Abstract
Understory vegetation diversity is the key indicator of ecological outcomes in the close-to-nature transformation of plantations, with its composition revealing successional dynamics and ecosystem functionality. In response to China’s “Green and Beautiful Guangdong” Initiative, enhancing the ecological quality of plantations has been established as a critical objective for sustainable forest management. This study assessed the understory vegetation in four representative transformed plantations in Guangdong Province, China, using Multi-Response Permutation Procedure (MRPP), Indicator Species Analysis (ISA), Detrended Correspondence Analysis (DCA), and Redundancy Analysis (RDA). The results showed that: (1) Species richness was highest in the Eucalyptus L’Hér plantation (102 species), followed by Pinus massoniana Lamb (94), Acacia mangium Willd (92), and Litchi chinensis Soon plantations (85). (2) MRPP analysis revealed significant differences in species composition among plantation types (A = 0.149, p < 0.001). ISA identified 5, 7, 3, and 5 indicator species for each type, respectively, predominantly light-demanding pioneers such as Dicranopteris dichotoma (Thunb.) Bernh and Microstegium vagans (Nees ex Steud.) A. Camus. (3) DCA ordination showed clear compositional segregation among the understory communities of Eucalyptus, Pinus massoniana, and Acacia mangium plantations, whereas the Litchi chinensis plantation exhibited substantial overlap with others. RDA further demonstrated a significant negative correlation between mean diameter at breast height (DBH) and understory diversity (p < 0.01) across all plantations except Litchi chinensis. These findings offer a quantitative basis for tailored management strategies. We recommend structural adjustments through target-tree thinning to optimize light availability by regulating DBH, combined with interplanting native understory species. This integrated approach can enhance structural heterogeneity and promote more effective and sustainable plantation restoration.
1. Introduction
In southern China, monoculture plantations (e.g., Eucalyptus and Pinus massoniana) have experienced understory vegetation degradation and reduced water and soil conservation capacity due to long-term single-species rotation [1]. Under the “Green Beauty Guangdong” initiative, an ecological construction plan aligned with smart forestry objectives—Guangdong Province, China aims to transform 100,000 hectares of low-efficiency forests by 2025 through intelligent monitoring and precision management strategies [2]. Therefore, under the goal of achieving multi-functional plantations, a key task for sustainable plantation management is to transform monospecific even-aged plantations into mixed-species, uneven-aged plantations [3]. This vision is central to the provincial “Eucalyptus Transformation” initiative and the “Green and Beautiful Guangdong” ecological campaign, which emphasize the use of smart forestry technologies (such as remote sensing and IoT sensors for monitoring) and ecological big data to support plantation quality improvement. Through the strategy of “Reducing Eucalyptus and Promoting Mixed Forests”, the focus is on rehabilitating low-efficiency plantations using precision restoration techniques (including targeted thinning and the planting of site-appropriate native species) [4]. Close-to-nature transformation has emerged as a viable approach for multi-objective management of plantations. It constitutes a forest management system focused on continuous forest cover and multi-functional cultivation [5,6]. The core of this approach involves enriching plantations with native tree species to establish multi-layered, mixed-species forests—converting even-aged monocultures into uneven-aged, stratified mixed forests dominated by indigenous species. Such transformation helps maintain fundamental plantation diversity patterns and enhances the provision of multiple ecosystem services. By leveraging natural processes and stimulating natural regeneration mechanisms, this transformation not only rapidly enhances stand productivity but also improves soil fertility, increases species diversity, and boosts biomass and carbon storage [7]. Previous studies have demonstrated that such practices significantly improve stand stability and ecological service functions [8], with an average increase of 32% in understory vegetation species richness after transformation [9]. Such improvements are critical for supporting ecological service valuation and carbon neutrality goals via data-driven forestry. Thus, changes in understory diversity serve as a key indicator for evaluating restoration effectiveness in the context of precision forestry and intelligent ecosystem management.
As an essential component of forest ecosystems, understory vegetation plays multiple critical roles: it enhances the vertical structural complexity of plantations [10], contributes to ecosystem stability, and supports biodiversity maintenance, soil and water conservation, and nutrient cycling. Consequently, changes in the structure and composition of understory vegetation can reflect successional dynamics and act as sensitive indicators of the ecological outcomes of close-to-nature transformation. Understory structure responds directly to overstory changes [11], species composition signals successional stages, and indicator species reflect microhabitat variations [12]. Shifts in tree species composition—through differences in canopy coverage, litter characteristics, and resultant understory microenvironments—significantly influence understory vegetation assembly [13]. Many plant species indicate specific environmental conditions; the presence or absence of such indicator species can reflect habitat quality and stability, while their distribution and community structure reveal patterns of biodiversity and species interactions [14]. However, most existing studies have focused on transformation effects within a single forest type [15,16] or were limited to changes in diversity indices [17], leaving a gap in the systematic comparisons of indicator species divergence and driving mechanisms across different transformed plantations. A comprehensive analysis of indicator species across multiple forest types is essential for understanding stand habitat heterogeneity and successional dynamics following transformation, and supports the development of predictive models for use in precision forestry.
This study investigates four typical transformed plantations in Dongguan City, Guangdong Province, China: Litchi chinensis plantation, Eucalyptus plantation, Pinus massoniana plantation, and Acacia mangium plantation. Using Multi-Response Permutation Procedure (MRPP), Indicator Species Analysis (ISA), and Redundancy Analysis (RDA), we aim to (1) compare post-transformation understory vegetation diversity among stands, (2) elucidate the ecological overlap between indicator and dominant species and their implications for successional stages, and (3) quantify the influence of stand structural factors on diversity indicators. The findings will provide scientific support for adjusting stand structure and implementing differentiated management strategies within plantation transformation efforts in Guangdong Province, China, and contribute to the development of sustainable forestry.
2. Materials and Methods
2.1. Overview of the Study Site
The study area is located in Dongguan City, Guangdong Province, China (113°31′–114°15′ E, 22°39′–23°09′ N), characterized by a subtropical monsoon climate with long summers, short winters, ample sunshine, abundant rainfall, minimal temperature variation, and distinct monsoonal patterns. The mean annual temperature is 22.1 °C, and the average annual precipitation reaches 1800 mm. Dongguan City has a terrain that is higher in the southeast and lower in the northwest. Its landforms are mainly hilly terraces and alluvial plains. The main soil types include red-yellow paddy soil, rice paddy soil, garden soil and alluvial soil. The soil texture is mainly sandy soil and loam. The natural soil layer is relatively deep. The zonal vegetation type is monsoon evergreen broad-leaved forest. However, due to rapid urbanization, frequent disturbances, and extensive deforestation for orchard establishment, the region now supports large areas of plantations, including Pinus massoniana, Cunninghamia lanceolata (Lamb.) Hook, Eucalyptus, Acacia mangium, and bamboo forests, as well as substantial orchards of Litchi chinensis, Dimocarpus longan Lour and Mangifera indica L [18]. Prior to data collection, distinct silvicultural interventions were applied: a 40% thinning in the Eucalyptus plantation; a 30%–60% thinning in the Acacia mangium plantation; crown pruning in the Litchi chinensis plantation to maintain an inter-crown distance of ~1.5 m (yielding a canopy density of ~0.4); and a final density of 150 trees per hectare in the Pinus massoniana plantation after thinning. Furthermore, a selection of fast-growing native broadleaf species suitable for the transformation of these respective low-efficiency plantations was introduced. For instance, Heptapleurum heptaphyllum (L.) Y.F. Deng and Litsea cubeba (Lour.) Pers were planted in the Eucalyptus plantation; Elaeocarpus sylvestris (Lour.) Poir was introduced to the Acacia mangium plantation; Litsea cubeba was added to the Litchi chinensis plantation; and Camphora officinarum Nees and Liquidambar formosana Hance were planted in the Pinus massoniana plantation. Following these close-to-nature transformed measures, the plots were not commercially exploited. The relatively small mean diameter at breast height (DBH) across all stands (Table 1) indicates that these are young plantations, likely in an early to mid-stage of their rotation.

Table 1.
Location and basic stand characteristics of the four plantation types.
This research focuses on typical sample plots of close-to-nature transformed plantations in Dongguan City, Guangdong Province, China: Litchi chinensis plantation, Eucalyptus plantation, Pinus massoniana plantation, and Acacia mangium plantation with investigations targeting understory vegetation.
2.2. Plot Setup and Vegetation Survey
Based on field surveys, one large permanent plot of 0.5 hectares was established for each of the four plantation types in Dongguan City, Guangdong Province, China: Litchi chinensis, Eucalyptus, Pinus massoniana, and Acacia mangium. Within each 0.5−ha plot, fifty 100-m2 quadrats (10 m × 10 m) were systematically established for the tree vegetation inventory. This resulted in a total of 200 tree survey quadrats across the study (4 plantations × 50 quadrats). Within each quadrat, all trees were measured for height (H), diameter at breast height (DBH), crown width (Cw), and height to the first live branch (Hb). For the understory vegetation survey, a nested sampling design was employed. Plot selection avoided areas with significant topographic variation to ensure uniformity in slope and aspect. Within each 100−m2 quadrat, five 1-m2 subplots were positioned: one at each of the four corners and one at the center. This design resulted in a total of 1000 understory subplots across all plantations (4 plantations × 50 quadrats × 5 subplots). In each 1−m2 subplot, all understory vascular plants were identified, and their abundance (number of individuals), coverage (%), and average height (cm) were documented. Data from the five subplots within a single 100-m2 quadrat were subsequently pooled (i.e., species abundances and cover values were summed) to represent the understory vegetation characteristics for that quadrat.
Species were categorized based on population density per hectare: those with no more than one individual were classified as rare species [19,20], those with 2–10 individuals as occasional species, 10–100 individuals as common species, and more than 100 individuals as high-density species [21].
2.3. Data Processing and Analysis
2.3.1. Species Diversity Indicators
The Species diversity index uses species richness (S), Simpson diversity index (D), Shannon-Wiener index (H′), and Pielou index (E):
- S is the total species richness (the number of species recorded in the quadrat);
- Pi is the relative abundance of species *i*, calculated as Pi = ni/N, where ni is the number of individuals of species *i*, and N is the total number of all individuals in the quadrat;
- ln denotes the natural logarithm.
2.3.2. Multi-Response Permutation Process
Multi-response permutation procedures (MRPPs) were employed to examine whether significant differences existed in understory species composition among the different plantation types. An MRPP is a non-parametric statistical method widely used in ecological studies to test for differences in community structure between two or more groups [22,23]. The key statistics generated by MRPPs include T (test statistic), A (agreement statistic), and p-value. The T statistic reflects the degree of separation between groups, with larger absolute values of T indicating greater between-group heterogeneity. The A statistic measures within-group homogeneity; higher values of A denote stronger agreement within groups. When all items within a group are identical, the observed delta equals zero and A reaches its maximum value of 1. If within-group heterogeneity matches expectation by chance, A = 0, while A < 0 indicates lower within-group consistency than expected randomly. In community ecology, A values are generally below 0.1, and values exceeding 0.3 suggest high within-group homogeneity [24].
2.3.3. Importance Value (IVI)
The Importance Value Index (IVI) is a composite metric used to evaluate the ecological dominance and structural significance of a species within a community. It was calculated for each understory plant species by integrating three key parameters: relative coverage, relative density, and relative height.
The importance value (Importance Value Index, IVI) was calculated using the following formula [25]:
IVI = Relative Height + Relative Coverage + Relative Density
The resulting IVI is an absolute value ranging from 0 to 300.
2.3.4. Indicator Species Analysis (ISA)
Indicator species analysis (ISA) was applied to identify diagnostic plant species and assess differences in indicator values (INVs) among the understory vegetation of the different plantation types. The indicator value (INV) combines a species’ relative abundance and relative frequency within each group. A Monte Carlo permutation test with 999 repetitions was used to evaluate the statistical significance of each species’ indicator value [25]. Species with an INV ≥ 30 and a significance level of p ≤ 0.05 were considered indicator species for the corresponding forest stand [26].
2.3.5. Data Statistics Software
Data organization and preliminary calculations were conducted using Microsoft Excel 2013 (Microsoft Corporation, Redmond, WA, USA). The composition and diversity of the understory vegetation across the four plantation types were assessed using a combination of analytical techniques.
Rank-abundance curves were plotted to visualize and compare the community structure and evenness across the four plantation types. In these curves, species are ranked from most to least abundant along the x-axis, while the y-axis represents their relative abundance (e.g., coverage or importance value). The shape of the curve provides insights into community heterogeneity: a flatter curve indicates higher evenness, whereas a steeper curve suggests stronger dominance by a few species.
To quantitatively test for differences in species composition among the plantation types, the Multi-Response Permutation Procedure (MRPP) was employed. Indicator Species Analysis (ISA) with a Monte Carlo permutation test (999 repetitions) was further applied to identify plant species that were significantly associated with a specific plantation type. Differences in species diversity indices, including Species richness, the Simpson diversity index, the Shannon-Wiener index, and the Pielou evenness index, were statistically evaluated using one-way analysis of variance (ANOVA). For indices where significant differences were detected, Tukey’s Honest Significant Difference (HSD) post hoc test was subsequently used to determine specific pairwise differences between plantation types. The patterns in understory community composition were visualized using Detrended Correspondence Analysis (DCA). Finally, the relationships between species diversity indices and stand structural factors (tree height, DBH, crown width, and underbranch height) were examined using Redundancy Analysis (RDA) and tested for significance with Monte Carlo permutations.
The MRPP, ISA, and DCA were performed using PC-ORD 7.0 (Wild Blueberry Media, LLC, Corvallis, OR, USA). ANOVA and post hoc tests were conducted using Statistica 8.0 (TIBCO Software Inc., Palo Alto, CA, USA), and the RDA was carried out in Canoco 5.0 (Biometris, Wageningen, The Netherlands).
3. Results
3.1. Understory Vegetation Species Composition of the Four Close-to-Nature Transformations of the Plantation
A total of 170 understory plant species were documented across the four plantation types (Table S1). Species richness was highest in the Eucalyptus plantation (102 species), followed by the Pinus massoniana (94), Acacia mangium (92), and Litchi chinensis (85) plantations (for the complete species list, see Supplementary File S1). The species abundance rank curves for the four forest types are presented in Figure 1. The curve of the Eucalyptus plantation exhibited the flattest slope, followed by those of the Litchi chinensis plantation and Pinus massoniana plantations, while the Acacia mangium plantation showed the steepest curve. As the slope of the curve increases, the disparity in species abundance within the stand becomes more pronounced, indicating higher species dominance and reduced evenness. As shown in Table 2, there were minimal differences in both the individual counts and species richness of rare species across the four stands. Among occasional species, the Litchi chinensis plantation had the lowest numbers of individuals and species, whereas the Eucalyptus plantation contained the highest. In terms of dominant species, the Eucalyptus plantation ranked highest in both individual and species counts, while the Pinus massoniana plantation accounted for the lowest percentages in both categories. The understory vegetation communities across all four stands were predominantly composed of common species in terms of individual counts, whereas occasional species contributed substantially to overall species richness.
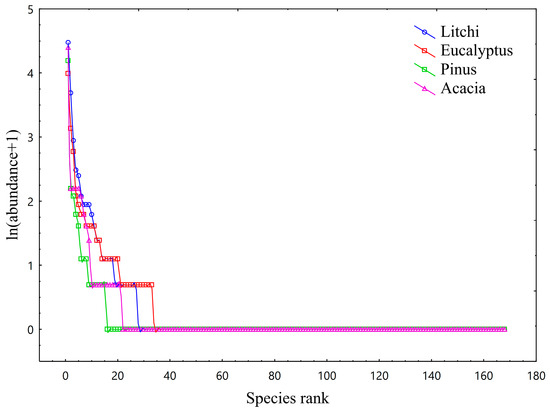
Figure 1.
Rank-abundance curves of understory vegetation in the four plantation types. The blue curve represents the Litchi chinensis plantation, the red curve represents the Eucalyptus plantation, the green curve represents the Pinus massoniana plantation, and the purple curve represents the Acacia mangium plantation. The relative steepness of the curves reveals differences in community evenness. The flat slope of the Eucalyptus curve indicates higher evenness, while the steeper slope for Acacia mangium suggests greater dominance by a few species.

Table 2.
Frequency distribution of tree species by silvicultural classification in the four plantation types.
3.2. Diversity Characteristics of Four Close-to-Nature Transformations of the Plantation
Differences in understory vegetation species diversity among the four forest types are shown in Figure 2. All five diversity indices differed significantly across stands. As depicted in Figure 2A, the abundance of individuals followed the order: Litchi chinensis plantation (188.82) > Eucalyptus plantation (124.84) > Acacia mangium plantation (98.92) > Pinus massoniana plantation (73.70). The abundance in the Litchi plantation was significantly higher than that in the other stands (p < 0.05); however, it exhibited the lowest species richness (S = 14.82), suggesting that the plant community is dominated by a limited number of species. In terms of species richness, the Eucalyptus plantation ranked highest (S = 21.60), followed by the Pinus massoniana plantation (S = 18.06), while the Litchi chinensis (S = 14.82) and Acacia mangium (S = 17.08) plantations showed relatively lower values (Figure 2B). The Shannon-Wiener index (H′, Figure 2D) was highest in the Pinus massoniana plantation, followed by the Eucalyptus plantation, with the Acacia mangium and Litchi plantations displaying lower diversity. Integrating both species richness (S) and Shannon-Wiener index (H′), the Pinus massoniana plantation demonstrated high species number and diversity, whereas the Eucalyptus plantation, though species-rich, had slightly lower diversity. The evenness index (Figure 2C) decreased in the order: Pinus massoniana plantation (E = 0.73) > Eucalyptus plantation (E = 0.66) ≈ Acacia mangium plantation (E = 0.66) > Litchi chinensis plantation (E = 0.48), indicating the most uniform species distribution in the Pinus massoniana plantation, intermediate evenness in the Eucalyptus and Acacia mangium plantations, and the lowest evenness in the Litchi plantation. Consistent with this, the Litchi chinensis plantation also showed the lowest Simpson diversity index (D) with a high coefficient of variation (Figure 2E), implying a loosely structured understory community likely dominated by a few superior species.
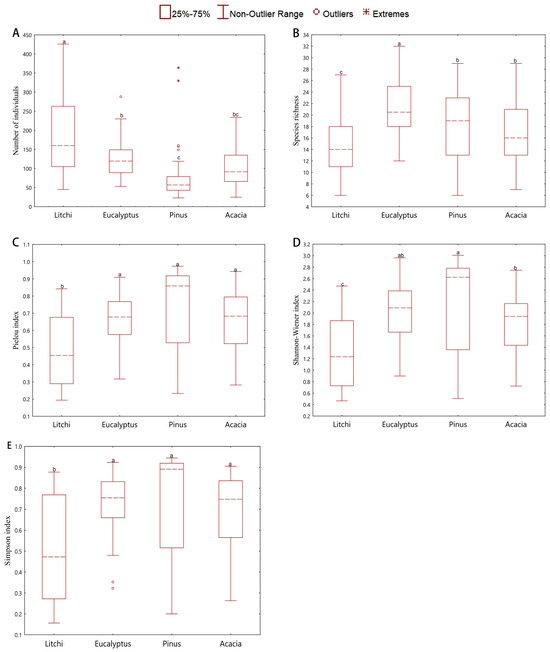
Figure 2.
Boxplots showing the biodiversity indices of different close-to-nature transformed forests. (A) Individual number, (B) Species richness index, (C) Evenness index, (D) Shannon-Wiener index, (E) Simpson diversity index. Different lowercase letters show significant differences in the four close-to-nature transformations of the plantation (p < 0.05). The dots represent Mild Outliers, and the star-shaped symbols represent Extreme Outliers. The key overall finding is that the Litchi chinensis plantation consistently exhibited the lowest diversity and evenness, while the other three types showed varied and generally higher profiles.
3.3. Differences in Species Diversity Among the Four Close-to-Nature Transformation of the Plantation
MRPP analysis revealed highly significant differences in understory species composition and distribution among the four plantation types (A = 0.149, T = −20.65, p < 0.001), indicating that between-group heterogeneity exceeded within-group homogeneity, thereby supporting distinct species assemblages across stands (Table 3). Specifically, within-group homogeneity was lowest between the Litchi chinensis and Pinus massoniana plantations (A = 0.071) and highest between the Acacia mangium and Pinus massoniana plantations (A = 0.151). All pairwise comparisons reached extreme significance (p < 0.0001). The smallest compositional difference was observed between the Litchi chinensis and Pinus massoniana plantations (|T| = 7.61), while the largest difference was between the Acacia mangium and Pinus massoniana plantations (|T| = 18.10). Gradient analysis of community similarity indicated that the Pinus massoniana plantation showed relatively minor differences from most of the other transformed stands. In contrast, the Acacia mangium plantation exhibited significant ecological differentiation, with markedly higher divergence from the Eucalyptus plantation (|T| = 15.74) and the Pinus massoniana plantation (|T| = 18.10) than from the Litchi chinensis plantation (|T| = 13.19).

Table 3.
Results of the Multi-Response Permutation Procedure (MRPP) testing for differences in species composition among the four plantation types.
3.4. ISA Indicator Species and Dominant Species Under Four Close-to-Nature Transformations of the Plantation
The close-to-nature transformation significantly influenced the composition and structure of understory vegetation across the four plantation types. In the Litchi chinensis plantation, the understory community was dominated by herbaceous plants. This is evidenced by the fact that all five indicator species were herbs, and four of the top five dominant species (based on IVI) within this plantation were also herbaceous (Table 4). However, the species with the highest indicator values—Lygodium japonicum (Thunb.) Sw (INV = 70.6) and Mikania micrantha Kunth (INV = 70.1)—differed from the dominant species Dicranopteris dichotoma (IVI = 79.47). The Eucalyptus plantation exhibited the highest number of indicator species (7 species, including 5 shrubs), with notably higher indicator values compared to other plantations, such as Evodia lepta (Spreng.) Merr (INV = 66.4) and Mussaenda pubescens W.T. Aiton (INV = 63.5). According to Table 4, the dominant species in this plantation were the herb Cyrtococcum patens (L.) A. Camus (IVI = 36.18) and the shrub Mussaenda pubescens (IVI = 28.47), yet their indicator values remained below 30. This discrepancy may be attributed to the high canopy light transmittance in Eucalyptus stands, which promotes the establishment of light-demanding shrubs (e.g., Litsea cubeba) and drought-tolerant herbs (e.g., Lygodium microphyllum (Cav.) R. Br). The Pinus massoniana plantation had the fewest indicator species (3 shrubs). Nonetheless, as shown in Table 4, its dominant species consisted of a mix of herbaceous plants (e.g., Dicranopteris dichotoma, IVI = 74.61) and shrubs (e.g., Ilex asprella (Hook. & Arn.) Champ. ex Benth, IVI = 17.43), and it also showed a relatively high Shannon-Wiener index (H′ = 2.14). These results suggest that the open canopy and natural litter layer in Pinus massoniana stands facilitate the coexistence of multiple vegetation strata—tree, shrub, and herb layers. In the Acacia mangium plantation, indicator species included four shrubs and one herb (Table 5), while the dominant species were primarily herbaceous (Microstegium vagans, IVI = 63.96) and arboreal (Aporosa dioica (Roxb.) Müll. Arg, IVI = 31.53) (Table 4). As a nitrogen-fixing species, Acacia mangium likely enhances soil nitrogen availability, thereby facilitating the establishment of early-successional shrubs such as Psychotria asiatica L (INV = 45.7).

Table 4.
Dominant understory vegetation (species with the top five in importance value, IVI) in the four close-to-nature plantation types.

Table 5.
Understory vegetation and indicator values (INV ≥ 30) in the four close-to-nature plantations.
3.5. DCA of Understory Vegetation Diversity in Four Types of Close-to-Nature Transformations of the Plantation
The DCA ordination revealed a clear pattern of understory community differentiation among the plantation types (Figure 3). A key finding was the strong segregation between the Eucalyptus, Pinus massoniana, and Acacia mangium plantations, with no overlap observed between Eucalyptus and Pinus massoniana, Eucalyptus and Acacia mangium, or Pinus massoniana and Acacia mangium. This suggests that each of these three plantation types has a highly distinct and specific understory community. Conversely, the Litchi chinensis plantation samples displayed a widely dispersed distribution that substantially overlapped with all other types, especially with Acacia mangium and Pinus massoniana. This indicates that the understory vegetation in Litchi chinensis plantations is less unique and shares considerable compositional similarity with the other plantations, possibly acting as a compositional intermediate or representing a more generalized habitat.
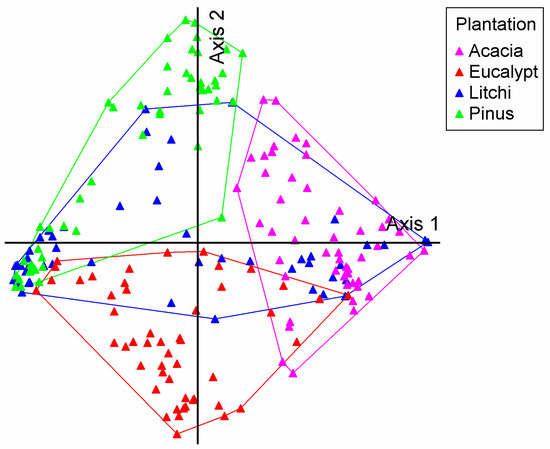
Figure 3.
Detrended correspondence analysis (DCA) ordination of understory vegetation communities across the four plantation types. The plot visualizes compositional differences based on data from 200 survey quadrats. Each point represents a single quadrat, colored according to its plantation type. The blue triangle represents the Litchi chinensis plantation, the red triangle represents the Eucalyptus plantation, the green triangle represents the Pinus massoniana plantation, and the purple triangle represents the Acacia mangium plantation. The DCA axes (axis 1 and axis 2) represent the primary gradients in species composition. The key finding is the clear separation among the Eucalyptus, Pinus massoniana, and Acacia mangium communities, while the Litchi chinensis quadrats show substantial overlap with others, indicating a less distinct species assemblage.
3.6. Redundancy Analysis of Species Diversity and Stand Factors in the Four Types of Close-to-Nature Transformation of the Plantation
Redundancy analysis (RDA) was performed using diversity indices of understory vegetation across the four stands as response variables and stand structural factors as explanatory variables (Tables S1 and S2). The results demonstrated that the first two RDA axes collectively explained a substantial proportion of the variance in understory diversity: 28.05% and 28.39% for the Litchi chinensis plantation, 41.55% and 41.62% for the Eucalyptus plantation, 22.60% and 22.65% for the Pinus massoniana plantation, and 46.58% and 46.78% for the Acacia mangium plantation. This indicates that the first two ordination axes effectively captured the relationships between species diversity and stand factors in the transformed plantations. The RDA biplot for the Litchi chinensis plantation (Figure S1A) revealed that the first axis (RDA1) was primarily associated with variations in H, DBH, Hb, Cw. According to Monte Carlo permutation tests (Table S1), mean DBH significantly influenced understory species diversity (p = 0.034), explaining 50.8% of the variance. It was negatively correlated with the Simpson (D), Evenness (E), and Shannon-Wiener (H′) indices. For the Eucalyptus plantation (Figure S1B), RDA1 mainly reflected gradients in DBH, Hb, and H, while RDA2 was associated with Cw. Monte Carlo tests indicated that both H and Cw significantly affected understory diversity (p = 0.002 and p = 0.004, respectively), accounting for 69.3% and 30.6% of the explained variance. H was negatively correlated with the D, E, and species richness (S) values, whereas Cw was negatively correlated with the evenness index (E).
The RDA biplot for the Pinus massoniana plantation (Figure S1C) indicated that the first axis (RDA1) was mainly associated with variations in H, DBH, Hb, and Cw. Monte Carlo permutation tests (Table S2) revealed that DBH significantly influenced understory species diversity (p = 0.002), accounting for 92.1% of the explained variance. DBH was negatively correlated with species richness (S), Simpson diversity index (D), evenness index (E), and Shannon-Wiener index (H′).
For the Acacia mangium plantation (Figure S1D), RDA1 primarily reflected changes in DBH, H, and Hb, while RDA2 was associated with Cw. Monte Carlo tests showed that both DBH and Cw significantly affected understory diversity (p = 0.002 and p = 0.014, respectively), explaining 76.5% and 18.1% of the variance, respectively. DBH was negatively correlated with the S, D, E, and H′values, whereas Cw was only negatively correlated with the evenness index (E).
4. Discussion
This study characterized the understory vegetation diversity in four close-to-nature transformed plantations, revealing distinct variations across plantation types. An integrated view of multiple diversity indices (Species richness S, Shannon-Wiener H′, Simpson D, and Pielou’s E) revealed distinct ecological patterns among the four transformed plantations. Specifically, the Pinus massoniana plantation exhibited the highest overall diversity profile, with the greatest values in both species richness (S) and the Shannon-Wiener index (H′ = 2.10), coupled with the highest evenness (E). The Eucalyptus plantation, while also species-rich, showed a slightly lower H′ (2.0). In contrast, the Litchi chinensis plantation was characterized by the lowest values across all indices (e.g., H′ = 1.30, E = 0.48), indicating a community dominated by few species. The Acacia mangium plantation presented an intermediate profile (H′ = 1.80). The Shannon-Wiener indices observed in our study fall within the upper range of values reported for various managed forest types in Guangdong Province, China, such as artificial broad-leaved and coniferous forests (H′ ≈ 1.06 − 1.19) [25], though they remain below that of old-growth forests in the region (H′ ≈ 3.0) [26,27,28], suggesting potential for further development. Furthermore, when compared to reports from untransformed monocultures [1], the diversity indices in our transformed stands (e.g., H′, E, and D for Eucalyptus and Acacia mangium) were greater.
The close-to-nature transformation significantly altered stand structure and species composition, creating distinct understory environments through differences in canopy cover and litter properties [7]. It is important to note that the exact timing of these transformation interventions was not available for our study sites, and the current stand age remains uncertain. Therefore, our interpretations of successional stages are based on the observed community composition and structure as a snapshot in time. In line with Clements’ organismic concept of succession, plant communities in this study displayed divergent compositions, indicating different successional stages [29]. The Litchi chinensis plantation, with its dominant species (high IVI) and indicator species (all five were herbs with INV ≥ 70) being highly overlapping, and its low evenness (E = 0.48), appears dominated by a few herbaceous species and likely arrested at an early pioneer stage. This might be a legacy issue from the past high-intensity weeding management, which suppressed the growth of woody plants and allowed herbaceous plants adapted to disturbances (such as Mikania micrantha, with an indicator value of INV = 70.1) to take the dominant position [30]. This could also be related to the dense invasion of this alien species. The Eucalyptus plantation had the highest number of indicator species (seven, five of which were shrubs) and a mix of shrubs and herbs as dominants. However, its lower Shannon-Wiener index (H′) compared to the Pinus massoniana plantation suggests a community in a state of dynamic competition. Light-demanding shrubs such as Litsea cubeba (INV = 55.0) and Evodia lepta (INV = 66.40) are associated with its open canopy [31]. Although Cyrtococcum patens is a dominant herb (IVI = 36.18), it is suppressed by shrub shading, resulting in a shrub-dominated community. In contrast, the Pinus massoniana plantation exhibited a more complex multi-layered structure. It demonstrated higher stability, as indicated by a higher H′, with Dicranopteris dichotoma (IVI = 74.61) as a dominant species and shrubs like Adina rubella Hance (INV = 35.60) as indicators. Although soil acidification from pine needle litter inhibits some herbs [32], the natural litter layer promotes saprotrophic fungal diversity and nutrient cycling, supporting community stability. The Acacia mangium plantation had fewer individual plants but comparable species richness. Its high-IV indicator species, including the tree Aporosa dioica (INV = 61.20) and the shrub Psychotria asiatica (INV = 45.70), suggest a successional stage characterized by a dynamic balance between trees and shrubs, The research results are consistent with the dominant species of the main natural forest communities of Aporosa dioica + Heptapleurum heptaphyllum in the same area [33], reflecting that there are commonalities between the characteristics of the transformed community and the natural forest community. High canopy closure causes light limitation, suppressing understory diversity. The co-occurrence of herbaceous and tree species in the dominant group (e.g., Microstegium vagans, IVI = 63.96; Aporosa dioica, IVI = 31.53) alongside the presence of woody indicator species with high indicator value (e.g., Aporosa dioica, INV = 61.20; Psychotria asiatica, INV = 45.70) reflects constrained niche competition in the high-density Acacia mangium plantation. Its lower H′ compared to the Pinus massoniana plantation indicates a community that is not yet fully stable, potentially transitioning from mid to late successional stages [34].
Consistent with previous studies [35,36], stand factors significantly influenced understory diversity, though the key drivers differed. Redundancy analysis identified DBH as the primary negative driver of diversity in all stands except Eucalyptus, as increased DBH enhances canopy closure and reduces light availability, limiting the establishment of light-demanding species [37,38,39,40]. This relationship indicates that canopy structure indicators can serve as reliable predictive indicators for the development of understory vegetation in intelligent forestry applications. We acknowledge that the lack of precise stand age data prevents us from disentangling the effects of stand development stage from the inherent properties of the tree species. The Acacia mangium plantation, with the largest mean DBH (11.5 ± 7.76 cm), was characterized by the prevalence of shade-tolerant woody species, including key indicators such as Aporosa dioica (INV = 61.20), Ficus hirta (INV = 41.4), and Psychotria asiatica (INV = 45.70). The dominance of these shade-tolerant plants under strong shading resulted in lower understory species diversity [41]. In contrast, the Pinus massoniana plantation, with the smallest mean DBH (7.13 ± 4.31 cm), allowed more light penetration, supporting higher diversity, though soil acidification from litter may still inhibit some herbs [42]. The nitrogen-fixing capacity of Acacia mangium appears to promote shrub growth, leading to the highest Simpson and Shannon-Wiener indices among the four stands and facilitating the dominance of light-adapted species, as indicated by high indicator values for species like Raphiolepis indica (INV = 43.40) and Adina rubella (INV = 35.60). Contrary to some previous findings [43], mean height to the first live branch (under-branch height) showed no significant correlation with species diversity in this study. This suggests that, under the predominant shading effect mediated by DBH (e.g., in the Acacia mangium plantation), adjusting the under-branch height alone may be insufficient to alter understory resource availability significantly or enhance diversity. The natural branching structure of certain species, like Litchi chinensis, may further limit its potential for understory regulation [43].
Future management of these transformed plantations under the Green Beauty Guangdong initiative should adopt differentiated strategies tailored to the specific successional stage and structural attributes of each forest type. A key limitation of this study lies in the absence of direct control sites (either pre-restoration data or single-species plantations that were not restored during the same period), which makes it impossible to conduct a strict comparison. Nevertheless, our findings provide valuable insights into the structural and compositional states achievable after close-to-nature transformation. Furthermore, long-term soil monitoring is essential, as variations in litter input among species can interact with soil properties to influence plant community assembly through complex synergistic effects.
5. Conclusions and Future Recommendations
This study demonstrates that the transformed Litchi chinensis plantation, which had previously been intensively managed as an orchard, exhibited the lowest species diversity (H′ = 1.30) and evenness (E = 0.48). Its indicator species were all herbaceous, suggesting that succession is arrested at a pioneer stage. The transformed Eucalyptus plantation had the highest number of indicator species (7), predominantly light-demanding shrubs and drought-tolerant herbs, but its Shannon-Wiener index (H′ = 2.00) was lower than that of the Pinus massoniana plantation, indicating a mid-successional dynamic state. The transformed Pinus massoniana plantation showed the highest Shannon-Wiener (H′ = 2.10) and evenness indices (E = 0.73), with a stable multi-layered structure comprising trees, shrubs, and herbs, forming a relatively complex community structure. However, its high canopy cover and acidic leaf litter depositions suppresses herbaceous diversity. The transformed Acacia mangium plantation exhibited intermediate species diversity, with the largest mean DBH (11.51 cm), fostered a shaded understory characterized by shade-tolerant woody indicator species (e.g., Aporosa dioica, INV = 61.2) and intermediate species diversity (H′ = 1.80) characterized by a community of shade-tolerant woody indicator species under a high-density canopy. This structure fosters intense niche competition, positioning it at a mid-to-late successional stage with potential for further development towards a more stable, complex forest. These findings provide an ecological basis for refining intelligent classification of plantation successional stages and support the development of tailored restoration protocols under the Green Beauty Guangdong framework.
For the four transformed plantation types examined in this study, differentiated management strategies should be implemented in conjunction with adjustments to DBH structure. In the future, an intelligent monitoring system combining remote sensing and stereoscopic observation can be utilized to quantify the parameters of forest stand structure and provide support for thinning decisions. In plantations with larger DBH (such as Acacia mangium and Eucalyptus plantations), targeted thinning may be applied to reduce stand density and simultaneously promote niche complementarity between light-demanding herbs and shade-tolerant shrubs. For stands with smaller DBH (e.g., Pinus massoniana plantation), the current high under-branch height can be maintained. In addition, interplanting under the forest canopy could be introduced to enhance vertical structural heterogeneity, improve the stability of the understory vegetation community, and further contribute to the precision upgrading of plantation quality. These methods are in line with the goals of smart forestry—to achieve precise and sustainable ecological management of the main types of plantations in Guangdong Province, China, through the use of data and technology.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f16111683/s1.
Author Contributions
Conceptualization, X.X. and X.J.; formal analysis, X.X. and Z.L.; resources, X.J.; investigation, X.X., Z.L. and R.H.; writing—original draft preparation, X.X.; writing—review and editing, R.H.; supervision, X.J.; funding acquisition, X.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and Technology Program from Forestry Administration of Guangdong Province (2023KJCX004).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy concerns.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Luo, Y.M.; Tan, X.P.; Zou, X.J.; Rao, X.Q.; Lin, Y.B.; Shen, W.J. Understory Vegetation Diversity Characteristics and Influencing Factors of Four Common Plantation Types in Southern China. J. Trop. Subtrop. Bot. 2022, 30, 1–10. [Google Scholar] [CrossRef]
- Lu, Y.L.; Yang, C.Y.; Jiang, J.; Zhong, H.; Chung, Y.-L. High-Quality Forest Management Path under the Background of Green and Beautiful Guangdong Ecological Construction. For. Investig. Des. 2024, 53, 32–37. [Google Scholar]
- Liu, L.X.; Du, M.Y.; Hu, X.F.; Jin, Y.S.; Zhao, Y.; Ji, X.Q.; Wang, Z.; Liu, W. Effects of Close-to-Nature Forest Management on Stand Structure and Understory Vegetation of Larix principis-rupprechtii Plantation. Guangxi For. Sci. 2025, 54, 160–166. [Google Scholar] [CrossRef]
- Li, H.L.; Li, Y.Q.; Dai, S.P.; Luo, H.X.; Li, M.F.; Fang, J.H. Research Progress on Key Technologies of Smart Forestry in China. Chin. J. Trop. Crops 2024, 45, 2476–2486. [Google Scholar]
- Emborg, J.; Christensen, M.; Heilmann-Clausen, J. The structural dynamics of Suserup Skov, a near-natural temperate deciduous forest in Denmark. For. Ecol. Manag. 2000, 126, 173–189. [Google Scholar] [CrossRef]
- Schweitzer, C.J.; Dey, D.C. Forest structure, composition, and tree diversity response to a gradient of regeneration harvests in the mid-Cumberland Plateau escarpment region, USA. For. Ecol. Manag. 2011, 262, 1729–1741. [Google Scholar] [CrossRef]
- Xie, Y.S.; Meng, J.H.; Zeng, J.; Ming, A.G.; Liu, X.Z.; Jia, H.Y.; Lei, X.D.; Lu, Y.C. Analysis of Close-to-Nature Transformation Effects in Pure Pinus massoniana Plantations. For. Res. 2023, 36, 31–38. [Google Scholar] [CrossRef]
- Ming, A.G.; Liu, S.R.; Li, H.; Zeng, J.; Sun, D.J.; Lei, L.Q.; Meng, M.J.; Tao, Y.; Ming, C.D. Effects of close-to-nature management on biomass and its allocation in Pinus massoniana and Cunninghamia lanceolata plantations. Acta Ecol. Sin. 2017, 37, 7833–7842. [Google Scholar] [CrossRef]
- Zhang, P.J.; Xu, J.M.; Lu, W.H.; Pan, S.; Chen, M.; Li, K.; Shang, X. Analysis of Plant Diversity and Soil Physicochemical Properties of Different Stand Ages of Eucalyptus urophylla × E. tereticornis Plantation in Leizhou Peninsula. J. Cent. South Univ. For. Technol. 2021, 41, 96–105. [Google Scholar]
- Deng, J.; Fang, S.; Fang, X.; Jin, Y.; Kuang, Y.; Lin, F.; Liu, J.; Ma, J.; Nie, Y.; Ouyang, S.; et al. Forest understory vegetation study: Current status and future trends. For. Res. 2023, 3, 6. [Google Scholar] [CrossRef]
- Siddig, A.A.; Ellison, A.M.; Ochs, A.; Villar-Leeman, C.; Lau, M.K. How do ecologists select and use indicator species to monitor ecological change? Insights from 14 years of publication in Ecological Indicators. Ecol. Indic. 2016, 60, 223–230. [Google Scholar] [CrossRef]
- Woziwoda, B.; Greda, A.; Dyderski, M.K. Effect of litter cover on acorn survival, seedling emergence, and early growth of introduced Quercus rubra. Eur. J. For. Res. 2025, 144, 283–294. [Google Scholar] [CrossRef]
- Ozdemir, S. Effects of environmental variables on taxonomic diversity and grouping of plant communities in the Mediterranean region (Antalya). Eur. J. For. Res. 2024, 143, 1903–1914. [Google Scholar] [CrossRef]
- Wang, S.J.; Yan, M.H.; Huang, Q.L.; Peng, W.-C.; Liao, L.-G.; Huang, S.-Q.; Song, L. A Preliminary Report on the Rapid Transformation of Acacia mangium Plantation into Natural Forest in Hainan Tropical Rainforest National Park. For. Res. 2025, 38, 23–33. [Google Scholar]
- He, Q.X.; Huang, H.M.; Li, J.Y.; Liang, Y.; Ma, H.; Huang, X.; You, Y. Effects of uneven-aged mixed transformation of Pinus massoniana plantation on understory vegetation diversity and functional groups. Chin. J. Ecol. 2025, 44, 3379–3388. [Google Scholar] [CrossRef]
- Luo, J.C.; Yang, S.M.; Lyu, Y.Z.; Liu, Y.; Wu, G.; Li, Z.; Tang, X.; Liu, J.; Li, X. Diversity characteristics of understory medicinal plant communities in Cunninghamia lanceolata forest after stand transformation. J. Anhui Agric. Univ. 2024, 51, 713–725. [Google Scholar]
- Wang, D.F.; Cao, H.L. Main Vegetation Types and Ecological Public Welfare Forest Construction in Dongguan City. Guangdong For. Sci. Technol. 1999, 15, 23–28. [Google Scholar]
- Zhou, J.G.; Li, L.; Wei, S.G. Functional diversity characteristics of common and rare species in South subtropical forests. Acta Ecol. Sin. 2024, 44, 699–711. [Google Scholar]
- Fangliang, H.; Legendre, P.; LaFrankie, J.V. Distribution patterns of tree species in a Malaysian tropical rain forest. J. Veg. Sci. 1997, 8, 105–114. [Google Scholar] [CrossRef]
- Ye, J.T.; Deng, T.; Cen, H.F.; Duan, Y.B.; Zhu, X.Z.; Hu, X.H. Niche and interspecific association of dominant tree species in the Abies ziyuanensis community of Yinzhulaoshan, Guangxi. Acta Ecol. Sin. 2025, 45, 3921–3932. [Google Scholar]
- Williams, M.A. Response of microbial communities to water stress in irrigated and drought-prone tallgrass prairie soils. Soil Biol. Biochem. 2007, 39, 2750–2757. [Google Scholar] [CrossRef]
- Stallins, J.A. Dune plant species diversity and function in two barrier island biogeomorphic systems. Plant Ecol. 2003, 165, 183–196. [Google Scholar] [CrossRef]
- Paluots, T.; Liira, J.; Leis, M.; Laarmann, D.; Põldveer, E.; Franklin, J.F.; Korjus, H. Long-Term Cumulative Effect of Management Decisions on Forest Structure and Biodiversity in Hemiboreal Forests. Forests 2024, 15, 2035. [Google Scholar] [CrossRef]
- Grandin, U. PC-ORD version 5: A user-friendly toolbox for ecologists. J. Veg. Sci. 2006, 17, 843–844. [Google Scholar]
- Zhang, L.F.; Liu, Z.G.; Dong, L.B. Division of development stages and stand structure characteristics of natural hardwood broadleaved forest in Mao’er Mountain. J. Beijing For. Univ. 2025, 47, 10–22. [Google Scholar]
- Yang, B.; Xue, W.Y.; Zhang, W.W.; Lu, Y.L.; Zhang, W.H. Management direction and target of secondary Quercus acutissima forest based on tree species regeneration characteristics and growth process model. Acta Ecol. Sin. 2025, 45, 3933–3945. [Google Scholar] [CrossRef]
- Chen, C.G.; Xue, C.Q.; Wang, Q.L.; Yang, Y.; Jiang, J.; Chen, Q.; Yang, C.; Zheng, J.; Zhang, Y. Plant Diversity of Typical Vegetation Types in Guangdong Province Based on 2017 Continuous Forest Inventory Samples. For. Environ. Sci. 2020, 36, 60–65. [Google Scholar]
- Cui, F.K.; Xu, X.Y.; Fu, L.Y.; Liang, J.; Molly Li, W.; Cai, Z. Analysis of Species Diversity and Change Characteristics of Understory Vegetation in Different Forest Types in Three Forest Areas. Anhui Agric. Sci. Bull. 2024, 30, 68–76. [Google Scholar] [CrossRef]
- Clements, F.E. Plant succession: An analysis of the development of vegetation. Carnegie Inst. 1916, 45, 339–341. [Google Scholar]
- Ni, G.; Zhao, P.; Huang, Q.; Zhu, L.; Hou, Y.; Yu, Y.; Ye, Y.; Ouyang, L. Mikania micrantha invasion enhances the carbon (C) transfer from plant to soil and mediates the soil C utilization through altering microbial community. Sci. Total Environ. 2020, 711, 135020. [Google Scholar] [CrossRef] [PubMed]
- Degefu, M.A.; Degefa, S.; Kebede, W.; Daba, D. Effect of complete abolition of Eucalyptus species on under canopy species diversity in Gullele Botanic Garden, Ethiopia. Environ. Chall. 2023, 11, 100701. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Charles McClaugherty Plant Litter; Springer: Cham, Switzerland, 2014. [Google Scholar]
- Li, G.H.; Zhang, S.K.; Ye, Y.X.; Chen, J. Species diversity and population dynamics of dominant species in the Heptapleurum heptaphyllum community in Yinxingshan Forest Park, Dongguan. J. For. Environ. Sci. 2018, 34, 65–72. [Google Scholar]
- Chazdon, R.L. Beyond deforestation: Restoring forests and ecosystem services on degraded lands. Science 2008, 320, 1458–1460. [Google Scholar] [CrossRef]
- Zhou, R.H.; Wang, M.; Li, J.; Zhai, B.; Wang, Z.; Qi, J.; Hao, J. Dynamic characteristics of species diversity and community structure of shrub and herb layers in eucalyptus plantations. J. Sichuan Agric. Univ. 2020, 38, 430–438. [Google Scholar]
- Su, T.C.; Wang, Y.Y.; Xiang, L.; Yu, J.; Chen, C.; Wang, Q.; Wang, F.; Hao, J. Effects of silvicultural measures on community structure and species diversity of poplar plantations along the Chengdu Ring Expressway. Chin. J. Appl. Environ. Biol. 2022, 28, 1144–1150. [Google Scholar]
- Fu, L.Y.; Sun, H.; Zhang, H.R.; Lei, X.; Lei, Y.; Tang, S. Effects of DBH on crown width characteristic factors of Cunninghamia lanceolata under different canopy densities. Acta Ecol. Sin. 2013, 33, 2434–2443. [Google Scholar]
- Tan, X.M.; Zhang, W.; Xiao, N.; Mo, X.Q.; Gao, G.N.; You, Y.M.; Ming, A.G.; Huang, X.M. Effects of the transformation of Cunninghamia lanceolata plantation into native broad-leaved forest on species composition and diversity of understory vegetation. Acta Ecol. Sin. 2022, 42, 2931–2942. [Google Scholar]
- Wang, L.; Lin, S.; Li, Y.H.; Chen, M.F.; He, K.N. Study on the relationship between canopy structure and understory vegetation species diversity of typical stands in Datong, Qinghai. Acta Bot. Boreali-Occident. Sin. 2019, 39, 524–533. [Google Scholar]
- Duan, W.J.; Li, D.; Li, C. Analysis of understory vegetation diversity and its influencing factors in five different stand ages of Eucalyptus urophylla × E. grandis plantation. Ecol. Environ. Sci. 2022, 31, 857–864. [Google Scholar]
- Liu, J.L.; Lyu, Q.; Liu, J.J.; Chen, X.H.; Fan, C.; Li, X.W. Early effects of gap size on the niche of dominant species in the understory shrub and herb layer of Pinus massoniana plantation. Acta Bot. Boreali-Occident. Sin. 2022, 42, 312–325. [Google Scholar]
- Wang, Y.H.; Chen, Z.; Zhou, J.G.; Zhang, Y.Y. Effects of pure Pinus massoniana forest transformation on soil acidification characteristics and aggregate stability in Chongqing acid rain area. Acta Ecol. Sin. 2021, 41, 5184–5194. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; He, Y.T.; Zhou, N.; Liu, H.X.; Hao, J.F. Effects of stand factors on understory vegetation diversity of different forest communities in Shenmu Lei. Acta Bot. Boreali-Occident. Sin. 2023, 43, 1950–1958. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).