Climate-Change Impacts on Distribution of Amazonian Woody Plant Species Key to Conservation, Restoration and Sustainable Use in the Colombian Amazon
Abstract
1. Introduction
2. Materials and Methods
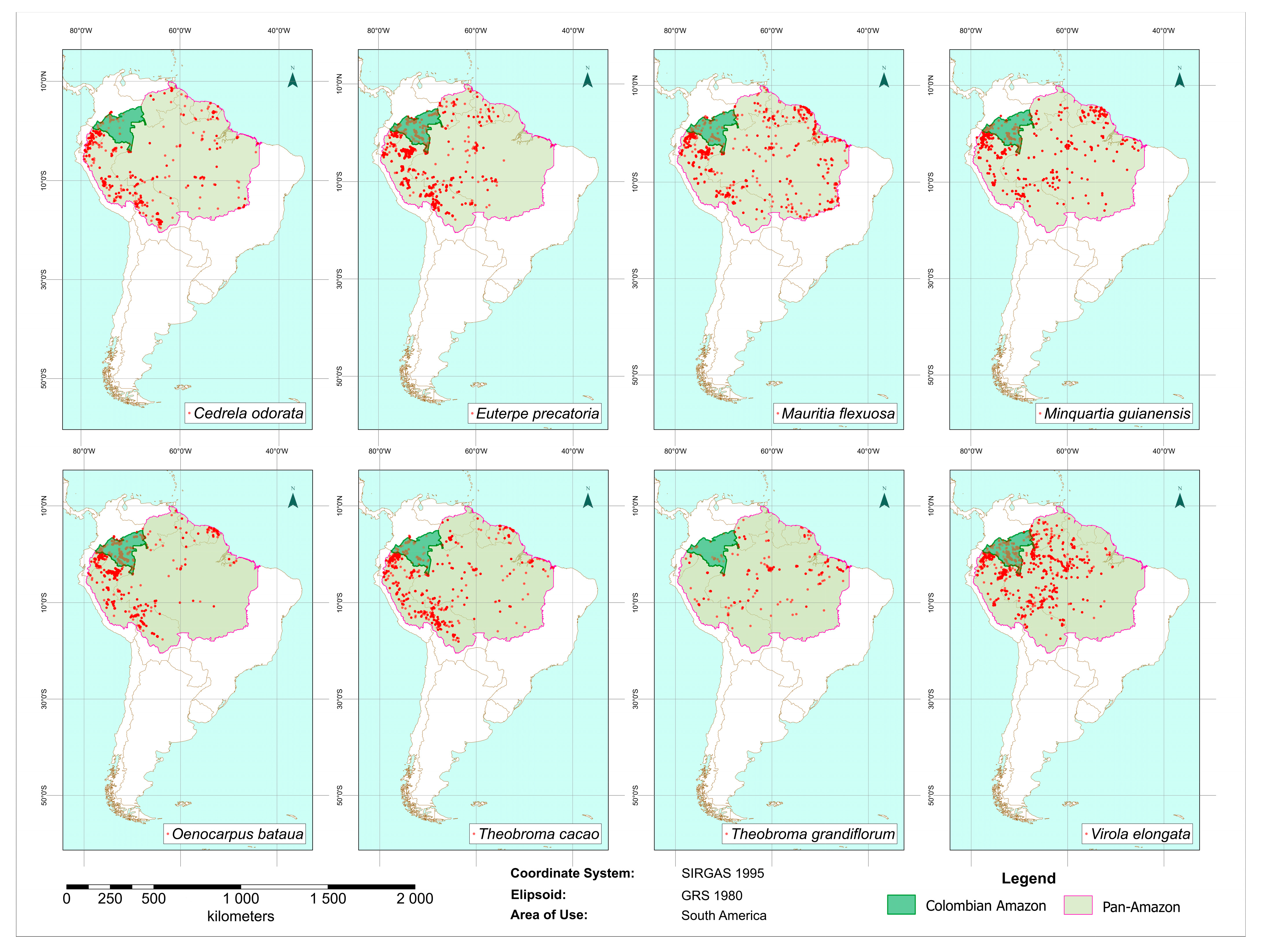
2.1. Species Occurrence Data Acquisition and Processing
2.2. Environmental Predictors
2.3. Species Distribution Modelling Framework
2.4. Analysis of Special Management Areas Under Climate Modeling Scenarios
3. Results
3.1. Occurrence Data Retrieval and Filtering
3.2. Overall Cross-Validation Performance
3.3. Algorithmic Performance and Trade-Offs
3.4. Robustness to Spatial/Environmental Partitioning
3.5. Cross-Species Synthesis of Variable Importance
3.6. Habitat Suitability for Baseline and Future Periods
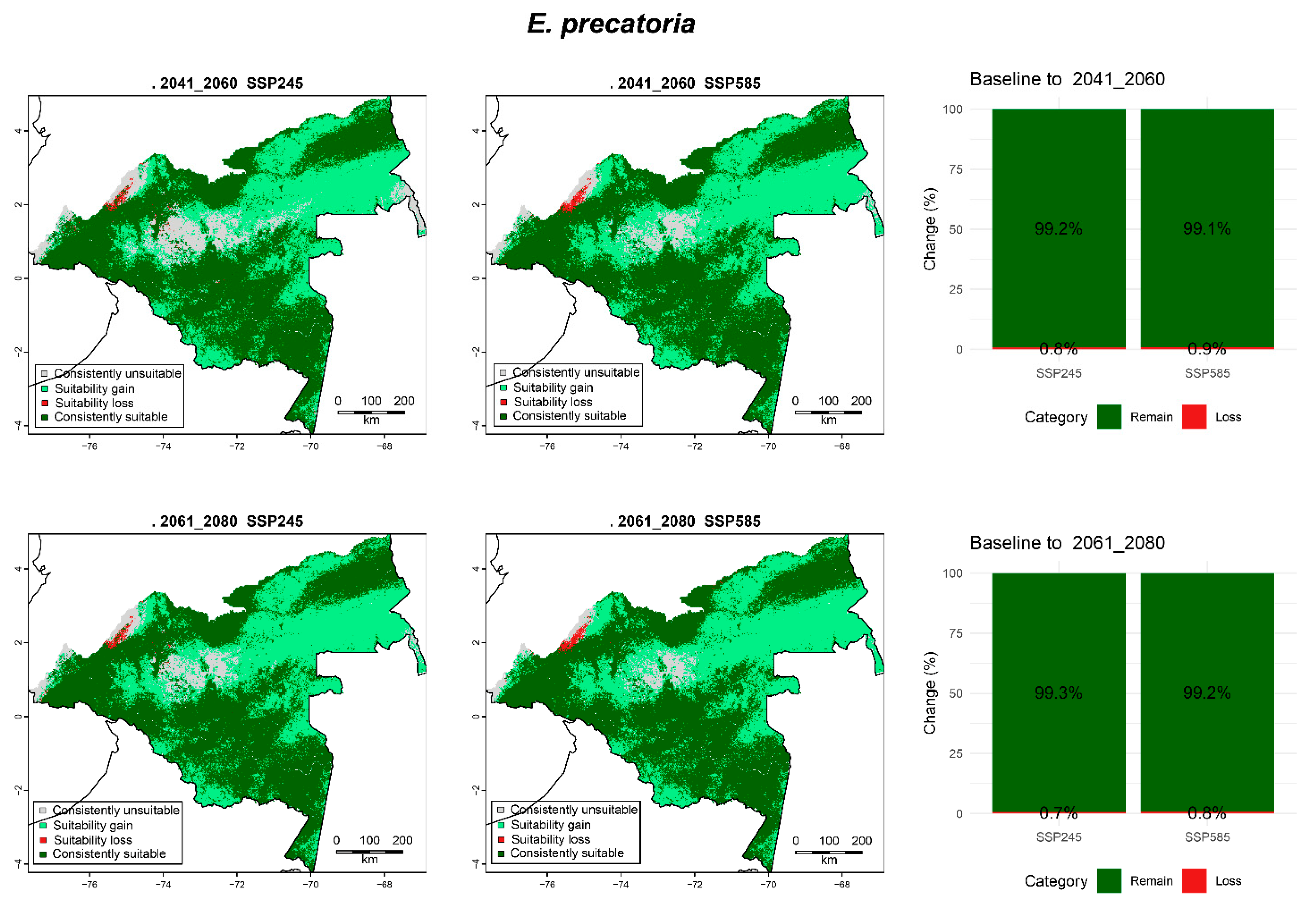
3.6.1. E. precatoria
3.6.2. O. bataua
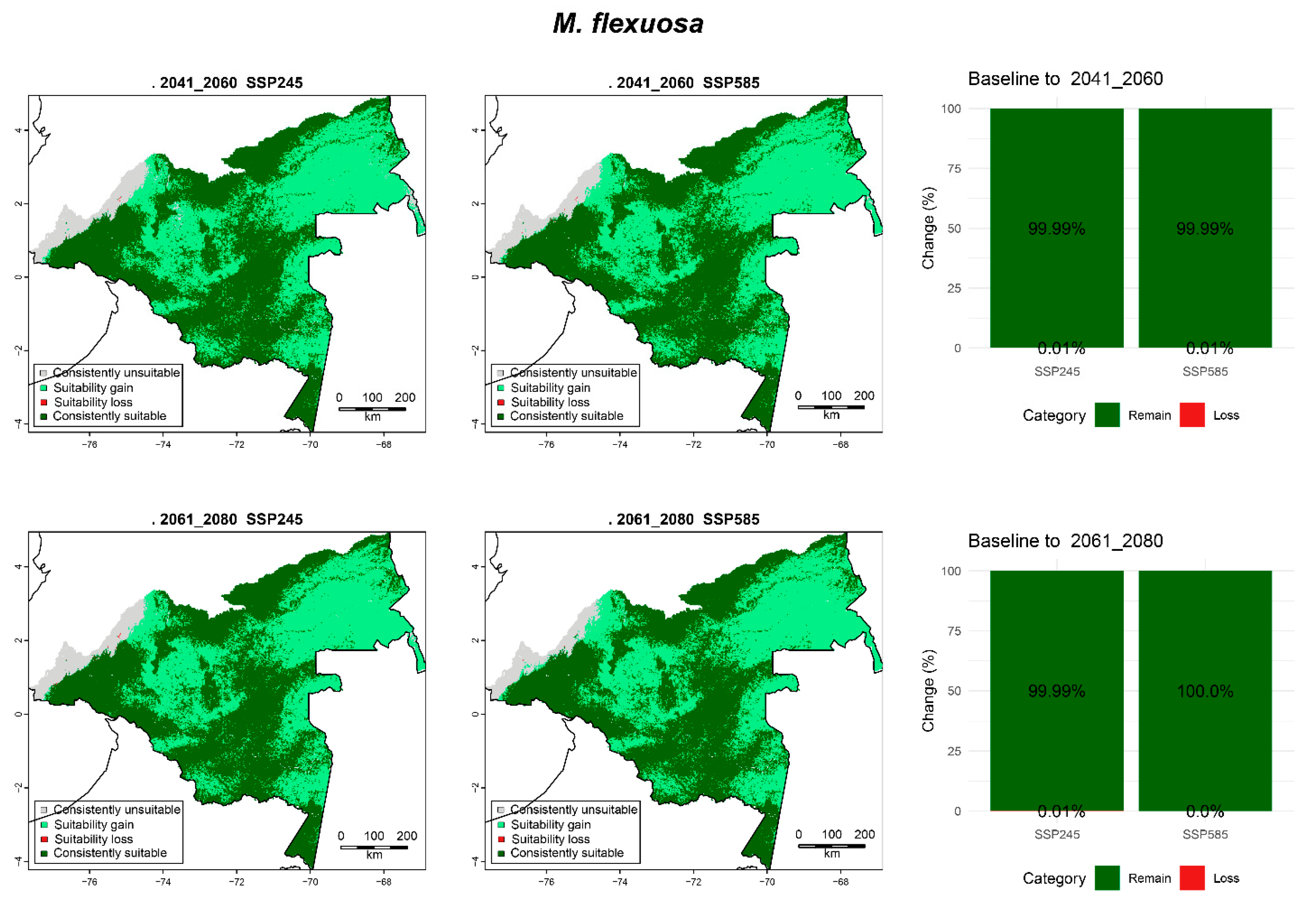
3.6.3. M. flexuosa
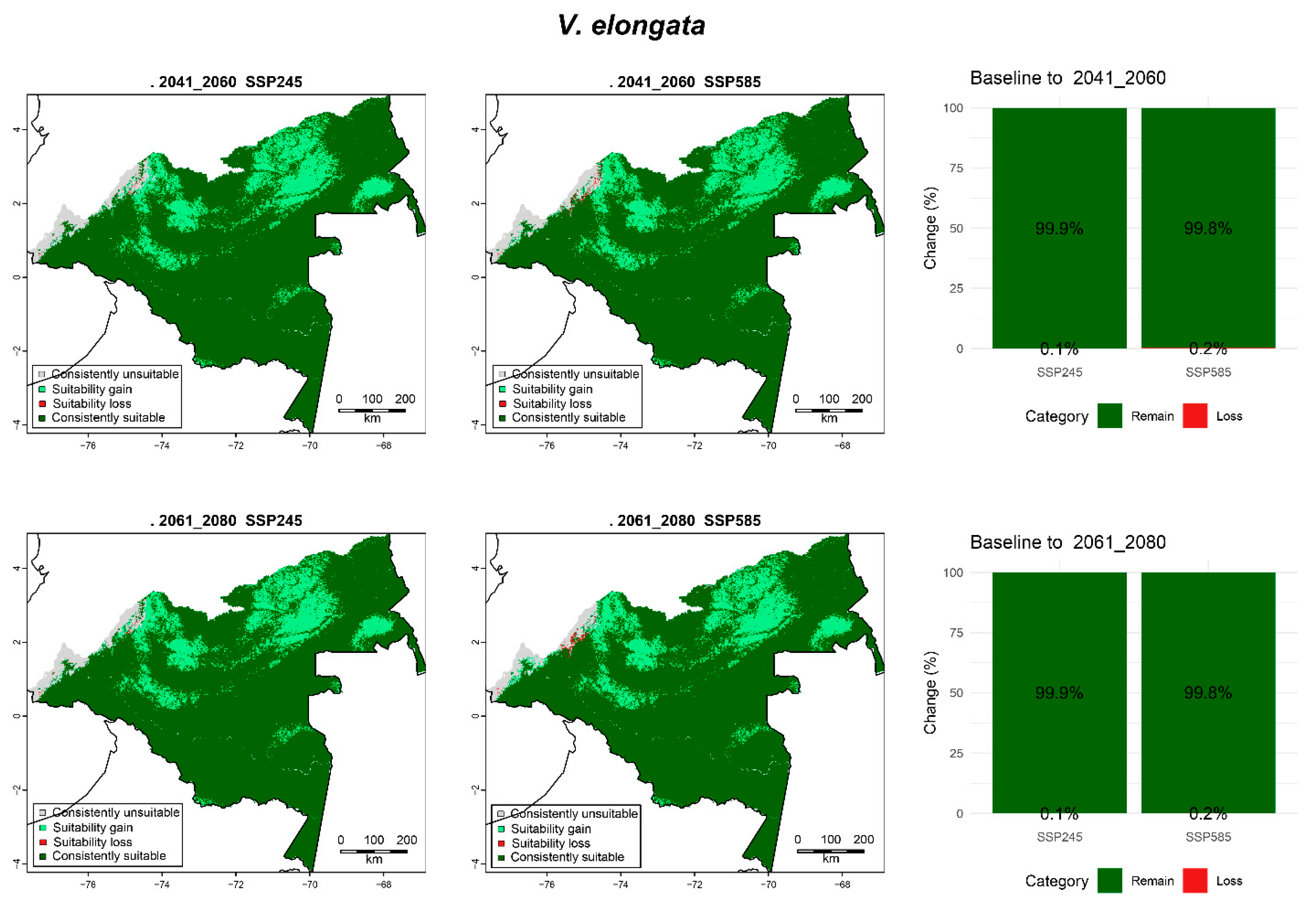
3.6.4. V. elongata
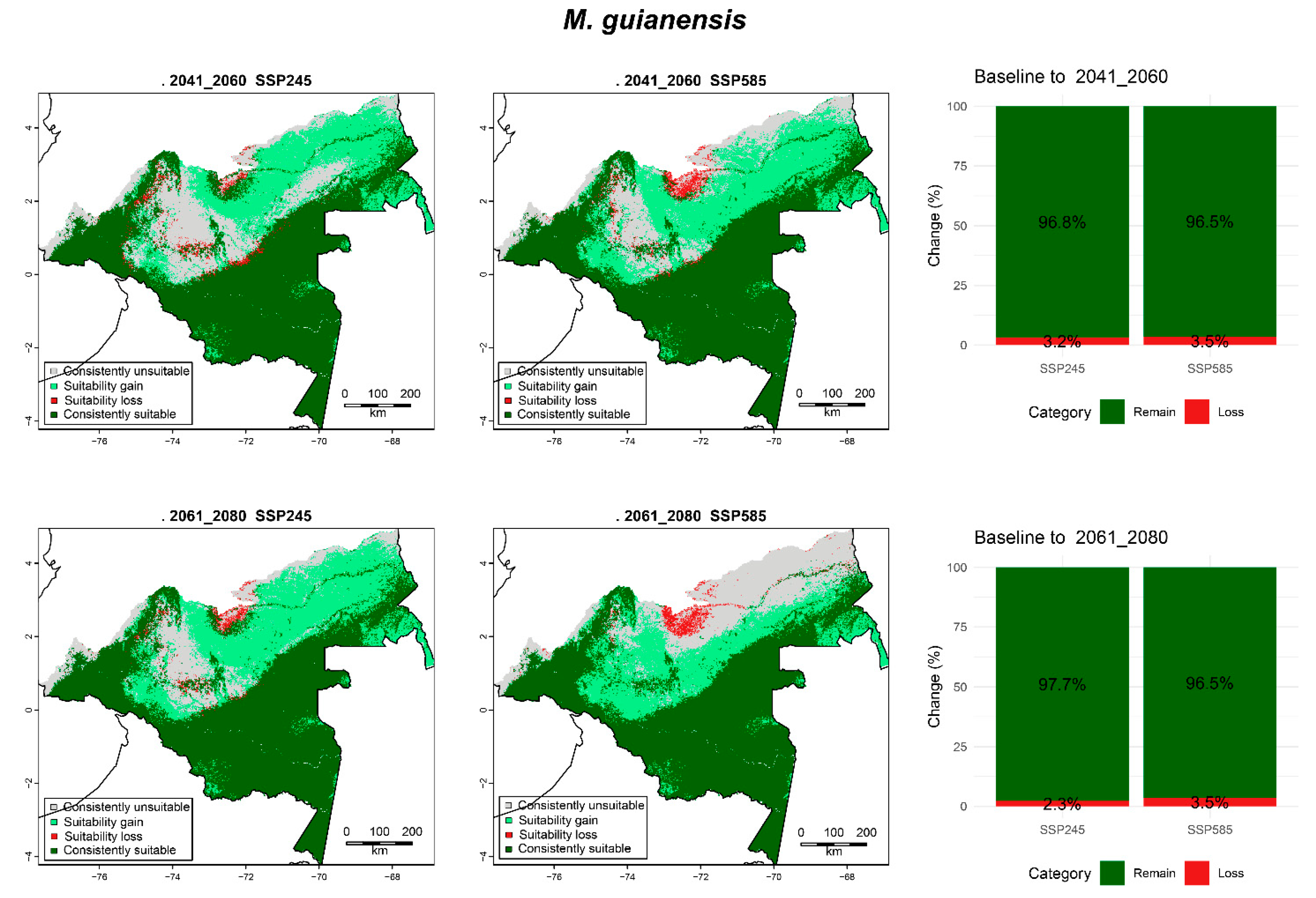
3.6.5. M. guianensis
3.6.6. C. odorata
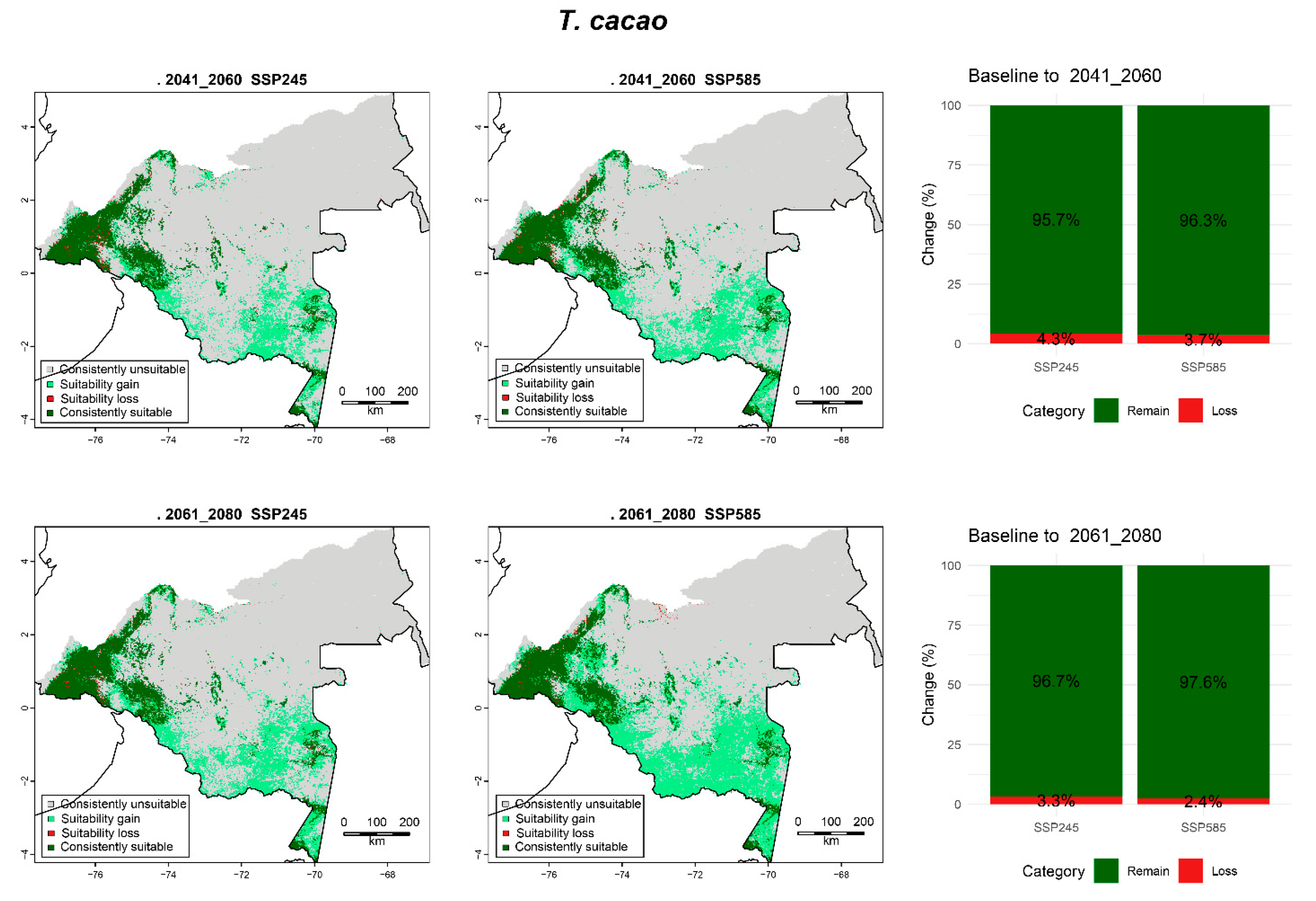
3.6.7. T. cacao
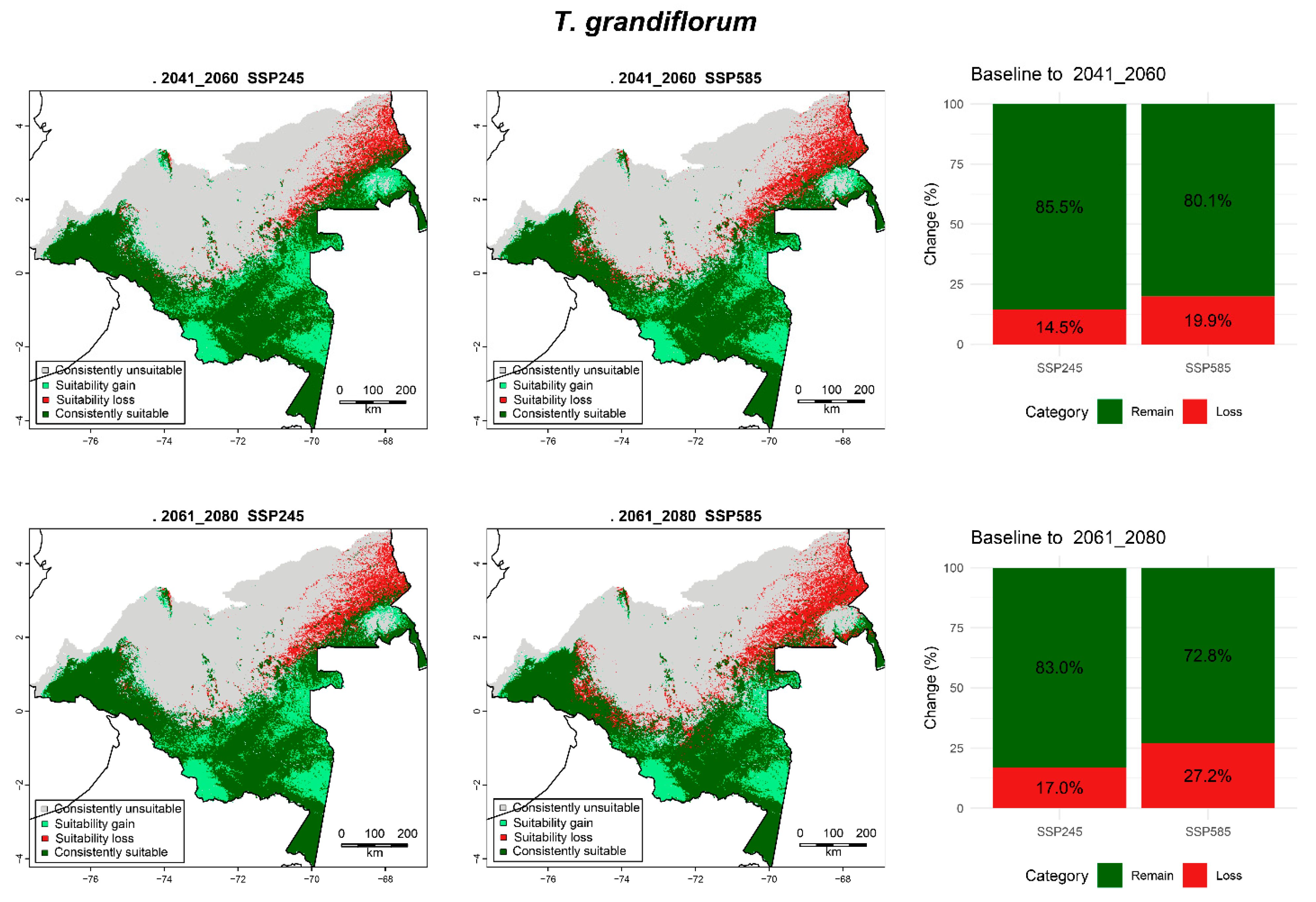
3.6.8. T. grandiflorum
3.7. Potential Habitat Changes Within Special Management Areas
4. Discussion
4.1. Environmental Drivers Shaping Species Suitability
4.2. Climate Impacts on Habitat Suitability and Distribution
4.3. Implications of Habitat Changes Within Special Management Areas
4.4. Methodological Strengths and Limitations of the SDM Approach
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nobre, C.A.; Peña-Claros, M.; Arieira, J.; Brandão, D.O.; Riveros, F.V. Climate Change and Sustainability in Amazonia 2025. In Oxford Research Encyclopedia of Climate Science; Oxford University Press: Oxford, UK.
- Artaxo, P.; Hansson, H.C.; Machado, L.A.T.; Rizzo, L. V Tropical Forests Are Crucial in Regulating the Climate on Earth. PLOS Clim. 2022, 1, e0000054. [Google Scholar] [CrossRef]
- Ter Steege, H. Hyperdominance in the Amazonian Tree Flora. Science 2013, 342, 1243092. [Google Scholar] [CrossRef]
- Crowther, T.W.; Glick, H.B.; Covey, K.R.; Bettigole, C.; Maynard, D.S.; Thomas, S.M.; Smith, J.R.; Hintler, G.; Duguid, M.C.; Amatulli, G.; et al. Mapping Tree Density at a Global Scale. Nature 2015, 525, 201–205. [Google Scholar] [CrossRef]
- Moraes, R.; Correa, S.; Doria, C.; Duponchelle, F. Amazonian Ecosystems and Their Ecological Functions. In Amazon Assessment Report 2021; Nobre, C., Encalada, A., Anderson, E., Alcazar, F., Bustamante, M., Mena, C., Peña-Claros, M., et al., Eds.; United Nations Sustainable Development Solutions: New York, NY, USA, 2021; pp. 1–33. [Google Scholar]
- Qin, Y.; Xiao, X.; Liu, F.; de Sa e Silva, F.; Shimabukuro, Y.; Arai, E.; Fearnside, P.M. Forest Conservation in Indigenous Territories and Protected Areas in the Brazilian Amazon. Nat. Sustain. 2023, 6, 295–305. [Google Scholar] [CrossRef]
- Donadio Linares, L.M. The Awkward Question: What Baseline Should Be Used to Measure Biodiversity Loss? The Role of History, Biology and Politics in Setting up an Objective and Fair Baseline for the International Biodiversity Regime. Environ. Sci. Policy 2022, 135, 137–146. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021. The Physical Science Basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC); IPCC: Geneva, Switzerland, 2021. [Google Scholar]
- Murcia, U.; Barón, O.; Leon, A.; Garcia, S.; Arias, J. Cambios Multitemporales En El Período 2014 al 2016 y Coberturas Del Año 2016; Instituto Amazónico de Investigaciones Científicas Sinchi: Bogotá, Colombia, 2017; ISBN 9789585427105. [Google Scholar]
- Ortega, M.A.; Cayuela, L.; Griffith, D.M.; Camacho, A.; Coronado, I.M.; del Castillo, R.F.; Figueroa-Rangel, B.L.; Fonseca, W.; Garibaldi, C.; Kelly, D.L.; et al. Climate Change Increases Threat to Plant Diversity in Tropical Forests of Central America and Southern Mexico. PLoS ONE 2024, 19, e0297840. [Google Scholar] [CrossRef] [PubMed]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.-C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity Redistribution under Climate Change: Impacts on Ecosystems and Human Well-Being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef]
- Habibullah, M.S.; Din, B.H.; Tan, S.-H.; Zahid, H. Impact of Climate Change on Biodiversity Loss: Global Evidence. Environ. Sci. Pollut. Res. 2022, 29, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- IPCC Summary for Policymakers. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2023: Synthesis Report; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 1–34. [Google Scholar]
- Shahzad, A. Climate Change and Its Impact on Global Biodiversity. Prem. J. Environ. Sci. 2025, 3, 100013. [Google Scholar] [CrossRef]
- Marengo, J.A.; Souza, C.M.; Thonicke, K.; Burton, C.; Halladay, K.; Betts, R.A.; Alves, L.M.; Soares, W.R. Changes in Climate and Land Use Over the Amazon Region: Current and Future Variability and Trends. Front. Earth Sci. 2018, 6, 228. [Google Scholar] [CrossRef]
- Gatti, L.V.; Melack, J.; Basso, L.S.; Restrepo-Coupe, N.; Aguiar, A.P.; Pangala, S.; Saleska, S.R.; Aragão, L.E.O.; Phillips, O.L.; Armenteras, D. The Amazon Carbon Budget. In Amazon Assessment Report 2021; Nobre, C., Encalada, A., Anderson, E., Alcazar, F., Bustamente, M., Mena, C., Peña-Claros, M., Eds.; UN Sustainable Development Solutions Network (SDSN): New York, NY, USA, 2021; pp. 1–9. [Google Scholar]
- Da Silva, J.M.C.; Rapini, A.; Barbosa, L.C.F.; Torres, R.R. Extinction Risk of Narrowly Distributed Species of Seed Plants in Brazil Due to Habitat Loss and Climate Change. PeerJ 2019, 7, e7333. [Google Scholar] [CrossRef]
- Marengo, J.; Souza, C., Jr. Climate Change: Impacts and Scenarios for the Amazon; University of São Paulo: São Paulo, Brazil, 2018. [Google Scholar]
- Lovejoy, T.E.; Nobre, C. Amazon Tipping Point. Sci. Adv. 2018, 4, eaat2340. [Google Scholar] [CrossRef]
- Nepstad, D.C.; Stickler, C.M.; Filho, B.S.-; Merry, F. Interactions among Amazon Land Use, Forests and Climate: Prospects for a near-Term Forest Tipping Point. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Gutiérrez, J.; Díaz, S.; Rifai, S.W.; Corral-Rivas, J.J.; Nava-Miranda, M.G.; González-M, R.; Hurtado-M, A.B.; Revilla, N.S.; Vilanova, E.; Almeida, E.; et al. Tropical Forests in the Americas Are Changing Too Slowly to Track Climate Change. Science 2025, 387, eadl5414. [Google Scholar] [CrossRef]
- Berenguer, E.; Armenteras, D.; Lees, A.C.; Smith, C.C.; Fearnside, P.; Nascimento, N.; Alencar, A.; Almeida, C.; Aragão, L.E.O.; Barlow, J.; et al. Drivers and Ecological Impacts of Deforestation and Forest Degradation. In Amazon Assessment Report 2021; Nobre, C., Encalada, A., Anderson, E., Alcazar, F., Bustamante, M., Mena, C., Peña-Claros, M., Eds.; UN Sustainable Development Solutions Network (SDSN): New York, NY, USA, 2021. [Google Scholar]
- Du, Y.; Gao, G.; Ma, X.; Xu, S.; Fu, B. Amazon Basin Shows Reduced Forest Loss but Increased Forest Spatial Fragmentation in 1992–2020. Sci. Total Environ. 2025, 990, 179917. [Google Scholar] [CrossRef]
- IPBES. Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Brondizio, E.S., Settele, J., Díaz, S., Ngo, H.T., Eds.; IPBES Secretariat: Bonn, Germany, 2019; 1148p. [Google Scholar] [CrossRef]
- Kouhestani, F. The Impact of Climate Change on Biological Systems and Biodiversity. Int. J. Sci. Res. Arch. 2025, 14, 1885–1900. [Google Scholar] [CrossRef]
- Gomes, V.H.F.; Vieira, I.C.G.; Salomão, R.P.; ter Steege, H. Amazonian Tree Species Threatened by Deforestation and Climate Change. Nat. Clim. Chang. 2019, 9, 547–553. [Google Scholar] [CrossRef]
- Bernal, R.; Gradstein, S.; Celis, M. Catálogo de Plantas y Líquenes de Colombia; Universidad Nacional de Colombia (Sede Bogotá): Bogotá, Colombia, 2016. [Google Scholar]
- SIAT-AC. Instituto Amazónico de Investigaciones Científicas SINCHI. Indicadores de Monitoreo Ambiental SIMAAC. Sistema Regional de Indicadores de Monitoreo Ambiental de La Amazonia Colombiana. Available online: https://siatac.co/monitoreo-ambiental-simaac/ (accessed on 10 August 2025).
- ter Steege, H.; Pitman, N.C.A.; do Amaral, I.L.; de Souza Coelho, L.; de Almeida Matos, F.D.; de Andrade Lima Filho, D.; Salomão, R.P.; Wittmann, F.; Castilho, C.V.; Guevara, J.E.; et al. Mapping Density, Diversity and Species-Richness of the Amazon Tree Flora. Commun. Biol. 2023, 6, 1130. [Google Scholar] [CrossRef]
- Instituto de Hidrología, Meteorología y Estudios Ambientales—Ideam. Escenarios de Cambio Climático de La Cuarta Comunicación de Colombia; Instituto de Hidrología, Meteorología y Estudios Ambientales—Ideam, Ministerio de Ambiente y Desarrollo Sostenible, Programa de las Naciones Unidas para el Desarrollo—PNUD, Fundación Natura y Proyecto GEF-CBIT “Transparencia Climática Colombia”: Bogotá, Colombia, 2024. [Google Scholar]
- Instituto Amazónico de Investigaciones Científicas SINCHI. Sistema de Información Ambiental Territorial de la Amazonia colombiana SIAT-AC Coberturas de La Tierra—SIMCOBA. Available online: https://siatac.co/simcoba/ (accessed on 15 August 2025).
- Ministerio de Ambiente y Desarrollo Sostenible Estrategia Nacional de Restauración 2023–2026. 2023. Available online: https://www.minambiente.gov.co/wp-content/uploads/2025/03/ENR_3032025-1.pdf (accessed on 20 August 2025).
- Williams, C.A.; de Almeida, C.T.; Silva Junior, C.H.L. Unraveling the Drivers and Feedbacks of Deforestation across the Amazon Basin. Nat. Sustain. 2024, 7, 110–120. [Google Scholar]
- de Andrade, A.F.A.; Velazco, S.J.E.; De Marco Júnior, P. ENMTML: An R Package for a Straightforward Construction of Complex Ecological Niche Models. Environ. Model. Softw. 2020, 125, 104615. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models with Applications in R. In Habitat Suitability and Distribution Models: With Applications in R; Ecology, Biodiversity and Conservation, Cambridge University Press: Cambridge, UK, 2017; ISBN 9780521765138. [Google Scholar]
- Zhao, Y.; Xu, L.; Zhang, Y.; Wen, Y.; Zhang, D. Climate-Change Impacts on Distribution, Habitats, Sustainability and Cultivation Planning of Two Economically and Ecologically Important Forest Trees (China Fir and Masson Pine) in China. Glob. Ecol. Conserv. 2025, 57, e03349. [Google Scholar] [CrossRef]
- González-Orozco, C.E.; Pesca, A. Regionalization of Cacao (Theobroma cacao L.) in Colombia. Front. Sustain. Food Syst. 2022, 6, 925800. [Google Scholar] [CrossRef]
- Igawa, T.K.; de Toledo, P.M.; Anjos, L.J.S. Climate Change Could Reduce and Spatially Reconfigure Cocoa Cultivation in the Brazilian Amazon by 2050. PLoS ONE 2022, 17, e0262729. [Google Scholar] [CrossRef]
- Nelson, J.T.; Motamayor, J.C.; Cornejo, O.E. Environment and Pathogens Shape Local and Regional Adaptations to Climate Change in the Chocolate Tree, Theobroma cacao L. Mol. Ecol. 2021, 30, 656–669. [Google Scholar] [CrossRef]
- Urrego, L.E.; Galeano, A.; Peñuela, C.; Sánchez, M.; Toro, E. Climate-Related Phenology of Mauritia Flexuosa in the Colombian Amazon. Plant Ecol. 2016, 217, 1207–1218. [Google Scholar] [CrossRef]
- Sanjurjo-Vílchez, J.; Soudre-Zambrano, M.; Bendayán-Acosta, L. Distribución Biogeográfica de Seis Especies de Frutales Nativos En La Región Loreto, Perú. Una Aproximación al 2020 Mediante Modelamiento y Simulación Usando DIVA-GIS. Folia Amaz. 2012, 21, 129–140. [Google Scholar] [CrossRef]
- Da Costa, W.P.L.B.; Pinheiro, É.F.M.; Latorraca, J.V.F.; Moutinho, V.H.P.; Carmo, F.H.D.J.; Ataíde, G.C.V.S.; Volpato, M.; Aguiar, D.L.; Andrade, F.W.C. The Climate Change Influence on Cedrela odorata L. Radial Growth in the Amazon. Sustainability 2023, 15, 16755. [Google Scholar] [CrossRef]
- Cotrina Sánchez, A.; Rojas Briceño, N.B.; Bandopadhyay, S.; Ghosh, S.; Torres Guzmán, C.; Oliva, M.; Guzman, B.K.; Salas López, R. Biogeographic Distribution of Cedrela spp. Genus in Peru Using MaxEnt Modeling: A Conservation and Restoration Approach. Diversity 2021, 13, 261. [Google Scholar] [CrossRef]
- Estrada-Contreras, I.; Equihua, M.; Laborde, J.; Martínez Meyer, E.; Sánchez-Velásquez, L.R. Current and Future Distribution of the Tropical Tree Cedrela odorata L. in Mexico under Climate Change Scenarios Using MaxLike. PLoS ONE 2016, 11, e0164178. [Google Scholar] [CrossRef]
- Marques, M.J.; de Souza Bezerra, C.; Tomaz, J.S.; Lopes, R.; Wrege, M.S.; de Aguiar, A.V.; Ramos, S.L.F.; Meneses, C.H.S.G.; de Jesus Pinto Fraxe, T.; Lopes, M.T.G. Prediction of Geographic Distribution and Ecological Niche Modeling of Açaí Palm Trees in the Amazon. Pesqui. Agropecu. Trop. 2024, 54, e78108. [Google Scholar] [CrossRef]
- Zizka, A.; Silvestro, D.; Andermann, T.; Azevedo, J.; Ritter, C.D.; Edler, D.; Farooq, H.; Herdean, A.; Ariza, M.; Scharn, R.; et al. CoordinateCleaner: Standardized Cleaning of Occurrence Records from Biological Collection Databases. Methods Ecol. Evol. 2019, 10, 744–751. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. SpThin: An R Package for Spatial Thinning of Species Occurrence Records for Use in Ecological Niche Models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Barve, N.; Barve, V.; Jiménez-Valverde, A.; Lira-Noriega, A.; Maher, S.P.; Peterson, A.T.; Soberón, J.; Villalobos, F. The Crucial Role of the Accessible Area in Ecological Niche Modeling and Species Distribution Modeling. Ecol. Modell. 2011, 222, 1810–1819. [Google Scholar] [CrossRef]
- Hijmans, R.J. Spatial Data Analysis [R Package Terra Version 1.8-60]. CRAN: Contributed Packages 2025. Available online: https://CRAN.R-project.org/package=mgcv (accessed on 15 August 2025).
- Farr, T.G.; Rosen, P.A.; Caro, E.; Crippen, R.; Duren, R.; Hensley, S.; Kobrick, M.; Paller, M.; Rodriguez, E.; Roth, L.; et al. The Shuttle Radar Topography Mission. Rev. Geophys. 2007, 45. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Poggio, L.; de Sousa, L.M.; Batjes, N.H.; Heuvelink, G.B.M.; Kempen, B.; Ribeiro, E.; Rossiter, D. SoilGrids 2.0: Producing Soil Information for the Globe with Quantified Spatial Uncertainty. SOIL 2021, 7, 217–240. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where Is Positional Uncertainty a Problem for Species Modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- R Core Team. R-4.5.0. A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2025; Available online: https://cran.r-project.org/bin/windows/base/old/4.5.0/ (accessed on 11 April 2025).
- Naimi, B.; Araujo, M.B. Sdm: Species Distribution Modelling v1.1-8. CRAN: Contributed Packages. 2021. Available online: https://cran.r-project.org/web/packages/sdm/index.html (accessed on 15 August 2025).
- Wood, S. Mgcv: Mixed GAM Computation Vehicle with Automatic Smoothness Estimation v1.9-1. CRAN: Contributed Packages. 2023. Available online: https://cran.r-project.org/web/packages/mgcv/index.html (accessed on 15 August 2025).
- Ridgeway, G.; Developers, G. Gbm: Generalized Boosted Regression Models v2.1-8. CRAN: Contributed Packages. 2020. Available online: https://cran.r-project.org/web/packages/gbm/index.html (accessed on 20 August 2025).
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Dismo: Species Distribution Modeling v.1.3-16. CRAN: Contributed Packages. 2024. Available online: https://cran.r-project.org/web/packages/dismo/dismo.pdf (accessed on 18 June 2024).
- Breiman, L.; Cutler, A.; Liaw, A.; Wiener, M. RandomForest: Breiman and Cutlers Random Forests for Classification and Regression v. 4.7-1.1. CRAN: Contributed Packages 2022. Available online: https://cran.r-project.org/web/packages/randomForest/randomForest.pdf (accessed on 22 May 2022).
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A Platform for Ensemble Forecasting of Species Distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Boisier, J.P.; Ciais, P.; Ducharne, A.; Guimberteau, M. Projected Strengthening of Amazonian Dry Season by Constrained Climate Model Simulations. Nat. Clim. Chang. 2015, 5, 656–660. [Google Scholar] [CrossRef]
- Environmental Systems Research Institute (Esri) How Pairwise Intersect Works—ArcGIS Pro Documentation. 2025. Available online: https://pro.arcgis.com/en/pro-app/latest/tool-reference/analysis/how-pairwise-intersect-works.htm (accessed on 15 May 2025).
- Quesada, C.A.; Phillips, O.L.; Schwarz, M.; Czimczik, C.I.; Baker, T.R.; Patiño, S.; Fyllas, N.M.; Hodnett, M.G.; Herrera, R.; Almeida, S.; et al. Basin-Wide Variations in Amazon Forest Structure and Function Are Mediated by Both Soils and Climate. Biogeosciences 2012, 9, 2203–2246. [Google Scholar] [CrossRef]
- Esquivel-Muelbert, A.; Baker, T.R.; Dexter, K.G.; Lewis, S.L.; Brienen, R.J.W.; Feldpausch, T.R.; Lloyd, J.; Monteagudo-Mendoza, A.; Arroyo, L.; Álvarez-Dávila, E.; et al. Compositional Response of Amazon Forests to Climate Change. Glob. Chang. Biol. 2019, 25, 39–56. [Google Scholar] [CrossRef]
- Fyllas, N.M.; Patiño, S.; Baker, T.R.; Bielefeld Nardoto, G.; Martinelli, L.A.; Quesada, C.A.; Paiva, R.; Schwarz, M.; Horna, V.; Mercado, L.M.; et al. Basin-Wide Variations in Foliar Properties of Amazonian Forest: Phylogeny, Soils and Climate. Biogeosciences 2009, 6, 2677–2708. [Google Scholar] [CrossRef]
- Nunes-Silva, P.; Acosta, A.L.; Borges, R.C.; Freitas, B.M.; Oliveira, R.C.; Giannini, T.C.; Imperatriz-Fonseca, V.L. Climate Change Will Alter Amazonian Bumblebees’ Distribution, but Effects Are Species-Specific. Front. Bee Sci. 2025, 3, 1510004. [Google Scholar] [CrossRef]
- Ceccarelli, V.; Fremout, T.; Zavaleta, D.; Lastra, S.; Correa, S.I.; Arévalo-Gardini, E.; Rodriguez, C.A.; Hilacondo, W.C.; Thomas, E. Climate Change Impact on Cultivated and Wild Cacao in Peru and the Search of Climate Change-Tolerant Genotypes. Divers. Distrib. 2021, 27, 1462–1476. [Google Scholar] [CrossRef]
- Sampayo-Maldonado, S.; Ordoñez-Salanueva, C.A.; Mattana, E.; Way, M.; Castillo-Lorenzo, E.; Dávila-Aranda, P.D.; Lira-Saade, R.; Téllez-Valdés, O.; Rodríguez-Arévalo, N.I.; Flores-Ortiz, C.M.; et al. Potential Distribution of Cedrela odorata L. in Mexico According to Its Optimal Thermal Range for Seed Germination under Different Climate Change Scenarios. Plants 2023, 12, 150. [Google Scholar] [CrossRef]
- de Morais, I.L.L.; de Lima, A.A.; dos Santos, I.N.L.; Meneses, C.; da Silva, R.F.; Lopes, R.; Ramos, S.L.F.; de Aguiar, A.V.; Wrege, M.S.; Lopes, M.T.G. Climate Change Impact on the Distribution of Forest Species in the Brazilian Amazon. Sustainability 2024, 16, 3458. [Google Scholar] [CrossRef]
- Osland, M.J.; Bradford, J.B.; Toth, L.T.; Germino, M.J.; Grace, J.B.; Drexler, J.Z.; Stagg, C.L.; Grossman, E.R.; Thorne, K.M.; Romañach, S.S.; et al. Ecological Thresholds and Transformations Due to Climate Change: The Role of Abiotic Stress. Ecosphere 2025, 16, e70229. [Google Scholar] [CrossRef]
- Schroth, G.; Läderach, P.; Martinez-Valle, A.I.; Bunn, C.; Jassogne, L. Vulnerability to Climate Change of Cocoa in West Africa: Patterns, Opportunities and Limits to Adaptation. Sci. Total Environ. 2016, 556, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Gollnow, F.; Cammelli, F.; Carlson, K.M.; Garrett, R.D. Gaps in Adoption and Implementation Limit the Current and Potential Effectiveness of Zero-Deforestation Supply Chain Policies for Soy. Environ. Res. Lett. 2022, 17, 114003. [Google Scholar] [CrossRef]
- Rodríguez-León, C.H.; Sterling, A.; Trujillo-Briñez, A.; Suárez-Córdoba, Y.D.; Roa-Fuentes, L.L. Forest Attribute Dynamics in Secondary Forests: Insights for Advancing Ecological Restoration and Transformative Territorial Management in the Amazon. Diversity 2025, 17, 39. [Google Scholar] [CrossRef]
- Rodríguez-León, C.H.; Sterling, A.; Daza-Giraldo, D.D.; Suárez-Córdoba, Y.D.; Roa-Fuentes, L.L. Scaling Plant Functional Strategies from Species to Communities in Regenerating Amazonian Forests: Insights for Restoration in Deforested Landscapes. Diversity 2025, 17, 570. [Google Scholar] [CrossRef]
- Brudvig, L.; Damschen, E.; Tewksbury, J.; Haddad, N.; Levey, D. Landscape Connectivity Promotes Plant Biodiversity Spillover into Non-Target Habitats. Proc. Natl. Acad. Sci. USA 2009, 106, 9328–9332. [Google Scholar] [CrossRef] [PubMed]
- Forest, F. Preserving the Evolutionary Potential of Floras in Biodiversity Hotspots. Nature 2007, 445, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Vitt, P.; Havens, K.; Kramer, A.T.; Sollenberger, D.; Yates, E. Assisted Migration of Plants: Changes in Latitudes, Changes in Attitudes. Biol. Conserv. 2010, 143, 18–27. [Google Scholar] [CrossRef]


| Species | AUC | TSS |
|---|---|---|
| E. precatoria | 0.762 | 0.440 |
| O. bataua | 0.796 | 0.530 |
| M. flexuosa | 0.722 | 0.345 |
| V. elongata | 0.738 | 0.407 |
| M. guianensis | 0.744 | 0.427 |
| C. odorata | 0.800 | 0.511 |
| T. cacao | 0.828 | 0.562 |
| T. grandiflorum | 0.729 | 0.434 |
| Species | Best Algorithm | AUC | TSS |
|---|---|---|---|
| E. precatoria | RF | 0.780 | 0.461 |
| O. bataua | RF | 0.807 | 0.546 |
| M. flexuosa | MaxEnt | 0.730 | 0.342 |
| V. elongata | RF | 0.754 | 0.434 |
| M. guianensis | BRT | 0.753 | 0.410 |
| C. odorata | BRT | 0.814 | 0.529 |
| T. cacao | RF | 0.846 | 0.585 |
| T. grandiflorum | BRT | 0.754 | 0.475 |
| Species | Period | Scenario | Consistently Unsuitable (Mha, %) | Suitability Gain (Mha, %) | Suitability Loss (Mha, %) | Consistently Suitable (Mha, %) |
|---|---|---|---|---|---|---|
| C. odorata | 2041_2060 | SPS245 | 41.69 (85.84) | 1.52 (3.13) | 0.19 (0.39) | 5.17 (10.64) |
| SSP585 | 40.70 (83.8) | 2.51 (5.17) | 0.25 (0.51) | 5.11 (10.52) | ||
| 2061_2080 | SPS245 | 40.97 (84.35) | 2.24 (4.61) | 0.21 (0.43) | 5.15 (10.6) | |
| SSP585 | 40.58 (83.56) | 2.63 (5.41) | 0.47 (0.96) | 4.89 (10.07) | ||
| E. precatoria | 2041_2060 | SPS245 | 4.43 (9.12) | 17.12 (35.26) | 0.21 (0.42) | 26.81 (55.2) |
| SSP585 | 2.47 (5.08) | 19.09 (39.3) | 0.23 (0.48) | 26.78 (55.14) | ||
| 2061_2080 | SPS245 | 2.75 (5.66) | 18.80 (38.72) | 0.19 (0.39) | 26.82 (55.23) | |
| SSP585 | 1.85 (3.82) | 19.70 (40.56) | 0.22 (0.46) | 26.79 (55.16) | ||
| M. flexuosa | 2041_2060 | SPS245 | 2.44 (5.02) | 21.04 (43.32) | 0.01 (0.02) | 25.08 (51.64) |
| SSP585 | 2.08 (4.28) | 21.40 (44.07) | 0.01 (0.02) | 25.08 (51.64) | ||
| 2061_2080 | SPS245 | 2.14 (4.41) | 21.34 (43.93) | 0.01 (0.02) | 25.08 (51.63) | |
| SSP585 | 1.84 (3.8) | 21.63 (44.55) | 0.00 (0.0) | 25.09 (51.65) | ||
| M. guianensis | 2041_2060 | SPS245 | 9.82 (20.22) | 11.96 (24.62) | 0.87 (1.78) | 25.92 (53.37) |
| SSP585 | 7.71 (15.88) | 14.07 (28.97) | 0.94 (1.95) | 25.84 (53.21) | ||
| 2061_2080 | SPS245 | 6.92 (14.25) | 14.86 (30.59) | 0.61 (1.25) | 26.18 (53.9) | |
| SSP585 | 10.54 (21.7) | 11.24 (23.14) | 0.94 (1.93) | 25.85 (53.23) | ||
| O. bataua | 2041_2060 | SPS245 | 5.58 (11.49) | 16.81 (34.62) | 0.18 (0.38) | 25.99 (53.51) |
| SSP585 | 3.02 (6.22) | 19.37 (39.89) | 0.22 (0.46) | 25.95 (53.43) | ||
| 2061_2080 | SPS245 | 3.61 (7.42) | 18.79 (38.69) | 0.16 (0.32) | 26.02 (53.57) | |
| SSP585 | 2.62 (5.4) | 19.77 (40.71) | 0.43 (0.89) | 25.74 (52.99) | ||
| T. cacao | 2041_2060 | SPS245 | 37.72 (77.66) | 5.34 (10.99) | 0.24 (0.49) | 5.27 (10.86) |
| SSP585 | 36.21 (74.55) | 6.85 (14.1) | 0.20 (0.42) | 5.31 (10.93) | ||
| 2061_2080 | SPS245 | 36.45 (75.05) | 6.61 (13.6) | 0.18 (0.37) | 5.33 (10.98) | |
| SSP585 | 31.82 (65.53) | 11.23 (23.12) | 0.13 (0.28) | 5.38 (11.08) | ||
| T. grandiflorum | 2041_2060 | SPS245 | 22.17 (45.65) | 5.93 (12.21) | 2.96 (6.09) | 17.50 (36.04) |
| SSP585 | 22.96 (47.28) | 5.14 (10.58) | 4.08 (8.4) | 16.38 (33.73) | ||
| 2061_2080 | SPS245 | 22.53 (46.4) | 5.57 (11.47) | 3.49 (7.18) | 16.98 (34.95) | |
| SSP585 | 23.78 (48.96) | 4.33 (8.91) | 5.56 (11.45) | 14.90 (30.68) | ||
| V. elongata | 2041_2060 | SPS245 | 1.60 (3.3) | 7.18 (14.79) | 0.04 (0.09) | 39.74 (81.82) |
| SSP585 | 1.47 (3.03) | 7.31 (15.06) | 0.09 (0.18) | 39.69 (81.73) | ||
| 2061_2080 | SPS245 | 1.45 (2.99) | 7.33 (15.1) | 0.04 (0.08) | 39.74 (81.83) | |
| SSP585 | 1.30 (2.68) | 7.48 (15.41) | 0.10 (0.2) | 39.69 (81.71) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murcia-García, U.G.; Sterling, A.; Rodríguez-Espinoza, J.; Carrero-Rincón, J.A.; Acosta-Salinas, M.I.; Rodríguez-León, C.H. Climate-Change Impacts on Distribution of Amazonian Woody Plant Species Key to Conservation, Restoration and Sustainable Use in the Colombian Amazon. Forests 2025, 16, 1640. https://doi.org/10.3390/f16111640
Murcia-García UG, Sterling A, Rodríguez-Espinoza J, Carrero-Rincón JA, Acosta-Salinas MI, Rodríguez-León CH. Climate-Change Impacts on Distribution of Amazonian Woody Plant Species Key to Conservation, Restoration and Sustainable Use in the Colombian Amazon. Forests. 2025; 16(11):1640. https://doi.org/10.3390/f16111640
Chicago/Turabian StyleMurcia-García, Uriel G., Armando Sterling, Jeferson Rodríguez-Espinoza, José A. Carrero-Rincón, María I. Acosta-Salinas, and Carlos H. Rodríguez-León. 2025. "Climate-Change Impacts on Distribution of Amazonian Woody Plant Species Key to Conservation, Restoration and Sustainable Use in the Colombian Amazon" Forests 16, no. 11: 1640. https://doi.org/10.3390/f16111640
APA StyleMurcia-García, U. G., Sterling, A., Rodríguez-Espinoza, J., Carrero-Rincón, J. A., Acosta-Salinas, M. I., & Rodríguez-León, C. H. (2025). Climate-Change Impacts on Distribution of Amazonian Woody Plant Species Key to Conservation, Restoration and Sustainable Use in the Colombian Amazon. Forests, 16(11), 1640. https://doi.org/10.3390/f16111640






