Effects of Rooting Substrates and Plant Growth Regulators on Rooting Performance, Photosynthetic Characteristics, and Soil Properties of Broussonetia × kazinoki Sieb. Cuttings
Abstract
1. Introduction
2. Materials and Methods
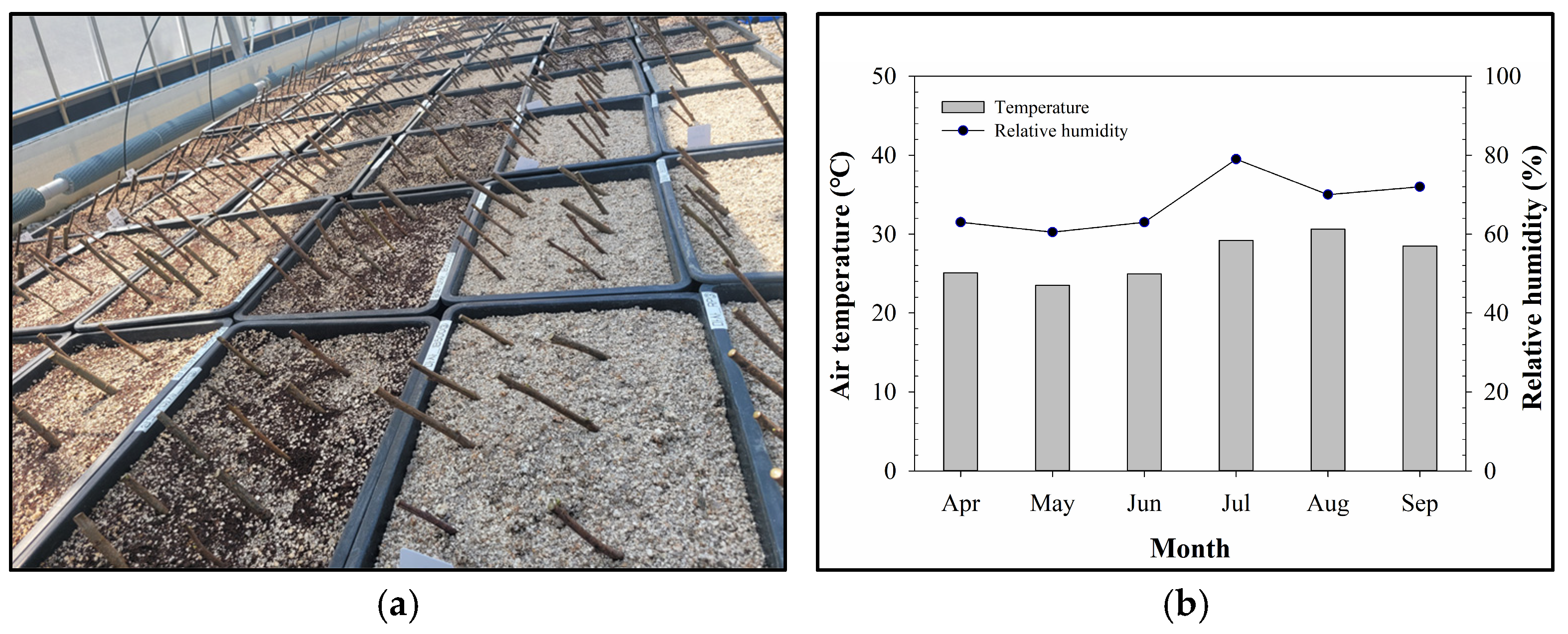
2.1. Plant Materials
2.2. Rooting Substrate Treatments
2.3. PGR Treatments
2.4. Leaf Gas Exchange Measurements
2.5. JIP-Test Chlorophyll Flourescence and SPAD Mesurements
2.6. Root Growth Measurement
2.7. Analysis of Rooting Substrates
2.8. Statistical Analysis
3. Results
3.1. Leaf Gas Exchange
3.2. SPAD and JIP Analyses of B. × kazinoki Cuttings
3.2.1. SPAD
3.2.2. JIP-Test Analysis
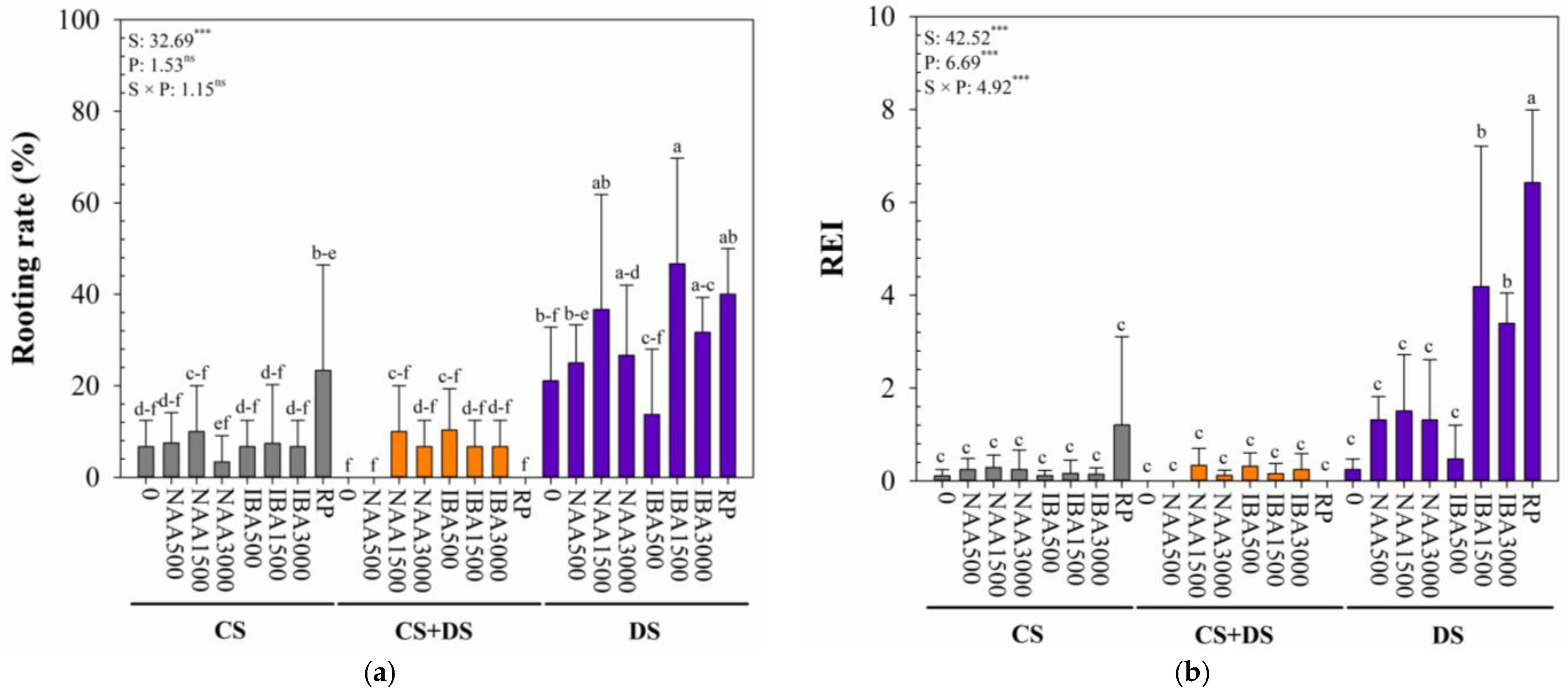
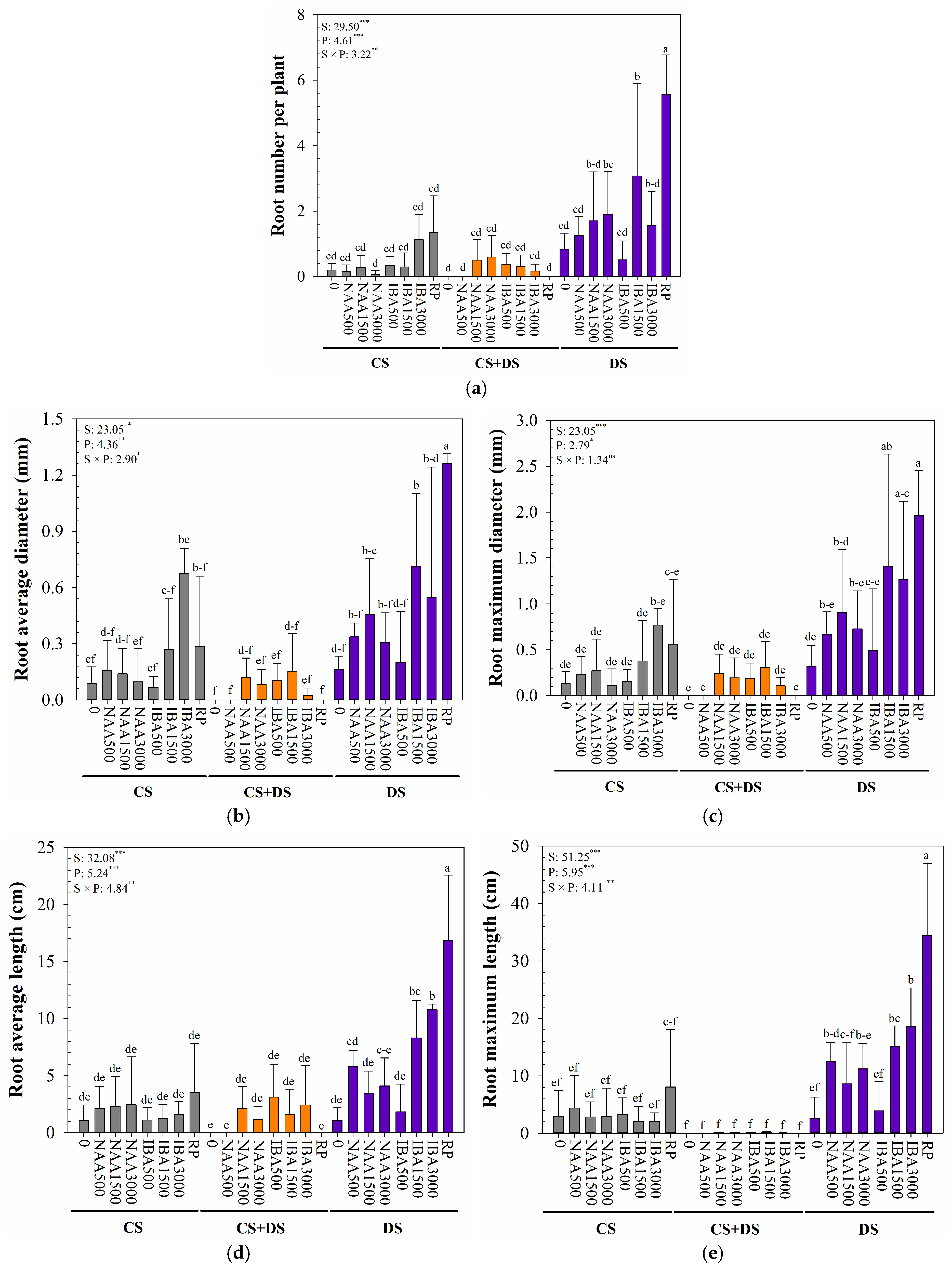
3.3. Rooting Characteristics of B. × kazinoki
3.4. Physicochemical Analysis of Rooting Substrates
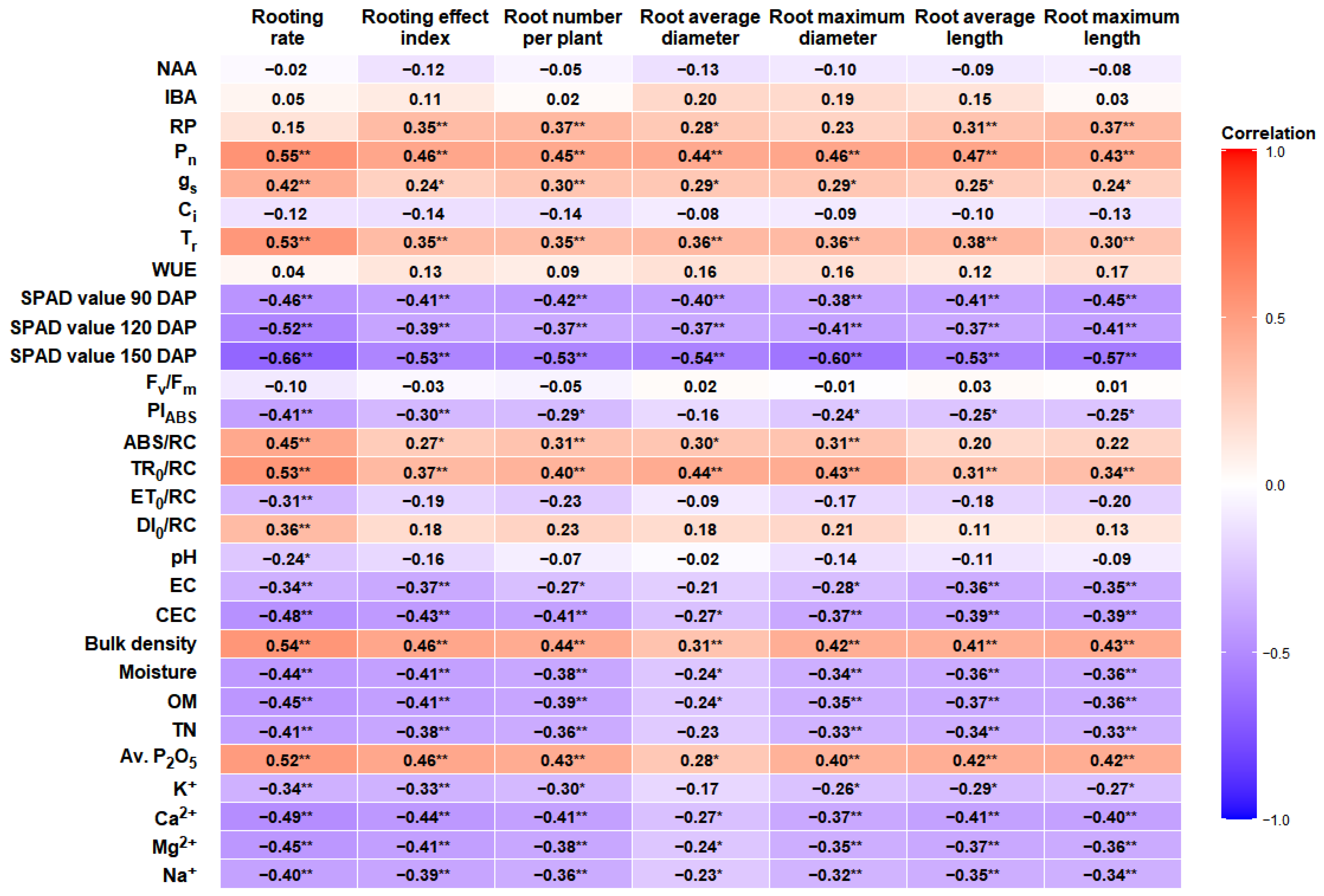
3.5. Correlations Among Gas Exchanges, Chlorophyll Fluorescence, Soil Factors, and Rooting Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABS/RC | Absorbed photon flux per reaction center |
| Av.P2O5 | Available phosphorus |
| Ci | Intercellular CO2 concentration |
| CEC | Cation exchange capacity |
| CS | Commercial substrate |
| DI0/RC | Dissipated energy flux per reaction center |
| DS | Decomposed granite soil. |
| EC | Electrical conductivity |
| ET0/RC | Electron transport flux per reaction center |
| Fv/Fm | Maximum quantum yield of PSII photochemistry |
| gS | Stomatal conductance |
| IBA | Indole-3-butyric acid |
| NAA | 1-Naphthaleneacetic acid |
| OM | Organic matter |
| PGR | Plant growth regulator |
| PIABS | Performance index for energy conservation from |
| Pn | Net photosynthetic rate |
| REI | Rooting efficiency index |
| RP | Rooting powder |
| TN | Total nitrogen |
| Tr | Transpiration rate |
| TR0/RC | Trapped exciton flux per reaction center |
References
- Kuo, W.H.; Liu, S.H.; Chang, C.C.; Hsieh, C.L.; Li, Y.H.; Ito, T.; Won, H.; Kokubugata, G.; Chung, K.F. Plastome phylogenomics of Allaeanthus, Broussonetia and Malaisia (Dorstenieae, Moraceae) and the origin of B. × kazinoki. J. Plant Res. 2022, 135, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.T.; Tanaka, K.; Takashima, A.; Shibutani, A.; Ishikawa, R. Genetic diversity on farm in Japanese paper mulberry. Ecol. Evol. 2025, 15, e70828. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Bowden, C. Handmade paper: A review of its history, craft, and science. BioResources 2009, 4, 1736–1792. [Google Scholar] [CrossRef]
- Jeong, S.H. A study on manufacturing technologies and excellence of Korean traditional paper. Korean J. Cult. Herit. Stud. 2015, 48, 96–131. [Google Scholar]
- Lee, O.K.; Kim, S.; Lee, H.W. Evolution of the hanji-making technology, from ancient times to the present. J. Korean Wood Sci. Technol. 2023, 51, 509–525. [Google Scholar] [CrossRef]
- National Institute of Biological Resources (NIBR). National Species List of Korea. National Institute of Biological Resources, Incheon, Korea. Available online: https://species.nibr.go.kr (accessed on 6 November 2025).
- Chung, K.F.; Kuo, W.H.; Hsu, Y.H.; Li, Y.H.; Rubite, R.R.; Xu, W.B. Molecular recircumscription of Broussonetia (Moraceae) and the identity and taxonomic status of B. kaempferi var. australis. Bot. Stud. 2017, 58, 11. [Google Scholar] [CrossRef]
- Won, H. Test of the hybrid origin of Broussonetia× kazinoki (Moraceae) in Korea using molecular markers. Korean J. Plant Taxon. 2019, 49, 282–293. [Google Scholar] [CrossRef]
- Song, J.E.; Kim, D.H.; Kim, H.J. A study on the characteristics of traditional Hanji, Xuan paper, and Washi for UNESCO registration. J. Korea Tappi 2023, 55, 92–102. [Google Scholar] [CrossRef]
- Go, I.H.; Jeong, S.H. Anatomical, morphological, and chemical characteristics of paper-mulberry wood and bast fiber for raw material of Korean paper (hanji). J. Conserv. Sci. 2018, 34, 517–524. [Google Scholar] [CrossRef]
- Lee, H.; Ha, H.; Lee, J.K.; Park, S.J.; Jeong, S.I.; Shin, H.K. The leaves of Broussonetia kazinoki Siebold inhibit atopic dermatitis-like response on mite allergen-treated Nc/Nga mice. Biomol. Ther. 2014, 22, 438–444. [Google Scholar] [CrossRef]
- Cha, J.Y.; Kim, Y.T.; Kim, H.S.; Cho, Y.S. Antihyperglycemic effect of stem bark powder from paper mulberry (Broussonetia kazinoki Sieb.) in Type 2 diabetic Otsuka Long-Evans Tokushima fatty rats. J. Med. Food 2008, 11, 499–505. [Google Scholar] [CrossRef]
- Lee, H.; Li, H.; Jeong, J.H.; Noh, M.; Ryu, J.H. Kazinol B from Broussonetia kazinoki improves insulin sensitivity via Akt and AMPK activation in 3T3-L1 adipocyte. Fitoterapia 2016, 12, 90–96. [Google Scholar] [CrossRef]
- Kang, H.M.; Koroki, K. A study on the cultivation and production of Korean paper Mulberries and Korean paper manufactures. J. Fac. Agric. Kyushu Univ. 2008, 53, 291–297. [Google Scholar] [CrossRef]
- Korea Forest Service. Forestry Management Status Survey Report 1; Korea Forest Service: Deajeon, Republic of Korea, 2013; pp. 65–74. [Google Scholar]
- Korea Forestry Promotion Institute (Koapi). Forest Products Production Survey. 2019. Available online: https://www.kofpi.or.kr/search/main.jsp (accessed on 1 September 2025).
- Korea Forestry Promotion Institute (Koapi). Forest Products Production Survey. 2020. Available online: https://www.kofpi.or.kr/search/main.jsp (accessed on 1 September 2025).
- Korea Forestry Promotion Institute (Koapi). Forest Products Production Survey. 2021. Available online: https://www.kofpi.or.kr/search/main.jsp (accessed on 1 September 2025).
- Zhang, J.; Chen, S.; Liu, R.; Jiang, J.; Chen, F.; Fang, W. Chrysanthemum cutting productivity and rooting ability are improved by grafting. Sci. World. J. 2013, 2013, 286328. [Google Scholar] [CrossRef]
- Wu, H.X. Benefits and risks of using clones in forestry—A review. Scand. J. For. Res. 2019, 34, 352–359. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.J. Effect of shading degree and rooting media on growth of cuttings in Caragana sinica (Buc’hoz) Rehder and Sedum middendorffianum Maxim. Korean J. Med. Crop Sci. 2015, 23, 271–276. [Google Scholar] [CrossRef]
- Kwon, H.H.; Cho, W. Effects of external treatment of auxins, substrate type, leaf attachment, and leaf cutting on stem cutting of Pseudolysimachion ovatum (Nakai) T. Yamaz. Flower Res. J. 2023, 31, 253–259. [Google Scholar] [CrossRef]
- Kim, C.; Kim, Z. Effects of cutting time, auxin treatment, and cutting position on rooting of the green-wood cuttings and growth characteristics of transplanted cuttings in the adult Prunus yedoensis. Hortic. Sci. Technol. 2012, 30, 129–136. [Google Scholar] [CrossRef]
- Mohammed, A.A. Role of cold storage in rooting of stem cuttings: A review. AJAAR Asian J. Adv. Agric. Res. 2023, 21, 1–6. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, K.D.; Davies, F.T.; Geneve, R.L. Hartmann and Kester’s Principles and Practices of Plant Propagation, 8th ed.; Pearson: London, UK, 2013; pp. 2–928. [Google Scholar]
- Campbell, S.M.; Anderson, S.L.; Brym, Z.T.; Pearson, B.J. Evaluation of substrate composition and exogenous hormone application on vegetative propagule rooting success of essential oil hemp (Cannabis sativa L.). PLoS ONE 2021, 16, e0249160. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Ohyama, K. Paper Mulberry (Broussonetia kazinoki Sieb.). In Trees II; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1989; pp. 402–411. [Google Scholar] [CrossRef]
- Park, B.; Lee, N. Vegetation propagation of Broussonetia kazinoki by rooted cuttings. J. Agric. Life Sci. 1979, 10, 45–48. [Google Scholar]
- Munkgerel, B. Effects of Shading Levels and Growth Regulators on the Rooting Characteristics of Broussonetia kazinoki. Master’s Thesis, Chungbuk National University, Cheongju, Republic of Korea, February 2019. [Google Scholar]
- Park, S. Rooting Characteristics of Broussonetia kazinoki According to Auxin Concentration and Cutting Length by Cutting Time. Master’s Thesis, Chungbuk National University, Cheongju, Republic of Korea, August 2021. [Google Scholar]
- Husen, A.; Iqbal, M.; Siddiqui, S.N.; Sohrab, S.S.; Masresha, G. Effect of indole-3-butyric acid on clonal propagation of mulberry (Morus alba L.) stem cuttings: Rooting and associated biochemical changes. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 161–166. [Google Scholar] [CrossRef]
- Qin, X.; Xing, D.; Wu, Y.; Wang, W.; Li, M.; Solangi, K. Diurnal variation in transport and use of intracellular leaf water and related photosynthesis in three karst plants. Agronomy 2022, 12, 2758. [Google Scholar] [CrossRef]
- Photon System Instruments. FluorPen FP 110 PAR-FluorPen FP 1100 Monitoring Pen MP. Volume 100. Available online: https://psi.cz/support/downloads/hd001/fp001/ (accessed on 1 September 2025).
- Ceusters, N.; Valcke, R.; Frans, M.; Claes, J.E.; Van den Ende, W.; Ceusters, J. Performance index and PSII connectivity under drought and contrasting light regimes in the CAM orchid Phalaenopsis. Front. Plant Sci. 2019, 10, 1012. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, H.; Chen, H.; Wang, T.; Quan, J.e.; Bi, H. The effect of hormone types, concentrations, and treatment times on the rooting traits of Morus ‘Yueshenda 10’ softwood cuttings. Life 2023, 13, 1032. [Google Scholar] [CrossRef]
- National Academy of Agricultural Science (NAAS). Methods of Soil Chemical Analysis; RDA: Suwon, Republic of Korea, 2010. [Google Scholar]
- De Vos, B.; Lettens, S.; Muys, B.; Deckers, J.A. Walkley–Black analysis of forest soil organic carbon: Recovery, limitations and uncertainty. Soil Use Manag. 2007, 23, 221–229. [Google Scholar] [CrossRef]
- Kirk, P.L. Kjeldahl method for total nitrogen. Anal. Chem. 1950, 22, 354–358. [Google Scholar] [CrossRef]
- Alban, L.A.; Vacharotayan, S.; Jackson, T.L. Phosphorus availability in reddish brown lateritic soils. I. Laboratory studies. Agron. J. 1964, 56, 555–558. [Google Scholar] [CrossRef]
- Mesén, F.; Newton, A.C.; Leakey, R.R.B. The effects of propagation environment and foliar area on the rooting physiology of Cordia alliodora (Ruiz & Pavon) Oken cuttings. Trees 1997, 11, 404–411. [Google Scholar] [CrossRef]
- Smalley, T.J.; Dirr, M.A.; Armitage, A.M.; Wood, B.W.; Teskey, R.O.; Severson, R.F. Photosynthesis and leaf water, carbohydrate, and hormone status during rooting of stem cuttings of Acer rubrum. J. Am. Soc. Hortic. Sci. 1991, 116, 1052–1057. [Google Scholar] [CrossRef]
- Svenson, S.E.; Davies, F.T., Jr.; Duray, S.A. Gas exchange, water relations, and dry weight partitioning during root initiation and development of poinsettia cuttings. J. Am. Soc. Hortic. Sci. 1995, 120, 454–459. [Google Scholar] [CrossRef]
- Wilkerson, E.G.; Gates, R.S.; Zolnier, S.; Kester, S.T.; Geneve, R.L. Transpiration capacity in poinsettia cuttings at different rooting stages and the development of a cutting coefficient for scheduling mist. J. Am. Soc. Hortic. Sci. 2005, 130, 295–301. [Google Scholar] [CrossRef]
- Gómez, S.; Gómez, C. Effects of blue-light percentage and carbon dioxide concentration on the water status and growth of chrysanthemum and begonia cuttings in vertical indoor propagation systems. Sci. Hortic. 2025, 347, 114201. [Google Scholar] [CrossRef]
- Tombesi, S.; Palliotti, A.; Poni, S.; Farinelli, D. Influence of light and shoot development stage on leaf photosynthesis and carbohydrate status during the adventitious root formation in cuttings of Corylus avellana L. Front. Plant Sci. 2015, 6, 973. [Google Scholar] [CrossRef] [PubMed]
- Sakai, A.; Sakai, S.; Akiyama, F. Do sprouting tree species on erosion-prone sites carry large reserves of resources? Ann. Bot. 1997, 79, 625–630. [Google Scholar] [CrossRef]
- Palacio, S.; Maestro, M.; Montserrat-Martí, G. Relationship between shoot-rooting and root-sprouting abilities and the carbohydrate and nitrogen reserves of Mediterranean dwarf shrubs. Ann. Bot. 2007, 100, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Zerche, S.; Druege, U. Nitrogen content determines adventitious rooting in Euphorbia pulcherrima under adequate light independently of pre-rooting carbohydrate depletion of cuttings. Sci. Hortic. 2009, 121, 340–347. [Google Scholar] [CrossRef]
- Dwumah, K.A.; Boadi, S.; Kyereh, B. Clonal propagation of Broussonetia papyrifera by stem and root cuttings. J. Sci. Technol. Ghana 2024, 1, 1–12. [Google Scholar] [CrossRef]
- Jiang, C.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Maruo, T. A correlation analysis on chlorophyll content and SPAD value in tomato leaves. Hortic. Res. 2017, 71, 37–42. [Google Scholar] [CrossRef]
- Kandel, B.P. Spad value varies with age and leaf of maize plant and its relationship with grain yield. BMC Res. Notes 2020, 13, 475. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Chrysargyris, A. Alternative growing media under the same fertigation scheme affected mineral accumulation and physiological parameters in grapevine cultivars. Horticulturae 2025, 11, 479. [Google Scholar] [CrossRef]
- Zakaria, N.I.; Ismail, M.R.; Awang, Y.; Megat Wahab, P.E.; Berahim, Z. Effect of root restriction on the growth, photosynthesis rate, and source and sink relationship of chilli (Capsicum annuum L.) grown in soilless culture. BioMed Res. Int. 2020, 2020, 2706937. [Google Scholar] [CrossRef]
- Lazár, D. Chlorophyll a fluorescence induction. Biochim. Biophys. Acta 1999, 1412, 1–28. [Google Scholar] [CrossRef]
- Park, J.Y.; Yoo, S.Y.; Kang, H.G.; Kim, T.W. Use of chlorophyll a fluorescence imaging for photochemical stress assessment in maize (Zea mays L.) leaf under hot air condition. Korean J. Crop Sci. 2016, 61, 270–276. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.; Nam, S.Y. Optimized concentrations of auxinic rooting promoters improve stem cutting propagation efficiency and morphophysiological characteristics in Hedera algeriensis cv. Gloire de Marengo. Hortic. Sci. Technol. 2025, 43, 357–372. [Google Scholar] [CrossRef]
- Lee, J.H.; Nam, S.Y. Growth and photosynthetic responses of Taraxacum coreanum Nakai Seedlings according to the shading levels. Hortic. Sci. Technol. 2024, 42, 214–224. [Google Scholar] [CrossRef]
- Srivastava, A.; Strasser, R.J.; Govindjee. Greening of peas: Parallel measurements of 77 K emission spectra, OJIP chlorophyll a fluorescence transient, period four oscillation of the initial fluorescence level, delayed light emission, and P700. Photosynthetica 1999, 37, 365–392. [Google Scholar] [CrossRef]
- Jedmowski, C.; Ashoub, A.; Momtaz, O.; Brüggemann, W. Impact of drought, heat, and their combination on chlorophyll fluorescence and yield of wild barley (Hordeum spontaneum). J. Bot. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, C.; Pei, W.; Fan, K.; Shen, W. Chlorophyll a fluorescence as a tool to monitor physiological status in the leaves of Artemisia ordosica under root cutting conditions. Front. Plant Sci. 2023, 14, 1308209. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kim, Y.; Bae, Y.; Hyeon, S.; Choi, M.; Jang, D. Investigating growth, root development, and chlorophyll fluorescence of tomato scions and rootstocks under UV-B stress in a plant factory with artificial lighting. Sci. Hortic. 2025, 347, 114191. [Google Scholar] [CrossRef]
- Rapacz, M.; Szewczyk-Taranek, B.; Bani, I.; Marcinkowski, P. The fitness of pelargonium cuttings affects the relationship between the photochemical activity of the photosynthetic apparatus and rooting ability. Sci. Rep. 2024, 14, 19716. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, S.; Wang, X.; Du, Y.; He, Q.; Zhang, Y.; Shen, L.; Hu, H.; Zhang, G.; Li, X. Adventitious root formation in cuttings: Insights from Arabidopsis and prospects for woody plants. Biomolecules 2025, 15, 1089. [Google Scholar] [CrossRef]
- Sedaghathoor, S.; Kayghobadi, S.; Tajvar, Y. Rooting of Mugo pine (Pinus mugo) cuttings as affected by IBA, NAA and planting substrate. For. Syst. 2016, 25, eSC08. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y.; Jiang, C.; Lu, M.Z.; Zhang, J. Exogenous hormones supplementation improve adventitious root formation in woody plants. Front. Bioeng. Biotechnol. 2022, 10, 1009531. [Google Scholar] [CrossRef]
- Pop, T.I.; Pamfil, D.; Bellini, C. Auxin control in the formation of adventitious roots. Not. Bot. Hort. Agrobot. Cluj. 2011, 39, 307–316. [Google Scholar] [CrossRef]
- Vielba, J.M.; Vidal, N.; José, M.C.S.; Rico, S.; Sánchez, C. Recent Advances in Adventitious Root Formation in Chestnut. Plants 2020, 9, 1543. [Google Scholar] [CrossRef]
- Bellamine, J.; Penel, C.; Greppin, H.; Gaspar, T. Confirmation of the role of auxin and calcium in the late phases of adventitious root formation. Plant Growth Regul. 1998, 26, 191–194. [Google Scholar] [CrossRef]
- Oh, H.J.; Lee, S.Y.; Shin, U.S.; Kim, H.C.; Kim, S.Y. Several factors affecting growth of Veronica rotunda var. subintegra (Nakai) T. Yamaz. stem cutting. Korean J. Plant Resour. 2021, 34, 270–277. [Google Scholar] [CrossRef]
- Qin, H.; Huang, R. Auxin controlled by ethylene steers root development. Int. J. Mol. Sci. 2018, 19, 3656. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Hayashi, T.; Harada, T.; Konishi, K. Effects of medium composition and pre-treatment on rooting of plug nursery plant. Acta Hortic. 1992, 319, 441–446. [Google Scholar] [CrossRef]
- Weil, R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Pearson Education: London, UK, 2017; pp. 1–1086. [Google Scholar]
- Hwang, H.S.; Jeong, H.W.; Jo, H.G.; Kang, J.H.; Hwang, S.J. Rooting and growth characteristics of ‘Maehyang’ strawberry cutting transplants affected by different growing media including decomposed granite. Rhizosphere 2022, 22, 100520. [Google Scholar] [CrossRef]
- Shanker, K.; Misra, S.; Topwal, M.; Singh, V. Research review on use of different rooting media in fruit crops. J. Pharmacogn. Phytochem. 2019, 8, 258–261. [Google Scholar]
- Brady, N.C. The Nature and Properties of Soils, 9th ed.; Macmillan: New York, NY, USA, 1984. [Google Scholar]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Life in the balance: A signaling network controlling survival of flooding. Curr. Opin. Plant Biol. 2010, 13, 489–494. [Google Scholar] [CrossRef]
- Lee, J.; Lee, D.; Seo, B.; Kang, I.; Yang, J.; Kim, E.; Kwak, K.; Kim, Y. Growth response to soil physical properties and flooding during silage corn paddy cultivation. Korean J. Crop. Sci. 2024, 69, 325–335. [Google Scholar] [CrossRef]
- Abrol, I.; Yadav, J.S.P.; Massoud, F. Salt-Affected Soils and Their Management; FAO United Nations: Rome, Italy, 1988; pp. 1–39. [Google Scholar]
- Ossom, E.M.; Kunene, S.S. Influence of growing media on stem diameter and ecological characteristics of Pinus patula seedlings in Swaziland. World J. Agric. Sci. 2010, 6, 652–659. [Google Scholar]
- Albrecht, B.A.; Benson, C.H. Effect of desiccation on compacted natural clays. J. Geotech. Geoenviron. Eng. 2001, 127, 67–75. [Google Scholar] [CrossRef]
- Lee, C.W.; Ok, J.H.; Kim, Y.M.; Song, Y.S.; Park, H.J.; Hyun, B.K.; Lee, Y.J.; Oh, T.K. Effect of soil physical properties on nitrogen leaching during sesame (Sesamum indicum L.) cultivation under lysimeter conditions. Korean J. Agric. Sci. 2022, 49, 379–387. [Google Scholar] [CrossRef]
- Zou, J.; Lin, J.; Zhang, B.; Que, Q.; Zhang, J.; Li, Y.; Liu, Y.; Zhou, X.; Chen, X.; Zhou, W. An efficient propagation system through root cuttings of an ecological and economic value plant—Broussonetia papyrifera (L.) L.’Hér. ex Vent. Plants 2022, 11, 1423. [Google Scholar] [CrossRef]
- Druege, U.; Zerche, S.; Kadner, R. Nitrogen- and storage-affected carbohydrate partitioning in high-light-adapted pelargonium cuttings in relation to survival and adventitious root formation under low light. Ann. Bot. 2004, 94, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.; Noctor, G. Photosynthetic Nitrogen Assimilation and Associated Carbon and Respiratory Metabolism; Springer: Dordrecht, The Netherlands, 2002. [Google Scholar]



| Parameter | Name and Basic Physiological Interpretation |
|---|---|
| Specific energy fluxes (per active PSII reaction center) | |
| ABS/RC = M0·(1/VJ)·(1/φP0) | Absorbed photon flux per reaction center (RC) |
| TR0/RC = M0·(1/VJ) | Trapped exciton flux (leading to QA reduction) of absorbed photons per RC |
| ET0/RC = M0·(1/VJ)·ψ0 | Electron transport flux (from reduced QA to QB) per RC |
| DI0/RC = (ABS/RC) − (TR0/RC) | Dissipated energy flux per RC |
| Performance index | |
| PIABS = (RC/ABS)·[φP0/(1 − φP0)]·[ψE0/(1 − ψE0)] | Performance index for energy conservation from photons absorbed by PSII antenna, to the reduction of QB |
| Quantum yields and probabilities | |
| φP0 = Fv/Fm | Maximum quantum efficiency of primary PSII photochemistry; maximum efficiency at which light absorbed by PSII is used for reduction of QA |
| ψE0 = 1 − VJ | Probability with which a PSII trapped election is transferred from reduced QA to QB |
| Soil | CS | CS + DS | DS | |
|---|---|---|---|---|
| pH [1:5] | v/v | 7.34 | 7.25 | 7.47 |
| EC | dS·m−1 | 0.47 | 0.26 | 0.06 |
| CEC | cmol+·kg−1 | 28.20 | 6.08 | 2.26 |
| Bulk density | g·mL−1 | 0.31 | 0.83 | 1.28 |
| Moisture | % | 61.04 | 33.19 | 5.90 |
| OM | 16.09 | 3.75 | 0.80 | |
| Total N | 0.14 | 0.04 | 0.01 | |
| Av.P2O5 | mg·kg−1 | 7.21 | 6.01 | 7.67 |
| K+ | cmol+·kg−1 | 2.19 | 0.24 | 0.05 |
| Ca2+ | 8.05 | 2.01 | 2.10 | |
| Mg2+ | 6.03 | 0.98 | 0.19 | |
| Na+ | 8.37 | 1.13 | 0.11 | |
| Substrate Type | Plant Growth Regulator (mg·L−1) | Pn | gs | Ci | Tr | WUE |
|---|---|---|---|---|---|---|
| (µmol CO2·m−2·s−1) | (mol m−2·s−1) | (µmol CO2·mol−1) | (mmol·m−2·s−1) | (µmolCO2·mmol H2O−1) | ||
| CS | 0 | 0.77 c,d,e | 0.017 a,b | 381.04 | 0.55 a,b | 2.22 |
| NAA500 | 1.27 b,c,d,e | 0.013 b | 270.09 | 0.76 a,b | 3.65 | |
| NAA1500 | 0.22 e | 0.000 b | 238.45 | 0.06 a,b | 2.86 | |
| NAA3000 | 0.38 d,e | 0.000 b | 533.93 | 0.09 a,b | 1.97 | |
| IBA500 | 0.36 d,e | 0.003 b | 302.72 | 0.14 a,b | 2.77 | |
| IBA1500 | 1.14 b,c,d,e | 0.017 a,b | 350.75 | 0.64 a,b | 1.97 | |
| IBA3000 | 0.96 b,c,d,e | 0.007 b | 399.21 | 0.31 a,b | 2.21 | |
| RP | 0.29 d,e | 0.003 b | 177.56 | 0.23 a,b | 2.54 | |
| CS + DS | 0 | 0.31 d,e | 0.000 b | 207.00 | 0.09 a,b | 1.26 |
| NAA500 | 1.23 b,c,d,e | 0.007 b | 89.63 | 0.15 a,b | 7.25 | |
| NAA1500 | 3.21 a,b,c,d | 0.027 a,b | 353.97 | 1.83 a,b | 1.26 | |
| NAA3000 | 0.63 c,d,e | 0.010 b | 193.65 | 0.32 a,b | 1.24 | |
| IBA500 | 2.19 a,b,c,d,e | 0.027 a,b | 380.43 | 1.56 a,b | 0.91 | |
| IBA1500 | 0.93 b,c,d,e | 0.017 a,b | 457.93 | 0.85 a,b | 1.20 | |
| IBA3000 | 0.71 c,d,e | 0.003 b | 392.48 | 0.09 a,b | 2.17 | |
| RP | 0.32 d,e | 0.003 b | 98.60 | 0.03 b | 0.73 | |
| DS | 0 | 2.05 a,b,c,d,e | 0.073 a | 256.73 | 1.63 a,b | 3.25 |
| NAA500 | 2.31 a,b,c,d,e | 0.037 a,b | 310.32 | 1.07 a,b | 1.99 | |
| NAA1500 | 2.73 a,b,c,d,e | 0.027 a,b | 202.02 | 1.32 a,b | 1.76 | |
| NAA3000 | 3.53 a,b,c | 0.006 b | 349.89 | 2.04 a | 1.75 | |
| IBA500 | 2.19 a,b,c,d,e | 0.023 a,b | 196.93 | 0.68 a,b | 3.96 | |
| IBA1500 | 4.54 a | 0.030 a,b | 186.88 | 2.03 a,b | 2.76 | |
| IBA3000 | 1.93 a,b,c,d,e | 0.020 a,b | 315.73 | 1.10 a,b | 4.94 | |
| RP | 3.83 a,b | 0.047 a,b | 232.95 | 1.68 a,b | 3.57 | |
| Substrate type (S) | 14.62 *** | 7.98 *** | ns | 7.84 *** | 7.84 *** | |
| Plant growth regulator (P) | ns | ns | ns | ns | ns | |
| (S) × (P) | ns | ns | ns | ns | ns | |
| Substrate Type | Plant Growth Regulator (mg·L−1) | Fv/Fm | PIABS | ABS/RC | TR0/RC | ET0/RC | DI0/RC |
|---|---|---|---|---|---|---|---|
| CS | 0 | 0.66 | 0.53 a | 2.60 c,d | 1.70 b,c,d | 0.66 | 0.90 b |
| NAA500 | 0.60 | 0.38 a,b,c | 3.16 a,b,c,d | 1.80 a,b,c | 0.63 | 1.36 a,b | |
| NAA1500 | 0.58 | 0.34 a,b,c | 3.14 a,b,c,d | 1.74 b,c,d | 0.64 | 1.40 a,b | |
| NAA3000 | 0.60 | 0.39 a,b,c | 3.04 a,b,c,d | 1.77 b,c,d | 0.62 | 1.27 a,b | |
| IBA500 | 0.65 | 0.47 a,b | 2.74 b,c,d | 1.75 b,c,d | 0.64 | 0.99 b | |
| IBA1500 | 0.62 | 0.39 a,b,c | 2.93 a,b,c,d | 1.80 a,b,c | 0.64 | 1.13 a,b | |
| IBA3000 | 0.63 | 0.50 a | 3.09 a,b,c,d | 1.90 a,b,c | 0.69 | 1.19 a,b | |
| RP | 0.60 | 0.41 a,b,c | 2.55 c,d | 1.64 c,d | 0.59 | 0.91 b | |
| CS + DS | 0 | 0.52 | 0.33 a,b,c | 2.37 d | 1.46 d | 0.56 | 0.90 b |
| NAA500 | 0.66 | 0.50 a | 2.47 c,d | 1.63 c,d | 0.62 | 0.84 b | |
| NAA1500 | 0.61 | 0.32 a,b,c | 3.02 a,b,c,d | 1.82 a,b,c | 0.59 | 1.19 a,b | |
| NAA3000 | 0.63 | 0.43 a,b,c | 2.77 b,c,d | 1.73 b,c,d | 0.64 | 1.05 a,b | |
| IBA500 | 0.66 | 0.55 a | 2.55 c,d | 1.67 c,d | 0.68 | 0.88 b | |
| IBA1500 | 0.64 | 0.46 a,b | 2.74 b,c,d | 1.71 b,c,d | 0.66 | 1.03 a,b | |
| IBA3000 | 0.64 | 0.45 a,b | 2.87 a,b,c,d | 1.80 a,b,c | 0.67 | 1.07 a,b | |
| RP | 0.62 | 0.37 a,b,c | 3.08 cd | 1.84 a,b,c | 0.65 | 1.23 a,b | |
| DS | 0 | 0.60 | 0.32 a,b,c | 3.27 a,b,c | 1.90 a,b,c | 0.63 | 1.37 a,b |
| NAA500 | 0.65 | 0.39 a,b,c | 2.83 a,b,c,d | 1.82 a,b,c | 0.62 | 1.01 b | |
| NAA1500 | 0.57 | 0.21 b,c | 3.68 a | 2.01 a,b | 0.50 | 1.67 a | |
| NAA3000 | 0.62 | 0.32 a,b,c | 3.12 a,b,c,d | 1.89 a,b,c | 0.65 | 1.23 a,b | |
| IBA500 | 0.65 | 0.40 a,b,c | 2.78 b,c,d | 1.80 a,b,c | 0.64 | 0.98 b | |
| IBA1500 | 0.59 | 0.18 c | 3.59 a,b | 2.11 a | 0.60 | 1.49 a,b | |
| IBA3000 | 0.61 | 0.41 a,b,c | 3.12 a,b,c,d | 1.88 a,b,c | 0.68 | 1.24 a,b | |
| RP | 0.62 | 0.28 a,b,c | 3.08 a,b,c,d | 1.89 a,b,c | 0.59 | 1.19 a,b | |
| Substrate type (S) | ns | 5.63 ** | 6.26 ** | 10.24 *** | ns | 3.41 * | |
| Plant growth regulator (P) | ns | ns | ns | ns | ns | ns | |
| (S) × (P) | ns | ns | ns | ns | ns | ns | |
| Substrate Type | Plant Growth Regulator (mg·L−1) | pH | EC | CEC | Bulk Density | Moisture | OM | TN | Av.P2O5 | K+ | Ca2+ | Mg2+ | Na+ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (dS·m−1) | (cmol+·kg−1) | (g·mL−1) | (%) | (mg·kg−1) | (cmol+·kg−1) | ||||||||
| CS | 0 | 7.40 b,c,d,e,f | 0.21 a,b,c,d,e | 18.83 a,b | 0.48 h | 76.65 a,b | 9.27 a | 0.08 c | 5.18 c | 1.34 a,b,c | 6.16 a,b | 3.93 a | 3.88 b,c |
| NAA500 | 7.41 b,c,d,e,f | 0.21 a,b,c,d | 18.55 b | 0.49 h | 78.91 a | 9.25 a | 0.09 b,c | 5.25 c | 1.07 c | 5.67 b | 3.91 a | 2.90 d | |
| NAA1500 | 7.48 a,b,c | 0.23 a,b | 19.60 a,b | 0.48 h | 75.21 b | 9.19 a | 0.11 a | 4.88 c | 1.52 a | 5.82 a,b | 3.92 a | 4.87 a | |
| NAA3000 | 7.50 a,b | 0.23 a | 20.19 a | 0.51 h | 76.59 a,b | 9.17 a | 0.09 c | 5.03 c | 1.44 a,b | 5.99 a,b | 3.85 a | 4.39 a,b | |
| IBA500 | 7.56 a | 0.23 a,b | 19.39 a,b | 0.50 h | 77.85 a,b | 9.33 a | 0.09 b,c | 4.99 c | 1.45 a,b | 5.89 a,b | 3.93 a | 4.21 a,b | |
| IBA1500 | 7.46 a,b,c,d,e | 0.23 a,b | 20.28 a | 0.49 h | 76.94 a,b | 9.44 a | 0.10 a,b | 5.10 c | 1.50 a | 6.19 a,b | 3.87 a | 4.35 a,b | |
| IBA3000 | 7.44 a,b,c,d,e | 0.22 a,b,c | 19.05 a,b | 0.50 h | 76.22 a,b | 9.24 a | 0.09 b,c | 5.07 c | 1.19 b,c | 6.39 a | 3.90 a | 3.45 c,d | |
| RP | 7.37 b,c,d,e,f,g,h | 0.23 a,b | 19.32 a,b | 0.48 h | 77.01 a,b | 8.46 b | 0.09 c | 5.28 c | 1.45 a,b | 6.24 a,b | 3.92 a | 4.18 a,b | |
| CS + DS | 0 | 7.34 e,f,g,h | 0.18 d,e,f,g,h | 9.87 c | 0.81 g | 32.61 c | 3.93 c | 0.03 d | 6.16 b | 0.39 d | 4.34 c | 1.40 c | 1.64 e |
| NAA500 | 7.39 b,c,d,e,f,g | 0.19 c,d,e,f,g,h | 7.93 d,e | 0.84 e,f | 30.46 c,d,e | 3.39 d,e | 0.03 d | 6.47 b | 0.34 d,e | 4.12 c,d | 1.67 b | 1.68 e | |
| NAA1500 | 7.39 b,c,d,e,f,g | 0.19 c,d,e,f,g,h | 7.72 e | 0.82 e,f,g | 30.93 c,d | 3.46 d | 0.03 d | 6.39 b | 0.33 d,e | 4.02 c,d | 1.42 c | 1.67 e | |
| NAA3000 | 7.36 c,d,e,f,g,h | 0.19 a,b,c,d,e,f,g | 7.99 d,e | 0.83 e,f,g | 27.76 e,f | 3.21 d,e | 0.03 d | 6.14 b | 0.45 d | 4.08 c,d | 1.43 c | 1.86 e | |
| IBA500 | 7.40 b,c,d,e,f | 0.20 a,b,c,d,e,f | 8.81 c,d,e | 0.85 e | 29.27 d,e | 3.56 c,d | 0.03 d | 6.60 b | 0.27 d,e | 3.60 d,e | 1.27 c | 1.28 e | |
| IBA1500 | 7.48 a,b,c,d | 0.19 b,c,d,e,f,g | 9.75 c | 0.82 f,g | 29.66 c,d,e | 3.96 c | 0.04 d | 6.09 b | 0.43 d | 3.67 d,e | 1.42 c | 1.87 e | |
| IBA3000 | 7.31 f,g,h | 0.19 c,d,e,f,g,h | 9.31 c,d | 0.85 e | 30.45 c,d,e | 3.98 c | 0.04 d | 6.18 b | 0.31 d,e | 3.37 e | 1.30 c | 1.48 e | |
| RP | 7.40 b,c,d,e,f | 0.17 d,e,f,g,h | 8.18 d,e | 0.84 e,f | 25.37 f | 3.00 e | 0.03 d | 6.63 b | 0.32 d,e | 3.70 d,e | 1.32 c | 1.46 e | |
| DS | 0 | 7.39 b,c,d,e,f | 0.16 f,g,h | 2.187 f | 1.25 a,b | 7.11 g | 0.65 f | 0.01 e | 7.93 a | 0.03 e | 1.98 e | 0.15 d | 0.22 f |
| NAA500 | 7.42 b,c,d,e,f | 0.16 f,g,h | 2.024 f | 1.24 a,b | 6.82 g | 0.70 f | 0.01 e | 7.56 a | 0.03 e | 2.15 e | 0.16 d | 0.24 f | |
| NAA1500 | 7.41 b,c,d,e,f | 0.17 e,f,g,h | 2.14 f | 1.25 a | 6.05 g | 0.68 f | 0.01 e | 7.50 a | 0.02 e | 1.99 e | 0.15 d | 0.23 f | |
| NAA3000 | 7.35 d,e,f,g,h | 0.16 f,g,h | 1.98 f | 1.22 b,c | 6.96 g | 0.72 f | 0.01 e | 7.93 a | 0.02 e | 2.16 e | 0.15 d | 0.27 f | |
| IBA500 | 7.30 f,g,h | 0.17 d,e,f,g,h | 2.06 f | 1.24 a,b | 6.21 g | 0.74 f | 0.01 e | 7.88 a | 0.02 e | 2.13 e | 0.16 d | 0.23 f | |
| IBA1500 | 7.26 h | 0.15 gh | 2.36 f | 1.21 c | 7.08 g | 0.77 f | 0.01 e | 7.78 a | 0.02 e | 2.01 e | 0.14 d | 0.22 f | |
| IBA3000 | 7.26 g,h | 0.15 h | 2.04 f | 1.20 c | 6.61 g | 0.76 f | 0.01 e | 8.12 a | 0.03 e | 2.19 e | 0.15 d | 0.24 f | |
| RP | 7.45 a,b,c,d,e | 0.16 f,g,h | 2.29 f | 1.15 d | 6.75 g | 0.74 f | 0.01 e | 7.55 a | 0.03 e | 2.11 e | 0.18 d | 0.26 f | |
| Substrate type (S) | 14.23 *** | 53.51 *** | 2564.06 *** | 13,842.11 *** | 10,764.60 *** | 7152.29 *** | 580.18 *** | 249.35 *** | 434.04 *** | 949.18 *** | 6670.14 *** | 500.68 *** | |
| Plant growth regulator (P) | ns | ns | ns | 7.10 *** | ns | 6.19 *** | ns | ns | ns | ns | ns | 2.56 * | |
| (S) × (P) | 3.19 ** | ns | ns | 6.69 *** | 2.22 ** | 2.36 * | ns | ns | ns | 2.27 * | ns | 2.25 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Moon, B.; Kim, S.; Lee, H.W. Effects of Rooting Substrates and Plant Growth Regulators on Rooting Performance, Photosynthetic Characteristics, and Soil Properties of Broussonetia × kazinoki Sieb. Cuttings. Forests 2025, 16, 1752. https://doi.org/10.3390/f16111752
Lee S, Moon B, Kim S, Lee HW. Effects of Rooting Substrates and Plant Growth Regulators on Rooting Performance, Photosynthetic Characteristics, and Soil Properties of Broussonetia × kazinoki Sieb. Cuttings. Forests. 2025; 16(11):1752. https://doi.org/10.3390/f16111752
Chicago/Turabian StyleLee, Sora, Bowook Moon, Seokju Kim, and Hyung Won Lee. 2025. "Effects of Rooting Substrates and Plant Growth Regulators on Rooting Performance, Photosynthetic Characteristics, and Soil Properties of Broussonetia × kazinoki Sieb. Cuttings" Forests 16, no. 11: 1752. https://doi.org/10.3390/f16111752
APA StyleLee, S., Moon, B., Kim, S., & Lee, H. W. (2025). Effects of Rooting Substrates and Plant Growth Regulators on Rooting Performance, Photosynthetic Characteristics, and Soil Properties of Broussonetia × kazinoki Sieb. Cuttings. Forests, 16(11), 1752. https://doi.org/10.3390/f16111752






