Study on Pyrolysis Characteristics and Combustibility of Typical Arbor Species Along Different Altitude Gradients in Southwestern Yunnan
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Sample Collection and Preparation
Sample Collection
2.3. Sample Preparation
2.4. Thermogravimetric Experiment
2.5. Data Processing and Analysis
2.6. Principal Component Analysis (PCA)
3. Results and Analysis
3.1. Analysis of TG-DTG Curve Characteristics
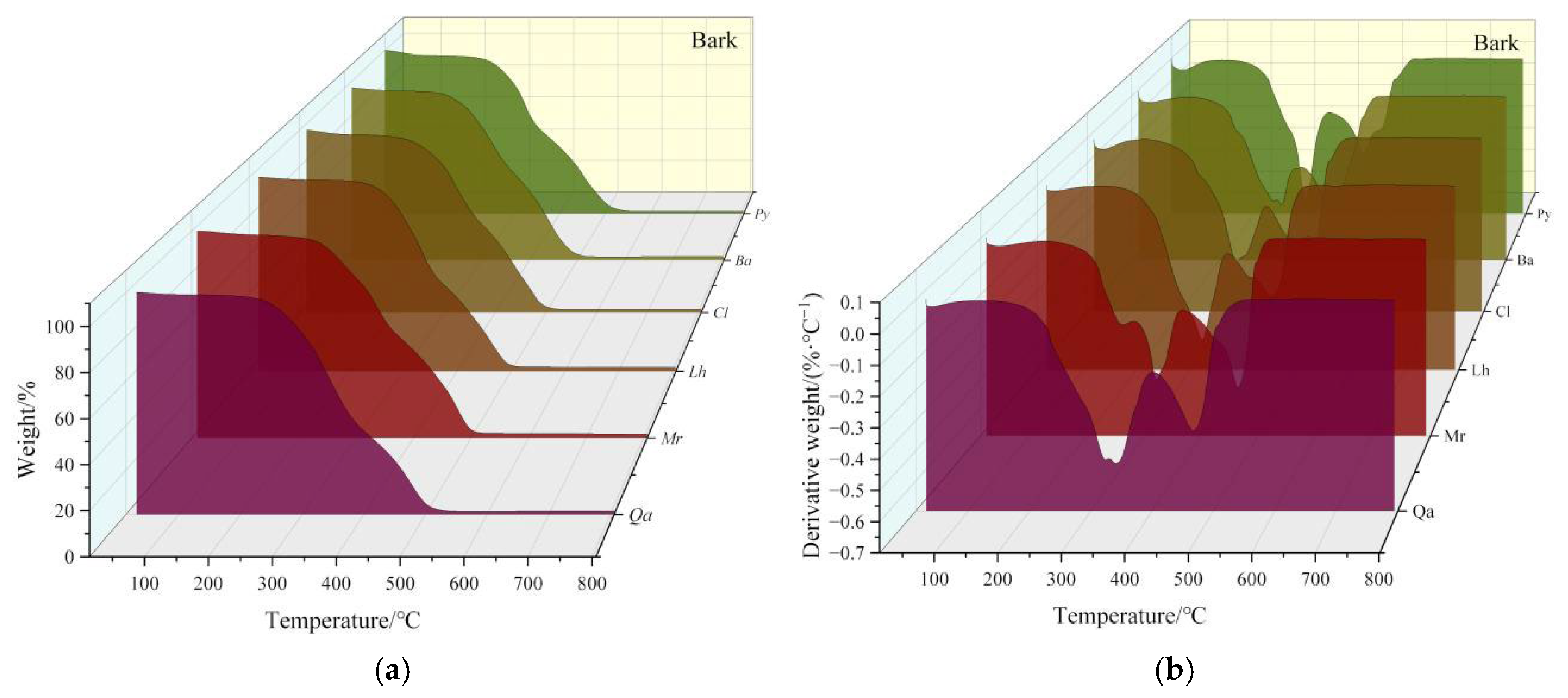
3.1.1. Analysis of Bark Pyrolysis Characteristics
- 1.
- Moisture Loss Stage
- 2.
- Holocellulose Decomposition Stage
- 3.
- Lignin Decomposition Stage
- 4.
- Ash Formation Stage
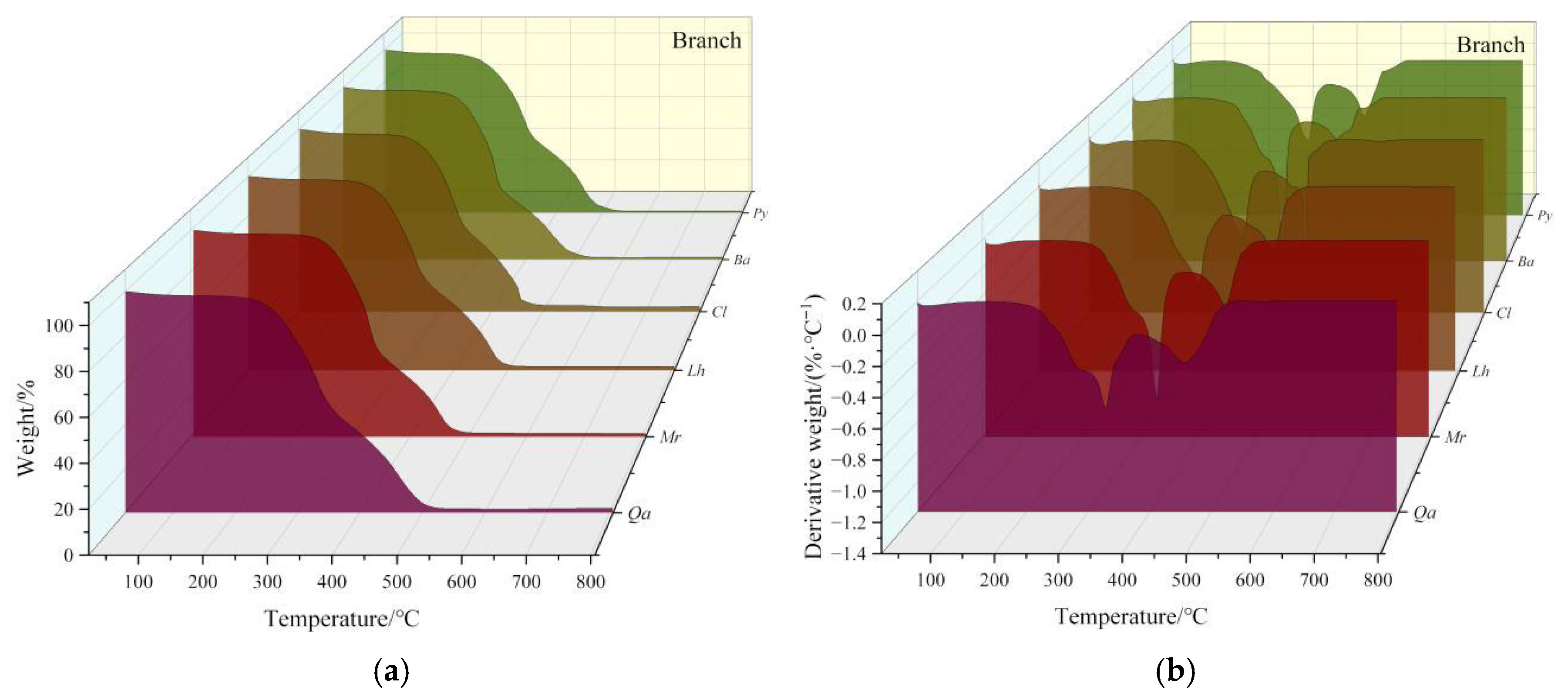
3.1.2. Analysis of Branch Pyrolysis Characteristics
- 1.
- Moisture Loss Stage
- 2.
- Holocellulose Decomposition Stage
- 3.
- Lignin Decomposition Stage
- 4.
- Ash Formation Stage
3.1.3. Analysis of Leaf Pyrolysis Characteristics
- 1.
- Moisture Loss Stage
- 2.
- Holocellulose Decomposition Stage
- 3.
- Lignin Decomposition Stage
- 4.
- Ash Formation Stage
3.2. Pyrolysis Kinetics Analysis
3.3. Principal Component Analysis Ordination
4. Discussion
5. Conclusions
- (1)
- The pyrolysis process of combustible materials is divided into four stages: moisture loss, holocellulose pyrolysis, lignin pyrolysis, and ash formation. The holocellulose decomposition stage accounted for the greatest mass loss, with rates exceeding 49%. Significant differences in pyrolysis characteristics were observed among parts: leaves and bark had lower initial pyrolysis temperatures and higher volatile matter content, presenting a higher combustion risk. The average holocellulose decomposition rate followed the order: bark < leaf < branch. The ash content of different parts ranged from 1.19% to 5.01%.
- (2)
- The activation energy during the holocellulose decomposition stage ranged from 60.47 to 75.89 kJ·mol−1 for bark, 65.23 to 86.41 kJ·mol−1 for branches, and 56.05 to 71.14 kJ·mol−1 for leaves. This indicates that branches require the highest energy for pyrolysis, followed by bark, with leaves requiring the least.
- (3)
- The comprehensive combustibility ranking derived from principal component analysis—integrating total mass loss rate, stage-specific mass loss, activation energy, and ash content—was: Pinus yunnanensis > Betula alnoides > Lithocarpus henryi > Quercus acutissima > Cunninghamia lanceolata > Myrica rubra. Beyond providing a combustibility order, this study offers mechanistic insights into the underlying causes, such as the presence of kinetic compensation effects and the antagonistic roles of volatile extracts versus ash content in influencing flammability.
- (4)
- High-altitude tree species such as Pinus yunnanensis and Betula alnoides exhibit strong flammability, necessitating enhanced fuel management strategies in high-elevation forests—such as branch pruning and litter clearance—to mitigate fire risk. In contrast, the low-altitude species Myrica rubra demonstrates consistently low flammability across all components, making it suitable for recommendation as a fire-resistant species for stand transformation or firebreak establishment in this region.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kelly, L.T.; Giljohann, K.M.; Duane, A.; Aquilué, N.; Archibald, S.; Batllori, E.; Bennett, A.F.; Buckland, S.T.; Canelles, Q.; Clarke, M.F.; et al. Fire and biodiversity in the Anthropocene. Science 2020, 370, eabb0355. [Google Scholar] [CrossRef]
- Romeo, F.; Marziliano, P.A.; Turrión, M.-B. Short-term effects of different fire severities on soil properties and Pinus halepensis regeneration. J. For. Res. 2020, 31, 1271–1282. [Google Scholar] [CrossRef]
- Baranovskiy, N.V.; Kirienko, V.A. Forest fuel drying, pyrolysis and ignition processes during forest fire: A review. Processes 2022, 10, 89. [Google Scholar] [CrossRef]
- Meng, Q.; Lu, H.; Huai, Y.; Xu, H.; Yang, S. Forest fire spread simulation and fire extinguishing visualization research. Forests 2023, 14, 1371. [Google Scholar] [CrossRef]
- Yuen, A.C.; Chen, T.B.; Yeoh, G.H.; Yang, W.; Cheung, S.C.; Cook, M.; Yu, B.; Chan, Q.N.; Yip, H.L. Establishing pyrolysis kinetics for the modelling of the flammability and burning characteristics of solid combustible materials. J. Fire Sci. 2018, 36, 494–517. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, T.; Song, X.; Wang, X.L.; Wang, Y.Z. Hazard evaluation of forest combustibles based on the correlation between pyrolysis products and combustion parameters. J. Hazard. Mater. 2024, 469, 133914. [Google Scholar] [CrossRef]
- Sun, J.; Qi, W.; Huang, Y.; Xu, C.; Yang, W. Facing the wildfire spread risk challenge: Where are we now and where are we going? Fire 2023, 6, 228. [Google Scholar] [CrossRef]
- Arromdee, P.; Ninduangdee, P. Combustion characteristics of pelletized-biomass fuels: A thermogravimetric analysis and combustion study in a fluidized-bed combustor. Energy Ecol. Environ. 2023, 8, 69–88. [Google Scholar] [CrossRef]
- Jia, G. Combustion characteristics and kinetic analysis of biomass pellet fuel using thermogravimetric analysis. Processes 2021, 9, 868. [Google Scholar] [CrossRef]
- Countryman, C.M.; Philpot, C.W. Physical Characteristics of Chamise as a Wildland Fuel; Pacific Southwest Forest and Range Experiment Station, Forest Service, US Department of Agriculture: Berkeley, CA, USA, 1970.
- Luo, J.Y.; Chen, Y.H.; Zhang, X.C. Flammability and chemical composition of forest fuels. J. Northeast For. Univ. 1992, 20, 35–42. [Google Scholar]
- Su, Y.; Luo, Y.H.; Wu, W.G. Characteristics of pine wood oxidative pyrolysis: Degradation behavior, carbon oxide production and heat properties. J. Anal. Appl. Pyrolysis 2012, 98, 137–143. [Google Scholar] [CrossRef]
- Wang, S.R.; Jin, S. Pyrolysis kinetics and flammability ranking of 8 combustibles in Nanchang area based on thermogravimetric analysis. J. Cent. South Univ. For. Technol. 2015, 35, 94–98. [Google Scholar]
- Song, Y.Y.; Jin, S.; Wang, Z.Y. Pyrolysis characteristics and kinetics of 4 herbaceous combustibles. J. Cent. South Univ. For. Technol. 2012, 32, 51–55. [Google Scholar]
- Gao, N.; Li, A.; Ouan, C. TG-FTIR and Py-GC/MS analysis on pyrolysis and combustion of pine sawdust. J. Anal. Appl. Pyrolysis 2013, 100, 26–32. [Google Scholar] [CrossRef]
- Man, Z.Y.; Sun, L.; Hu, H.Q. Combustion spread rate and prediction model of 8 forest surface dead fuels in southern China under flat and windless conditions. Sci. Silvae Sin. 2019, 55, 197–204. [Google Scholar]
- Hu, H.Q.; Gao, J.; Hu, T.X. Flammability ranking of 7 common arbor species in Yanbian Prefecture based on thermogravimetric analysis. J. Cent. South Univ. For. Technol. 2020, 40, 1–10. [Google Scholar]
- Paredes, R.; Castells, B.; Tascón, A. Thermogravimetric assessment of biomass: Unravelling kinetic, chemical composition and combustion profiles. Fire 2024, 7, 396. [Google Scholar] [CrossRef]
- Viera, K.; Katarina, D.; Tatiana, H.; Jaroslava, S. The Effect of Chemical Components of Thermally Treated Meranti Wood on the Higher Heating Value. Fire 2025, 8, 394. [Google Scholar] [CrossRef]
- Zhang, G.L.; Ci, X.L.; Yang, X.Q. Study on spatio-temporal distribution characteristics and flammability of forest fires. For. Grassl. Resour. Res. 2023, 5, 48–55. [Google Scholar]
- Sun, Y.P. Study on Combustion Emission Characteristics of Typical Forest Vegetation in Southwest China. Master’s Thesis, University of Science and Technology of China, Hefei, China, 2023. [Google Scholar]
- Zhao, F.; Shu, L.F.; Zhou, R.L. Evaluation of forest fire behavior simulation models in southwest forest areas. Chin. J. Appl. Ecol. 2017, 28, 3144–3154. [Google Scholar]
- Shu, L.F.; Tian, X.R.; Lin, Q.Z. Theory and application of fire prevention forest belts. J. Northeast For. Univ. 1999, 27, 71–75. [Google Scholar]
- Zhai, Z.G.; Liu, N.A. Study on flammability of leaf samples based on thermogravimetry and factor analysis. Fire Saf. Sci. 2008, 17, 67–72. [Google Scholar]
- Chen, Y.; Sun, T.L.; Li, P.H. Study on kinetic and thermodynamic properties of baked rice straw. Chem. Ind. For. Prod. 2024, 44, 20–30. [Google Scholar]
- Zhang, L.L.; Tian, D.Q.; Wan, X. Comprehensive evaluation of shade tolerance of 11 colored-leaf plants. J. Zhejiang A F Univ. 2025, in press.
- Bär, A.; Mayr, S. Bark insulation: Ten Central Alpine tree species compared. For. Ecol. Manag. 2020, 474, 118361. [Google Scholar] [CrossRef]
- Chen, B.X.; Guo, Y.; Fan, J.L. Pyrolysis characteristics and gas release characteristics of leaves of 6 arbor species in Heilongjiang Province. Chin. J. Appl. Ecol. 2022, 33, 76–84. [Google Scholar]
- Hu, Y.M. Study on Pyrolysis Process and Thermodynamic Properties of Each Component of Lignocellulosic Biomass. Master’s Thesis, Chinese Academy of Forestry, Beijing, China, 2013. [Google Scholar]
- Niu, H.C. Study on Pyrolysis Kinetics and Flammability of Forest Fuels. Ph.D. Thesis, University of Science and Technology of China, Hefei, China, 2014. [Google Scholar]
- Jin, S.; Yang, Y.B. Pyrolysis characteristics and flammability analysis of leaves of 7 typical arbor species in southern China based on thermogravimetry. J. Cent. South Univ. For. Technol. 2015, 35, 58–63. [Google Scholar]
- Yang, H.W. Study on Tree Species Flammability and Fire Behavior in Liaozhong Area. Master’s Thesis, Shenyang Aerospace University, Shenyang, China, 2023. [Google Scholar]
- Yang, Y.B. Study on Flammability of 7 Typical Forest Fuels in Southern China. Master’s Thesis, Northeast Forestry University, Harbin, China, 2015. [Google Scholar]
- Chen, H.X. Study on Physicochemical Models and Analytical Methods for Biomass Pyrolysis. Ph.D. Thesis, University of Science and Technology of China, Hefei, China, 2006. [Google Scholar]
- Ge, W.W.; Zhang, H.Y.; Tang, C.G. Thermogravimetric analysis of leaves of 16 broad-leaved tree species in Kunming area. Chem. Ind. For. Prod. 2010, 30, 77–81. [Google Scholar]
- Zhang, Y.X.; Sun, C.Y. Thermogravimetric analysis of leaves of 10 common tree species in Heilongjiang area. For. Fire Prev. 2014, 1, 16–20. [Google Scholar]
- Jin, S.; Song, Y.Y.; Sun, C.Y. Slow heating pyrolysis characteristics of 12 herbaceous combustibles in Maoershan, Heilongjiang. Sci. Silvae Sin. 2012, 48, 101–108. [Google Scholar]
- Song, Y.Y. Study on Pyrolysis Characteristics and Kinetics of Typical Forest Fuels in Maoershan. Master’s Thesis, Northeast Forestry University, Harbin, China, 2012. [Google Scholar]
- Gao, J. Study on Flammability of Main Arbor Species in Yanbian Area Based on Thermogravimetric Analysis. Master’s Thesis, Northeast Forestry University, Harbin, China, 2020. [Google Scholar]
- Yan, X.X.; Wang, Q.H.; Li, X.N. Study on combustion characteristics of surface fuels in main forest types around Kunming. J. Southwest For. Univ. (Nat. Sci. Ed.) 2020, 40, 135–142. [Google Scholar]
- Hong, P.T.; Guo, Y.; Hu, H.Q. Flammability of arbor and shrub species in Greater Khingan Mountains based on thermogravimetric analysis. J. Cent. South Univ. For. Technol. 2022, 42, 80–93. [Google Scholar]
- Zhang, J.Q.; Zhou, M.; Zhao, P.W. Flammability of 5 typical tree species in the eastern foot of Greater Khingan Mountains, Inner Mongolia. J. Temp. For. Res. 2021, 4, 27–33. [Google Scholar]
- Lin, C. Analysis of Fire Resistance of 15 Arbor Species in Hohhot. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2021. [Google Scholar]
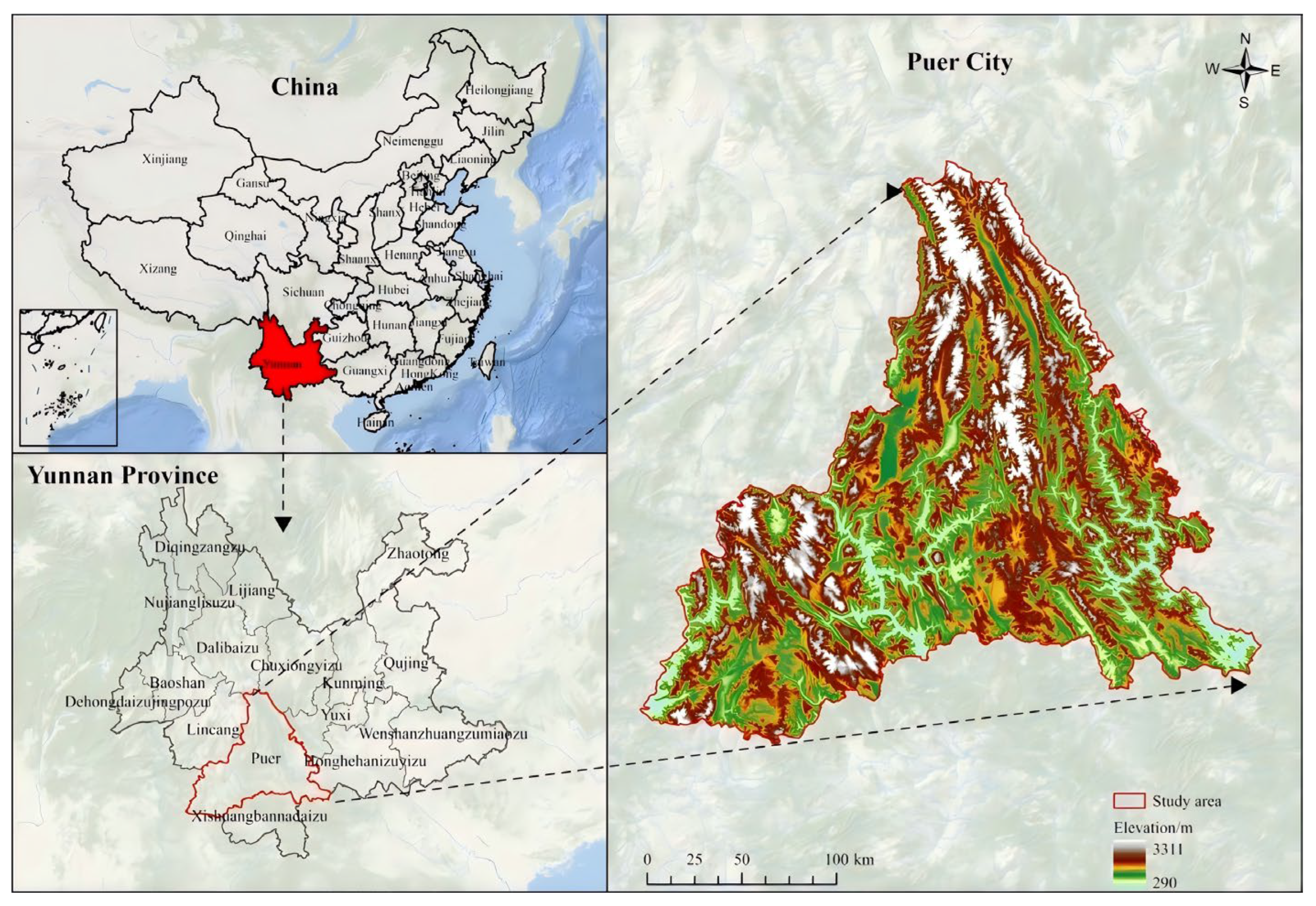

| Altitudinal Distribution | Sample Name | Mean Tree Height (m) | Mean DBH (cm) | Distribution Environment and Growth Characteristics |
|---|---|---|---|---|
| Low altitude (ca. 500–1500 m) | Quercus acutissima | 11.5 ± 2.1 | 17.2 ± 3.5 | Low mountain and hilly areas, drought-tolerant |
| Myrica rubra | 8.3 ± 1.7 | 15.8 ± 2.9 | Low mountain and hilly areas, prefers acidic soil | |
| Medium altitude (ca. 800–2000 m) | Lithocarpus henryi | 13.8 ± 2.4 | 21.5 ± 4.2 | Subtropical mountainous areas, strong adaptability |
| Cunninghamia lanceolata | 15.2 ± 2.8 | 19.3 ± 3.8 | Subtropical mountainous areas, prefers warm and humid conditions | |
| High altitude (ca. 1500–3000 m) | Betula alnoides | 14.6 ± 2.3 | 18.7 ± 3.2 | Temperate and cold–temperate mountainous areas, prefers humid environment |
| Pinus yunnanensis | 16.3 ± 2.9 | 24.8 ± 4.5 | Subtropical and temperate mountainous areas, barren soil-tolerant |
| Reaction Mechanism | Symbols | f(α) | G(α) |
|---|---|---|---|
| First-order reaction | F1 | 1 − α | −ln(1 − α) |
| Second-order reaction | F2 | (1 − α)2 | (1 − α) − 1 − 1 |
| Third-order reaction | F3 | (1 − α)3 | [(1 − α) − 2 − 1]/2 |
| Two-dimensional diffusion | D2 | [−ln(1 − α)] − 1 | α + (1 − α) ln(1 − α) |
| Three-dimensional diffusion | D3 | [3/2(1 − α)2/3]/[1 − (1 − α)1/3] | [1 − (1 − α)1/3]2 |
| Nucleation and growth | A2 | 2(1 − α) [−ln(1 − α)]1/2 | [−ln(1 − α)]1/2 |
| Nucleation and growth | A3 | 3(1 − α) [−ln(1 − α)]2/3 | [−ln(1 − α)]1/3 |
| Nucleation and growth | A4 | 4(1 − α) [−ln(1 − α)]3/4 | [−ln(1 − α)]1/4 |
| Phase boundary reaction | R1 | 1 | α |
| Phase boundary reaction | R2 | 2(1 − α)1/2 | 1 − (1 − α)1/2 |
| Phase boundary reaction | R3 | 3(1 − α)2/3 | 1 − (1 − α)1/3 |
| Part | Arbor Species | Fitting Equation | Activation Energy (E) (kJ/mol) | Pre-Exponential Factor (A) (min−1) | Frequency Factor R2 |
|---|---|---|---|---|---|
| Bark | Betula alnoides | Y = −7876.71X + 0.401 | 65.49 | 3.92 × 102 | 0.99883 |
| Lithocarpus henryi | Y = −9028.14X + 2.156 | 75.06 | 2.6 × 104 | 0.99907 | |
| Quercus acutissima | Y = −7605.27X − 0.620 | 63.23 | 1.36 × 103 | 0.99803 | |
| Cunninghamia lanceolata | Y = −7694.79X − 0.013 | 63.97 | 2.53 × 103 | 0.99447 | |
| Myrica rubra | Y = 7273.08X − 0.644 | 60.47 | 1.27 × 103 | 0.99076 | |
| Pinus yunnanensis | Y = −9128.42X + 2.444 | 75.89 | 3.51 × 104 | 0.99822 | |
| Branch | Betula alnoides | Y = −10,393X + 4.361 | 86.41 | 2.71 × 105 | 0.9933 |
| Lithocarpus henryi | Y = −9734.19X + 3.483 | 80.93 | 1.06 × 105 | 0.99934 | |
| Quercus acutissima | Y = −8882.19X − 1.967 | 73.85 | 2.12 × 104 | 0.99822 | |
| Cunninghamia lanceolata | Y = −10,131.7X + 4.168 | 84.24 | 2.18 × 105 | 0.99854 | |
| Myrica rubra | Y = −10,336X + 4.417 | 85.93 | 2.86 × 105 | 0.99576 | |
| Pinus yunnanensis | Y = −7845.4X + 0.381 | 65.23 | 3.83 × 103 | 0.99396 | |
| Leaf | Betula alnoides | Y = −8557.05X + 1.598 | 71.14 | 1.41 × 104 | 0.99614 |
| Lithocarpus henryi | Y = −8490.94X + 1.384 | 70.59 | 1.13 × 104 | 0.99892 | |
| Quercus acutissima | Y = −8312.92X + 1.206 | 69.11 | 9.26 × 103 | 0.99783 | |
| Cunninghamia lanceolata | Y = −7682.11X + 0.163 | 63.87 | 3.01 × 103 | 0.99952 | |
| Myrica rubra | Y = −8372.97X + 1.083 | 69.62 | 8.24 × 103 | 0.99851 | |
| Pinus yunnanensis | Y = −6741.59X − 1.388 | 56.05 | 5.61 × 103 | 0.99257 |
| Part | Samples | X1 | X2 | X3 | X4 | X5 |
|---|---|---|---|---|---|---|
| Bark | Betula alnoides | 97.74 | 49.93 | 46.23 | 65.49 | 2.26 |
| Lithocarpus henryi | 97.95 | 55.21 | 41.18 | 75.06 | 2.05 | |
| Quercus acutissima | 98.68 | 63.84 | 33.79 | 63.23 | 1.32 | |
| Cunninghamia lanceolata | 98.37 | 50.15 | 45.47 | 63.97 | 1.63 | |
| Myrica rubra | 98.28 | 49.78 | 46.07 | 60.47 | 1.72 | |
| Pinus yunnanensis | 97.98 | 49.69 | 45.53 | 75.89 | 1.32 | |
| Leaf | Betula alnoides | 94.99 | 53.23 | 39.86 | 71.14 | 5.01 |
| Lithocarpus henryi | 96.37 | 57.05 | 37.86 | 70.59 | 3.63 | |
| Quercus acutissima | 96.37 | 52.38 | 42.27 | 69.11 | 3.16 | |
| Cunninghamia lanceolata | 95.38 | 56.82 | 34.97 | 63.87 | 4.62 | |
| Myrica rubra | 97.48 | 58.39 | 36.34 | 69.62 | 2.52 | |
| Pinus yunnanensis | 97.98 | 60.12 | 35.98 | 56.05 | 2.02 | |
| Branch | Betula alnoides | 98.47 | 66.50 | 30.52 | 86.41 | 1.33 |
| Lithocarpus henryi | 98.25 | 59.49 | 37.10 | 80.93 | 1.75 | |
| Quercus acutissima | 97.99 | 58.39 | 37.78 | 73.85 | 2.01 | |
| Cunninghamia lanceolata | 97.15 | 58.12 | 35.94 | 84.24 | 2.85 | |
| Myrica rubra | 98.47 | 65.74 | 30.74 | 85.93 | 1.53 | |
| Pinus yunnanensis | 98.82 | 58.53 | 38.24 | 65.23 | 1.19 |
| Species Name | Bark | Branch | Leaf | Comprehensive Score | Ranking |
|---|---|---|---|---|---|
| Pinus yunnanensis | −0.23 | 0.38 | 0.39 | 0.54 | 1 |
| Betula alnoides | −0.42 | 1.02 | −0.21 | 0.39 | 2 |
| Lithocarpus henryi | −0.26 | 0.43 | −0.09 | 0.08 | 3 |
| Quercus acutissima | −0.08 | 0.20 | −0.05 | 0.07 | 4 |
| Cunninghamia lanceolata | −0.40 | 0.35 | 0.01 | −0.04 | 5 |
| Myrica rubra | −0.49 | 0.39 | 0.03 | −0.07 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Q.; Li, W.; Wu, Y.; Wei, Y.; Nuerlan, J.; Wang, M.; Shu, L.; Hu, T.; Ning, J.; Yang, G.; et al. Study on Pyrolysis Characteristics and Combustibility of Typical Arbor Species Along Different Altitude Gradients in Southwestern Yunnan. Forests 2025, 16, 1727. https://doi.org/10.3390/f16111727
Du Q, Li W, Wu Y, Wei Y, Nuerlan J, Wang M, Shu L, Hu T, Ning J, Yang G, et al. Study on Pyrolysis Characteristics and Combustibility of Typical Arbor Species Along Different Altitude Gradients in Southwestern Yunnan. Forests. 2025; 16(11):1727. https://doi.org/10.3390/f16111727
Chicago/Turabian StyleDu, Qiuyang, Weike Li, Yingda Wu, Yiqi Wei, Jianati Nuerlan, Mingyu Wang, Lifu Shu, Tongxin Hu, Jibin Ning, Guang Yang, and et al. 2025. "Study on Pyrolysis Characteristics and Combustibility of Typical Arbor Species Along Different Altitude Gradients in Southwestern Yunnan" Forests 16, no. 11: 1727. https://doi.org/10.3390/f16111727
APA StyleDu, Q., Li, W., Wu, Y., Wei, Y., Nuerlan, J., Wang, M., Shu, L., Hu, T., Ning, J., Yang, G., & Li, K. (2025). Study on Pyrolysis Characteristics and Combustibility of Typical Arbor Species Along Different Altitude Gradients in Southwestern Yunnan. Forests, 16(11), 1727. https://doi.org/10.3390/f16111727






