 43, 380–387. [Google Scholar]
- Ahammed, G.J.; Gantait, S.K.; Mitra, M.; Yang, Y.; Li, X. Role of ethylene crosstalk in seed germination and early seedling development: A review. Plant Physiol. Biochem. 2020, 151, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.Z.; Han, X.; Wang, H.; Fan, K.; Wang, Y.; Shen, J.Z.; Li, H.; Song, D.; Ding, S.; Ding, Z. Full-length transcriptome and metabolism revealed the difference of callus formation of tea cutting under white, red, and blue light. Plant Growth Regul. 2023, 101, 715–726. [Google Scholar] [CrossRef]
- Wang, L.B.; Qian, Y.H.; Wu, L.Y.; Wei, K.; Wang, L.Y. The MADS-box transcription factor CsAGL9 plays essential roles in seed setting in Camellia sinensis. Plant Physiol. Biochem. 2024, 207, 108301. [Google Scholar] [CrossRef]
- Liu, T.D.; Zhang, X.W. Comparative transcriptome and metabolome analysis reveal glutathione metabolic network and functional genes underlying blue and red-light mediation in maize seedling leaf. BMC Plant Biol. 2021, 21, 593. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Ni, M.; Zhang, Z.H.; Yu, F.Y. Research progress of brassinolide in regulating plant growth and development. Sci. Silvae Sin. 2022, 58, 144–155. [Google Scholar]
- Liu, Y. CWGCNA: An R package to perform causal inference from the WGCNA framework. NAR Genom. Bioinform. 2024, 6, lqae042. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.X.; Zeng, X.P.; Liu, Y.C.; Zhang, H.; Hu, Q.C. An orthogonal design of light factors to optimize growth, photosynthetic capability and metabolite accumulation of Anoectochilus roxburghii (Wall.) Lindl. Sci. Hortic. 2021, 288, 110272. [Google Scholar] [CrossRef]
- Ye, Y.Q.; Luo, H.Y.; Li, M.; Liu, X.X.; Cao, G.Q.; Xu, S.S. Effects of nitrogen forms on lateral roots development and photosynthetic characteristics in leaves of Cunninghamia lanceolata seedlings. Acta Bot. Boreali-Occident. Sin. 2018, 38, 2036–2044. [Google Scholar]
- Mao, W.; Bao, C.Y.; Cheng, Q.; Liang, N.; Wang, L.C.; Yang, H.Q. All-year high IAA and ABA contents in rhizome buds may contribute to natural four-season shooting in woody bamboo Cephalostachyum pingbianense. Plants 2024, 13, 410. [Google Scholar] [CrossRef]
- Xu, H.; Deng, L.; Zhou, X.; Xing, Y.; Li, G.; Chen, Y.; Huang, Y.; Ma, X.; Liu, Z.-J.; Li, M.; et al. Unveiling the PHR-centered regulatory network orchestrating the phosphate starvation signaling in Chinese fir (Cunninghamia lanceolata). bioRxiv 2024. bioRxiv:06.10.598158. [Google Scholar] [CrossRef]
- Guo, Y.J.; Su, A.; Tian, H.H.; Zhai, M.X.; Li, W.T.; Tian, Y.D.; Li, K.; Sun, G.; Jiang, R.; Han, R.; et al. Transcriptomic analysis of spleen revealed mechanism of dexamethasone-induced immune suppression in chicks. Genes 2020, 11, 513. [Google Scholar] [CrossRef]
- Makino, M.; Shimizu, K.; Kadota, K. Enhanced clustering-based differential expression analysis method for RNA-seq data. MethodsX 2024, 12, 102518. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, J.X.; Zhang, J.P.; You, W.; Hu, Q.L. Development of a TaqMan real-time PCR for detection of the Mycoplasma mycoides subsp. capri. Small Rumin. Res. 2019, 175, 31–36. [Google Scholar] [CrossRef]
- Xie, L.B.; Song, X.; Lei, L.; Chen, C.; Zhao, H.; Hu, J.Y.; Yu, Y.; Bai, X.; Wu, X.; Li, X.; et al. Exploring the potential mechanism of Heng-Gu-Gu-Shang-Yu-He-Ji therapy for osteoporosis based on network pharmacology and transcriptomics. J. Ethnopharmacol. 2024, 321, 117480. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Y.C.; Yin, L.Q.; Li, Q.Z.; Yang, L.Y.; Li, X. Effects of LED light on proliferation and physiological characteristics of Gerbera jamesonii plantlets. Acta Bot. Boreali-Occident. Sin. 2017, 37, 2419–2426. [Google Scholar]
- Liu, Q.; Lian, H.F.; Liu, S.Q.; Sun, Y.L.; Yu, X.H.; Guo, H.P. Effects of different LED light qualities on photosynthetic characteristics, fruit production and quality of strawberry. Chin. J. Appl. Ecol. 2015, 26, 1743–1750. [Google Scholar]
- Hernandez-Adasme, C.; Silva, H.; Escalona, V. In-door germination and seedling growth of green and red lettuce under LED-light spectrum and subsequent effect on baby leaf lettuce. Ital. J. Agron. 2022, 17, 1982. [Google Scholar] [CrossRef]
- Chen, J.C.; Chen, G.; Dai, J.H.; Fan, F.J.; Cao, G.Q.; Cao, S.J. Effects of different light quality ratios on photosynthetic and physiological characteristics of Chinese fir seedlings. J. Agric. Sci. 2022, 35, 2319–2325. [Google Scholar]
- Seki, S.; Yoshida, K.; Sugisaki, M.; Yamano, N.; Fujii, R. Characterization of the ultraviolet-B absorption band of carotenoids using solvent-dependent shifts in steady-state and transient absorption spectra. J. Phys. Chem. B 2024, 128, 5623–5629. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wonder, J.; Helming, T.; van Asselt, G.; Pantazopoulou, C.; van de Kaa, Y.; Kohlen, W.; Pierik, R.; Kajala, K. Evaluation of the roles of brassinosteroid, gibberellin and auxin for tomato internode elongation in response to low red: Far-red light. Physiol. Plant. 2024, 176, e14558. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, M.; Kesy, J.; Trejgell, A. Abscisic acid in preservation of Taraxacum pieninicum in the form of synthetic seeds in slow growth conditions. Plant Cell Tissue Organ Cult. 2021, 144, 295–312. [Google Scholar] [CrossRef]
- Peng, M.X.; Chen, R.M.; Wei, Q.; Guo, H.R.; Zeng, R.Z.; Xie, L.; Zhang, Z.-S.; Chen, J. Effects of genotype, light, and plant growth regulators on rhizome browning, proliferation, and sprouting in Cymbidium. HortScience 2023, 58, 671–676. [Google Scholar] [CrossRef]
- Fan, X.X.; Bian, Z.H.; Song, B.; Xu, H. Transcriptome analysis reveals the differential regulatory effects of red and blue light on nitrate metabolism in pakchoi (Brassica campestris L.). J. Integr. Agric. 2022, 21, 1015–1027. [Google Scholar] [CrossRef]
- Dong, T.Y.; Zhang, P.A.; Hakeem, A.; Liu, Z.J.; Su, L.Y.; Ren, Y.H.; Pei, D.; Xuan, X.; Li, S.; Fang, J. Integrated transcriptome and metabolome analysis reveals the physiological and molecular mechanisms of grape seedlings in response to red, green, blue, and white LED light qualities. Environ. Exp. Bot. 2023, 213, 105441. [Google Scholar] [CrossRef]
- Gu, H.; Ding, W.J.; Shi, T.T.; Ouyang, Q.X.; Yang, X.L.; Yue, Y.Z.; Wang, L. Integrated transcriptome and endogenous hormone analysis provides new insights into callus proliferation in Osmanthus fragrans. Sci. Rep. 2022, 12, 7609. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.Y.; Hu, D.H.; Wei, R.P.; Yan, S.; Wang, R.H.; Zheng, H.Q. Global transcriptome analysis reveals genes associated with seedling advance growth traits in a selfed family of Chinese fir (Cunninghamia lanceolata). Dendrobiology 2022, 87, 27–46. [Google Scholar] [CrossRef]
- Chen, X.T.; Zhao, G.Y.; Li, Y.L.; Wei, S.M.; Dong, Y.H.; Jiao, R.Z. Integrative analysis of the transcriptome and metabolome reveals the mechanism of Chinese fir seed germination. Forests 2023, 14, 676. [Google Scholar] [CrossRef]
- Zheng, Z.; Gao, S.; Wang, G. Far red light induces the expression of LHCSR to trigger nonphotochemical quenching in the intertidal green macroalgae Ulva prolifera. Algal Res.-Biomass Biofuels Bioprod. 2019, 40, 101512. [Google Scholar] [CrossRef]
- Ptushenko, V.V.; Bondarenko, G.N.; Vinogradova, E.N.; Glagoleva, E.S.; Karpova, O.V.; Ptushenko, O.S.; Shibzukhova, K.A.; Solovchenko, A.E.; Lobakova, E.S. Chilling upregulates expression of the PsbS and LhcSR genes in the chloroplasts of the green microalga Lobosphaera incisa IPPAS C-2047. Biochemistry 2022, 87, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.; Kim, E.; Ishii, A.; Ruban, A.V.; Minagawa, J. Overexpression of LHCSR and PsbS enhances light tolerance in Chlamydomonas reinhardtii. J. Photochem. Photobiol. B 2023, 244, 112718. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, L.; Luo, R.; Zhang, Q.; Wang, X.; Lin, F.; Wang, P. Genome-wide analysis of filamentous temperature-sensitive H protease (ftsH) gene family in soybean. BMC Genom. 2024, 25, 524. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Zhou, B.H.; Wang, Y.X.; Hao, F.Y.; Zhu, L.; Liu, Y.H.; Wang, N.; Wang, F.; Li, X.; Dong, Y.; et al. Genome-wide identification and comprehensive analysis of the FtsH gene family in Soybean (Glycine max). Int. J. Mol. Sci. 2023, 24, 16996. [Google Scholar] [CrossRef]
- Zaltsman, A.; Feder, A.; Adam, Z. Developmental and light effects on the accumulation of FtsH protease in Arabidopsis chloroplasts–implications for thylakoid formation and photosystem II maintenance. Plant J. 2005, 42, 609–617. [Google Scholar] [CrossRef]
- Zhong, M.; Zeng, B.J.; Tang, D.Y.; Yang, J.X.; Qu, L.N.; Yan, J.D.; Wang, X.; Li, X.; Liu, X.; Zhao, X.; et al. The blue light receptor CRY1 interacts with GID1 and DELLA proteins to repress GA signaling during photomorphogenesis in Arabidopsis. Mol. Plant 2021, 14, 1328–1342. [Google Scholar] [CrossRef]
- Yan, B.Q.; Yang, Z.J.; He, G.H.; Jing, Y.X.; Dong, H.X.; Ju, L.; Zhang, Y.W.; Zhu, Y.F.; Zhou, Y.; Sun, J.Q. The blue light receptor CRY1 interacts with GID1 and DELLA proteins to repress gibberellin signaling and plant growth. Plant Commun. 2021, 2, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.W.; Liu, M.J.; Chen, I.C.; Huang, C.H.; Chao, L.Y.; Hsieh, H.L. A glutathione S-transferase regulated by light and hormones participates in the modulation of Arabidopsis seedling development. Plant Physiol. 2010, 154, 1646–1658. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Yang, W.; Chen, J.; Peng, F.M. Effect of light quality on physiological characteristics and expression of antioxidant enzyme genes in Bletilla striata L. in vitro. J. Trop. Subtrop. Bot. 2016, 24, 665–670. [Google Scholar]
- Tian, Y.Y.; Wang, H.Y.; Zhang, Z.Q.; Zhao, X.X.; Wang, Y.; Zhang, L.X. An RNA-seq analysis reveals differential transcriptional responses to different light qualities in leaf color of Camellia sinensis cv. Huangjinya. J. Plant Growth Regul. 2022, 41, 612–627. [Google Scholar] [CrossRef]
- Su, Y.T.; Zeeshan, U.; Haq, M.; Liu, X.F.; Li, Y.; Yu, J.; Yang, D.; Wu, Y.; Liu, Y. A genome-wide identification and expression analysis of the Casparian strip membrane domain protein-like gene family in Pogostemon cablin in response to p-HBA-induced continuous cropping obstacles. Plants 2023, 12, 3901. [Google Scholar] [CrossRef] [PubMed]
- Erdayani, E.; Nagarajan, R.; Grant, N.P.; Gill, K.S. Genome-wide analysis of the HSP101/CLPB gene family for heat tolerance in hexaploid wheat. Sci. Rep. 2020, 10, 3948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, M.L.; Jiang, Z.X.; Liu, L.; Pu, J.B.; Zhang, W.J.; Wang, S.; Xu, F. The xyloglucan endotransglucosylase/hydrolase gene XTH22/TCH4 regulates plant growth by disrupting the cell wall homeostasis in Arabidopsis under boron deficiency. Int. J. Mol. Sci. 2022, 23, 1250. [Google Scholar] [CrossRef]
- Sgamma, T.; Forgione, I.; Luziatelli, F.; Iacona, C.; Mancinelli, R.; Thomas, B.; Ruzzi, M.; Muleo, R. Monochromic radiations provided by light emitted diode (LED) modulate infection and defense response to fire blight in pear trees. Plants 2021, 10, 1886. [Google Scholar] [CrossRef] [PubMed]



| Treatment | Light Quality | Abbreviation | Peak Wavelength (nm) | Half Wave Width (nm) | Light Intensity (μmol m−2 s−1) |
|---|---|---|---|---|---|
| Tr1 | Composite | Comp | 450 (B), 532 (G), 660 (R) | 20 (B), 30 (G), 20 (R) | 20 (B) + 2 (G) + 30 (R) |
| Tr2 | Blue | B | 450 | 20 | 52 |
| Tr3 | Green | G | 532 | 30 | 52 |
| Tr4 | Red | R | 660 | 20 | 52 |
| Control | White | W | — | — | 52 |
| Treatment | Light Quality | Proliferation Coefficient | Shoot Height /cm | Leaf Area /mm2 | Fresh Weight /mg | Dry Weight /mg | Dry Matter Ratio/% |
|---|---|---|---|---|---|---|---|
| Tr1 | Comp | 3.68 ± 0.12 a | 2.91 ± 0.20 ab | 7.84 ± 0.40 b | 194 ± 14 a | 20.1 ± 1.0 a | 10.3 ± 0.2 b |
| Tr2 | B | 3.85 ± 0.17 a | 2.53 ± 0.20 b | 7.27 ± 0.42 b | 152 ± 13 b | 18.1 ± 1.5 a | 11.8 ± 0 a |
| Tr3 | G | 2.12 ± 0.10 c | 2.97 ± 0.17 ab | 5.14 ± 0.23 c | 127 ± 12 c | 9.9 ± 1.1 b | 7.9 ± 0.2 e |
| Tr4 | R | 1.74 ± 0.10 c | 3.03 ± 0.40 a | 9.02 ± 0.47 a | 123 ± 14 c | 10.9 ± 1.5 b | 8.9 ± 0.4 d |
| Control | W | 2.85 ± 0.18 b | 2.61 ± 0.18 ab | 7.12 ± 0.41 b | 116 ± 12 c | 10.8 ± 1.2 b | 9.5 ± 0.1 c |
| F value | 32.366 | 2.473 | 38.142 | 19.420 | 39.571 | 150.312 | |
| p-value | 0.0001 | 0.1119 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Xu, S.; Ye, Y.; Lin, K.; Lan, W.; Cao, G. Integrative Analysis of Transcriptome and Metabolome Reveals Light Quality-Mediated Regulation of Adventitious Shoot Proliferation in Chinese Fir. Forests 2025, 16, 486. https://doi.org/10.3390/f16030486
Chen M, Xu S, Ye Y, Lin K, Lan W, Cao G. Integrative Analysis of Transcriptome and Metabolome Reveals Light Quality-Mediated Regulation of Adventitious Shoot Proliferation in Chinese Fir. Forests. 2025; 16(3):486. https://doi.org/10.3390/f16030486
Chicago/Turabian StyleChen, Meixiang, Shanshan Xu, Yiquan Ye, Kaimin Lin, Weili Lan, and Guangqiu Cao. 2025. "Integrative Analysis of Transcriptome and Metabolome Reveals Light Quality-Mediated Regulation of Adventitious Shoot Proliferation in Chinese Fir" Forests 16, no. 3: 486. https://doi.org/10.3390/f16030486
APA StyleChen, M., Xu, S., Ye, Y., Lin, K., Lan, W., & Cao, G. (2025). Integrative Analysis of Transcriptome and Metabolome Reveals Light Quality-Mediated Regulation of Adventitious Shoot Proliferation in Chinese Fir. Forests, 16(3), 486. https://doi.org/10.3390/f16030486






