Abstract
Studying the response of plant leaf functional traits to elevation helps us understand plant adaptation to the environment and their distribution trends under global climate change. Currently, how plant leaf functional traits respond to elevation across different scales or among different species remains controversial. Quercus rehderiana Hand.-Mazz. is widely distributed across various altitude ranges in southwestern China, making it an ideal species to address this question. Therefore, this study established three 20 × 20 m quadrats at each of five altitude gradients (2000, 2200, 2400, 2600, and 2800 m). By measuring morphological and nutrient indicators in leaves from five individuals of Quercus rehderiana in each quadrat, we analyzed the response of leaf functional traits to elevation. The results showed that leaf thickness (LT), specific leaf area (SLA), phosphorus (P), potassium (K) concentrations, carbon phosphorus ratio (C:P ratio), and nitrogen phosphorus ratio (N:P ratio) of Quercus rehderiana varied significantly across different elevations. Regression analysis revealed that leaf area (LA), K concentration, and carbon nitrogen ratio (C:N ratio) decreased with increasing elevation, while LT and nitrogen (N) concentration increased. Correlation analysis indicated that LA was significantly negatively correlated with LT and leaf P concentration, but positively correlated with carbon (C) concentration and stoichiometric ratios (C:N, C:P, N:P). Leaf thickness (LT) was significantly negatively correlated with K and calcium (Ca) concentration. Specific leaf area (SLA) and K concentration were significantly negatively correlated with leaf dry matter content (LDMC). The leaves of Quercus rehderiana mainly adapt to different elevations through trade-offs among different morphological and chemical traits. These findings can support the conservation of germplasm resources and forest management.
1. Introduction
Plant functional traits—encompassing morphological, physiological, and developmental characteristics—can serve as valuable indicators for identifying which plant species or groups are capable of withstanding current severe and rapidly shifting environmental conditions [1,2,3,4]. Understanding the mechanisms underlying the relationships between these traits and environmental factors is of significant relevance [5]. In particular, altitudinal gradients, which bring about variations in environmental conditions such as light and temperature, offer a useful natural platform for investigating these mechanisms. Such insights will further our understanding of which plant phenotypes are likely to adapt or even thrive under ongoing and future climate change [6,7]. Leaves, having the largest contact area with the external environment, are most sensitive to external changes and best reflect plant adaptability [8,9]. Thus, leaves have become a key organ in eco-physiological studies of plant environmental adaptation [6,10,11]. Long-term survival in extreme environments leads to the development of unique leaf morphological structures and physiological mechanisms [12,13]. Therefore, analyzing how leaf functional traits respond to various environmental factors is crucial for understanding plant adaptability.
Leaf functional traits encompass a series of measurable morphological, structural, and physiological indicators [14]. Leaf area (LA) relates to light capture capacity and photosynthetic rate [15,16]. Leaf thickness (LT) is associated with mechanical resistance, drought and cold tolerance, water retention, and transpiration [17,18]. Specific leaf area (SLA), representing the leaf area per unit dry mass investment, is a key trait influencing growth rate, as it allows plants to expose a larger area to light and CO2 for a given dry mass input [19]. Leaf dry matter content (LDMC) reflects the carbon investment strategy in structural tissues and is related to plant defense [14]. Chemical elements in plants, such as C, N, P, K, Ca, participate in growth, development, and physiological regulation [20]. Moreover, the correlations among leaf element concentrations reflect adaptation mechanisms, regulatory processes, and environmental feedback [21,22]. Specifically, the C:N and C:P ratios are closely linked to carbon sequestration capacity, nitrogen and phosphorus use efficiency, and growth rate [21,23], while the N:P ratio serves as an important indicator of nutrient limitation [24,25,26].
In recent years, studies on alpine plants across elevation gradients have become a research focus in global change science, offering insights into local environmental variations [27,28,29,30]. Variations in plant morphological and physiological traits along elevation gradients provide valuable opportunities to examine plant responses to environmental changes. Thus, investigating leaf functional traits across elevations helps clarify the relationship between plant growth and the environment, and reveals long-term adaptation mechanisms. Quercus rehderiana Hand.-Mazz., belonging to the Fagaceae family, is widely distributed in Tibet, Sichuan, Yunnan, and Guizhou, and plays a significant role in biodiversity conservation and water resource maintenance in southwestern China [31,32]. Additionally, Quercus rehderiana possesses significant economic value, serving as an important source for various production uses such as fodder, firewood, charcoal, farming tools, and bee boxes [33]. However, recent studies indicate that the suitable habitat for Quercus is expanding to relatively higher altitudes in Yunnan and Guizhou under global climate change [34]. It is noteworthy that Quercus rehderiana is distributed across various altitude gradients in Guizhou Province (ranging from 2000 to 2800 m) [32,35]. The physio-ecological mechanisms of leaf adaptation behind this range expansion of Quercus remain unclear. Recent studies have explored leaf morphology, anatomy, and nutrient adaptation of Quercus across elevations on the Tibetan Plateau [36,37]. However, the lower elevation limit of Quercus rehderiana in the Yunnan–Guizhou Plateau is much lower than that on the Tibetan Plateau. Thus, how leaf traits of Quercus rehderiana vary at low to mid-elevations remains poorly understood. This study aims to examine the leaf traits of Quercus rehderiana across different elevations in the Guizhou Plateau, to enhance understanding of leaf trait variation at mid-low elevations and improve knowledge of vulnerable ecosystems in high-elevation areas of the region. The findings may also support germplasm conservation and forest management, and predictions of forest dynamics under global climate change.
To adapt to low temperatures, drought, and high light at high elevations, plants typically reduce leaf area and SLA, while increasing LT and LDMC. Therefore, we first hypothesize that LA and SLA decrease with elevation, while LT and LDMC increase. According to the Temperature–Plant Physiology Hypothesis [38], decreasing temperatures at higher elevations lead to increased leaf N concentration. Furthermore, previous studies found no consistent trends in N:P and C:P ratios with elevation, whereas the C:N ratio decreased with increasing elevation. Thus, we hypothesize that leaf N concentration increases with elevation, while the C:N ratio decreases. Previous studies have indicated that K, as a major intracellular element, has a demand directly correlated with growth rate. Due to low temperatures in high-elevation areas limiting plant growth, the requirement for K decreases, leading to reduced K concentrations in leaves [39,40,41]. Therefore, we hypothesize that the K concentration in plant leaves decreases with increasing elevation.
2. Materials and Methods
2.1. Study Sites
The study area is located in Weining County and Hezhang County of Bijie City in northwestern Guizhou Province. These two adjacent counties are situated in the heart of the Wumeng Mountains [42]. The region experiences a subtropical monsoon climate characterized by mild summers and cool winters, with an average annual temperature ranging from 10 to 15 °C [43]. Precipitation is abundant, with an average annual rainfall of approximately 1000 mm. The frost-free period exceeds 180 days. Due to the significant elevation variations within the study area, the vertical climatic gradient supports the growth of diverse flora and fauna. Well-preserved forests of Quercus rehderiana Hand.-Mazz. are distributed at elevations between 2000 and 2800 m, making this an ideal area for investigating the ecological adaptation patterns of leaf functional traits across different altitude gradients [32,44].
2.2. Plot Establishment and Sampling
Five 20 m × 20 m quadrats were set up at the 2200 m elevation gradient in July 2021 [35]. In July 2023, we established three 20 m × 20 m survey quadrats for Quercus rehderiana at each of four elevation gradients (2000 m, 2400 m, 2600 m, 2800 m). All quadrats were established in areas with comparable slope gradients, aspects, and soil types to ensure environmental consistency, with altitude being the sole major variable. Each 20 m × 20 m quadrat was divided into four 10 m × 10 m sub-quadrats to facilitate precise sampling. In July 2023, we employed a five-point sampling method to select five Quercus rehderiana individuals from each 20 m × 20 m plot. The selected trees were located near the center of each of the four 10 m × 10 m subplots and near the center of the 20 m × 20 m plot. Thus, a total of 15 Quercus rehderiana individuals were selected across the three 20 m × 20 m plots at each elevation gradient. From each sampled tree, three healthy, intact, mature, and sun-exposed leaves were collected. This resulted in 15 leaves per 20 m × 20 m plot, and 45 leaves per elevation gradient (across three plots), which were used for morphological trait measurements. Additionally, five more leaves were collected from each tree, totaling 25 leaves per 20 m × 20 m plot. These leaves were pooled into one composite sample per plot for elemental content analysis. Therefore, three elemental content values were obtained for each elevation gradient.
2.3. Trait Measurements
Following the removal of petioles, 15 leaves were scanned with an HP Scanjet M231 scanner. The LA (cm2) was measured using ImageJ software (https://imagej.net/software/imagej/, accessed on 10 October 2023). Leaf fresh weight was determined using an electronic balance (accuracy: 0.0001 g), after which the leaf samples were oven-dried at 70 °C for 48 h. Specific leaf area (SLA, cm2 g−1) and LDMC (g g−1) were calculated accordingly. SLA was defined as leaf area divided by leaf dry weight, while LDMC was calculated as dry weight divided by fresh weight. Leaf cross-sections were imaged using an Ultra-depth Digital Microscope (Yiweishike Technology Co., Ltd., Chengdu, China), and LT (µm) was measured with ImageJ software (https://imagej.net/software/imagej/, accessed on 10 October 2023).
The dried mixed leaf samples were ground into fine powder using a crusher and then passed through a 0.25 mm mesh screen. The N (mg g−1) and C (mg g−1) concentrations were measured using a Dumas combustion-type C-N elemental analyzer (Vario MAX CN, Elementar Analysensysteme GmbH, Hanau, Germany). The P (mg g−1), K (mg g−1), and Ca (mg g−1) were determined by inductively coupled plasma atomic emission spectrometry (iCAP 7400, Thermo Fisher Scientific, Bremen, Germany). Additionally, we calculated the elemental stoichiometric ratio. The C:P and C:N ratios in leaves can reliably reflect a plant’s growth rate and nutrient use efficiency, illustrating the physiological strategies plants employ in utilizing N and P [23,45]. The N:P ratio as an indicator of nutrient limitation [24,46].
2.4. Data Analyses
At each elevation gradient, the values of leaf functional traits represent the mean of three measurements taken from three mixed leaf samples. We used the Kruskal–Wallis test to examine differences in leaf functional traits among elevation gradients, and conducted Dunn’s test for post hoc pairwise comparisons. Linear regression analysis was applied to assess the relationship between leaf functional traits and elevation gradients. Pearson correlation analysis was conducted to evaluate correlations among the traits. All analyses were performed using R version 4.4.0.
3. Results
Kruskal–Wallis tests revealed that LT was significantly lower at 2000 m than at 2800 m (Figure 1B); SLA was significantly higher at 2000 m than at 2200 m (Figure 1C); leaf P concentration was significantly lower at 2200 m than at 2600 m (Figure 1G); leaf K concentration was significantly higher at 2000 m than at 2400 m (Figure 1H); the C:P ratio was higher at 2200 m than at 2600 m (Figure 1K) and the N:P ratio was significantly higher at 2200 m than at both 2000 m and 2600 m (Figure 1L). In contrast, no significant differences across elevation gradients were detected for LA, LDMC, C, N, Ca concentrations or the C:N ratio (Figure 1A,D–F,I,J).
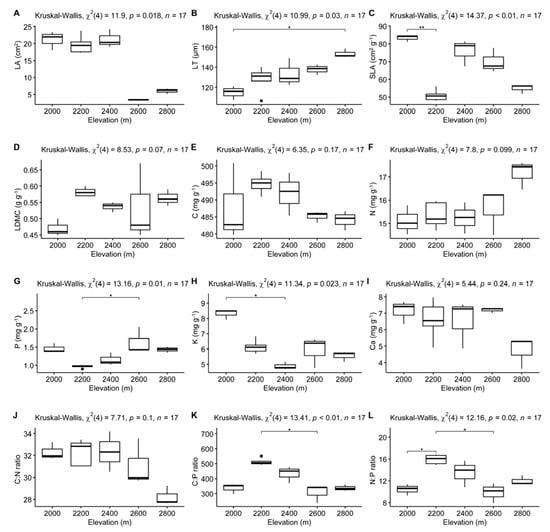
Figure 1.
Kruskal–Wallis test of leaf traits of Quercus rehderiana across different elevation gradients. Significant differences based on Dunn’s post hoc test (Bonferroni-adjusted) are indicated on the plot. * p < 0.05, ** p < 0.01. LA, leaf area; LT, leaf thickness; SLA, specific leaf area; LDMC, leaf dry matter content; C, carbon concentration; N, nitrogen concentration; P, phosphorus concentration; K, potassium concentration; Ca, calcium concentration; C:N, carbon nitrogen ratio; C:P, carbon phosphorus ratio; N:P, nitrogen phosphorus ratio. (A–L) indicates the serial number of figures.
The results of the linear regression analysis indicated a significant decline in LA, K concentration, and the C:N ratio as elevation increased (Figure 2A,H,J). Conversely, LT and N concentration demonstrated a significant upward trend (Figure 2B,F). Other leaf traits, including SLA, LDMC, C, P, Ca concentrations, and C:P and N:P ratios, showed no clear trend along the elevation gradient (Figure 2C–E,G,I,K,L).
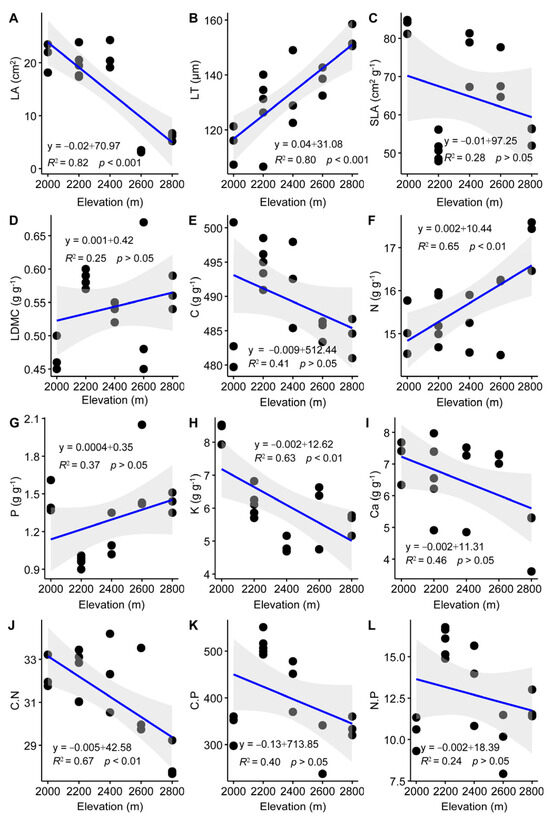
Figure 2.
Linear regression analysis on leaf functional traits of Quercus rehderiana across an elevation gradient. LA, leaf area; LT, leaf thickness; SLA, specific leaf area; LDMC, leaf dry matter content; C, carbon concentration; N, nitrogen concentration; P, phosphorus concentration; K, potassium concentration; Ca, calcium concentration; C:N, carbon nitrogen ratio; C:P, carbon phosphorus ratio; N:P, nitrogen phosphorus ratio. (A–L) indicates the serial number of figures. p < 0.05, significant difference; p > 0.05, no significant difference; r, correlation coefficient.
Correlation analysis (Figure 3) revealed that LA was significantly positively correlated with C concentration (r = 0.52, p < 0.05), C:N (r = 0.53, p < 0.05), C:P (r = 0.68, p < 0.01), and N:P (r = 0.57, p < 0.05) ratios, but significantly negatively correlated with P concentration (r = −0.67, p < 0.01). LT was significantly negatively correlated with LA (r = −0.55, p < 0.05), K concentration (r = −0.50, p < 0.05), and Ca concentration (r = −0.63, p < 0.01). LDMC was significantly negatively correlated with SLA (r = −0.66, p < 0.01) and K (r = −0.61, p < 0.01).
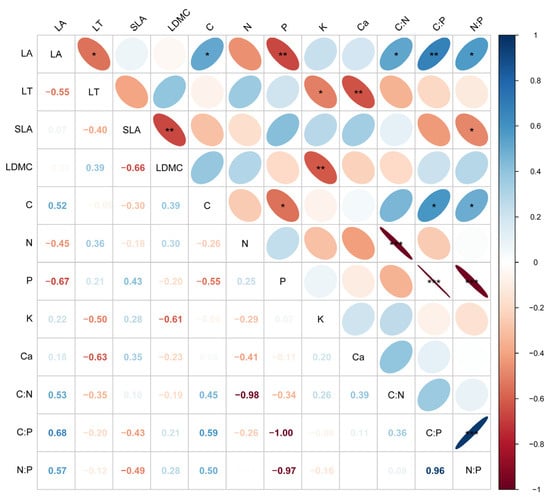
Figure 3.
Pearson correlation analysis of leaf functional traits. LA, leaf area; LT, leaf thickness; SLA, specific leaf area; LDMC, leaf dry matter content; C, carbon concentration; N, nitrogen concentration; P, phosphorus concentration; K, potassium concentration; Ca, calcium concentration; C:N, carbon nitrogen ratio; C:P, carbon phosphorus ratio; N:P, nitrogen phosphorus ratio. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
4. Discussion
In this study, we found that LA concentration increased with elevation, whereas LT decreased. These patterns were partly consistent with our first hypothesis. The results indicate that elevation is a key environmental factor driving leaf trait variation, but different traits exhibit significantly different response patterns to altitudinal changes [47,48]. The reduction in LA is generally considered an adaptive strategy of plants to harsh high-altitude conditions such as strong winds and low temperatures, which helps reduce water transpiration and physical damage [37,39,48,49]. The leaf C:N ratio is a reliable indicator of plant growth rate and nutrient use efficiency, reflecting the plant’s physiological strategy for N and P utilization [23,50]. The decrease in C:N ratio strongly suggests a shift in leaf nutrient use strategy. This is due to increased N concentration (see below) and/or reduced non-structural carbon accumulation, indicating that plants may allocate more resources to nitrogen-rich photosynthetic organs under high-altitude conditions to enhance photosynthetic efficiency [38]. Consistent with our third hypothesis, we observed a significant decrease in leaf K concentration with increasing elevation. The findings suggest that plant growth may be limited by low temperature at high elevations [39,41]. Previous studies found that K primarily functions as an osmotic regulator and enzyme activator in plants, and its demand may be directly related to the plant’s growth rate [51,52]. In high-altitude environments characterized by low temperatures and intense radiation, plant growth and metabolism tend to shift from a “growth-centered” strategy to one focused on “maintenance and defense”. This shift in metabolic activity implies that plants may reduce investment in certain nutrient elements, such as K, which is otherwise utilized for rapid growth [39]. Previous studies on nutrients in soil and leaves across different elevation gradients found that although soil potassium availability showed little change, leaf potassium content still decreased significantly with increasing elevation, further supporting the view that temperature limits plant uptake rather than soil supply [40].
In contrast to the above trends, LT and N concentration showed significant linear increases with altitude. Leaf thickness (LT) tends to increase with rising elevation, which not only enhances the cold resistance of plants but also improves the heat retention capacity of the leaves [53,54]. Meanwhile, the increased LT at high altitudes helps protect against wind-induced damage, reduces water transpiration, and improves water retention in plants [13,14]. These adaptations are crucial for enabling high-altitude plants to survive in harsh ecological environments [55]. The increase in N concentration is a particularly critical finding, as it supports the “Temperature-Plant Physiology Hypothesis” [38] and is consistent with our second hypothesis. In low-temperature, high-altitude environments, plants enhance the nitrogen content in their leaves—likely allocated primarily to ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), which is involved in photosynthesis—to compensate for the rate-limiting effects of low temperatures on biochemical processes, thereby maintaining a high photosynthetic capacity and adapt to environmental stresses characteristic of high altitudes, such as low temperatures, intense light, and short growing seasons [38,56,57].
However, not all traits were sensitive to the altitudinal gradient. Specific leaf area (SLA), LDMC, C, P, and Ca concentrations, as well as C:P and N:P ratios, did not show significant trends with elevation. The stability of SLA and LDMC suggests that, within the altitudinal range of this study, the trade-off between leaf construction cost (dry matter investment) and light capture efficiency may be relatively constant or influenced by other local factors [58]. The stability of P concentration and the resulting lack of trends in C:P and N:P ratios indicate that phosphorus availability in the region may not be a major limiting factor for plant growth. In this study, the leaf N:P ratios of Quercus rehderiana Hand.-Mazz. across different elevation gradients were consistently below 16, providing further evidence that the growth of Quercus rehderiana in this area is not limited by P.
Correlation analysis in this study revealed a series of significant and strong relationships between plant leaf structural traits (e.g., LA, LT, LDMC) and chemical elements (C, N, P) as well as their stoichiometric ratios. The positive correlations of LA with C concentration, C:N ratio, C:P ratio, and N:P ratio. High LA is considered indicative of a plant’s fast resource acquisition strategy, characterized by low tissue construction cost, rapid growth, and short lifespan [58,59]. However, our results indicate that leaves with high LA are not “impoverished” in all elements; rather, they exhibit higher C concentration and lower P concentration. This suggests that these leaves might invest more in structural carbon (e.g., cellulose, lignin) to support their larger area, while simultaneously being relatively “economical” in allocating phosphorus (which is primarily involved in metabolic functions like nucleic acids and ATP) [60]. This trade-off between C and P directly leads to significantly increased C:P and N:P ratios. Therefore, high-LA species represents a strategic endpoint characterized by “high C construction and low P metabolism,” aligning with “fast–slow” plant economics spectrum [61]. The negative correlations of LT with LA, K, and Ca concentrations further corroborate the coordinated variation among plant traits. Thicker leaves are generally associated with enhanced mechanical support and stress resistance, which contrasts with the fast resource acquisition strategy [37,62]. The negative correlations between LT and K and Ca concentrations may indicate differing relative demands or accumulation patterns for these cationic nutrients—involved in physiological regulation and cell wall formation—during the construction of thick-walled, dense leaf tissues [52,63,64]. The strong negative correlation between LDMC and SLA is one of the most robust relationships in the leaf economics spectrum [65], a finding reaffirmed by our results. High LDMC signifies denser leaf tissue with lower water content, associated with slower growth and longer leaf lifespan [14,66].
In summary, Quercus rehderiana adapts to different elevation gradients through alterations in leaf traits. As altitude increases, changes in soil and climatic environmental factors may influence the functional traits and adaptability of plant leaves [36,47,67]. However, this study did not account for the effects of specific soil nutrient contents or environmental variables such as temperature, precipitation, and solar radiation under varying altitude gradients on the leaf functional traits of Quercus rehderiana. Future research should further analyze the impact of these individual factors on leaf functional traits and identify the specific environmental factors that affect leaf functional traits across different altitude gradients.
5. Conclusions
This study investigated the variation in leaf functional traits of Quercus rehderiana Hand.-Mazz. along five elevation gradients by measuring key leaf characteristics. The results showed that LA and K concentration decreased with increasing elevation, while LT and N concentration increased. In addition, close relationships were observed between leaf morphological traits and nutrient-related traits. These findings indicate that Quercus rehderiana adapts to different elevation conditions through modifications in leaf traits and synergistic trait combinations. The results provide valuable insights for concentration, forest management, and conservation. This study did not consider the effects of soil nutrients and global climate change on plant leaf traits. Future research should incorporate long-term monitoring of trait variations across different organs and soil nutrient conditions, which would contribute to a comprehensive understanding of plant adaptation and distribution along elevation gradients under global climate change.
Author Contributions
Conceptualization, X.-L.B. and W.-J.L.; investigation, X.-L.B., B.H., S.Z. and W.-J.L.; methodology, X.-L.B. and B.H.; formal analysis, X.-L.B., S.Z. and W.-J.L.; writing—original draft preparation, X.-L.B. and W.-J.L.; writing—review and editing, X.-L.B. and W.-J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Project of Guizhou Science and Technology Fund (qiankehejichu-QN-[2025]278), the Science and Technology Project of Bijie city of open competition mechanism to select the best candidates (BKHZDZX[2023]1), the Dongfeng Lake and Liuchong River Basin of Observation and Research Station of Guizhou Province (QKHPT YWZ[2025]002), and the Project of Guizhou Science and Technology Fund (qiankehejichu-ZK-[2024]key077).
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank the Weining County Forestry Bureau for providing logistic support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Reich, P.B.; Wright, I.J.; Cavender-Bares, J.; Craine, J.M.; Oleksyn, J.; Westoby, M.; Walters, M.B. The evolution of plant functional variation: Traits, spectra, and strategies. Int. J. Plant Sci. 2003, 164, S143–S164. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Ciccarelli, D.; Bona, C. Exploring the functional strategies adopted by coastal plants along an ecological gradient using morpho-functional traits. Estuaries Coasts 2022, 45, 114–129. [Google Scholar]
- Heilmeier, H. Functional traits explaining plant responses to past and future climate changes. Flora 2019, 254, 1–11. [Google Scholar] [CrossRef]
- Kühn, N.; Tovar, C.; Carretero, J.; Vandvik, V.; Enquist, B.J.; Willis, K.J. Globally important plant functional traits for coping with climate change. Front. Biogeogr. 2021, 13, e53774. [Google Scholar] [CrossRef]
- Henn, J.J.; Anderson, K.E.; Brigham, L.M.; Bueno de Mesquita, C.P.; Collins, C.G.; Elmendorf, S.C.; Green, M.D.; Huxley, J.D.; Rafferty, N.E.; Rose-Person, A.; et al. Long-term alpine plant responses to global change drivers depend on functional traits. Ecol. Lett. 2024, 27, e14518. [Google Scholar] [CrossRef]
- Andrew, S.C.; Gallagher, R.V.; Wright, I.J.; Mokany, K. Assessing the vulnerability of plant functional trait strategies to climate change. Glob. Ecol. Biogeogr. 2022, 31, 1194–1206. [Google Scholar]
- Gratani, L. Plant phenotypic plasticity in response to environmental factors. Adv. Bot. 2014, 2014, 208747. [Google Scholar] [CrossRef]
- Zirbel, C.R.; Bassett, T.; Grman, E.; Brudvig, L.A. Plant functional traits and environmental conditions shape community assembly and ecosystem functioning during restoration. J. Appl. Ecol. 2017, 54, 1070–1079. [Google Scholar] [CrossRef]
- Westoby, M.; Wright, I.J. Land-plant ecology on the basis of functional traits. Trends Ecol. Evol. 2006, 21, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü.; Keenan, T.F.; Hallik, L. A worldwide analysis of within-canopy variations in leaf structural, chemical and physiological traits across plant functional types. New Phytol. 2015, 205, 973–993. [Google Scholar] [CrossRef]
- Li, S.; Lu, S.; Wang, J.; Chen, Z.; Zhang, Y.; Duan, J.; Liu, P.; Wang, X.; Guo, J. Responses of physiological, morphological and anatomical traits to abiotic stress in woody plants. Forests 2023, 14, 1784. [Google Scholar] [CrossRef]
- Yavas, I.; Jamal, M.A.; Ul Din, K.; Ali, S.; Hussain, S.; Farooq, M. Drought-induced changes in leaf morphology and anatomy: Overview, implications and perspectives. Pol. J. Environ. Stud. 2024, 33, 1517–1530. [Google Scholar] [CrossRef]
- Perez-Harguindeguy, N.; Diaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Cornwell, J.M.; Gurvich, D.E.; et al. Corrigendum to: New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2016, 64, 715–716. [Google Scholar] [CrossRef]
- Yin, Q.; Tian, T.; Han, X.; Xu, J.; Chai, Y.; Mo, J.; Lei, M.L.; Wang, L.; Wang, L. The relationships between biomass allocation and plant functional trait. Ecol. Indic. 2019, 102, 302–308. [Google Scholar] [CrossRef]
- Guimarães, Z.T.M.; Dos Santos, V.A.H.F.; Nogueira, W.L.P.; de Almeida Martins, N.O.; Ferreira, M.J. Leaf traits explaining the growth of tree species planted in a Central Amazonian disturbed area. For. Ecol. Manag. 2018, 430, 618–628. [Google Scholar] [CrossRef]
- Sun, M.; Tian, K.; Zhang, Y.; Wang, H.; Guan, X.; Yue, H. Research on leaf functional traits and their environmental adaptation. Plant Sci. J. 2017, 35, 940–949. [Google Scholar]
- Onoda, Y.; Richards, L.; Westoby, M. The importance of leaf cuticle for carbon economy and mechanical strength. New Phytol. 2012, 196, 441–447. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Plant Physiological Ecology; Springer: New York, NY, USA, 1998. [Google Scholar]
- Bhatla, S.C.; Lal, M.A. Plant Physiology, Development and Metabolism; Springer: Singapore, 2023. [Google Scholar]
- Ågren, G.I. The CN:P stoichiometry of autotrophs–theory and observations. Ecol. Lett. 2004, 7, 185–191. [Google Scholar] [CrossRef]
- McGroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C:N:P stoichiometry in forests worldwide: Implications of terrestrial redfield-type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]
- Minden, V.; Kleyer, M. Internal and external regulation of plant organ stoichiometry. Plant Biol. 2014, 16, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Koerselman, W.; Meuleman, A.F. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef]
- Slot, M.; Cala, D.; Aranda, J.; Virgo, A.; Michaletz, S.T.; Winter, K. Leaf heat tolerance of 147 tropical forest species varies with elevation and leaf functional traits, but not with phylogeny. Plant Cell Environ. 2021, 44, 2414–2427. [Google Scholar] [CrossRef]
- Krishna, M.; Winternitz, J.; Garkoti, S.C.; Penuelas, J. Functional leaf traits indicate phylogenetic signals in forests across an elevational gradient in the central Himalaya. J. Plant Res. 2021, 134, 753–764. [Google Scholar] [CrossRef]
- de Freitas, G.V.; Da Cunha, M.; Vitória, A.P. A systematic review of leaf and wood traits in the Neotropics: Environmental gradients and functionality. Trees 2024, 38, 551–572. [Google Scholar] [CrossRef]
- Ren, Y.; Li, J.; Zhang, S.; Shao, J.; Li, X.; Zhong, Q.; Hu, D.; Cheng, D. Leaf trait networks of subtropical woody plants weaken along an elevation gradient. Plant Sci. 2025, 352, 112340. [Google Scholar] [CrossRef]
- Lu, L.M.; Mao, L.F.; Yang, T.; Ye, J.F.; Liu, B.; Li, H.L.; Sun, M.; Miller, J.T.; Mathews, S.; Hu, H.H.; et al. Evolutionary history of the angiosperm flora of China. Nature 2018, 554, 234–238. [Google Scholar] [CrossRef]
- Wang, X.F.; Duan, Y.X.; Jin, L.L.; Wang, C.Y.; Peng, M.C.; Li, Y.; Wang, X.H.; Ma, Y.F. Prediction of historical, present and future distribution of Quercus Sect. Heterobalanus based on the optimized MaxEnt model in China. Acta. Ecol. Sin. 2023, 43, 6590–6604. [Google Scholar]
- Rawat, B.; Rawat, J.M.; Purohit, S.; Singh, G.; Sharma, P.K.; Chandra, A.; Begum, J.P.S.; Venugopal, D.; Jaremko, M.; Qureshi, K.A. A comprehensive review of Quercus semecarpifolia Sm.: An ecologically and commercially important Himalayan tree. Front. Ecol. Evol. 2022, 10, 961345. [Google Scholar] [CrossRef]
- Zhang, Z.; Jian, Y.; Wang, L.; Chen, S.; Li, J.; Yuan, Y.; Li, H.; Tan, B.; Xu, Z. Impact of climate change on the potential suitable habitat of Quercus aquifoliodes in the four southwest provinces of China. J. Northeast For. Univ. 2025, 53, 1–11. [Google Scholar]
- Bai, X.L.; Feng, T.; Zou, S.; He, B.; Chen, Y.; Li, W.J. Differences in Leaf Functional Traits of Quercus rehderiana Hand.-Mazz. in Forests with Rocky and Non-Rocky Desertification in Southwest China. Forests 2024, 15, 1439. [Google Scholar] [CrossRef]
- Wang, C.; Lu, J.; Zhou, C.; Yao, H.; Jiayang, L.; La, B. Altitude distribution of leaf functional traits of Quercus aquifolioides in southeastern Tibet. J. Fores. Environ. 2021, 41, 366–372. [Google Scholar]
- Guo, W.; Zhuo, M.; He, Z.; Ren, Y.; Qu, X.; Fang, J. Anatomical characteristics and environmental adaptability of Quercus aquifolioides leaf in Sejila mountain, Southeastern Tibet. J. Southwest For. Univ. 2022, 42, 33–38. [Google Scholar]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef]
- Körner, C.; Kèorner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Soethe, N.; Lehmann, J.; Engels, C. Nutrient availability at different altitudes in a tropical montane forest in Ecuador. J. Trop. Ecology 2008, 24, 397–406. [Google Scholar] [CrossRef]
- Zhang, S.B.; Zhang, J.L.; Slik, J.F.; Cao, K.F. Leaf element concentrations of terrestrial plants across China are influenced by taxonomy and the environment. Glob. Ecol. Biogeogr. 2012, 21, 809–818. [Google Scholar] [CrossRef]
- Cao, Y. Development status of forest and grass resources in the context of sustainable development and high-quality development countermeasures. For. Sci. Inf. 2024, 56, 178–180. [Google Scholar]
- He, Y.; Zhao, Y.; Zhao, C. Risk assessment of flood in Wumeng Mountainous Area: A case study on Bijie, Guizhou. Guizhou Sci. 2021, 39, 53–59. [Google Scholar]
- Bai, X.; Zou, S.; Feng, T.; He, B.; Li, W. Ecological stoichiometry comparison of the plant-litter-soil system of Quercus rehderiana in different rocky desertification habitats. BMC Plant Biol. 2025, 25, 1021. [Google Scholar] [CrossRef]
- Andersen, T.; Elser, J.J.; Hessen, D.O. Stoichiometry and population dynamics. Ecol. Lett. 2004, 7, 884–900. [Google Scholar] [CrossRef]
- Sardans, J.; Alonso, R.; Janssens, I.A.; Carnicer, J.; Vereseglou, S.; Rillig, M.C.; Fernández-Martínez, M.; Sanders, T.G.M.; Penuelas, J. Foliar and soil concentrations and stoichiometry of nitrogen and phosphorous across European Pinus sylvestris forests: Relationships with climate, N deposition and tree growth. Funct. Ecol. 2016, 30, 676–689. [Google Scholar] [CrossRef]
- Wang, Y.; He, M.; Jiang, G.; Yin, P.; Ying, W.; Yang, Q. Characteristics of leaf functional traits of Quercus spinosa and their response to environmental factors at different altitude gradients in Wumeng township. Acta Ecol. Sin. 2024, 44, 7238–7248. [Google Scholar]
- Chen, J.; Tian, Z.; Zhang, Y.; Wang, J.; Zeng, Z.; La, Q. Response of leaf functional traits of Quercus aquifolioides to environmental factors in the Niyang River. J. Anhui Agric. Sci. 2025, 53, 103–108. [Google Scholar]
- Larcher, W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Zhang, J.; Li, M.; Xu, L.; Zhu, J.; Dai, G.; He, N. C: N:P stoichiometry in terrestrial ecosystems in China. Sci. Total Environ. 2021, 795, 148849. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Peñuelas, J. Potassium control of plant functions: Ecological and agricultural implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, X.; Huang, X.; Cheng, X. An eco-anatomical study on Abies fabri leaves at gradient elevation in Gongga Mountain. J. Southwest For. Univ. 2020, 40, 160–165. [Google Scholar]
- Liu, W.; Zheng, L.; Qi, D. Variation in leaf traits at different altitudes reflects the adaptive strategy of plants to environmental changes. Ecol. Evol. 2020, 10, 8166–8175. [Google Scholar] [CrossRef]
- Miao, Y.; Zong, N.; Li, Z. Research progress on trade-off strategies for functional traits in alpine plants. Chin. J. Appl. Environ. Biol. 2025, 31, 1157–1172. [Google Scholar]
- Reich, P.B.; Walters, M.B. Photosynthesis-nitrogen relations in Amazonian tree species: II. Variation in nitrogen vis-a-vis specific leaf area influences mass-and area-based expressions. Oecologia 1994, 97, 73–81. [Google Scholar] [CrossRef]
- He, J.S.; Wang, Z.; Wang, X.; Schmid, B.; Zuo, W.; Zhou, M.; Zheng, C.; Wang, M.; Fang, J. A test of the generality of leaf trait relationships on the Tibetan Plateau. New Phytol. 2006, 170, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The global spectrum of plant form and function: Enhanced species-level trait dataset. Sci. Data 2022, 9, 755. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Fagan, W.F.; Kerkhoff, A.J.; Swenson, N.G.; Enquist, B.J. Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. New Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide ‘fast-slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Jin, S. Research on the response of plant leaf functional traits to environment. Anhui Agric. Sci. Bull. 2025, 31, 54–58. [Google Scholar]
- Zhou, W.; Wang, H. The physiological and molecular mechanisms of calcium uptake, transport, and metabolism in plants. Chin. Bull. Bot. 2007, 24, 762–778. [Google Scholar]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Wilson, P.J.; Thompson, K.E.N.; Hodgson, J.G. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol. 1999, 143, 155–162. [Google Scholar] [CrossRef]
- Shipley, B.; Vu, T.T. Dry matter content as a measure of dry matter concentration in plants and their parts. New Phytol. 2002, 153, 359–364. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, X.; Liu, X.; Wen, Z.; Liu, S. Characteristics of macronutrient variation in leaves of Quercus aquifolioides along with the altitudinal gradients on the Balangshan Mountain in Wolong Nature Reserve, China. J. Sichuan For. Sci. Technol. 2012, 33, 1–6. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).