Abstract
Forest biodiversity is of particular importance for the world’s natural resources and for humans, so it is essential to observe the impact of forest management on its state. In this paper, the effects of Poland’s forestry evolution after 1945 on the forest biodiversity at the species level are interpreted based on four indicators (deadwood, species composition of forest stands, introduced tree species, and common forest bird species) and considering the two politically and economically different eras. In the era of socialism (1945–1989), the area share of broadleaved tree species increased intensively, with a steady predominance of single-species stands, the ongoing removal of deadwood, and a relatively slow increase in the area occupied by alien tree species. In the era of democracy (ongoing since 1990), there was a less intensive increase in the proportion of broadleaved species, an intensive increase in the stock of deadwood, an increase in the area of multi-species stands over that of single-species stands, as well as an increase in the value of the Forest Bird Index. However, an intensive increase in the area occupied by alien tree species, especially invasive ones, which negatively impact native wildlife, was also noticeable in the era of democracy. The dilemmas and problems related to the amount of deadwood in forests; the continued conversion of stands towards more complex ones; the presence of other invasive species; the consequences of climate change; the reduction in migration barriers for forest animals; and the provision of funding for biodiversity conservation activities need to be discussed and resolved or implemented further.
1. Introduction
The delivery of ecosystem services by forests, e.g., the maintenance of species biodiversity [1,2], depends on how forests are handled, including the forest management model (e.g., [3]). The latter, in turn, depends on a country’s historical, political, economic, and social conditions. Therefore, their change over time will affect the biodiversity status of local forests. Analysing the directions and dynamics of changes occurring in a long-term perspective and large spatial scale, interpreting their causes and determinants, as well as pointing out possible further consequences and problems, can serve to improve the principles of local forest management and also be a valuable record of experience for forest managers from other countries and regions of the world. Research to date has tended to focus on the impact of one element of forest management [4] or on the effects of forest management on one group of organisms [4,5,6,7] or another parameter [8], often referring to an uncommonly long time horizon or a limited area. In this article, the relationship between forest management and the species level of forest biodiversity will be examined in the example of the whole country of Poland (Central Europe), covering the relatively long period from 1945 (end of World War II) to the present and referring to the different parameters related to species diversity.
Due to Poland’s climatic and geographic location, forests are the most natural and potentially dominant natural formation, occupying almost the entire area of the country in the past [9,10]. As a result of the development of agriculture and the growing demand for raw wood materials, Poland’s forest cover has gradually decreased. The situation worsened further between 1772 and 1918, when Poland was gradually split between the bordering empires (Russia, Prussia, and Austria), which led to the intensive use of the resources of the local environment [10,11,12]. At the same time, from the 19th century onwards, both in Poland and Europe, coniferous tree species were promoted in forests as being particularly useful in the wood processing industry [12,13,14,15,16,17]. The depletion of the species structure of stands has consequently reduced the overall/species biodiversity in forests [9].
In the inter-war period (1918–1939), when Poland was already independent, the foundations of a Polish model of multifunctional forestry were developed. A state forest management enterprise was also established at that time (in 1924), which is now called the State Forests National Forest Holding (in short: the State Forests, SF). However, further forestry development was interrupted by the Second World War, which further destroyed Polish forests [18].
The period analysed in this article, from the end of the Second World War to the present day, is highly differentiated, especially politically and economically. The first part of it (1945–1989) falls into the era of socialism. It was characterised by a centrally planned and regulated economy, extensive nationalisation and industrialisation [19,20], severe environmental pollution, the extinction of natural habitats [21], a substantial weakening of forest health [16,22] and a resource-based economic model of forestry promoting Scots pine Pinus sylvestris L. and Norway spruce Picea abies (L.) H. Karst [13,16]. The measures taken by foresters at that time to protect biodiversity and improve the condition of forest stands were not able to have a real and radical impact on the overall condition of Polish forests [22].
The situation changed only in the second part of the analysed period, i.e., after 1989 (the era of democracy). Although the change of the political system in Poland did not affect the ownership structure of forested land (almost 80% of the area of all Polish forests was still under the management of the State Forests [23,24]), it did, however, enable the intensification of work on the transformation of the forest management model as opposed to the forests remaining in private ownership. The transformation of the forest management model was implemented at the legal level (the Forest Act [25]: the concept of sustainable forest management (SFM); the orders by the Director General of the SF: the best ecological practices, new guidelines for forest management) and at the policy level (Polish Policy for Comprehensive Forest Resource Protection [26] and National Forest Policy [27]: efforts towards improving forest biodiversity). Not insignificant for this process was the simultaneous active involvement of Polish representatives in conceptual work within the Ministerial Conferences on the Protection of Forests in Europe (MCPFE—currently, FOREST EUROPE) and the Convention on Biological Diversity (CBD). Further essential events for the evolution of the forest management model in Poland are related to the ratification of the Aarhus Convention in 2003 (socialisation of environmental management and protection [28]) and Poland’s accession to the European Union in 2004 (implementation of the provisions of the Habitats Directive and the Birds Directive of fundamental importance for the protection of biodiversity [29]).
The described transformations have influenced and continue to influence the biodiversity status of Polish forests. This problem has so far been analysed on two levels—that of the landscape [24] and that of the ecosystem [30]. This article will extend this analysis to another level of biodiversity—the species level, which, so far, has not yet seen such an analysis in Poland. It should be noted that about 60%–65% of all species found in Poland are permanently or periodically associated with Polish forests, with fungi being the most dependent [31]. Therefore, it is very important to control the interrelationship between the implemented management and the state of species biodiversity in Polish forests.
Given the above, the main aim of the present study was to evaluate the effects of forestry evolution under Poland’s conditions on the state of forest biodiversity at the species level, using selected indicators. From the perspective of a period of 75 years (1945–2020), the following specific objectives were distinguished: (1) to determine the direction and dynamics of changes with regard to forest species, (2) to identify the determinants of the observed changes and their effects, and (3) to identify threats to the forest species and the direction of further action for the benefit of forest biodiversity at the species level.
2. Materials and Methods
2.1. Indicators
For the present study, the influence of forestry evolution in Poland, especially in forests administered by the State Forests, on forest biodiversity at the species level was primarily based on selected indicators related to SFM developed by FOREST EUROPE [23,32].
The four selected indicators relate in part to different levels of forest biodiversity, but specifically to biodiversity at the species level:
- Deadwood—C&I for SFM: Indicator 4.5, deadwood, is understood as the ‘volume of standing deadwood and of lying deadwood on forest and other wooded land’ [23,32]. Although this indicator has a much broader meaning than just for species biodiversity (deadwood also affects landscape appearance and habitat conditions [33,34,35]), it is assumed in this article to be particularly important for its formation. Indeed, deadwood resources are indispensable and of exceptional importance for many species that cannot properly exist without them [33,34,36,37]. Due to their availability, the data on deadwood presented in this article refer to timber/coarse wood (m3) and, therefore, do not consider all available resources. However, from the point of view of the needs of species, these smallest/thin fragments of deadwood are sometimes less helpful [35,38,39]. In addition, deadwood in the data from the period up to and including 2001 was referred to as ‘dead trees’ in the source materials, which may suggest a further limitation of the reported volume to standing dead trees. Data on the average amount of deadwood (timber gross) per 1 ha of forest (m3/ha) were calculated by dividing the total volume of this wood by the area of forests administered in a given year by the State Forests.
- Species composition of forest stands—This indicator covers a complex issue. Firstly, a qualitative factor was considered, i.e., the diversity of tree species (coniferous and deciduous) in the forests. The diversity of tree species has a powerful influence on the diversity of other species associated with them and the forest ecosystem [23,40,41]. In doing so, the analysis considered the proportion (%) of dominant (prevalent) species in the stand rather than the actual species, which was dictated by the availability of data from the entire study period and attention to the comparability of the information collected. Secondly, a quantitative factor was considered, i.e., the species diversity (number of species) of trees within individual stands. It refers to C&I for SFM: Indicator 4.1, diversity of tree species, understood as the ‘area of forest and other wooded land, classified by number of tree species occurring’ [23,32]. Information on the number of tree species forming tree stands, and the area occupied by them (ha) is not published in publicly available statistical reports and yearbooks in Poland, only periodically provided for MCPFE/FOREST EUROPE reports. The first such data only date back to 2000 [23,42,43].
- Introduced tree species—C&I for SFM: Indicator 4.4, introduced tree species, is understood as an ‘area of forest and other wooded land dominated by introduced tree species’ [23,32], which means that in this area, they have the most significant impact on the biocoenosis, usually due to their greatest abundance or highest mass. Introduced tree species, used in European forestry, among others, in the hope of good results of wood production, are a geographically alien element of forest ecosystems. In some situations, they pose a threat to them in the form of invasive spread [23,44]. Updated knowledge of their occurrence is, therefore, essential. However, publicly available data for Polish forests—as in the case of tree species diversity—are compiled for the State of Europe’s Forests reports only from 2000 onwards [42]. This paper analyses both the total area (ha) on which introduced tree species predominate and the area (ha) on which those introduced species that have acquired invasive characteristics predominate.
- Common forest bird species—C&I for SFM: Indicator 4.10, common forest bird species, is understood as the ‘occurrence of common breeding bird species related to forest ecosystems’ [23,32]. The described indicator, which still requires further development and testing for consideration, presents changes in breeding populations of 34 bird species that are both common and characteristic for forest habitats in Europe (the reference year is given as 1980). The indicator describes the proportion of the annual population of the studied species in a given year compared to the population of these species assessed in the reference year. The Forest Bird Index (FoBI) in Poland has been reported since 2000. It includes 34 bird species, 22 of which are shared with the common forest bird index of the MCPFE [23,45] (see Table A1 in Appendix A). To assess the situation of common forest bird species before 2000, three consecutive studies with a uniform method of determining bird abundance were analysed [46,47,48]. For all 34 bird species included in the FoBI, abundance information published in 1972, 1990, and 2003 (the latter year relatively coinciding with the start of official monitoring for the FoBI) was collated. Then, the trends in abundance between these dates (increase, stabilisation, and decrease) and how many species they affected were assessed. These data allowed for a simplified generalisation of the assessment for the whole group of birds for which the FoBI is defined in Poland today. Although it is not a perfect solution due to the sometimes wide ranges of the individual original abundance categories, in the absence of other precise sources of information, it was the only one possible.
2.2. Scope of Analyses
In this article, the temporal scope of the analyses covers the period 1945–2020, spanning 75 years, which is consistent with previous analyses referring to the landscape level [24] and the ecosystem level [30]. However, wherever possible, reference was also made to the most up-to-date data in the range under study. The period studied consists of two distinct political and economic eras: the era of socialism (1945–1989) and the era of democracy (1989–2020 and contemporary), with the year 1990 assumed as the breakthrough year in the adopted timeline.
It has been assumed that the impact of the evolution of Polish forestry on forest species diversity will be examined based on data compiled at 10-year intervals, related below to the history of Poland and the forest management implemented on its territory:
- 1950: post-war national reconstruction; socialist economy model forced (centrally planned, also in forest management);
- 1960: Socialist economy model fully implemented (resource-based economic model in forestry);
- 1970: continuation of the socialist economy model (including forest management), deterioration of forest health;
- 1980: forthcoming political and forest management changes; starting point for environmental protection and increasing public awareness of the environment;
- 1990: transformation of the political system and economy; new priorities for national development; key changes in forest management; participation in MCPFE and in the development of the Convention on Biological Diversity;
- 2000: preparations for Poland’s accession to the EU and adoption of EU legislation (m.in. Natura 2000); implementation of nature conservation programmes in each forest district of the SF;
- 2010: establishing Natura 2000 sites; implementing the Aarhus Convention (public consultation of forest management plan); further greening of forest management; intensification of actions against climate change impacts;
- 2020: enhancing measures implemented since 1990; increased pressure from society on the protection of national forests.
The tables show data referring to the end of a given year. If data were unavailable for a given study year, if possible, data from contiguous years were used, and relevant information was then indicated in the footnotes in the tables.
The analyses included forest areas managed by the State Forests, and references to all forests in Poland were made if information regarding the SF was unavailable. This approach was based on the easier access to detailed and reliable information and data (mainly historical), as well as the homogeneity of forest management objectives pursued on a large spatial scale (today, the SF manage above 77% of the total forest area in Poland [49]). Additionally, the State Forests are influenced by pressures exerted by the government and decisions on land management policy because of managing state land.
The substantive scope of the work included evaluating information/data compiled in terms of the characteristics of the feature analysed, taking into account variation over time as well as the direction and dynamics of changes; providing a comprehensive analytical commentary; identifying threats to the forest species; and indicating directions of beneficial actions.
2.3. Sources and Analysis of Information
The presented numerical data came from statistical yearbooks on forestry and environmental protection published mainly by Statistics Poland (GUS); reports published by State Forests National Forest Holding (Poland) [50,51,52,53,54]; the Forest Data Bank [55]; database with results of bird monitoring in Poland [56]; monographs and databases prepared for the needs of the reports State of Europe’s Forests by MCPFE/FOREST EUROPE [23,42,43]; relevant available monographs and articles. Due to the lack of pertinent studies/reports for some study years (especially for those at the beginning of the study period), several statistics were unavailable; nevertheless, the trend and dynamics of changes could still be determined, at least for the era of democracy. To discuss the results obtained, the results of several articles from the Scopus (Elsevier) database (keywords: “Polish forestry” and other more detailed keywords), as well as those found using a snowballing approach, were used.
3. Results and Discussion
3.1. Deadwood
Deadwood has four main ecological functions [33,34], and the importance of each depends on the biogeographical location, geomorphological and hydrological conditions, ecosystem type, and the type and amount of deadwood stock [34]. Firstly, it modifies the habitat conditions in the forest community. There is an increase in the mosaicity of these conditions, with variations in light conditions, temperature, humidity, and forest floor micro-relief [34,35,57]. Secondly, it directly impacts the species diversity and population conditions of some plant, animal, and fungal species. It provides a substrate for the establishment of certain groups of organisms while supplying them with the necessary energy and chemicals; provides moist microhabitats; enables nesting, burrowing, and shelter sites; provides migration routes and allows for survival under adverse (extreme) thermal and water conditions (e.g., [33,34,35,38]). Considering this more broadly, it is worth noting that the amount of dead coarse woody debris also strongly influences, among other things, the overall species structure of the herb layer [58], causing a reduction in the number of oligotrophic species [59]. Thirdly, deadwood in watercourses and reservoirs located in forest complexes influences habitat conditions (e.g., by slowing water flow) and biodiversity (e.g., as a source of organic matter for aquatic organisms) [34,57,60]. Fourthly, it modifies the circulation of primary nutrients in the forest ecosystem and is a source of nutrients (N, P, Ca, and Mg), contributing actively to soil development [23,61,62].
Available data on the volume of deadwood resources in the State Forests during the period under review are summarised in Table 1.

Table 1.
Deadwood resources in the State Forests *.
The dynamics of changes in the amount of deadwood. The lack of initial data (Table 1) makes it impossible to determine precise quantitative trends for the entire period under study. However, the difficult time just after the end of the Second World War was associated with considerable pressure on deadwood for fuel and, therefore, its small quantities on the ground in the forest. Based on the available data (Table 1), it can be seen that the amount of deadwood declined at the end of the era of socialism, remained at a similar level for at least the first decade of the era of democracy, and then increased relatively rapidly until the present day. This trend refers to the total volume of deadwood resources and its average amount per 1 ha of forest, bearing in mind that throughout the analysed period, the forest area has been steadily increasing [24]. Thus, considering the era of democracy (Table 1—1990–2020), on average, 1.44 million m3 of deadwood resources increased annually in the State Forests, growing the amount of deadwood per statistical hectare of forest by 0.2 m3. However, these are indicative figures due to the evolving methodology of determining deadwood resources over time [36]. If we take into account the most up-to-date data available (at the end of 2022 [49,55]), according to which, gross deadwood was estimated at 76,076 thousand m3, a rapid progression in the size of the total resources can be observed, translating at the same time into an increase in the average amount of deadwood per 1 ha of forest to 10.7 m3.
Factors influencing the total volume of deadwood. The deadwood volume is a function of the supply and decomposition rate. However, it is also influenced by the microclimate of the forest floor, geomorphic processes affecting slopes, soil characteristics, tree species composition, stand structure and development stage, the ecological health status of the forest stand (e.g., the frequency of the disturbances in a natural stand’s development), the type of felling, and the frequency of improvement cutting, as well as by the stand age [23,62]. In the case of forests managed by the State Forests in Poland, several intertwining factors appear to have been particularly important in shaping the amount of deadwood in the study period (Table 1): the forest management model (approach to the discussed issue), the sanitary condition and stability of forests, changes in the age structure of forests, the development of research on the inventory and significance of deadwood, and the execution of the forest management certification process.
In the socialist economy model, leaving deadwood in the forest was considered in terms of economic loss, danger of fires, and secondary pests and diseases [62]. At the same time, this was a period of increasing problems with the health of forest stands, mainly due to the low resilience and stability of monocultures, as well as significant environmental pollution (see Section 3.2), so substantial amounts of dead trees appeared (Table 1—data for 1980). However, they were generally removed [67], so the stock of deadwood in the era of socialism varied in size and distribution and, most often, was impermanent. At the same time, it should be emphasised that the arguments of Polish scientists in favour of leaving at least some of the dead trees in forests due to their biocenotic role were already prevalent in the 1970s, but they remained without a practical response at that time [68]. This had a chance to be implemented only after the change in the political and economic system in Poland (late 1980s and early 1990s), which was also associated with a significant acceleration of the transformation of the forest management model [69].
In 1995 (the era of democracy), guidelines for improving forest management on an ecological basis were introduced in the State Forests. They recommended leaving some old trees until their biological death and also leaving selected dead trees, especially hollow trees [70], but no specific indications were formulated. At that time, deadwood was also recognised as a helpful resource from the point of view of forest protection, an area of forest management that focuses on protecting the forest from, among others, pests and diseases [67]. However, there was a lack of accurate knowledge of its quantity and quality in Polish forests [62]. In addition, the problem of the poor health of some stands was still being faced (e.g., [71]), resulting from old management decisions taken during the Partitions of Poland or the socialist economy (see Section 3.2), which involved the need to remove dead trees that threatened the stability of forests (Table 1—progressive decrease in deadwood volume between 1990 and 2000).
The then-first principles of handling down deadwood in Polish forests were developed only in the extensive scientific research carried out in 2000–2002 [62]. Over time, they have been included in SF’s forest management documents (from 2012 [72,73,74]). At the same time, deadwood resources started to be commonly inventoried, although only for dead trees [55]; however, deadwood also includes, among others, branches lying on the forest floor and roots [62]. The determination of the deadwood volume (in a broad sense) was carried out as part of the BioSoil Forest Biodiversity module. The average value in Polish forests in 2008 was 9.6 m3/ha [75], while for dead trees alone, it was 5.2 m3/ha [55]. Currently, larger branches are also inventoried on sample plots for the purpose of preparing forest management plans [74,76].
The increase in the amount of deadwood in forests (State Forests—Table 1) during the period under study was also influenced by a gradual change in the age structure of forests—the proportion of area covered by old/mature stands (>80 years old) and stands with old trees kept increasing from 14.3% to 33.5%, with a higher rate in the era of democracy than in the era of socialism [30]. This generates the potential for a greater volume of dead trees, significantly affecting the size of local deadwood resources. The requirements of the FSC (Forest Stewardship Council) and PEFC (Programme for the Endorsement of Forest Certification) forest management certification schemes are also not insignificant for the preservation of these resources—although only in the democratic era [72].
Demand for deadwood. It is difficult to determine unequivocally how much deadwood is needed in a forest [36,62]. It should be based on scientific knowledge of the biology of the individual species that depend on these resources, the successional changes taking place in the forest ecosystem, and economic issues, and be determined individually at the site where these species occur [36]. Depending on the study, however, specific generalised values emerge, such as 5% of the volume of a mature stand in a given habitat in a commercial forest (in selected protective forests—15%–20%), no less than five thick whole lying dead logs or standing trees per 1 ha of forest [38], 10–30 m3/ha to provide suitable conditions for most organisms, and >30 m3/ha to make these conditions very good [75]. The current Forest Protection Manual recommends at least three dead trees per ha of forest [73], and the Regulation on Good Forest Management Practices recommends three to five dead trees [77]. For example, the abundance of mature single-species Scots pine Pinus sylvestris stands in the State Forests averages around 370 m3/ha [78]. If the aforementioned value of 5% is adopted, the recommended volume of deadwood in a mature commercial pine forest would be 18.5 m3/ha on average (this would be a dozen or so large trees—Based on Grzywacz [79]), and 15%–20% in the case of a protective forest—55.5–74 m3/ha. In the former case, the given value would meet the criterion of suitable conditions, and in the latter case, very good conditions for the development of organisms associated with deadwood. At the same time, these values significantly exceed those given in Table 1. However, it should be noted that the data in Table 1 refer to forests of different ages and species compositions, additionally taking into account only timber gross.
Quality and distribution of deadwood. To ensure that the above-mentioned functions of deadwood are fulfilled, especially in providing living conditions for numerous organisms, in addition to quantity, the quality of the deadwood left behind is also essential. It depends, among other things, on the size of the woody pieces (it is optimal if woody pieces of all diameter classes are present in the forest [61], although, from the point of view of the needs of many rare species, large-sized deadwood is more valuable [38,39]), as well as from their degree of decomposition [34,35]. The spatial distribution of deadwood is also important [62], especially for species with low migratory capacity [38]. However, this does not imply that it is (always) necessary to have a regular distribution of, e.g., dead trees; their resources may be more concentrated, for example, within the boundaries of protected areas [72], usually with limited/modified interference from forest management. Another parameter of relevance to the suitability of deadwood for providing a habitat for species is its degree of insolation [80]. Current guidelines for forest management in State Forests recommend leaving deadwood in various stages of decomposition, dead trees with the highest biocenotic potential, and a large number of dead trees in buffer zones around watercourses and eutrophic lakes and in swampy habitats [73,74].
The situation in Poland compared to the situation in Europe. At the European scale, where the average amount of deadwood is 11.5 m3/ha, its Polish resources per 1 ha of forest are not very impressive, although they are higher than those of Belarus (1.5 m3/ha), Portugal (2.3 m3/ha), Republic of Moldova (3.5 m3/ha), and Denmark (4.9 m3/ha). However, there is a group of countries where these stocks exceed 20 m3/ha: Austria, the Czech Republic, Germany, Latvia, Lithuania, the Russian Federation, Slovakia, Slovenia, and Switzerland [23]. This value is more favourable from the point of view of securing the needs of saproxylic organisms [75].
3.2. Species Composition of Forest Stands
The species composition of forest stands and the accompanying vegetation can be shaped in different ways due to human use of the forest (e.g., [3,81,82]). The resulting specific forest composition decisively influences the quantity and quality of forest resources [40].
Forest management has a direct impact mainly on the diversity of species of trees [83]. Their selection should consider the properties of individual species in terms of their position in succession, growth trajectory, role in maintaining biotic balance, and persistence and flexibility under given conditions [84]. The species composition of a stand may determine how many and which functions and ecosystem services (i.e., services related to biodiversity) a given forest can offer [23,85,86]. Indeed, the selection of trees in a stand is of great importance for the overall biodiversity of a forest due to the shaping of different habitat conditions (depending, e.g., on canopy density, spatial structure, and properties of the litter) and the various linkages between species [41,59,83,87,88]. The diversity of macrofungi and lichens, for example, depends on the regional diversity of tree species. In the Białowieża Forest in eastern Poland, the greatest diversity of these organisms is associated with pedunculate oak Quercus robur L. (96 and 92 species, respectively), common hornbeam Carpinus betulus L. (84 and 79) and silver/downy birch Betula pendula Roth./pubescens Ehrh. (101/60). In contrast, the relatively lowest diversity is associated with Norway maple Acer platanoides L. (38 and 39) and aspen Populus tremula L. (16 and 34) [89]. In Switzerland, for example, the highest number of fungal species is associated with spruce Picea (813) and beech Fagus (735), followed by alder Alnus (555), oak Quercus (446), silver fir Abies alba Mill. (414), and pine Pinus (392) [90]. A relationship between the forest type and bat activity is also noted. In Northern Ireland, the Karkonosze Mountains, or the Mediterranean region, higher activity is recorded in deciduous and mixed forests [91,92,93], and in Finland in coniferous forests [94]. However, it is worth noting, not only in the context of bats, that deciduous and mixed forests are usually characterised by a greater availability of roosts than in coniferous forests [95].
Forest management implemented in Poland during the period under study since the end of the Second World War has influenced changes in the proportion of dominant species (or groups of species) [49], and those that have occurred in areas managed by the State Forests are shown in Table 2.

Table 2.
Changes in the species composition of stands in SF (by area share of dominant species) *.
The dynamics of changes in the species composition of forest stands. In the studied period, a steady increase in the area share of deciduous tree species in the Polish State Forests can be observed simultaneously with a decrease in coniferous tree species (Table 2, Figure 1). The average annual change in the share of deciduous or coniferous species over the whole period for which data are presented (1948–2020) was 0.16%, with a higher change in the era of socialism (0.19%) than in the era of democracy (0.12%). The latest available data (at the end of 2022) show a further decrease in the proportion of coniferous species (75.1%) and an increase in the proportion of deciduous species (24.9%) [55].
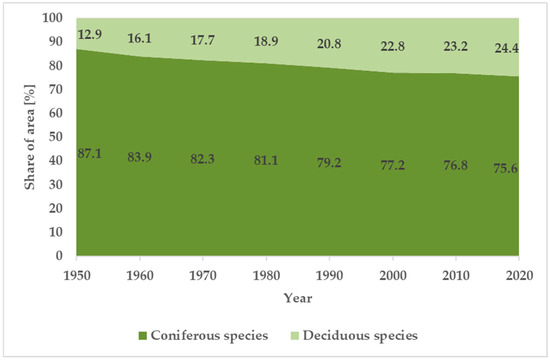
Figure 1.
Changes in the area share of coniferous and deciduous species in the State Forests (based on Table 2).
Historical context. The low species diversity of forests at the beginning of the period in question (Table 2) is due to historical conditions. Firstly, it was led by the long-term exploitation of forests during the 123 years of the entire partition of Poland, between 1795 and 1918 [12], followed by both World Wars and the reconstruction of the country following its destruction after World War II. The second factor was—as mentioned in the introduction—the long-lasting propagation of coniferous species during the partitions [84,100], and also later, in forest renewals and intensive post-war afforestation, which, by the early 1970s, covered 933,500 ha [10,101]. Mainly Scots pine Pinus sylvestris was introduced in the area-dominant lowlands, and Norway spruce Picea abies was introduced in the relatively small-area-occupying mountains, irrespective of the habitat type, as the species with the best economic return [12,14,15,102], with pine—as a species used in the afforestation of former agricultural land—dominating practically until the change of the political system from socialist to democratic [102]. In the case of Scots pine, however, it should be emphasised that in Poland, it has so far found particularly favourable habitat and climatic conditions for its development, producing many valuable ecotypes [10,101]. In very poor habitats (in lowlands), it is practically the only valuable pioneer species to be introduced by foresters [103]. However, the other habitats in Poland could potentially have a much greater diversity of native forest tree species (see Table A2 in Appendix A).
Consequences of using a limited tree species pool. The listed set of coniferous tree species (Scots pine Pinus sylvestris and Norway spruce Picea abies—Table 2), formerly introduced in the form of monocultures in Poland, including in the State Forests, combined with inappropriate habitats (often too fertile) and the geographic regions in which they were introduced, air pollution, and naturally significant variability in climatic conditions, have failed from an ecological point of view. Coniferous stands may exert a negative impact on forest sites (acidification—also through the transformation of precipitation in the canopy [104]; slow decomposition of fallen needles due to an unfavourable C/N ratio [105]), causing their degradation [14,84,106]. In addition, the simplification of tree species composition caused the lower stability of stands [14,107], causing, since the 1970s, many problems related to the health of this type of artificial ecosystem [21,84,99,108]. Pine stands on post-agricultural lands were and are commonly oppressed by disasters, among which root system diseases are of the highest importance for the survival of forest stands [109]. They are also subject to more significant pressure from the spread of alien tree species [110].
On the other hand, plantations of non-native spruce species, introduced in the Carpathians (not only in the Polish part of the Carpathians but also in the other countries of this mountain chain) in place of beech-dominated, deciduous, and mixed forests, were and are highly susceptible to storm damage [15]. It should be emphasised here that the problem of wind damage affects coniferous stands more than deciduous or mixed stands [111]. The aforementioned spruce plantations in the Carpathians [15], as well as stands with a high proportion of spruce in north-eastern Poland [112,113], are additionally highly susceptible to pest outbreaks, especially those of the eight-toothed spruce bark beetle (Ips typographus L.).
It should also be noted that Norway spruce Picea abies and Scots pine Pinus sylvestris are species sensitive to air pollution [108,114,115,116]. This pollution was particularly troublesome during the socialist economy [116,117,118]. In extreme situations, a combination of the circumstances mentioned above, with a particularly negative impact of air pollution and acid rain, followed by fungi and other pest outbreaks, led to the death of entire stands, e.g., in the Sudetes [116,118,119] and the Carpathians [40,114,116]. In addition to tree health deterioration, these pollutants (especially in the period 1970–1990) also significantly reduced tree mass growth in parts of Polish forests [108,115,117]. Since 1990—due to a change in the political and, above all, the economic system—air pollution in Poland has been lower, and therefore its impact on stands has been less pronounced [104,108,117]. However, the effects of this past pollution have led to further significant deterioration of older spruce stands in southern Poland due to the exhaustion of the self-regulation capacity of older trees and the destabilisation of the sensitive spruce ecosystem [116]. For this reason, further negative changes (stand dieback) occurred in the 21st century, to a greater extent in the Western Carpathians than in the Sudetes [118]. In the case of fir Abies (Table 2), older stands also suffered more from air pollution than younger stands (strongly reduced thickness increments and dying). Still, they were better able than spruce to regenerate when the air quality improved [117].
The large proportion of coniferous species (currently almost 76%—Table 2), as well as the pattern of climatic conditions in Poland, also have an impact on increased fire risk [17,99,120], as does the specific vegetation in southern Europe in France, Greece, Italy, Portugal, and Spain [121]. In addition, this is compounded by the increasing pressure from people, since the change of the political system, enjoying tourism and recreation in Polish forests [22]. As a result, e.g., in the first half of the 1990s, twice as many fires were reported in the State Forests as in the corresponding period of, e.g., the 1980s, and three times as many as, e.g., in the years 1951–1955 (own calculations based on [22]). The situation has improved somewhat, with fewer and fewer such incidents being reported on average during the following decades of the 21st century (based on [10]). However, the fact that most fires are caused by deliberate arson remains worrying. In 1960, for example, arson was the cause of 7.4% of fires in the State Forests [122]; in 2000, for example, arson was already the cause of 40.1% of fires [65]. This trend continues (40.9% in 2022 [10]). A similar phenomenon has been recorded in Spain and Italy [123]. Despite such pressure, fires in Polish forests (including State Forests) rarely develop over an area larger than 100 ha; the average area of one fire in individual years of the 21st century in SF did not exceed 0.5 ha [10]. In the latter case, the ever-improving fire protection system is decisive (e.g., [10,124,125,126]), but also the pattern of weather conditions and gradual changes in forest species composition (see Table 2). Still, fires are a significant threat to species diversity in forests [127,128], especially as over 80% of the forest area in Poland is threatened by fire [124].
The process of restoring proper species composition of stands. The visible transformation of the tree species composition in the State Forests over the 75 years analysed (Table 2—coniferous versus broadleaf species) is the aftermath of conscious decisions dictated by the need to restore the stability of forest ecosystems, taken both during the era of socialism and the era of democracy. Although the first work on increasing the share of deciduous tree species in Polish forests began after the Second World War [101], the initial extent of this work (during renewals) was so small that it did not significantly change the situation of forests. For this reason, in the 1970s (the era of socialism), a parallel process of reconstruction of forest stands began [16,22], i.e., in fact, the creation of new stands, with a species composition adapted to the habitat conditions, consisting of species indigenous to a given forest site [14], especially in regions affected by emissions and disasters. In the era of democracy, these activities primarily involved the Promotional Forest Complexes (PFCs), which were intended to be a place to implement a new model of pro-ecological forest management. During the reconstruction of stands, valuable broadleaves, then underrepresented in the forest, were introduced [129]. At this stage, it was essential to adopt tree site requirements at the scale of the entire State Forests as the most crucial factor in making decisions on the forest regeneration process [14,70]. Forest stand reconstruction continues to this day, inside and outside the PFCs [10,126].
The reconstruction process, often stretched over time, can be implemented at different stages of stand development. The moment of its commencement should be determined individually, considering the degree of incompatibility of its species composition with the habitat, age, mechanical stability, technical quality, productivity, threats, and the benefit–loss calculus [84]. Converting artificial stands to more natural forest types has also been (and still is) implemented outside Poland, for example, in Germany and the UK [16], as well as within the previously mentioned Carpathian ecoregion [15,16,40].
In addition to forest stand reconstruction and renewal method modification, new afforestation rules, introduced after the change of the political system from socialist to democratic, became an element positively influencing the increase in the share of deciduous tree species [70,102], approved and implemented since the second half of the 1990s. The National Programme of the Augmentation of Forest Cover [101,130] assumed, among other things, that the species composition of afforestation would be adapted to microhabitats [131], and one of its objectives was to achieve an increased share of broadleaved species and improved forest resilience, thanks to the use of a choice of species adapted to different natural site conditions. The result of these transformations is a high share of broadleaved species, as for Polish conditions, in the youngest stands on former farmland (37%). Among them, oak species (Quercus petraea (Matt.) Liebl. and Q. robur) and beech Fagus sylvatica L. dominate [102]. The same proportion of stands dominated by broadleaved trees is recorded on average across Europe [23].
Tree species diversity at stand level. The earliest available data on the number of tree species forming stands refers to the first decade of the era of democracy [42]. According to them, stands with a simplified species structure—single species—still had the largest share in all Polish forests (Table 3). Since then, the species structure of Polish forests has been gradually evolving towards a more complex one (from single-species towards two or three species), with a negative phenomenon of a decrease in the area of the most complex stands in terms of species composition between 2000 and 2010 (Table 3).

Table 3.
Tree species composition in Polish forests *.
The initial dominant share of the area of single-species stands (Table 3) resulted from the previous resource-based forest management model from the period of partitions and the era of socialism, in which admixture species were mainly ignored [84]. Meanwhile, promoting a limited number of tree species regardless of habitat conditions, combined with the dominance of a single management mode, is associated with simplifying forest communities and their biotic homogenisation [59,69]. Under disturbed, distorted conditions, stenotopic, specialist species, called ‘loser species’, give way, while more eurytopic, generalist species, called ‘winner species’, win out [59,69,82]. Such ecosystems are also subjected to the more significant negative pressure from the biotic, abiotic, and anthropogenic factors described earlier. Despite remedial work over the years, the proportion of single-species stands is still high. However, it is meeting the target set in the 1997 National Forest Policy [27] to reduce this proportion to 52% by 2050.
On the other hand, under extreme climatic and soil conditions, single-species stands may be more stable (e.g., spruce forests in the upper montane and pine forests in very dry habitats of coniferous forest [84]). There are also recorded situations where the proportion of one species increases spontaneously over time, and the proportion of other species decreases more and more, both in forests under a more sustainable (than before) management system [59,83] but also in forests excluded from forest management, strictly protected, having a limiting effect on the biodiversity of the entire biocenosis [41,59,85,132]. Another reason for a decrease in the number of species within a stand can be the process of species extinction in a region [10] or, for example, the removal of an alien/invasive species from the existing species composition, especially in an area of protected natural habitats [10,126].
Compared to other European countries with similar data, the current species diversity of Polish stands is similar to that of Austria and Norway. The highest proportion of single-species forests (monocultures)—much higher than that in Poland—is in Iceland (60.7%) and Turkey (72.7%), while the lowest is in Sweden (14.6%) and Slovenia (10.8%). In Europe, forests consisting of at least two species dominate (67% of the forest area [23]), and against this background, Polish forests appear much worse (56.5%—Table 3). In Poland, we can also observe a decrease in the area of the most complex forests (six or more species—Table 3), which aligns with the trend across Europe [23].
3.3. Introduced Tree Species
European forestry became increasingly interested in the acclimatisation of trees of foreign origin at the turn of the 19th century. A species widely introduced in forests then was Douglas fir Pseudotsuga menziesii [133], valued for its high growth rates, timber quality, and resistance to pests and diseases. Further species have been imported over time, the most important of which are Sitka spruce Picea sitchensis (Bong.) Carrière, lodgepole pine Pinus contorta Dougl. ex Loud., larch Larix spp., Populus hybrids and clones, black locust Robinia pseudoacacia L., red oak Quercus rubra L., and several Eucalyptus species [23]. In the case of Polish forests (Figure 2), the most crucial alien tree species include Douglas fir Pseudotsuga menziesii, black pine Pinus nigra Arn., Jack pine Pinus banksiana Lamb., black Locust Robinia pseudoacacia, red oak Quercus rubra, box elder Acer negundo L., and black cherry Prunus serotina Ehrh. [42]. Depending on the species, their presence was intended to improve the quantity and quality of wood, enrich poor or degraded habitats, diversify conifer monocultures, or protect the forest from insect infestations or chemical air and soil pollution [44].
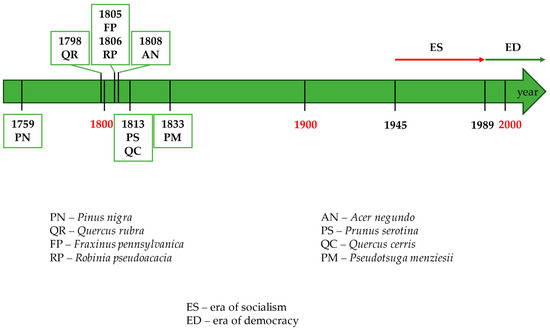
Figure 2.
Timeline of the introduction of alien tree species in Poland. The green boxes show the dates of introduction and symbols of alien tree species, which are explained below the timeline. The coloured arrows above the timeline show the duration of the two political eras analysed; the red arrow is the era of socialism, while the green arrow is the era of democracy.
However, alien species—whether plants, animals, or fungi—can, under favourable circumstances, begin to occupy new areas en masse and rapidly, bringing natural, economic, and social losses, threatening, in the latter case, human health and life [44,134]. Also, some introduced tree species have started to show a tendency to invade, exerting negative pressure on biodiversity and the function, structure, and dynamics of forest ecosystems [23].
The data on introduced tree species summarised in Table 4 refer to all Polish forests, with larger-scale introductions having been undertaken since the turn of the 19th and 20th centuries, mainly in the State Forests and outside them also in other wooded lands from which, however, alien tree species could migrate into forests [44,135]. On the other hand, the presence of introduced tree species, e.g., in national parks, which are excluded from the administration of the State Forests, is because previously, these areas were most often managed by the SF.

Table 4.
The area of stands in Poland dominated by introduced tree species *.
The dynamics of changes in the presence of alien tree species. The lack of initial data (Table 4) makes it impossible to determine precise quantitative trends for the entire period under study and individual political/economic eras. However, it can be noted without any doubt—assuming a hypothetical area of 0 ha in 1945 (although it was somewhat larger)—that between 1945 and 2000 (the era of socialism and a fragment of the era of democracy), the average annual increase in the area occupied by alien tree species was much lower (max. 364 ha/year) than in the rest of the era of democracy (2000–2020—1650 ha/year). Based on the available/confirmed data (Table 4), one can see an increase in the total area occupied by alien tree species and in its part dominated by invasive species. Of the two phenomena mentioned, the increase in the area occupied by invasive tree species, which is greater than the increase in the area occupied by all introduced tree species, may be more worrying in Poland (Table 4—2010–2020).
Neutral alien species. Of the previously mentioned most important alien tree species, Douglas fir Pseudotsuga menziesii has been introduced in Poland since 1833 on a smaller scale than in other parts of Europe and not in all regions of the country—primarily in the western part [44,133]. It has been recognised as a ‘safe’, non-invasive species [44]. Douglas fir is proposed as a component of forests (a so-called admixture species) in all regions of Poland/State Forests and for use in fast-growing tree plantations [136]. Regarding black pine Pinus nigra, planted, e.g., in areas polluted by industrial emissions and on dunes [137,138], its invasiveness has also not been demonstrated in Poland [44]. The current Silvicultural Principles [136] mention black pine only in the context of seed-sowing standards in forest nurseries. The use of only ‘safe’ non-native species in Polish forests is a requirement of both forest management certification schemes operating in the country: the FSC and PEFC [139].
Invasive alien species. Originally from North America, the black locust Robinia pseudacacia has been recorded in Poland since at least 1806 [44]. It was planted most intensively just after World War II on post-agricultural lands (the era of socialism) [102], with its impact on forests assessed negatively already in the 1970s [135]. Later, especially after the change of the political system from socialist to democratic, its use in forests was significantly reduced [102], and in the 21st century, its cultivation in forest areas was abandoned [140]. In 2015, it occupied 2000 ha in post-agricultural forests in Poland as the dominant species [102]. However, Tokarska-Guzik et al. [44] estimate this to be several thousand hectares on the scale of all forests, and according to Danielewicz et al. [140], there are 273 thousand hectares of forests where the black locust occurs, even in small numbers. It is currently classified in Poland and Europe as an invasive species [23,44], although, at the same time, it has no such status in European Union legislation [141], as well as in Polish legislation [142]. In Poland, it particularly threatens old acidophilous oak woods with Quercus robur on sandy plains. It also negatively affects Euro-Siberian steppe woods with Quercus spp. as well as oak-hornbeam forests Galio-Carpinetum and Stellario-Carpinetum [44]. However, the current Silvicultural Principles [136] mention the black locust in the context of seed-sowing standards in forest nurseries and the establishment of fast-growing tree plantations.
Red oak Quercus rubra, also native to North America, was the alien species most commonly used in forest management in many regions of Central Europe. At the same time, its capacity for spontaneous reproduction and expansion in forests [110] and for depleting the forest flora [135] was recognised quite early. It has been present in Poland since 1798 and, according to 2018 estimates, occupies over 14,000 hectares in State Forests and thousands of hectares in private forests [143]. The red oak is nowadays classified in Poland as an invasive species [44], as it is in Europe [23]. However, no such status exists in European Union legislation [141], as well as in Polish legislation [142]. In Poland, it particularly threatens old acidophilous oak woods with Quercus robur on sandy plains and also negatively affects, among others, the oak-hornbeam forests Galio-Carpinetum and Stellario-Carpinetum [44]. In the current Silvicultural Principles [136], it is not listed as a valuable species for forest management.
The box elder Acer negundo, originally from North America, has been recorded in Poland since 1808 as a species introduced mainly in wooded lands, from where it penetrated forests [44]. Today, it is classified as an invasive species in Poland and Europe [23,44]. However, it has no such status in European Union legislation [141] or Polish legislation [142]. The box elder is widespread in Poland [144]. In the case of forest ecosystems, it particularly threatens alluvial forests with common alder Alnus glutinosa (L.) Gaertn. and ash Fraxinus excelsior L. and, to a lesser extent, riparian mixed forests along the major rivers [44]. In the current Silvicultural Principles [136]—like red oak Quercus rubra—it is not listed as a valuable species for forest management.
Black cherry Prunus serotina, initially growing in North and Central America, has been recorded in Poland since 1813 [44]. Actually, it occurs throughout the country, particularly abundant in the central and south-western regions. As a dominant or only associated species, it is recorded in the State Forests on approximately 100,000 ha [145], including 42% of the fresh mixed coniferous forest habitat area and 36% of the fresh mixed broadleaved forest habitat [146]. Nowadays, it is classified in Poland and Europe as an invasive species [23,44], whereas such a status does not exist either in European Union legislation [141] or in Polish legislation [142]. The black cherry particularly threatens old acidophilous oak woods with Quercus robur on sandy plains in Poland. It also negatively affects, among others, the oak-hornbeam forests Galio-Carpinetum and Stellario-Carpinetum [44]. In the current Silvicultural Principles [136], it is also not listed as a valuable species for forest management.
In addition to the taxa described above, there are also examples of tree species that have rarely been tested in Polish forestry, which does not mean that there are no problems associated with them today. An example of such a species is the Turkey oak Quercus cerris L., from southern Europe and Asia Minor. At sites in Poland, it has sometimes been reported to be more effective (invasive) at becoming a permanent component of the forest ecosystem than the red oak Quercus rubra [110]. The green ash Fraxinus pennsylvanica Marsh., although having a lower invasiveness category than, for example, red oak and box elder Acer negundo, nevertheless also poses a threat locally, especially to riparian mixed forests along the major rivers [44]. On the other hand, Jack pine Pinus banksiana, also tested in Polish forestry, is dangerous due to interbreeding with native Scots pine Pinus sylvestris, analogous to non-native Japanese larch Larix kaempferi (Lambert) Carriere interbreeding with native European larch Larix decidua Mill. [147].
The situation in Poland compared to the situation in Europe. The not-very-intensive use of non-native tree species in the past, the recognition of the problems associated with them, and the greening of forest management have resulted in non-native tree stands accounting for only 0.6% of the area of Polish forests, with an average for European forests of 3.1% [23]. From the point of view of maintaining Poland’s native species diversity, this result is undoubtedly more favourable than, for example, in Hungary, where the area share of non-native tree species constituting the main component of plantations is 29.6%, in Denmark (44.4%) or in Ireland (63.0%) [23]. On the other hand, this result is worse than, e.g., in Belarus, Estonia, Finland, Latvia, Liechtenstein, Lithuania, and Montenegro, where the share of alien tree species is, at most, 0.1%. At the same time, it is estimated that alien invasive species dominate 0.5 per cent of the forest area on a European scale, gradually increasing this area [23]. This percentage is analogous at the scale of Polish forests—0.53% (own calculations based on Table 4 and Referowska-Chodak and Kornatowska [24]). However, a disadvantageous difference can be observed when comparing the share of alien and invasive tree species in Europe and Poland. In Poland, the vast majority (92%—based on Table 4) of the area occupied by alien species is simultaneously occupied by invasive species, while in Europe, this is only 16% on average (based on [23]). This problem needs particular attention. On the other hand, it is worth noting that the introduction of new tree species may become helpful for enhancing the resilience of forests to the impacts of climate change [23], which will, however, require considerable caution to ensure that the effects do not turn out to be worse than anticipated.
3.4. Common Forest Bird Species
Forest management is not without impact on the species of plants, animals, and fungi that occur in forest ecosystems. An assessment of this impact can be formulated based on systematic monitoring of the species status, although with the caveat that species populations are also influenced by many factors other than forest management, e.g., other land uses and practices, climate change impacts, or the conditions during migration [23]. Among the different groups of organisms, birds are, on the one hand, widespread and sensitive to changes in the environment (relevant indicators of biodiversity) and, on the other hand, widespread among the public (citizen science initiatives). The data collected allow us to conclude that on the European scale, populations of 34 common forest bird species remained relatively stable for almost 40 years, as in 2017, they were 102.8% of the populations assessed in 1980 [23]. This suggests that forest management did not negatively impact the analysed bird species populations.
Poland’s Forest Bird Index (FoBI) is not calculated separately for the State Forests. Hence, the data in Table 5 refer to all Polish forests, of which the State Forests are the dominant manager [49].

Table 5.
Forest Bird Index values in Poland *.
The dynamics of changes in the situation of common forest bird species. Due to limited data availability, resulting from the relatively late implementation of monitoring of common forest birds in Poland [45], the data in Table 5 cover only a fragment of the era of democracy. A clear upward trend is noticeable, averaging 1.32% per year for the 20 years over 2000–2020, with a slightly more intense increase in the first decade (1.36% per year on average) than in the second (1.27%). The latest available indicator value (for 2023) is 1.4029 [45], indicating a continuing upward trend (Figure 3). On the other hand, taking into account older literature data, it can be observed that in the period 1972–1990 (the era of socialism), the trend in bird abundance change was, in similar proportions, horizontal (17 species) and upward (15 species), with two species whose situation worsened (based on [46,47]). In the period 1990–2003 (the beginning of the era of democracy), the situation was more stable—a horizontal trend (stabilisation) was recorded in the case of 28 species—an upward trend was recorded in the case of 5 species, and a downward trend was recorded in the case of 1 species (based on [47,48]). Therefore, it can be concluded that the FoBI index would also have an increasing value for the period 1972–2003 and an increasing trend at least for the period 1972–2023.
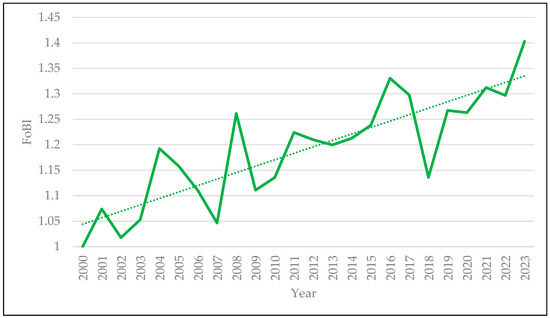
Figure 3.
Forest Bird Index values from 2000 to 2023 with a dotted trend line (based on [45]).
Factors influencing the situation of birds. The upward trend in the size of monitored populations of common forest birds shown above may be due to several reasons. The first factor may be a gradual increase in the proportion of deciduous tree species (see Section 3.2), which, as already mentioned, increases the availability of various types of shelters [95], increasing, at the same time, the diversity of food or materials necessary directly or indirectly for the proper functioning of birds (e.g., fruit, seeds, insects, and twigs). A second positive factor may be the gradual enrichment of Polish forests’ spatial structure, resulting from management system changes: reduction in the clearcutting system and promotion of more complex systems (shelter-wood cutting, selection cutting, and special cutting) [30]. This increases the number of available ecological niches. A third positive factor is the increasing proportion of older stands [30], offering specific microhabitats supporting numerous species’ existence [148]. This is partly due to a fourth positive factor, the increasing biomass of lying and standing dead trees (see Section 3.1), particularly often present in older stands and enabling, for example, nesting or food acquisition in the form of wood-dwelling insects. A fifth positive factor is reducing the use of chemicals in forest protection (mainly plant protection products) and, in particular, eliminating dangerous ones that negatively affect other organisms [67], like birds, which are often downstream consumers in trophic chains. A sixth positive factor is the increase in forest area covered by various forms of nature conservation [24]. Forest management restrictions specific to such sites reduce the direct pressure on birds (noise, human presence, and the removal of some trees). They may also imply a beneficial (for birds) modification of the spatial structure of forests. This is confirmed by the results of FoBI monitoring, divided into Natura 2000 areas (Special Protection Areas for birds) and areas outside them, where, in the former, the rate of change in abundance shows about a twice as high an increase as in the latter [45]. As a final factor, special actions can be mentioned that support the occurrence of birds identified as helpful from the point of view of forest management (insectivores, including those taken into account in the FoBI indicator), e.g., hanging nest boxes, preserving hollow trees and sites with nests, shaping special enclaves favourable for the presence of birds [67,77,149]. All the elements mentioned are specifically related to the forest management model adopted for widespread implementation in the era of democracy (sustainable management [25] and good forest management practices [70,77,149]). It does not mean, however, that during the era of socialism, which was more oriented towards the productive functions of forests, the above-mentioned measures beneficial to birds were not taken, although they were usually to a (much) lesser extent [24,30,67].
The above-mentioned positive factors do not only benefit the monitored common forest bird species (Table 5) but also many others, as well as other systematic groups of animals, plants, or fungi (e.g., [10,23,34,35,41,86,95,150]). However, the status of forest bird species in Poland varies depending on their group, e.g., a worse situation is recorded for gallinaceous birds (western capercaillie Tetrao urogallus L. and black grouse Lyrurus tetrix L.). While active conservation projects allow for maintaining the capercaillie population, they are not yet sufficiently effective for black grouse [10,151]. In the case of birds of prey, their protection in forests (especially in the era of democracy) has mostly had positive effects (e.g., [56,150,152]), but some of them are negatively affected by changes in other environments, especially in agricultural areas, where they forage [153]. The intensification of agriculture (or its abandonment) and water reclamation (drainage) limit the ability of bi-environmental birds to function correctly and, in the case of common farmland birds, cause a steady downward trend in the value of the Farmland Bird Index (FBI) [154].
The situation in Poland compared to the situation in Europe. Between 2000 and 2020, the abundance of common forest birds in Poland increased from a level of 100.0% to a level of 126.3% (Table 5). In a similar period (2000–2017), the population abundance of common forest birds in Europe increased from about 98% to 103% (based on [23]). Thus, one can observe a much higher rate of increase in the abundance of common forest birds in Poland than in Europe in general. It testifies positively to the quality of the changes in the forest management model in Poland, especially in the State Forests, although at the same time, there needs to be more analogous older data to confirm how much better it is than before.
3.5. Issues and Directions of the Protection of Forest Species in Poland
In the protection of biodiversity at the ecosystem level in Poland’s forests, problems and dilemmas remain to be solved (Figure 4).
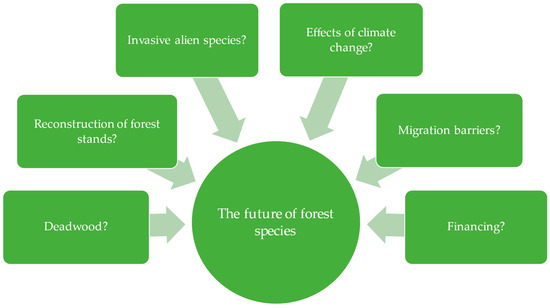
Figure 4.
Problems and dilemmas of enhancing forest biodiversity at the species level in Polish forests/forests administrated under State Forests.
Deadwood. The increase in the stock of deadwood in the State Forests is a positive trend from the point of view of maintaining/enhancing biodiversity, although it is met with different social perceptions. This is because the presence of deadwood, falling trees, or broken boughs and branches in the forest is perceived by some people as a lack of reasonable care for it—the forest is, in their view, ‘neglected’, ‘ugly’, and causes concern. Such a social assessment of a forest with deadwood does not occur only in Poland but also, for example, in the United Kingdom, Norway, Finland, Sweden, Slovenia, and other regions of Europe or the world [155]. Therefore, there is a need to educate society about the value of deadwood in the forest to increase the acceptance of foresters’ actions. On the other hand, proponents of a significant amount of deadwood are pessimistic about the fact that the final decision on this amount is left to the forest district manager [62,73], which, according to them, may have a limiting effect on the presence of this resource in the forest. Another problem that needs to be considered when increasing the deadwood stock is the simultaneous increase in the risk of forest fires and insect outbreaks and difficulties in conducting forest operations [23]. Grzywacz [79] additionally draws attention to the high financial value of timber that is not used in this way. Undoubtedly, however, the low amount and quality of deadwood may endanger forest biodiversity and harm the services provided by forest ecosystems [23].
Reconstruction of forest stands. The proportion of deciduous stands (currently 24.9%—see Section 3.2) is still below the potential resulting from the habitat structure [9,30,129]. Therefore, the continuation of stand reconstruction is still needed. This is because it conditions a return to the natural/proper species composition and thus increases the stability of forests and their resistance to negative biotic, abiotic, and anthropogenic factors [84,100]. Indeed, it should be noted that the threat to Polish forests, especially by biotic factors (pests and diseases), is among the highest in Europe [10,101,156]. It is influenced, among other things, by the large share of afforested former agricultural land (in 2015, it was 22.1% of the forest area administered by the State Forests [102]). The selection of tree species should consider the various interactions that may occur between them, such as growth inhibition and environmental changes [84]. However, it is noted that the stand reconstruction rate is insufficient in relation to needs [99]. Indeed, plans formulated in the mid-1990s assumed that an increase in the proportion of broadleaved species to 33% should be achieved, and the year 2050 was given as the cut-off date for reaching this goal [27]. However, it seems that this will be difficult to fulfil—if, in the thirty years 1990–2020, the share of deciduous species could only be increased by 3.6% (based on Table 2), it is difficult to expect a change of a further 8.6% (to 33%) in the subsequent thirty-year period 2020–2050. On the other hand, the acceleration of this process may be facilitated by the trend of coniferous species declining and deciduous species expanding under the influence of global climate change [136]. However, increasing the proportion of deciduous tree species may also have negative repercussions. The resulting increase in habitat shading may lead to the homogenisation of the undergrowth vegetation [157]. The preferences of the Polish public also indicate that mixed and coniferous stands are more attractive to them than broadleaved stands [158]. Therefore, there is a need for information/education activities provided by foresters, explaining the direction and reason for changes in the selection of tree species in Polish forests.
Invasive alien species. The significant increase in the area occupied by invasive tree species in Polish forests in recent years (see Section 3.3) should be considered a very worrying phenomenon, especially as it may accelerate due to environmental and climatic changes. In addition to trees, a similar problem of invasive occurrence concerns selected shrubs (in Poland, e.g., Amelanchier spicata (Lam.) K.Koch, Cornus sericea L., and Spiraea tomentosa L.) and annual or perennial plants (in Poland, e.g., Impatiens parviflora DC. and Echinocystis lobata (Michx.) Torr. &. Gray) [44]. Invasive animal species are also present in Polish forests, including sika deer Cervus nippon Temminck [159], common raccoon dog Nyctereutes procyonoides J. E. Gray [160], and raccoon Procyon lotor L. [161]. Invasive species in forests, considered particularly dangerous in both European Union and Polish legislation, are the common raccoon dog and the raccoon [141,142], and in Poland itself, additionally, the sika deer, which should be subject to rapid eradication [142]. It can be noted that this is a heavily narrowed list in relation to the actual state of invasion, and it does not include any plant species. Meanwhile, the problem of invasion of both plants and animals should be given special attention, as it threatens the natural diversity of species associated with Polish forests, similarly to other regions of the world (e.g., [134,162,163,164,165]).
Minimising the introduction of exotic tree species has been implemented in the era of democracy, primarily in the forest districts of Promotional Forest Complexes, since the mid-1990s [129]. Nowadays, detailed information on the occurrence of alien tree and shrub species (including invasive ones), their role in shaping the forest environment, and the possibility of their elimination should be collated in each forest district of the State Forests [74]. At the same time, there are no such direct guidelines about the other plant groups. This is still more than the official lists of species considered dangerous to Polish or EU nature and their recommended management [142]. Regarding invasive alien species (without reservation about their type), preventive measures in State Forests include monitoring their appearance [73]. In turn, mitigation of their negative impact on forest ecosystems should consider—after removing these species (especially from the aforementioned lists)—the improvement of conditions for native species and their reintroduction [142,166]. The question arises, however, of whether it is not too late to take rescue actions and whether—with mostly limited financial resources and organisational capacities—they will be effective, as local, selective measures will not solve the global and nationwide problem of rapidly spreading alien species.
Consequences of climate change. One of the most critical global problems that Polish forestry is also facing is climate change [167], which may significantly affect not only the status of species biodiversity but also the landscape, ecosystem, and genetic diversity. Temperature changes (including the presence of temperature extremes), warm, snowless winters, periods of drought interspersed with periods of torrential rain, and lowering groundwater levels are worsening the resilience of forest tree species. Combined with other factors, such as insects or fungi, they sometimes cause tree dieback [10,125,167,168]. In Poland, the dieback phenomenon has affected oak Quercus, ash Fraxinus, beech Fagus, birch Betula, and alder Alnus to varying degrees [10,101]. The persistent drought since 2015 has negatively impacted the main forest-forming species in Poland—Scots pine Pinus sylvestris [156,169], and also Norway spruce Picea abies [169]. In addition to the tree species mentioned above, many more organisms will be affected by climate change [170].
In addition to the increased fire risk (see Section 3.2), the new climatic conditions also favour the aforementioned invasions of alien species, including pathogens/pests, or the intensive growth of native species, which, until recently, had only a marginally negative impact. However, combined with the weakening of forest ecosystems due to extreme weather conditions, they are starting to affect the health of trees significantly [10,73,171]. Such species in Poland include the sharp-toothed bark beetle Ips acuminatus Gyll. and the common mistletoe Viscum album L., which are currently causing the weakening/decay of forest stands over significant areas: in the case of the sharp-toothed bark beetle, this was 26,000 ha in 2019 [169] (currently—1400 ha [10]), and in the case of the mistletoe, over 128,000 ha in 2022 [10].
It is still an open question of how to shape future forests best to be resilient, productive, and functional in the face of the climate change challenge [23]. Examples of projects implemented in the 21st century in the State Forests in Poland to adapt forests to climate change include increasing the water retention capacity (e.g., water damming steps) and counteracting water erosion, as well as developing a fire protection system [10,125,126,172]. The aforementioned measures for the restoration of small-scale retention are at the same time of great importance for the restoration and maintenance of the specific species diversity of wetlands and swamps, in both forest and non-forest areas [125,173]. The process of adapting the species composition of forest stands to the capacity of the habitat (mainly through the introduction of deciduous species in place of coniferous ones) is also being implemented, as mentioned earlier (see Section 3.2 and Section 3.5), thus increasing their resistance to harmful factors, including fire [17,100,125,126]. The continuation of these activities is essential for the maintenance of forests and the associated diversity of plant, animal, and fungal species. Concerning fires, additional efforts should be made to recognise the socio-economic aspects that influence their occurrence more fully (as has been realised, e.g., in Austria [174]) and to educate the public due to the high percentage of arson mentioned in Section 3.2 (40.9% of fires in 2022), but also fires resulting from carelessness and negligence on the part of humans (27.9%) [10].
Migration barriers. Another problem of forest biodiversity conservation at the species level is anthropogenic barriers that impede animal migration. In the case of forests fragmented by transport routes (railway lines and wide expressways), a solution to partially reduce the negative effect of such barriers is the construction of various types of animal crossings. Properly prepared wildlife overpasses and underpass crossings facilitate the migration of different groups of animals, reducing the threat of population isolation [125]. Considering the increasing numbers of ungulates and predators [49,151] and other groups of organisms, such solutions should be promoted and implemented to maintain high biodiversity in forests/state forests. It is all the more important as further expansion of Poland’s high-speed road system is planned [175] and, at the same time, since 2003, facilities for felling state-owned forests for this purpose have been present in law [29]. Unfortunately, these plans and solutions that foresters have little influence over strongly affect populations of forest species (e.g., vehicle collisions, reduced recombination, genetic isolation, limited responses to multiple stresses, and possible extinction [176,177,178,179]).
Costs and financing of species diversity conservation. A distinction can be made between direct, indirect, and alternative/opportunity costs of nature conservation in forests (State Forests) [180]. Direct costs can be associated, among other things, with the implementation of projects for the active protection of selected species—such initiatives and activities of the SF foresters have concerned, for example, European bison Bison bonasus L., amphibians, and bats in recent years. Some are financed with external funds, e.g., from the European Union [10], while the State Forests cover the rest. Undoubtedly, a significant direct cost will be associated with the eradication of invasive species. Indirect costs are mainly due to decisions that are more favourable from the point of view of forest ecology (e.g., protecting/restoring stand species diversity) than forest economics [180,181]. On the other hand, the opportunity costs mainly relate to excluding forest area/resources from production or its restriction due to species protection. It is the case, for example, in protection zones designated around sites of selected animal, plant, and fungal species, which cover 180.6 thousand hectares in the State Forests, in nature reserves (125.0 thousand hectares), which are a refugium for forest species, as well as in some Natura 2000 areas established for the protection of selected species of birds, other animals and plants, and covering a total of almost 2.9 million hectares [10]. Opportunity costs should also include the previously mentioned high financial value of unused deadwood (currently about 76 million m3—see Section 3.1). Grzywacz [181] estimated that the amount of full costs (direct costs, indirect costs, and lost benefits) incurred by the State Forests in connection with nature conservation at all levels of biodiversity could be as high as PLN 1.2 billion per year, which is a higher amount than the profit of this institution achieved, for example, in 2021, 2022, or 2023 [126,182,183]. Meanwhile, according to the law [25], the State Forests should operate on the principle of self-financing and cover the costs of its activities from its revenues. The primary source of revenue for the State Forests (in recent years, at 86%–89%) is the sale of timber [126]. Hence, the aforementioned restrictions on its harvesting could potentially significantly impact the situation and stability of this institution’s operation. This would require looking for legal and financial solutions that allow for a compromise between the needs of nature and the possibilities of forest management.
4. Conclusions
In the era of socialism (1945–1989), the area share of broadleaved tree species in stands increased intensively, with a still constant prevalence of single-species stands, ongoing removal of deadwood, and a relatively slow average increase in the area occupied by alien tree species. In the era of democracy (ongoing since 1990), there was observed a further increase in the proportion of broadleaved species (although less intensive than in the era of socialism), an intensive increase in the stock of deadwood left in the forest for natural decomposition, an increase in the area of multi-species stands up to an advantage over single-species stands, as well as an increase in the value of the Forest Bird Index (especially in the 21st century). These trends in the era of democracy, in the case of the share of deciduous tree species already initiated in the era of socialism, should be considered a positive effect of the evolution of the forest management model in Poland. Directly or indirectly, they also indicate the restoration of forest biodiversity at the species level.
In the era of democracy, however, an intensive increase in the area occupied by alien tree species, especially invasive ones, is also noticeable. Thus, not all directions of changes throughout the studied period should be assessed as positive for biodiversity conservation. Although the proportion of the area occupied by invasive tree species is not yet significant, it requires considerable vigilance on the part of forest managers, as the situation may change dynamically to a disadvantageous one.
The dilemmas, problems, and needs of current and future forest management in Poland in terms of the protection of species biodiversity are related to the amount of deadwood in forests and its natural, social, and financial determinants; the continued conversion of stands towards more species- (and structurally) complex stands; the presence of other invasive plant species, as well as animals, and the need to mitigate their negative impact on forest species and ecosystems; the consequences of climate change for forest species (tree dieback, fire risk, invasions, and water shortage) and the need to counteract and mitigate it; reducing migration barriers for forest animal species; and securing funding for biodiversity conservation activities at the species level. Maintaining the quantity and quality of forest species in Poland will require further efforts from foresters, as well as legal, financial, and educational solutions at a national level, as well as cooperation from communities worldwide to reduce the causes of global climate change.
The results and their interpretation presented in this paper, referring to the long-term perspective and large spatial scale, provide helpful information that can be used to improve forest management on a local, national, continental, and global scale with regard to its impact on biodiversity at the species level.
Funding
The APC was funded by the Institute of Forest Sciences, Warsaw University of Life Sciences (SGGW).
Data Availability Statement
Publicly available datasets were analysed in this study. These data can be found through the following links: https://stat.gov.pl/obszary-tematyczne/rolnictwo-lesnictwo/lesnictwo (accessed on 10 November 2024), https://stat.gov.pl/obszary-tematyczne/srodowisko-energia/srodowisko (accessed on 30 October 2024), https://www.bdl.lasy.gov.pl/portal/publikacje (accessed on 5 November 2024), https://www.bdl.lasy.gov.pl/portal/tworzenie-zestawienia-ru (accessed on 5 November 2024), https://monitoringptakow.gios.gov.pl (accessed on 5 December 2024), and https://fra-data.fao.org/assessments/panEuropean/2020/FE/home/overview (accessed on 5 December 2024).
Acknowledgments
The author wishes to thank the anonymous reviewers for their comments, which greatly helped to improve the paper.
Conflicts of Interest
The author declares no conflicts of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| CBD | Convention on Biological Diversity |
| C&I | Criteria and Indicators |
| EU | European Union |
| FBI | Farmland Bird Index |
| FoBI | Forest Bird Index |
| FSC | Forest Stewardship Council |
| MCPFE | Ministerial Conferences on the Protection of Forests in Europe |
| PEFC | Programme for the Endorsement of Forest Certification |
| PFC | Promotional Forest Complex |
| PLN | Polish zloty (Polish currency) |
| SF | State Forests National Forest Holding |
| SFM | Sustainable Forest Management |
Appendix A

Table A1.
List of common forest bird species included in MCPFE index and in Polish Forest Bird Index (FoBI).
Table A1.
List of common forest bird species included in MCPFE index and in Polish Forest Bird Index (FoBI).
| MCPFE Index | FoBI Index |
|---|---|
| Anthus trivialis L. | |
| Carduelis spinus L. | |
| Certhia brachydactyla Brehm | |
| Certhia familiaris L. | |
| Coccothraustes coccothraustes L. | |
| Columba oenas L. | |
| Dendrocopos medius L. | |
| Dryocopus martius L. | |
| Ficedula hypoleuca Pallas | |
| Garrulus glandarius L. | |
| Lophophanes cristatus L. = Parus cristatus L. | |
| Periparus ater L. = Parus ater L. | |
| Phoenicurus phoenicurus L. | |
| Phylloscopus collybita Vieillot | |
| Phylloscopus sibilatrix Bechstein | |
| Poecile montanus Conrad = Parus montanus Conrad | |
| Poecile palustris L. = Parus palustris L. | |
| Pyrrhula pyrrhula L. | |
| Regulus ignicapilla Temminck | |
| Regulus regulus L. | |
| Sitta europaea L. | |
| Turdus viscivorus L. | |
| Accipiter nisus L. | Aegithalos caudatus L. |
| Bombycilla garrulus L. | Erithacus rubecula L. |
| Bonasa bonasia L. | Ficedula parva Bechstein |
| Cyanopica cyanus Pallas | Fringilla coelebs L. |
| Dendrocopos minor L. | Lullula arborea L. |
| Emberiza rustica Pallas | Parus major L. |
| Ficedula albicollis Temminck | Phylloscopus trochilus L. |
| Nucifraga caryocatactes L. | Prunella modularis L. |
| Phylloscopus bonelli Vieillot | Sylvia atricapilla L. |
| Picus canus Gmelin | Troglodytes troglodytes L. |
| Serinus citrinella Pallas | Turdus merula L. |
| Tringa ochropus L. | Turdus philomelos Brehm |

Table A2.
The main native forest tree species in Poland (based on [9]).
Table A2.
The main native forest tree species in Poland (based on [9]).
| Tree Species | Tree Species | Tree Species |
|---|---|---|
| Abies alba Mill. | Fagus sylvatica L. | Quercus petraea Liebl. |
| Acer campestre L. | Fraxinus excelsior L. | Quercus robur L. |
| Acer platanoides L. | Larix decidua Mill. | Salix alba L. |
| Acer pseudoplatanus L. | Picea abies (L.) H.Karst | Sorbus aucuparia L. |
| Alnus glutinosa (L.) Gaertn. | Pinus sylvestris L. | Tilia cordata Mill. |
| Alnus incana (L.) Moench | Populus alba L. | Tilia platyphyllos Scop. |
| Betula pendula Roth. | Populus nigra L. | Ulmus glabra Huds. |
| Betula pubescens Ehrh. | Populus tremula L. | Ulmus laevis Pall. |
| Carpinus betulus L. | Prunus padus L. | Ulmus minor Mill. |
References
- Ecosystems and Human Well-Being: A Framework for Assessment; Alcamo, J., Bennett, E.M., Millennium Ecosystem Assessment (Program), Eds.; Island Press: Washington, DC, USA, 2003; ISBN 978-1-55963-402-1. [Google Scholar]
- Millenium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005; ISBN 1-59726-040-1. [Google Scholar]
- Van Calster, H.; Baeten, L.; De Schrijver, A.; De Keersmaeker, L.; Rogister, J.E.; Verheyen, K.; Hermy, M. Management Driven Changes (1967–2005) in Soil Acidity and the Understorey Plant Community Following Conversion of a Coppice-with-Standards Forest. For. Ecol. Manag. 2007, 241, 258–271. [Google Scholar] [CrossRef]
- Ludwiczak, E.; Nietupski, M.; Kosewska, A. Impact of Water Retention Practices in Forests on the Biodiversity of Ground Beetles (Coleoptera: Carabidae). Sustainability 2022, 14, 15068. [Google Scholar] [CrossRef]
- Iglesias-Carrasco, M.; Medina, I.; Ord, T.J. Global Effects of Forest Modification on Herpetofauna Communities. Conserv. Biol. 2023, 37, e13998. [Google Scholar] [CrossRef] [PubMed]
- Rudawska, M.; Leski, T.; Stasińska, M.; Karliński, L.; Wilgan, R.; Kujawska, M. The Contribution of Forest Reserves and Managed Forests to the Diversity of Macrofungi of Different Trophic Groups in European Mixed Coniferous Forest Ecosystem. For. Ecol. Manag. 2022, 518, 120274. [Google Scholar] [CrossRef]
- Hellberg, E.; Josefsson, T.; Östlund, L. The Transformation of a Norway Spruce Dominated Landscape since Pre-Industrial Times in Northern Sweden: The Influence of Modern Forest Management on Forest Structure. Silva Fenn. 2009, 43, 173. [Google Scholar] [CrossRef][Green Version]
- Angelstam, P.; Manton, M. Effects of Forestry Intensification and Conservation on Green Infrastructures: A Spatio-Temporal Evaluation in Sweden. Land 2021, 10, 531. [Google Scholar] [CrossRef]
- Tomanek, J.; Witkowska-Żuk, L. Botanika Leśna [Forest Botany]; PWRiL: Warsaw, Poland, 2008; ISBN 978-83-09-01018-0. (In Polish) [Google Scholar]
- Zajączkowski, G.; Jabłoński, M.; Jabłoński, T.; Szmidla, H.; Kowalska, A.; Małachowska, J.; Piwnicki, J.; Kaliszewski, A. Raport o Stanie Lasów w Polsce 2022 [Report on the Condition of Forests in Poland in 2022]; CILP: Warsaw, Poland, 2023; p. 108. (In Polish) [Google Scholar]
- Jażdżewski, K.; Modrzejewski, A. Polityka leśna państwa pruskiego na obecnych ziemiach polskich w latach 1772−1914 [Forest policy of Prussian state in the contemporary Polish lands in years 1772−1914]. Sylwan 2013, 157, 63–70. [Google Scholar] [CrossRef]
- Nienartowicz, A.; Lewandowska-Czarnecka, A.; Ortega, E.; Deptuła, M.; Filbrandt-Czaja, A.; Kownacka, M. Afforestation of Heathlands and Its Influence on the Land Cover, Accumulation of Plant Biomass and Energy Flow in the Landscape: An Example from Zaborski Landscape Park. EQ 2015, 21, 91–99. [Google Scholar] [CrossRef]
- Rykowski, K. Forest Policy Evolution in Poland. J. Sustain. For. 1997, 4, 119–126. [Google Scholar] [CrossRef]
- Węgiel, A.; Jaszczak, R.; Rączka, G.; Strzeliński, P.; Sugiero, D.; Wierzbicka, A. An Economic Aspect of Conversion of Scots Pine (Pinus Sylvestris L.) Stands in the Polish Lowland. J. For. Sci. 2009, 55, 293–298. [Google Scholar] [CrossRef]
- Keeton, W.S.; Crow, S.M. Sustainable Forest Management Alternatives for the Carpathian Mountain Region: Providing a Broad Array of Ecosystem Service. In Ecological Economics and Sustainable Forest Management: Developing a Trans-Disciplinary Approach for the Carpathian Mountains; Soloviy, I., Keeton, W.S., Eds.; Ukrainian National Forestry University Press: Lviv, Ukraine, 2009; pp. 109–127. ISBN 978-966-397-109-0. [Google Scholar]
- Mederski, P.; Jakubowski, M.; Karaszewski, Z. The Polish Landscape Changing Due to Forest Policy and Forest Management. iForest 2009, 2, 140–142. [Google Scholar] [CrossRef]
- Dietze, E.; Brykała, D.; Schreuder, L.T.; Jażdżewski, K.; Blarquez, O.; Brauer, A.; Dietze, M.; Obremska, M.; Ott, F.; Pieńczewska, A.; et al. Human-Induced Fire Regime Shifts during 19th Century Industrialization: A Robust Fire Regime Reconstruction Using Northern Polish Lake Sediments. PLoS ONE 2019, 14, e0222011. [Google Scholar] [CrossRef] [PubMed]
- Łuczak, M.; Paschalis-Jakubowicz, P. Uwarunkowania rynku drzewnego w Lasach Państwowych w latach 1918−2008 [Determinants of the wood market of the State Forests in the years 1918−2008]. Sylwan 2013, 157, 506–515. [Google Scholar] [CrossRef]
- Bałtowski, M. Gospodarka Socjalistyczna w Polsce: Geneza—Rozwój—Upadek [The Socialist Economy in Poland: Genesis—Development—Decline], 1st ed.; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2009; ISBN 978-83-01-16044-9. (In Polish) [Google Scholar]
- Swadźba, S. System Gospodarczy Polski w Latach 1918-2018 [Poland’s Economic System from 1918 to 2018]. Optimum 2019, 1, 19–31. [Google Scholar] [CrossRef]
- Effects of Air Pollution on Forest Health and Biodiversity in Forests of the Carpathian Mountains; Szaro, R.C., Bytnerowicz, A., Oszlányi, J., Godzik, B., Eds.; NATO Science Series; IOS and Ohmsha: Amsterdam, The Netherlands; Washington, DC, USA; Tokyo, Japan, 2002; ISBN 978-1-58603-258-6. [Google Scholar]
- Las w Liczbach [Forest in Numbers]; Rozwałka, Z., Ed.; ARW A. Grzegorczyk: Warsaw, Poland, 1997; ISBN 83-86902-11-6. (In Polish) [Google Scholar]
- FOREST EUROPE. State of Europe’s Forests 2020; Ministerial Conference on the Protection of Forests in Europe FOREST EUROPE, Liaison Unit Bratislava: Bratislava, Slovakia, 2020. [Google Scholar]
- Referowska-Chodak, E.; Kornatowska, B. Effects of Forestry Transformation on the Landscape Level of Biodiversity in Poland’s Forests. Forests 2021, 12, 1682. [Google Scholar] [CrossRef]
- The Forest Act, 1991 [Dz. U. 1991.101.444 as Amended, in Polish]. Available online: http://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU19911010444/U/D19910444Lj.pdf (accessed on 4 March 2020).
- Polska Polityka Kompleksowej Ochrony Zasobów Leśnych [Polish Policy for Comprehensive Forest Resource Protection]; Grzywacz, A., Ed.; SGGW: Warsaw, Poland, 1994. (In Polish) [Google Scholar]
- Ministerstwo Ochrony Środowiska, Zasobów Naturalnych i Leśnictwa Polityka Leśna Państwa [National Forest Policy]. Document Approved by the [Polish] Council of Ministers on 22 April 1997, 1st ed.; MOŚZNiL: Warsaw, Poland, 1997. [Google Scholar]
- Aarhus Convention: Convention on Access to Information, Public Participation in Decision-Making, and Access to Justice in Environmental Matters, Adopted on 25 June 1998 in Aarhus. Polish Version: 2003 [Dz. U. 2003.78.706]. Available online: http://isap.sejm.gov.pl/DetailsServlet?id=WDU20030780706 (accessed on 10 November 2021).
- Czerepko, J.; Geszprych, M.; Gołos, P. Basic Assumptions for Forest Management and Nature Conservation from Axiological, Legal, and Economic Perspective. Folia For. Pol. 2017, 59, 68–78. [Google Scholar] [CrossRef][Green Version]
- Referowska-Chodak, E.; Kornatowska, B. Effects of Forestry Transformation on the Ecosystem Level of Biodiversity in Poland’s Forests. Forests 2023, 14, 1739. [Google Scholar] [CrossRef]
- Grzywacz, A.; Referowska-Chodak, E. Gatunkowa Różnorodność Biologiczna Terenów Leśnych w Polsce [Species Biodiversity of Forest Areas in Poland]. In Zastosowanie Geoinformatyki w Leśnictwie [Application of Geoinformatics in Forestry]; Stereńczak, K., Ed.; IBL: Sękocin Stary, Poland, 2020; pp. 193–216. ISBN 978-83-62830-81-7. (In Polish) [Google Scholar]
- MCPFE Updated Pan-European Indicators for Sustainable Forest Management. Annex 1 to Madrid Ministerial Declaration. In Proceedings of the 7th Ministerial Conference, Madrid, Spain, 20–21 October 2015. [Google Scholar]
- Maser, C.; Anderson, R.G.; Cromack, K., Jr.; Williams, J.T.; Martin, R.E. Dead and down Woody Material. In Wildlife Habitats in Managed Forests: The Blue Mountains of Oregon and Washington; Thomas, J.W., Ed.; Agricultural Handbook; USDA Forest Service, Pacific Northwest Forest and Range Experiment Station: Portland, OR, USA, 1979; pp. 78–95. [Google Scholar]
- Stevens, V. The Ecological Role of Coarse Woody Debris: An Overview of the Ecological Importance of CWD in BC Forests; Working Paper—Ministry o Forests Research Program; Ministry of Forests, British Columbia: Victoria, BC, Canada, 1997.
- Solon, J. Ekologiczna Rola Martwego Drewna w Ekosystemach Leśnych—Dyskusja Wybranych Zagadnień w Świetle Literatury [Ecological Role of Deadwood in Forest Ecosystems—A Discussion of Selected Issues in the Light of the Literature]; Polska Akademia Nauk: Warsaw, Poland, 2002. [Google Scholar]
- Czerepko, J.; Hilszczański, J.; Jabłoński, M. Martwe drewno—żywy problem [Dead wood—a living problem]. Stud. I Mater. Cent. Edukac. Przyr.-Leśnej 2014, 41, 36–45. [Google Scholar]
- Jancewicz, E.; Kielan, E. Znaczenie martwego drewna w funkcjonowaniu populacji małych ssaków [Importance of coarse woody debris in the functioning of small mammals populations]. Sylwan 2017, 161, 519–528. [Google Scholar] [CrossRef]
- Gutowski, J.M.; Bobiec, A.; Zub, K. Drugie Życie Drzewa [The Second Life of a Tree]; WWF Poland: Warsaw-Hajnówka, Poland, 2004; ISBN 83-916021-6-8. [Google Scholar]
- Borowski, J. Pozostawianie drzew do ich naturalnego rozkładu, jako forma ochrony chrząszczy (Insecta, Coleoptera) [Leaving trees to their natural decay as a form of protection for beetles (Insecta, Coleoptera)]. Stud. I Mater. Cent. Edukac. Przyr.-Leśnej 2006, 11, 115–120. [Google Scholar]
- Griffiths, P.; Kuemmerle, T.; Baumann, M.; Radeloff, V.C.; Abrudan, I.V.; Lieskovsky, J.; Munteanu, C.; Ostapowicz, K.; Hostert, P. Forest Disturbances, Forest Recovery, and Changes in Forest Types across the Carpathian Ecoregion from 1985 to 2010 Based on Landsat Image Composites. Remote Sens. Environ. 2014, 151, 72–88. [Google Scholar] [CrossRef]
- Brzeziecki, B. Wieloletnia dynamika drzewostanów w Puszczy Białowieskiej (w warunkach ochrony ścisłej) [Multi-year stand dynamics in the Bialowieza Forest (under strict protection)]. In Stan Ekosystemów Leśnych Puszczy Białowieskiej [State of the Forest Ecosystems of the Bialowieza Forest]; Wikło, A., Ed.; CILP: Warsaw, Poland, 2016; pp. 45–58. ISBN 978-83-63895-38-9. (In Polish) [Google Scholar]
- FAO, UNECE, FOREST EUROPE. Joint Pan-European Data Collection. Available online: https://fra-data.fao.org/assessments/panEuropean/2020/FE/home/overview (accessed on 5 December 2024).
- FOREST EUROPE. State of Europe’s Forests 2015; Ministerial Conference on the Protection of Forests in Europe FOREST EUROPE, Liaison Unit Madrid: Madrid, Spain, 2015. [Google Scholar]
- Tokarska-Guzik, B.; Dajdok, Z.; Zając, M.; Zając, A.; Urbisz, A.; Danielewicz, W.; Hołdyński, C. Rośliny Obcego Pochodzenia w Polsce ze Szczególnym Uwzględnieniem Gatunków Inwazyjnych [Plants of Foreign Origin in Poland with Particular Reference to Invasive Species]; GDOŚ: Warsaw, Poland, 2012; ISBN 978-83-62940-34-9. (In Polish) [Google Scholar]
- GIOŚ. Poland Forest Bird Index in Poland. Available online: https://monitoringptakow.gios.gov.pl/lasy-zadrzewienia.html (accessed on 5 December 2024).
- Tomiałojć, L. Ptaki Polski—Wykaz Gatunków i Rozmieszczenie [Birds of Poland—List of Species and Distribution]; PWN: Warsaw, Poland, 1972. (In Polish) [Google Scholar]
- Tomiałojć, L. Ptaki Polski—Rozmieszczenie i Liczebność [Birds of Poland—Distribution and Abundance], 2nd ed.; PWN: Warsaw, Poland, 1990; ISBN 83-01-09080-4. (In Polish) [Google Scholar]
- Tomiałojć, L.; Stawarczyk, T. Awifauna Polski. Rozmieszczenie, Liczebność i Zmiany [Avifauna of Poland. Distribution, Abundance and Changes]; PTPP “Pro Natura”: Wrocław, Poland, 2003; ISBN 978-83-919626-1-9. (In Polish) [Google Scholar]
- Leśnictwo [Forestry]. Statistical Yearbook of 2023. 2024. Available online: https://stat.gov.pl/obszary-tematyczne/roczniki-statystyczne/roczniki-statystyczne/rocznik-statystyczny-lesnictwa-2024,13,7.html (accessed on 5 December 2024).
- BULiGL. Wyniki Aktualizacji Stanu Powierzchni Leśnej i Zasobów Drzewnych w Lasach Państwowych Na Dzień 1 Stycznia 1991 r. [Results of the Update of Forest Area and Timber Resources in the State Forests as of 1 January 1991]; BULiGL: Raszyn, Poland, 1991. [Google Scholar]
- BULiGL. Wyniki Aktualizacji Stanu Powierzchni Leśnej i Zasobów Drzewnych w Lasach Państwowych Na Dzień 1 Stycznia 2001 r. [Results of the Update of Forest Area and Timber Resources in the State Forests as of 1 January 2001]; BULiGL: Raszyn, Poland, 2001. [Google Scholar]
- BULiGL. Wyniki Aktualizacji Stanu Powierzchni Leśnej i Zasobów Drzewnych w Lasach Państwowych Na Dzień 1 Stycznia 2011 r. [Results of the Update of Forest Area and Timber Resources in the State Forests as of 1 January 2011]; BULiGL: Raszyn, Poland, 2011. [Google Scholar]
- BULiGL. Wyniki Aktualizacji Stanu Powierzchni Leśnej i Zasobów Drzewnych w Lasach Państwowych Na Dzień 1 Stycznia 2021 r. [Results of the Update of Forest Area and Timber Resources in the State Forests as of 1 January 2021]; BULiGL: Raszyn, Poland, 2022. [Google Scholar]
- BULiGL. Wyniki Aktualizacji Stanu Powierzchni Leśnej i Zasobów Drzewnych w Lasach Państwowych Na Dzień 1 Stycznia 2023 r. [Results of the Update of Forest Area and Timber Resources in the State Forests as of 1 January 2023]; BULiGL: Raszyn, Poland, 2024. [Google Scholar]
- BULiGL. Bank Danych o Lasach [Forest Data Bank]—A Database on Resources and Condition of Polish Forests. Available online: https://www.bdl.lasy.gov.pl (accessed on 5 December 2024).
- GIOŚ. Poland Wyniki Monitoringu Ptaków w Polsce [Results of Bird Monitoring in Poland]. Available online: https://monitoringptakow.gios.gov.pl (accessed on 5 December 2024).
- Franklin, J.F.; Cromack, K., Jr.; Denison, W.; McKee, A.; Maser, C.; Sedell, J.; Swanson, F.; Juday, G. Ecological Characteristics of Old-Growth Douglas-Fir Forests; U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station: Portland, OR, USA, 1981; p. 48.
- Scheller, R.M.; Mladenoff, D.J. Understory Species Patterns and Diversity in Old-Growth and Managed Northern Hardwood Forest. Ecol. Appl. 2002, 12, 1329–1343. [Google Scholar] [CrossRef]
- Durak, T. Long-Term Trends in Vegetation Changes of Managed versus Unmanaged Eastern Carpathian Beech Forests. For. Ecol. Manag. 2010, 260, 1333–1344. [Google Scholar] [CrossRef]
- Lofroth, E. The Dead Wood Cycle. In Conservation Biology Principles for Forested Landscapes; Voller, J., Harrison, S., Eds.; UBC Press: Vancouver, BC, Canada, 1998; pp. 185–214. [Google Scholar]
- Kruys, N.; Jonsson, B.G. Fine Woody Debris Is Important for Species Richness on Logs in Managed Boreal Spruce Forests of Northern Sweden. Can. J. For. Res. 1999, 29, 1295–1299. [Google Scholar] [CrossRef]
- Wolski, J. Down Dead Wood in a Forest—Still an Obstacle to Forest Management or Already an Ecological Issue? Geogr. Pol. 2012, 85, 97–121. [Google Scholar] [CrossRef]
- Leśnictwo [Forestry]. Statistical Yearbook of 1990; GUS: Warsaw, Poland, 1991.
- Leśnictwo [Forestry]. Statistical Yearbook of 1991; GUS: Warsaw, Poland, 1992.
- Leśnictwo [Forestry]. Statistical Yearbook of 2001; GUS: Warsaw, Poland, 2002.
- Leśnictwo [Forestry]. Statistical Yearbook of 2011; GUS: Warsaw, Poland, 2012.
- Grzywacz, A.; Referowska-Chodak, E. Ochrona lasu a ochrona przyrody [Forest protection versus nature conservation]. In Wyzwania Leśnictwa Wobec Zachodzących Zmian w Środowisku Przyrodniczym, Oczekiwań Społecznych, Uwarunkowań Ekonomicznych i Prawnych [Challenges of Forestry in the Face of Environmental Change, Social Expectations, Economic and Legal Conditions]; Gil, W., Ed.; IBL: Sękocin Stary, Poland, 2017; pp. 21–43. ISBN 978-83-62830-63-3. (In Polish) [Google Scholar]
- Piotrowski, W.; Wołk, K. O biocenotycznej roli martwych drzew w ekosystemach leśnych [On the biocoenotic role of dead trees in forest ecosystems]. Sylwan 1975, 119, 31–35. [Google Scholar]
- Durak, T.; Holeksa, J. Biotic Homogenisation and Differentiation along a Habitat Gradient Resulting from the Ageing of Managed Beech Stands. For. Ecol. Manag. 2015, 351, 47–56. [Google Scholar] [CrossRef]
- Director-General of the SF Zarządzenie Nr 11 Dyrektora Generalnego Lasów Państwowych z Dnia 14 Lutego 1995 r. w Sprawie Doskonalenia Gospodarki Leśnej Na Podstawach Ekologicznych [Order No. 11 of the Director General of the State Forests of 14 February 1995 on Improving Forest Management on an Ecological Basis]. Signature: ZZ-710-13/95 1995. Available online: https://www.wroclaw.lasy.gov.pl/documents/21578137/0/Zarzadzenie+Nr+11.pdf/3b10b05b-1803-46b9-9461-d76d2bb20c5f (accessed on 1 January 2025).
- Modrzyński, J. Dynamika Defoliacji i Zamierania Świerczyn w Sudetach i Karpatach [Dynamics of Defoliation and the Decline of Norway Spruce Stands in the Sudety and Carpathian Mountains]. Stud. Naturae 2008, 54, 175–183. [Google Scholar]
- Referowska-Chodak, E. Problematyka Martwego Drewna i Drzew Dziuplastych w Systemach Certyfikacji FSC i PEFC [Dead Wood and Cavity Trees Issues in FSC and PEFC Certification Systems]. Stud. I Mater. Cent. Edukac. Przyr.-Leśnej 2014, 41, 98–115. [Google Scholar]
- Instrukcja Ochrony Lasu [Forest Protection Instruction]; Haze, M., Ed.; CILP: Warsaw, Poland, 2024; ISBN 978-83-65659-85-9. (In Polish) [Google Scholar]
- Instrukcja Urządzania Lasu [Forest Management Planning Instruction]; Haze, M., Ed.; CILP: Warsaw, Poland, 2024; Volume 1, ISBN 978-83-946400-9-5. (In Polish) [Google Scholar]
- Stan Różnorodności Biologicznej Lasów w Polsce na Podstawie Powierzchni Obserwacyjnych Monitoringu. Synteza Wyników Uzyskanych w Ramach Realizacji Projektu BioSoil Forest Biodiversity [State of Forest Biodiversity in Poland Based on Monitoring Observation Plots. Synthesis of Results Obtained in the Framework of the BioSoil Forest Biodiversity Project]; Czerepko, J., Ed.; IBL: Sękocin Stary, Poland, 2008; ISBN 978-83-87647-75-9. (In Polish) [Google Scholar]
- Instrukcja Urządzania Lasu [Forest Management Planning Instruction]; Święcicki, Z., Ed.; CILP: Warsaw, Poland, 2012; Volume 1, ISBN 978-83-61633-69-3. (In Polish) [Google Scholar]
- Regulation on Good Forest Management Practices, 2023 [Dz.U. 2023.0.672, in Polish]. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20230000672/O/D20230672.pdf (accessed on 5 December 2024).
- Parzych, S.; Mandziuk, A.; Wysocka-Fijorek, E. Wpływ zasobności drzewostanów sosnowych na ustalanie ekonomicznego wieku dojrzałości rębnej [Impact of Scots pine stand growing stock on determining the optimal economic rotation age]. Sylwan 2018, 162, 671–678. [Google Scholar] [CrossRef]
- Grzywacz, A. Drewno Dominującym Składnikiem Biomasy Ekosystemów Leśnych [Wood Biomass Dominant Component of Forest Ecosystems]. Stud. I Mater. Cent. Edukac. Przyr.-Leśnej 2014, 41, 30–35, (In Polish with English Summary). [Google Scholar]
- Hilszczański, J.; Jaworski, T.; Plewa, R. Dlaczego Owady Saproksyliczne “Znikają” z Naszych Lasów, Czyli o Wyższości Jakości Martwego Drewna Nad Jego Ilością [Why Do Saproxylic Insects Disappear from Our Forests, or about the Superiority of the Quality of Dead Wood over Its Quantity]. Stud. I Mater. Cent. Edukac. Przyr.-Leśnej 2011, 27, 200–206, (In Polish with English Summary). [Google Scholar]
- Decocq, G.; Aubert, M.; Dupont, F.; Bardat, J.; Wattez-Franger, A.; Saguez, R.; de Foucault, B.; Alard, D.; Delelis-Dusollier, A. Silviculture-Driven Vegetation Change in a European Temperate Deciduous Forest. Ann. For. Sci. 2005, 62, 313–323. [Google Scholar] [CrossRef]
- Naaf, T.; Wulf, M. Traits of Winner and Loser Species Indicate Drivers of Herb Layer Changes over Two Decades in Forests of NW Germany: Traits Indicate Drivers of Change. J. Veg. Sci. 2011, 22, 516–527. [Google Scholar] [CrossRef]
- Durak, T. Changes in Diversity of the Mountain Beech Forest Herb Layer as a Function of the Forest Management Method. For. Ecol. Manag. 2012, 276, 154–164. [Google Scholar] [CrossRef]
- Jaworski, A. Sposoby Zagospodarowania, Odnawianie Lasu, Przebudowa i Przemiana Drzewostanów [Management Practices, Regeneration, Conversion and Transformation of Forest Stands], 2nd ed.; Hodowla lasu [Silviculture]; PWRiL: Warsaw, Poland, 2018; Volume 1, ISBN 978-83-09-01113-2. (In Polish) [Google Scholar]
- Brzeziecki, B. Puszcza Białowieska jako ostoja różnorodności biologicznej [Białowieża Forest as a biodiversity hotspot]. Sylwan 2017, 161, 971–981. [Google Scholar] [CrossRef]
- Jonsson, M.; Bengtsson, J.; Moen, J.; Gamfeldt, L.; Snäll, T. Stand Age and Climate Influence Forest Ecosystem Service Delivery and Multifunctionality. Environ. Res. Lett. 2020, 15, 0940a8. [Google Scholar] [CrossRef]
- Barbier, S.; Gosselin, F.; Balandier, P. Influence of Tree Species on Understory Vegetation Diversity and Mechanisms Involved—A Critical Review for Temperate and Boreal Forests. For. Ecol. Manag. 2008, 254, 1–15. [Google Scholar] [CrossRef]
- Jacob, M.; Weland, N.; Platner, C.; Schaefer, M.; Leuschner, C.; Thomas, F.M. Nutrient Release from Decomposing Leaf Litter of Temperate Deciduous Forest Trees along a Gradient of Increasing Tree Species Diversity. Soil Biol. Biochem. 2009, 41, 2122–2130. [Google Scholar] [CrossRef]
- Bujakiewicz, A.; Chlebicki, A.; Chmiel, M.; Lisiewska, M.; Majewski, T.; Mułenko, W.; Skirgiełło, A. Fungi: Summary. In Cryptogamous Plants in the Forest Communities of Białowieża National Park. General Problems and Taxonomic Group Analysis (Project CRYPTO [2]); Faliński, J.B., Mułenko, W., Eds.; Archivum Geobotanicum; Białowieska Stacja Geobotaniczna UW: Warsaw, Poland, 1995; pp. 159–164. [Google Scholar]
- Senn-Irlet, B. Welches Sind Pilzreiche Holzarten? Wald Und Holz 2008, 10, 57–59. [Google Scholar]
- Russ, J.M.; Montgomery, W.I. Habitat Associations of Bats in Northern Ireland: Implications for Conservation. Biol. Conserv. 2002, 108, 49–58. [Google Scholar] [CrossRef]
- Russo, D.; Jones, G. Use of Foraging Habitats by Bats in a Mediterranean Area Determined by Acoustic Surveys: Conservation Implications. Ecography 2003, 26, 197–209. [Google Scholar] [CrossRef]
- Bartonička, T.; Řehák, Z.; Flousek, J.; Furmankiewicz, J. Netopýři Českých a Polských Krkonoš/Nietoperze Czeskich i Polskich Karkonoszy; Správa KRNAP Vrchlabí, Dyrekcja KPN: Vrchlabí, Czech Republic; Jelenia Góra, Poland, 2015; ISBN 978-80-87706-90-9. [Google Scholar]
- Wermundsen, T.; Siivonen, Y. Foraging Habitats of Bats in Southern Finland. Acta Theriol. 2008, 53, 229–240. [Google Scholar] [CrossRef]
- Perry, R.W.; Thill, R.E.; Leslie, D.M. Scale-Dependent Effects of Landscape Structure and Composition on Diurnal Roost Selection by Forest Bats. J. Wildl. Manag. 2008, 72, 913–925. [Google Scholar] [CrossRef]
- Rocznik Statystyczny Leśnictwa i Przemysłu Drzewnego 1964 [Statistical Yearbook of Forestry and Wood Industry 1964]; PWRiL: Warsaw, Poland, 1966. (In Polish)
- Rocznik Statystyczny Leśnictwa i Gospodarki Drewnem 1981 [Statistical Yearbook of Forestry and Wood Economy]; GUS: Warsaw, Poland, 1981. (In Polish)
- Lasy Państwowe w Polsce w Latach 1944–1990 [State Forests in Poland 1944–1990], 1st ed.; Broda, J., Ed.; PWN: Warsaw-Poznań, Poland, 1997; ISBN 83-01-12359-1. [Google Scholar]
- Rozwałka, Z. Leśna Statystyka 1997–2007 [Forestry Statistics 1997–2007]; CILP: Warsaw, Poland, 2010; ISBN 978-83-61633-24-2. (In Polish) [Google Scholar]
- Bernadzki, E. Cele hodowli wczoraj i dziś [Silvicultural objectives yesterday and today]. Sylwan 1997, 141, 23–31. [Google Scholar]
- Forest Research Institute (Poland) Raport o Stanie Lasów w Polsce 2010 [Report on the Condition of Forests in Poland in 2010]; CILP: Warsaw, Poland, 2011.
- Sewerniak, P. Survey of Some Attributes of Post-Agricultural Lands in Polish State Forests. EQ 2015, 22, 9–16. [Google Scholar] [CrossRef]
- Barzdajn, W. Znaczenie hodowli lasu dla ochrony przyrody [Importance of silviculture for nature conservation]. In Gospodarka Leśna a Ochrona Przyrody [Forest Management and Nature Conservation]; Gwiazdowicz, D.J., Ed.; Ornatus, Wydawnictwo PTL: Poznań, Poland, 2006; pp. 31–50. ISBN 978-83-921460-7-0. [Google Scholar]
- Kowalska, A.; Astel, A.; Boczoń, A.; Polkowska, Ż. Atmospheric Deposition in Coniferous and Deciduous Tree Stands in Poland. Atmos. Environ. 2016, 133, 145–155. [Google Scholar] [CrossRef]
- Wittich, W. Der Einfluss Der Baumart Auf Den Bodenzustand. Allg. Forstz. 1961, 16, 41–45. [Google Scholar]
- Szymański, S. Siedlisko jako podstawa planowania hodowlanego [Site as base of silvicultural planning]. Sylwan 1985, 129, 13–21. [Google Scholar]
- Mazurski, K. Industrial Pollution: The Threat to Polish Forests. Ambio 1990, 19, 70–74. [Google Scholar]
- Cedro, A.; Cedro, B. Wpływ Warunków Klimatycznych i Zanieczyszczenia Powietrza Na Reakcję Przyrostową Sosny Zwyczajnej (Pinus sylvestris L.) Rosnącej w Lasach Miejskich Szczecina [Influence of Climatic Conditions and Air Pollution on Radial Growth of Scots Pine (Pinus sylvestris L.) in Szczecin’s City Forests]. For. Res. Pap. 2018, 79, 105–112. [Google Scholar] [CrossRef]
- Rykowski, K. Problemy ochrony lasu na gruntach porolnych [Problems of forest protection on afforested agricultural grounds]. Sylwan 1990, 134, 75–88. [Google Scholar]
- Danielewicz, W.; Kiciński, P.; Wiatrowska, B. Symptoms of the Naturalisation of the Turkey Oak (Quercus cerris L.) in Polish Forests. Folia For. Pol. 2016, 58, 147–162. [Google Scholar] [CrossRef][Green Version]
- Gardiner, B.; Byrne, K.; Hale, S.; Kamimura, K.; Mitchell, S.J.; Peltola, H.; Ruel, J.-C. A Review of Mechanistic Modelling of Wind Damage Risk to Forests. Forestry 2008, 81, 447–463. [Google Scholar] [CrossRef]
- Hilszczański, J.; Negron, J.; Janiszewski, W.; Munson, A.S. Stand Characteristics and Ips typographus (L.) (Col., Curculionidae, Scolytinae) Infestation during Outbreak in Northeastern Poland. Folia For. Pol.-Ser. A-For. 2006, 48, 53–64. [Google Scholar]
- Brzeziecki, B.; Hilszczański, J.; Kowalski, T.; Łakomy, P.; Małek, S.; Miścicki, S.; Modrzyński, J.; Sowa, J.; Starzyk, J.R. Problem masowego zamierania drzewostanów świerkowych w Leśnym Kompleksie Promocyjnym “Puszcza Białowieska” [Problem of a massive dying−off of Norway spruce stands in the ‘Białowieża Forest’ Forest Promotional Complex]. Sylwan 2018, 162, 373–386. [Google Scholar] [CrossRef]
- Oszlányi, J. Forest Health and Environmental Pollution in Slovakia. Environ. Pollut. 1997, 98, 389–392. [Google Scholar] [CrossRef]
- Gawrońska, G. Wpływ Zanieczyszczenia Atmosfery Na Lasy Krainy Karpackiej [Atmospheric Pollution and Its Impact on the Carpathian Country]. Rocz. Ochr. Sr. 2000, 2, 195–204. [Google Scholar]
- Modrzyński, J. Defoliation of Older Norway Spruce (Picea abies/L./Karst.) Stands in the Polish Sudety and Carpathian Mountains. For. Ecol. Manag. 2003, 181, 289–299. [Google Scholar] [CrossRef]
- Podlaski, R. Can Forest Structural Diversity Be a Response to Anthropogenic Stress? A Case Study in Old-Growth Fir Abies Alba Mill. Stands. Ann. For. Sci. 2018, 75, 99. [Google Scholar] [CrossRef]
- Bałazy, R.; Zasada, M.; Ciesielski, M.; Waraksa, P.; Zawiła-Niedźwiecki, T. Forest Dieback Processes in the Central European Mountains in the Context of Terrain Topography and Selected Stand Attributes. For. Ecol. Manag. 2019, 435, 106–119. [Google Scholar] [CrossRef]
- Sobik, M.; Błaś, M. Natural and Human Impact on Pollutant Deposition in Mountain Ecosystems with the Sudetes as an Example; University of Cambridge: Cambridge, UK, 2008; pp. 355–359. [Google Scholar]
- Zając, S.; Kaliszewski, A.; Młynarski, W. Forests and Forestry in Poland and Other EU Countries. Folia For. Pol. 2014, 56, 185–193. [Google Scholar] [CrossRef]
- Sieghardt, M.; Mursch-Radlgruber, E.; Paoletti, E.; Couenberg, E.; Dimitrakopoulus, A.; Rego, F.; Hatzistathis, A.; Randrup, T.B. The Abiotic Urban Environment: Impact of Urban Growing Conditions on Urban Vegetation. In Urban Forests and Trees; Konijnendijk, C., Nilsson, K., Randrup, T., Schipperijn, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 281–323. ISBN 978-3-540-25126-2. [Google Scholar]
- Rocznik Statystyczny Leśnictwa i Przemysłu Drzewnego 1961 [Statistical Yearbook of Forestry and Wood Industry 1961]; PWRiL: Warsaw, Poland, 1963. (In Polish)
- Tedim, F.; Xanthopoulos, G.; Leone, V. Forest Fires in Europe: Facts and Challenges. In Wildfire Hazards, Risks and Disasters; Shroder, J.F., Paton, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 77–99. ISBN 978-0-12-410434-1. [Google Scholar]
- Grajewski, S. Effectiveness of Forest Fire Security Systems in Poland; Infrastruktura i Ekologia Terenów Wiejskich/Infrastructure and Ecology of Rural Areas: Krakow, Poland, 2017; pp. 1563–1576. [Google Scholar] [CrossRef]
- Czerniak, A.; Grajewski, S.; Krysztofiak-Kaniewska, A.; Kurowska, E.E.; Okoński, B.; Górna, M.; Borkowski, R. Engineering Methods of Forest Environment Protection against Meteorological Drought in Poland. Forests 2020, 11, 614. [Google Scholar] [CrossRef]
- Directorate-General of the State Forests Sprawozdanie Finansowo-Gospodarcze za 2023 Rok [Report on Financial and Economic State of State Forests in 2023]; DGLP: Warsaw, Poland, 2024; p. 58. (In Polish)
- Brown, J.K.; Smith, J.K. Wildland Fire in Ecosystems: Effects of Fire on Flora; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Ft. Collins, CO, USA, 2000; p. RMRS-GTR-42-V2.
- Smith, J.K. Wildland Fire in Ecosystems: Effects of Fire on Fauna; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Ft. Collins, CO, USA, 2000; p. RMRS-GTR-42-V1.
- Blicharska, M.; Angelstam, P.; Elbakidze, M.; Axelsson, R.; Skorupski, M.; Węgiel, A. The Polish Promotional Forest Complexes: Objectives, Implementation and Outcomes towards Sustainable Forest Management? For. Policy Econ. 2012, 23, 28–39. [Google Scholar] [CrossRef]
- Forest Research Institute (Poland). Krajowy Program Zwiększania Lesistości [The National Programme of the Augmentation of Forest Cover]; Forest Research Institute: Sękocin Stary, Poland, 1995. [Google Scholar]
- Skolud, P. Zalesianie Gruntów Rolnych i Opuszczonych Terenów Rolniczych—Poradnik Właściciela [Afforestation of Agricultural Land and Abandoned Agricultural Sites—An Owner’s Guide], 2nd ed.; CILP: Warsaw, Poland, 2008; ISBN 978-83-89744-82-1. (In Polish) [Google Scholar]
- Schmidt, W. Herb Layer Species as Indicators of Biodiversity of Managed and Unmanaged Beech Forests. For. Snow Landsc. Res. 2005, 79, 111–125. [Google Scholar]
- Feliksik, E.; Wilczyński, S. Diversification of Increment Reactions of Douglas Fir (Pseudotsuga menziesii Franco) from Mountainous Regions of Southern Poland. J. For. Sci. 2003, 49, 552–558. [Google Scholar] [CrossRef]
- Solarz, W. Zagrożenie lasów ze strony inwazyjnych obcych gatunków grzybów, roślin i zwierząt [Threat to forests from invasive alien species of fungi, plants and animals]. In Zagrożenia Lasu Oraz Jego Funkcji—Przyczyny, Konsekwencje i Szanse dla Gospodarki Leśnej [Threats to the Forest and Its Functions—Causes, Consequences and Opportunities for Forest Management]; Gil, W., Ed.; IBL: Sękocin Stary, Poland, 2016; pp. 177–188. ISBN 978-83-62830-54-1. (In Polish) [Google Scholar]
- Bellon, S.; Tumiłowicz, J.; Król, S. Obce Gatunki Drzew w Gospodarstwie Leśnym [Alien Tree Species in Forest Management]; PWRiL: Warsaw, Poland, 1977. (In Polish) [Google Scholar]
- Kacprzak, P.; Drozdowski, S.; Grodziński, D.; Kowalkowski, W.; Łopiński, Ł.; Łukaszewicz, J.; Magnuszewski, M.; Małek, S.; Musiał, P.; Pabian, R.; et al. Zasady Hodowli Lasu [Silvicultural Principles]; PGL Lasy Państwowe, Poland: Warsaw, Poland, 2023. (In Polish) [Google Scholar]
- Król, S.; Ostrowicz, J. Sosna czarna (Pinus nigra Arn.) w lasach ochronnych Wybrzeża Koszalińskiego nad Bałtykiem [Black pine (Pinus nigra Arn.) in protection forests of the Koszalin sea-coast on Baltic]. Sylwan 1976, 120, 87–90. [Google Scholar]
- Kowalski, T. Porażenie Sosny Czarnej Przez Gremmeniella Abietina (=Scleroderris Lagerbergii) w Lasach Górnośląskiego Okręgu Przemysłowego [Infection of Pinus Nigra with Gremmeniella Abietina (=Scleroderris Lagerbergii) in Forest of the Upper-Silesian Industrial Region]. Sylwan 1987, 131, 31–39. [Google Scholar]
- Referowska-Chodak, E. Problematyka gatunków obcych w systemach certyfikacji FSC i PEFC [Alien species issues in FSC and PEFC certification systems]. Stud. I Mater. Cent. Edukac. Przyr.-Leśnej 2012, 33, 15–25. [Google Scholar]
- Danielewicz, W.; Mirski, P.; Gazda, A. Karta Informacyjna Gatunku—Robinia Akacjowa Robinia Pseudoacacia [Species Information Sheet—Black locust Robinia Pseudoacacia]. Available online: www.gov.pl/web/gdos/robinia-pseudoacacia---robinia-akacjowa (accessed on 7 November 2024).
- European Commission, Directorate-General for Environment. Commission Implementing Regulation (EU) 2016/1141 of 13 July 2016 Adopting a List of Invasive Alien Species of Union Concern Pursuant to Regulation (EU) No 1143/2014 of the European Parliament and of the Council (OJ L 189, 14.7.2016)—A Consolidated Version of the Union List 2016. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02016R1141-20220802&from=EN (accessed on 2 November 2024).
- Regulation of the Council of Ministers of 9 December 2022 on the List of Invasive Alien Species Posing a Threat to the Union and the List of Invasive Alien Species Posing a Threat to Poland, Remedial Action and Measures to Restore the Natural State of Ecosystems, 2022 [Dz.U. 2022.0.2649, in Polish]. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20220002649 (accessed on 5 December 2024).
- Woziwoda, B.; Chmura, D.; Danielewicz, W. Karta Informacyjna Gatunku—Dąb Czerwony Quercus Rubra [Species Information sheet—Red Oak Quercus Rubra]. Available online: www.gov.pl/web/gdos/quercus-rubra---dab-czerwony (accessed on 7 November 2024).
- Chmura, D.; Chmiel, J.; Danielewicz, W. Karta Informacyjna Gatunku—Klon Jesionolistny Acer Negundo [Species Information Sheet—Box Elder Acer negundo]. Available online: www.gov.pl/web/gdos/acer-negundo---klon-jesionolisty-2 (accessed on 7 November 2024).
- Bijak, S.; Czajkowski, M.; Ludwisiak, Ł. Occurrence of Black Cherry (Prunus serotina Ehrh.) in the State Forests in Poland. For. Res. Pap. 2015, 75, 359–365. [Google Scholar] [CrossRef]
- Halarewicz, A.; Otręba, A.; Danielewicz, W. Karta Informacyjna Gatunku—Czeremcha Amerykańska Padus serotina [Species Information Sheet—Black Cherry Padus serotina]. Available online: www.gov.pl/web/gdos/padus-serotina---czeremcha-amerykanska (accessed on 7 November 2024).
- Przybylski, T. Bioróżnorodność—szansa czy przeszkoda dla gospodarki leśnej? [Biodiversity—a chance or an impediment for forest management?]. Sylwan 1997, 141, 51–57. [Google Scholar]
- Humphrey, J.W. Benefits to Biodiversity from Developing Old-Growth Conditions in British Upland Spruce Plantations: A Review and Recommendations. Forestry 2005, 78, 33–53. [Google Scholar] [CrossRef]
- Regulation on Good Forest Management Practices. 2017. [Dz.U. 2017.0.2408, in Polish]. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20170002408 (accessed on 5 December 2024).
- Cenian, Z.; Lontkowski, J.; Mizera, T. Wzrost Liczebności i Ekspansja Terytorialna Bielika Haliaeetus Albicilla Jako Przykład Skutecznej Ochrony Gatunku [Increase in Numbers and Territorial Expansion of the White-Tailed Eagle Haliaeetus Albicilla as an Example of Successful Conservation of the Species]. Stud. I Mater. Cent. Edukac. Przyr.-Leśnej 2006, 12, 55–63. [Google Scholar]
- Ochrona Środowiska [Environment]. Statistical Yearbook of 2023. Available online: https://stat.gov.pl/obszary-tematyczne/srodowisko-energia/srodowisko/ochrona-srodowiska-2024,1,25.html (accessed on 1 December 2024).
- Mizera, T. 20 Lat Funkcjonowania Ochrony Strefowej w Polsce [20 Years of Zonal Protection in Poland]. Stud. I Mater. Cent. Edukac. Przyr.-Leśnej 2006, 12, 29–53. [Google Scholar]
- Rogoziński, R.; Referowska-Chodak, E. Strefowa Ochrona Ptaków w Nadleśnictwie Augustów—Historia, Stan Aktualny, Problemy, Perspektywy [Zonal Protection of Birds in Augustów Forest District—the Histo-Ry, Actual State, Problems, Prospects]. Stud. I Mater. Cent. Edukac. Przyr.-Leśnej 2011, 27, 22–33. [Google Scholar]
- GIOŚ. Poland Farmland Bird Index in Poland. Available online: https://monitoringptakow.gios.gov.pl/ptaki-krajobrazu-rolniczego.html (accessed on 5 December 2024).
- Referowska-Chodak Management and Social Problems Linked to the Human Use of European Urban and Suburban Forests. Forests 2019, 10, 964. [CrossRef]
- Matyjaszczyk, E.; Karmilowicz, E.; Skrzecz, I. How European Union Accession and Implementation of Obligatory Integrated Pest Management Influenced Forest Protection against Harmful Insects: A Case Study from Poland. For. Ecol. Manag. 2019, 433, 146–152. [Google Scholar] [CrossRef]
- Keith, S.A.; Newton, A.C.; Morecroft, M.D.; Bealey, C.E.; Bullock, J.M. Taxonomic Homogenization of Woodland Plant Communities over 70 Years. Proc. R. Soc. B 2009, 276, 3539–3544. [Google Scholar] [CrossRef] [PubMed]
- Gołos, P. Społeczne Znaczenie Publicznych Funkcji Lasu—Pożądany Dla Rekreacji i Wypoczynku Model Drzewostanu i Lasu [Social Importance of Public Forest Functions—Desirable for Recreation Model of Tree Stand and Forest]. For. Res. Pap. 2010, 71, 149–164, (In Polish with English Summary). [Google Scholar] [CrossRef][Green Version]
- Solarz, W.; Okarma, H.; Mazurska, K. Karta Informacyjna Gatunku—Jeleń Sika Cervus nippon [Species Information Sheet—Sika Deer Cervus nippon]. Available online: www.gov.pl/web/gdos/cervus-nippon---jelen-sika (accessed on 7 November 2024).
- Kowalczyk, R.; Zalewski, A.; Okarma, H. Karta Informacyjna Gatunku—Jenot Nyctereutes procyonoides [Species Information Sheet—Common Raccoon Dog Nyctereutes procyonoides]. Available online: www.gov.pl/web/gdos/nyctereutes-procyonoides---jenot (accessed on 7 November 2024).
- Bartoszewicz, M.; Zalewski, A.; Okarma, H. Karta Informacyjna Gatunku—Szop Pracz Procyon lotor [Species Information Sheet—Racoon Procyon lotor]. Available online: www.gov.pl/web/gdos/procyon-lotor---szop-pracz (accessed on 7 November 2024).
- Jaafar, S.M.d.; Metali, F.; Nafiah, S.N.S.; Supri, N.E.; Ahmad, N.; Burslem, D.F.R.P.; Sukri, R.S. Differential Impacts of Acacia Invasion on Nutrient Fluxes in Two Distinct Bornean Lowland Tropical Rain Forests. Forests 2022, 13, 2101. [Google Scholar] [CrossRef]
- Reczyńska, K.; Orczewska, A.; Yurchenko, V.; Wójcicka-Rosińska, A.; Świerkosz, K. Changes in Species and Functional Diversity of the Herb Layer of Riparian Forest despite Six Decades of Strict Protection. Forests 2022, 13, 747. [Google Scholar] [CrossRef]
- Guo, Q.; Potter, K.M.; Ren, H.; Zhang, P. Impacts of Exotic Pests on Forest Ecosystems: An Update. Forests 2023, 14, 605. [Google Scholar] [CrossRef]
- Gryz, J.; Krauze-Gryz, D.; Jasińska, K.D. Alien vs. Native—Influence of Fallow Deer (Dama Dama) Introduction on the Native Roe Deer (Capreolus Capreolus) Population. Forests 2024, 15, 1014. [Google Scholar] [CrossRef]
- Alien Species Act, 2021 [Dz. U. 2021.0.1718, in Polish]. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20210001718 (accessed on 6 December 2024).
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate Change Impacts, Adaptive Capacity, and Vulnerability of European Forest Ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Keča, N.; Koufakis, I.; Dietershagen, J.; Nowakowska, J.A.; Oszako, T. European Oak Decline Phenomenon in Relation to Climatic Changes. Folia For. Pol. 2016, 58, 170–177. [Google Scholar] [CrossRef]
- Zajączkowski, G.; Jabłoński, M.; Jabłoński, T.; Szmidla, H.; Kowalska, A.; Małachowska, J.; Piwnicki, J. Raport o Stanie Lasów w Polsce 2019 [Report on the Condition of Forests in Poland in 2019]; CILP: Warsaw, Poland, 2020; p. 105. (In Polish) [Google Scholar]
- Bartosz, R.; Bukowska, M.; Chylarecki, P.; Ignatowicz, A.; Puzio, A.; Wilińska, A. Ocena Wpływu Zmian Klimatu Na Różnorodność Biologiczną Oraz Wynikające z Niej Wytyczne Dla Działań Administracji Ochrony Przyrody Do Roku 2030 [Assessment of the Impact of Climate Change on Biodiversity and the Resulting Guidelines for Conservation Administration Action up to 2030]; GDOŚ: Warsaw, Poland, 2012. (In Polish) [Google Scholar]
- Santini, A.; Ghelardini, L.; De Pace, C.; Desprez-Loustau, M.L.; Capretti, P.; Chandelier, A.; Cech, T.; Chira, D.; Diamandis, S.; Gaitniekis, T.; et al. Biogeographical Patterns and Determinants of Invasion by Forest Pathogens in Europe. New Phytol. 2013, 197, 238–250. [Google Scholar] [CrossRef]
- Centrum Koordynacji Projektów Środowiskowych [Environmental Projects Coordination Centre]. Available online: http://ckps.lasy.gov.pl (accessed on 6 December 2024).
- Miler, A.T. Mała Retencja Wodna w Polskich Lasach Nizinnych [Small Water Retention in Polish Lowland Forest]; Infrastruktura i Ekologia Terenów Wiejskich/Infrastructure and Ecology of Rural Areas: Krakow, Poland, 2015; pp. 979–992. [Google Scholar] [CrossRef]
- Arndt, N.; Vacik, H.; Koch, V.; Arpaci, A.; Gossow, H. Modeling Human-Caused Forest Fire Ignition for Assessing Forest Fire Danger in Austria. iForest 2013, 6, 315–325. [Google Scholar] [CrossRef]
- Resolution No. 253/2022 of the Council of Ministers of 13 December 2022 on the Establishment of a Multiannual Programme Entitled ‘Government Programme for the Construction of National Roads Until 2030 (with an Outlook Until 2033)’, 2022 [RM-06111-259-22, In Polish]. Available online: www.gov.pl/web/infrastruktura/rzadowy-program-budowy-drog-krajowych-do-2030-r-z-perspektywa-do-2033-r (accessed on 13 November 2024).
- Ledig, F.T. Human Impacts on Genetic Diversity in Forest Ecosystems. Oikos 1992, 63, 87–108. [Google Scholar] [CrossRef]
- Kapos, V.; Lysenko, I.; Lesslie, R. Assessing Forest Integrity and Naturalness in Relation to Biodiversity. Forest Resources Assessment Programme, Working Paper 54; Forestry Department FAO: Rome, Itay, 2002. [Google Scholar]
- Langbein, J.; Putman, R.; Pokorny, B. Traffic Collisions Involving Deer and Other Ungulates in Europe and Available Measures for Mitigation. In Ungulate Management in Europe; Putman, R., Apollonio, M., Andersen, R., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 215–259. ISBN 978-0-511-97413-7. [Google Scholar]
- Thompson, I.D.; Guariguata, M.R.; Okabe, K.; Bahamondez, C.; Nasi, R.; Heymell, V.; Sabogal, C. An Operational Framework for Defining and Monitoring Forest Degradation. Ecol. Soc. 2013, 18, 20. [Google Scholar] [CrossRef]
- Referowska-Chodak, E. Ochrona Przyrody w Lasach Państwowych—Potrzeby i Oczekiwania Różnych Grup Społecznych Oraz ich Konsekwencje [Nature Protection in the State Forests—Needs and Expectations of Various Social Groups and Their Consequences], 1st ed.; SGGW: Warsaw, Poland, 2020; ISBN 978-83-7583-976-0. [Google Scholar]
- Grzywacz, A. Szacunek kosztów ochrony przyrody w lasach oraz w stosunku do przyszłych propozycji i oczekiwań [Estimate of conservation costs in forests and in relation to future proposals and expectations]. In Wielofunkcyjna Gospodarka Leśna Wobec Oczekiwań Przemysłu Drzewnego i Ochrony Przyrody [Multifunctional Forest Management in the Face of the Expectations of the Timber Industry and Nature Conservation]; Szabla, K., Ed.; PTL: Darłówko, Poland, 2019; pp. 69–88. ISBN 978-83-954196-0-7. [Google Scholar]
- Directorate-General of the State Forests Sprawozdanie Finansowo-Gospodarcze za 2022 Rok [Report on Financial and Economic State of State Forests in 2022]; DGLP: Warsaw, Poland, 2023; p. 57. (In Polish)
- Directorate-General of the State Forests Sprawozdanie Finansowo-Gospodarcze za 2021 Rok [Report on Financial and Economic State of State Forests in 2021]; DGLP: Warsaw, Poland, 2022; p. 51. (In Polish)
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).