Integrative Transcriptomic and Metabolomic Approaches to Deep Pink Flower Color in Prunus campanulata and Insights into Anthocyanin Biosynthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Sample Collection
2.2. Floral Morphometric Analysis
2.3. Physiological Index Measurement
2.4. Effects of Physico-Chemical Factors on Petal Color
2.5. RNA Extraction, Testing, and Transcriptome Sequencing
2.6. Transcriptome Assembly and Annotation
2.7. Data Processing
3. Results
3.1. Analysis of Growth Indicators
3.2. Analyses of Physiological Parameters and Color-Related Substance Contents in the Process of Petal Development
3.3. Correlation Analysis of Flower Pigmentation
3.4. Effects of Physicochemical Factors on the Coloration of Flavonoid Extracts from Petals
3.5. Metabolome Analysis of Prunus campanulata Petals
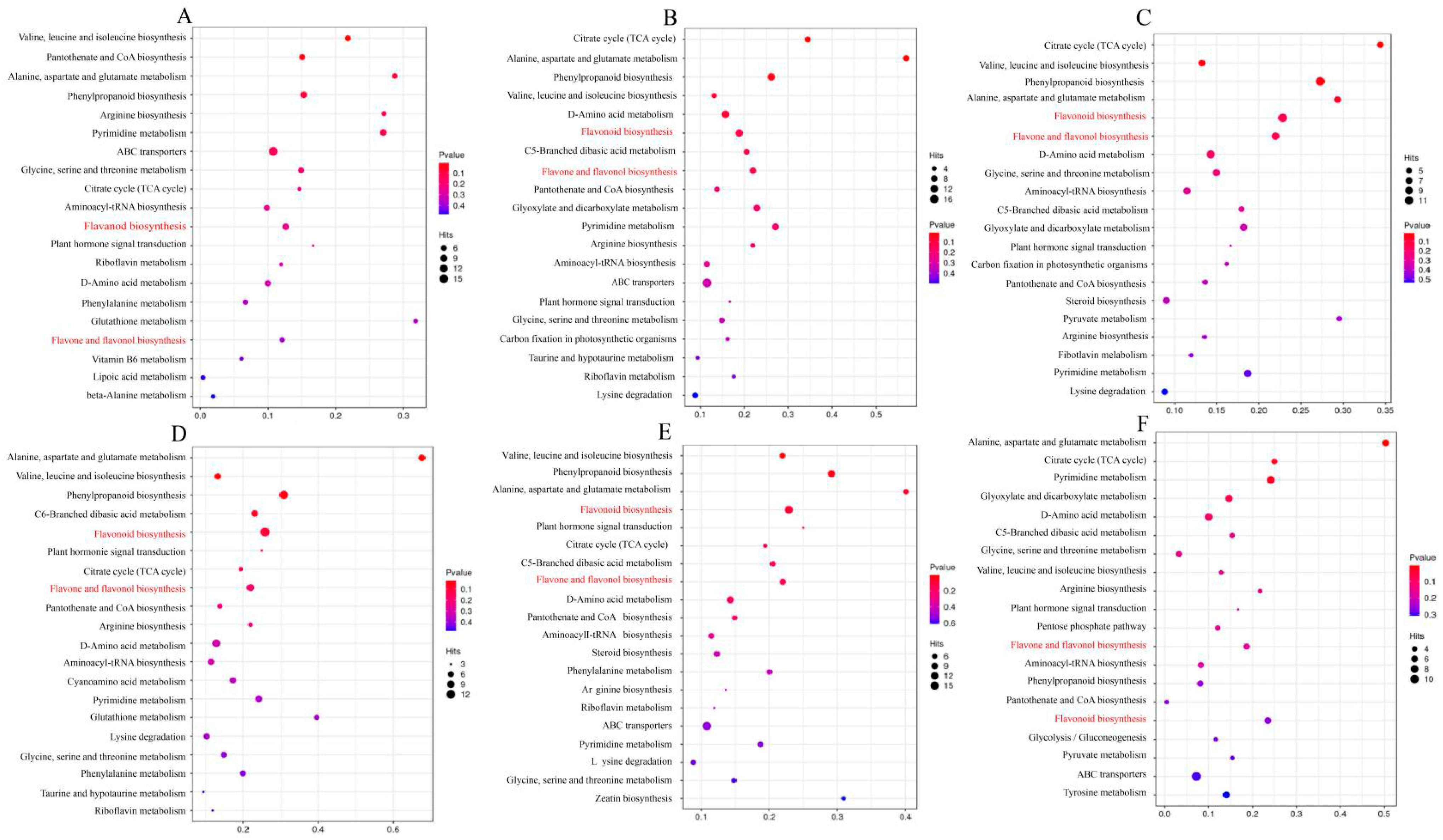
3.6. Functional Enrichment Analysis of Differential Metabolites
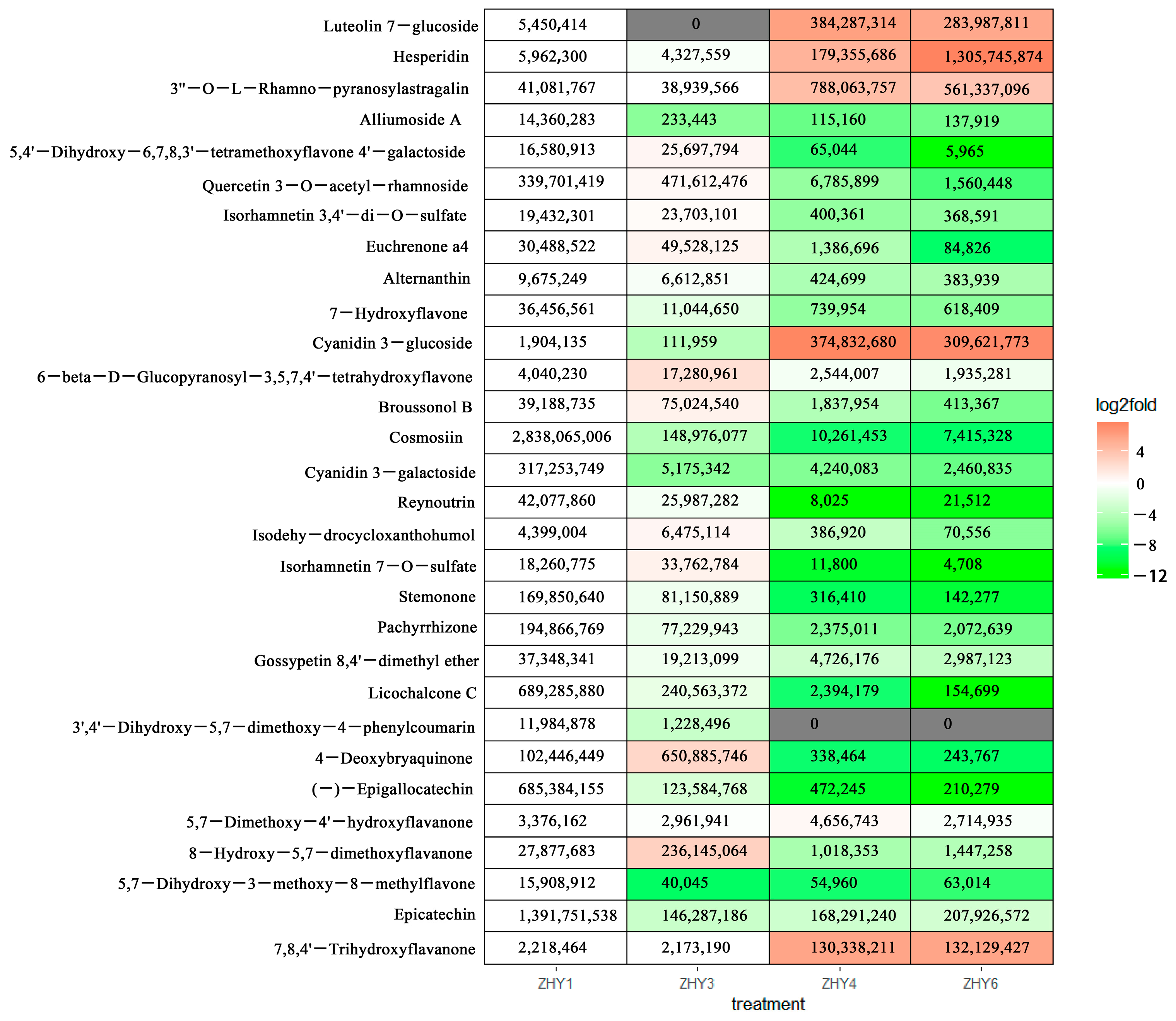
3.7. Analysis of Differential Flavonoid Metabolites
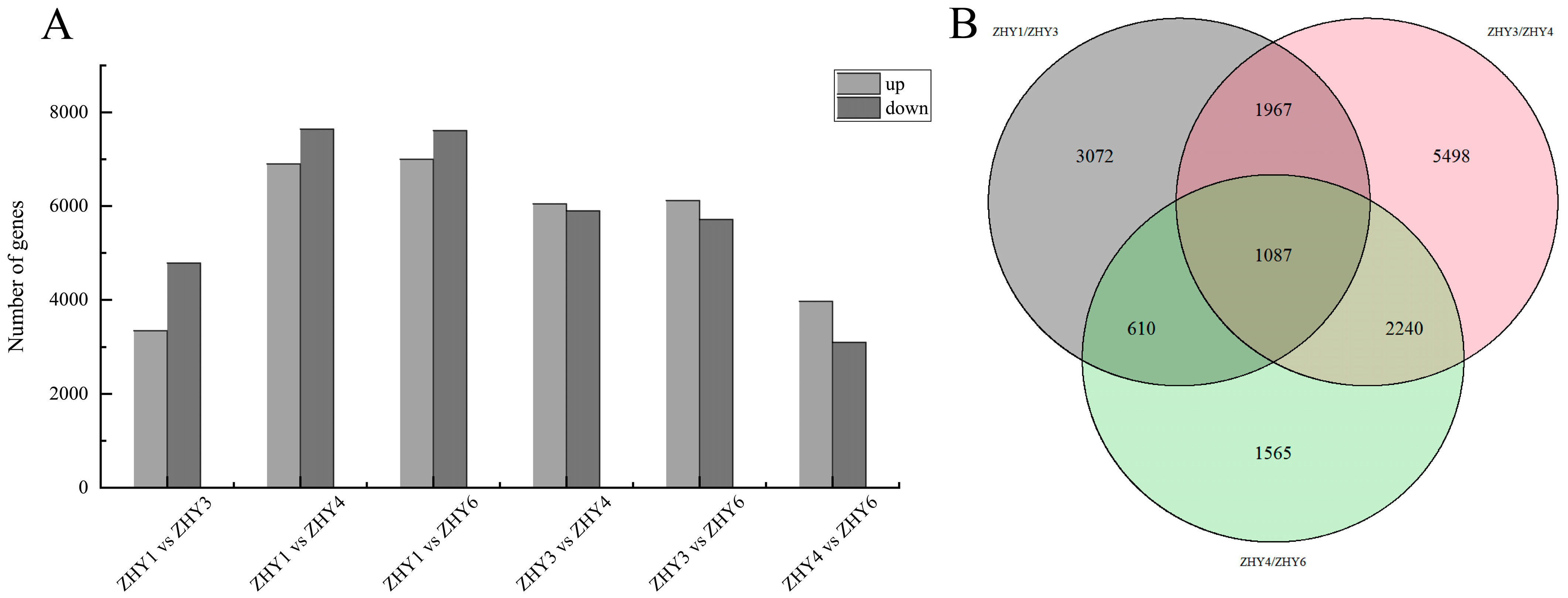
3.8. Transcriptome Sequencing Analysis over the Course of Petal Development
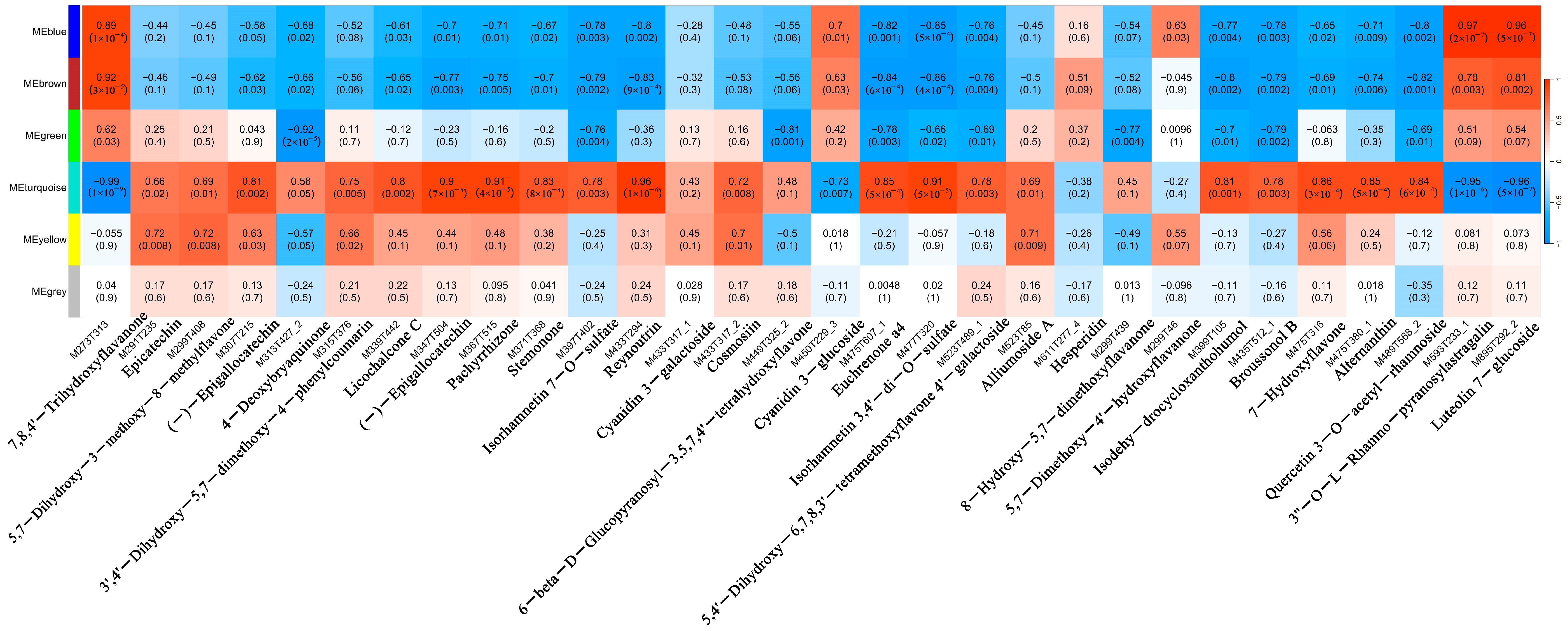
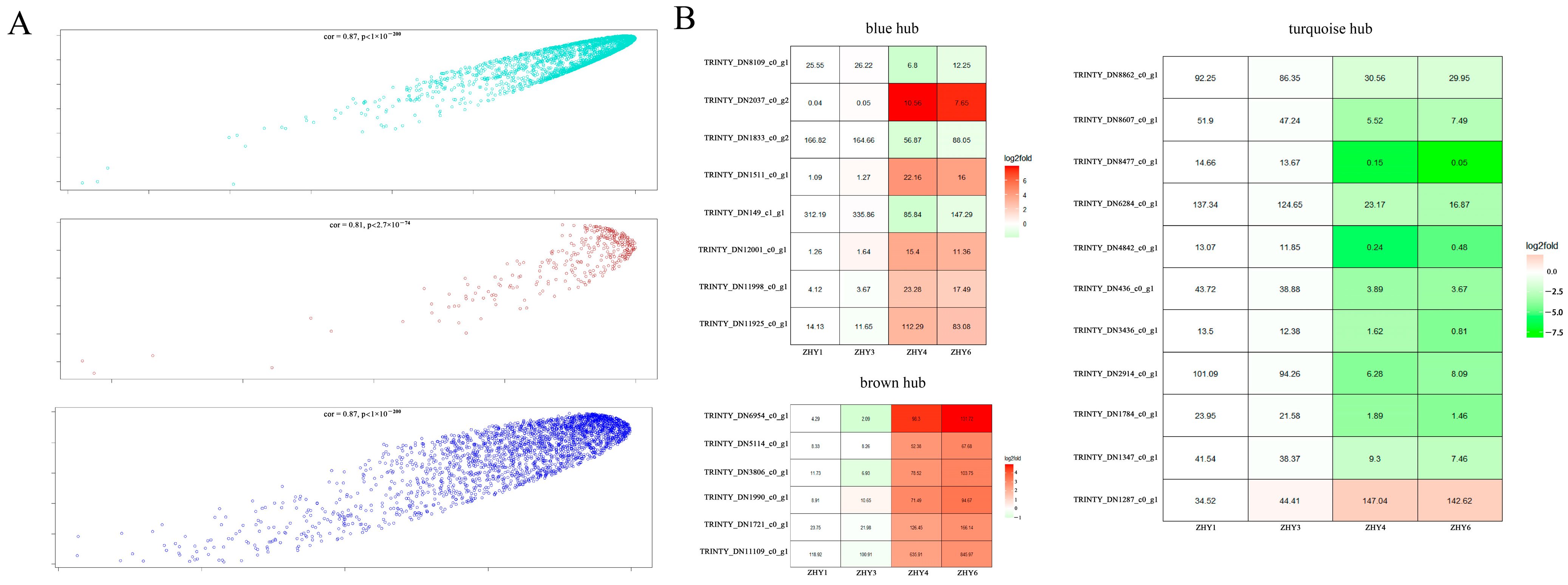
3.9. Analysis of Key Gene Modules and Their Association with Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCA | Principal component analysis |
| CHS | chalcone synthase |
| CHI | chalcone isomerase |
| F3′H | flavonoid-3′-hydroxylase |
| F3′5′H | flavonoid-3′,5′-hydroxylase |
| bHLH | basic Helix–Loop–Helix |
| UFGT | UDP-glucose flavonoid 3-O-glucosyltransferase |
| WGCNA | Weighted gene co-expression network analysis |
| TIC | Total ion chromatogram |
| OPLS-DA | Orthogonal partial least squares-discriminant analysis |
| Cy3G | Cyanidin-3-O-glucoside |
| Cy3Gal | Cyanidin-3-O-galactoside |
References
- Dorin, A.; Shrestha, M.; Garcia, J.E.; Burd, M.; Dyer, A.G. Ancient Insect Vision Tuned for Flight among Rocks and Plants Underpins Natural Flower Colour Diversity. Proc. R. Soc. B Biol. Sci. 2023, 290, 20232018. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, P.; Jiang, Y.; Li, J.; Chang, J.; Zhang, H.; Shao, H.; Zhou, Y. Integrated Metabolome and Transcriptome Analysis of Petal Anthocyanin Accumulation Mechanism in Gloriosa superba ‘Rothschildiana’ during Different Flower Development Stages. Int. J. Mol. Sci. 2023, 24, 15034. [Google Scholar] [CrossRef]
- Ou, Z.; Luo, J.; Qu, Y. Exploring the Molecular Mechanism of Coloration Differences in Two Meconopsis wilsonii Subspecies: Australis and Orientalis. Dev. Biol. 2024, 505, 1–10. [Google Scholar] [CrossRef]
- Van Der Kooi, C.J.; Dyer, A.G.; Kevan, P.G.; Lunau, K. Functional Significance of the Optical Properties of Flowers for Visual Signalling. Ann. Bot. 2019, 123, 263–276. [Google Scholar] [CrossRef]
- Whitney, H.M.; Bennett, K.M.V.; Dorling, M.; Sandbach, L.; Prince, D.; Chittka, L.; Glover, B.J. Why Do So Many Petals Have Conical Epidermal Cells? Ann. Bot. 2011, 108, 609–616. [Google Scholar] [CrossRef]
- Morita, Y.; Hoshino, A. Recent Advances in Flower Color Variation and Patterning of Japanese Morning Glory and Petunia. Breed. Sci. 2018, 68, 128–138. [Google Scholar] [CrossRef] [PubMed]
- De Jager, M.L.; Dreyer, L.L.; Ellis, A.G. Do Pollinators Influence the Assembly of Flower Colours within Plant Communities? Oecologia 2011, 166, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.T.; Yokoyama, J. Nonrandom Composition of Flower Colors in a Plant Community: Mutually Different Co-Flowering Natives and Disturbance by Aliens. PLoS ONE 2015, 10, e0143443. [Google Scholar] [CrossRef]
- Prieto-Benítez, S.; Dötterl, S.; Giménez-Benavides, L. Circadian Rhythm of a Silene Species Favours Nocturnal Pollination and Constrains Diurnal Visitation. Ann. Bot. 2016, 118, 907–918. [Google Scholar] [CrossRef]
- Akita, Y.; Morimura, S.; Loetratsami, P.; Ishizaka, H. A Review of Research on Flower-Colored Mutants of Fragrant Cyclamens Induced by Ion-Beam Irradiation. Hortic. Int. J. 2017, 1, 90–91. [Google Scholar] [CrossRef]
- Nakatsuka, T.; Sasaki, N.; Nishihara, M. Transcriptional Regulators of Flavonoid Biosynthesis and Their Application to Flower Color Modification in Japanese Gentians. Plant Biotechnol. 2014, 31, 389–399. [Google Scholar] [CrossRef]
- Conrad, A.C.; Mathabatha, M.F. Elucidating Variable Traits of Flower Pigments in Clivian Plants’ Species. Vegetos Int. J. Plant Res. 2017, 30, 9. [Google Scholar] [CrossRef]
- Verma, D.; Sharma, N.; Malhotra, U. Structural Chemistry and Stability of Anthocyanins. Pharma Innov. 2023, 12, 1366–1373. [Google Scholar] [CrossRef]
- Davies, K.M.; Landi, M.; Van Klink, J.W.; Schwinn, K.E.; Brummell, D.A.; Albert, N.W.; Chagné, D.; Jibran, R.; Kulshrestha, S.; Zhou, Y.; et al. Evolution and Function of Red Pigmentation in Land Plants. Ann. Bot. 2022, 130, 613–636. [Google Scholar] [CrossRef] [PubMed]
- Chandler, S.F.; Senior, M.; Nakamura, N.; Tsuda, S.; Tanaka, Y. Expression of Flavonoid 3′,5′-Hydroxylase and Acetolactate Synthase Genes in Transgenic Carnation: Assessing the Safety of a Nonfood Plant. J. Agric. Food Chem. 2013, 61, 11711–11720. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zheng, T.; Yang, Y.; Li, P.; Qiu, L.; Li, L.; Wang, J.; Cheng, T.; Zhang, Q. Meta-Analysis of the Effect of Overexpression of MYB Transcription Factors on the Regulatory Mechanisms of Anthocyanin Biosynthesis. Front. Plant Sci. 2021, 12, 781343. [Google Scholar] [CrossRef]
- Guo, J.; Han, W.; Wang, M.-H. Ultraviolet and Environmental Stresses Involved in the Induction and Regulation of Anthocyanin Biosynthesis: A Review. Afr. J. Biotechnol. 2008, 7, 4966–4972. [Google Scholar]
- Zhang, D.; Teng, Y. Germplasm Resources of Red Pears and Research Progress in Pear Coloring Mechanism. J. Fruit. Sci. 2011, 28, 485–492. [Google Scholar]
- Zumajo-Cardona, C.; Gabrieli, F.; Anire, J.; Albertini, E.; Ezquer, I.; Colombo, L. Evolutionary Studies of the bHLH Transcription Factors Belonging to MBW Complex: Their Role in Seed Development. Ann. Bot. 2023, 132, 383–400. [Google Scholar] [CrossRef]
- Hao, Y.; Zong, X.; Ren, P.; Qian, Y.; Fu, A. Basic Helix-Loop-Helix (bHLH) Transcription Factors Regulate a Wide Range of Functions in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 7152. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, L.; Liu, W.; Zhang, J.; Wang, N.; Chen, X. Research Progress of Fruit Color Development in Apple (Malus domestica Borkh.). Plant Physiol. Biochem. 2021, 162, 267–279. [Google Scholar] [CrossRef]
- Yousuf, B.; Gul, K.; Wani, A.A.; Singh, P. Health Benefits of Anthocyanins and Their Encapsulation for Potential Use in Food Systems: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2223–2230. [Google Scholar] [CrossRef]
- Alam, M.A.; Islam, P.; Subhan, N.; Rahman, M.M.; Khan, F.; Burrows, G.E.; Nahar, L.; Sarker, S.D. Potential Health Benefits of Anthocyanins in Oxidative Stress Related Disorders. Phytochem. Rev. 2021, 20, 705–749. [Google Scholar] [CrossRef]
- Liu, W.; Nan, G.; Nisar, M.F.; Wan, C. Chemical Constituents and Health Benefits of Four Chinese Plum Species. J. Food Qual. 2020, 2020, 8842506. [Google Scholar] [CrossRef]
- Winter, A.N.; Bickford, P.C. Anthocyanins and Their Metabolites as Therapeutic Agents for Neurodegenerative Disease. Antioxidants 2019, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- De Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The Pharmacokinetics of Anthocyanins and Their Metabolites in Humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef] [PubMed]
- Cásedas, G.; Les, F.; López, V. Anthocyanins: Plant Pigments, Food Ingredients or Therapeutic Agents for the CNS? A Mini-Review Focused on Clinical Trials. Curr. Pharm. Des. 2020, 26, 1790–1798. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Qian, Y.; Shan, L.; Zhao, R.; Tang, J.; Zhang, C.; Chen, M.; Duan, Y.; Zhu, F. Recent Advances in Flower Color and Fragrance of Osmanthus fragrans. Forests 2023, 14, 1403. [Google Scholar] [CrossRef]
- Choudhury, M.R.; Islam, M.S.; Ahmed, Z.U.; Nayar, F. Phytoremediation of Heavy Metal Contaminated Buriganga Riverbed Sediment by Indian Mustard and Marigold Plants. Environ. Prog. Sustain. Energy 2016, 35, 117–124. [Google Scholar] [CrossRef]
- Zhukov, A.V. Palmitic Acid and Its Role in the Structure and Functions of Plant Cell Membranes. Russ. J. Plant Physiol. 2015, 62, 706–713. [Google Scholar] [CrossRef]
- Roidoung, S.; Dolan, K.D.; Siddiq, M. Estimation of Kinetic Parameters of Anthocyanins and Color Degradation in Vitamin C Fortified Cranberry Juice during Storage. Food Res. Int. 2017, 94, 29–35. [Google Scholar] [CrossRef]
- Hosoda, K.; Sasahara, H.; Matsushita, K.; Tamura, Y.; Miyaji, M.; Matsuyama, H. Anthocyanin and Proanthocyanidin Contents, Antioxidant Activity, and in Situ Degradability of Black and Red Rice Grains. Asian-Australas J Anim Sci 2018, 31, 1213–1220. [Google Scholar] [CrossRef]
- Qiao, H.; Zhao, W.; Tian, S.; Wang, D.; Wu, H.; Wang, C.; Zhu, J.; Li, N.; Zhu, X.; Mu, S.; et al. Exploring the Mechanisms Underlying Petal Pigmentation Differences in Two Cultivars of Physalis philadelphica Based on HPLC and NGS. Horticulturae 2024, 10, 507. [Google Scholar] [CrossRef]
- Yuan, Y.-W.; Rebocho, A.B.; Sagawa, J.M.; Stanley, L.E.; Bradshaw, H.D. Competition between Anthocyanin and Flavonol Biosynthesis Produces Spatial Pattern Variation of Floral Pigments between Mimulus Species. Proc. Natl. Acad. Sci. USA 2016, 113, 2448–2453. [Google Scholar] [CrossRef]
- Li, B.-J.; Zheng, B.-Q.; Wang, J.-Y.; Tsai, W.-C.; Lu, H.-C.; Zou, L.-H.; Wan, X.; Zhang, D.-Y.; Qiao, H.-J.; Liu, Z.-J.; et al. New Insight into the Molecular Mechanism of Colour Differentiation among Floral Segments in Orchids. Commun. Biol. 2020, 3, 89. [Google Scholar] [CrossRef]
- Tanaka, Y.; Brugliera, F. Metabolic Engineering of Flower Color Pathways Using Cytochromes P450. In Fifty Years of Cytochrome P450 Research; Springer: Tokyo, Japan, 2014; pp. 207–229. [Google Scholar] [CrossRef]
- Sánchez-Cabrera, M.; Jiménez-López, F.J.; Narbona, E.; Arista, M.; Ortiz, P.L.; Romero-Campero, F.J.; Ramanauskas, K.; Igić, B.; Fuller, A.A.; Whittall, J.B. Changes at a Critical Branchpoint in the Anthocyanin Biosynthetic Pathway Underlie the Blue to Orange Flower Color Transition in Lysimachia Arvensis. Front. Plant Sci. 2021, 12, 633979. [Google Scholar] [CrossRef]
- Meng, X.; Li, G.; Gu, L.; Sun, Y.; Li, Z.; Liu, J.; Wu, X.; Dong, T.; Zhu, M. Comparative Metabolomic and Transcriptome Analysis Reveal Distinct Flavonoid Biosynthesis Regulation Between Petals of White and Purple Phalaenopsis Amabilis. J. Plant Growth Regul. 2020, 39, 823–840. [Google Scholar] [CrossRef]
- Yan, H.; Pei, X.; Zhang, H.; Li, X.; Zhang, X.; Zhao, M.; Chiang, V.L.; Sederoff, R.R.; Zhao, X. MYB-Mediated Regulation of Anthocyanin Biosynthesis. Int. J. Mol. Sci. 2021, 22, 3103. [Google Scholar] [CrossRef]
- Gou, J.-Y.; Felippes, F.F.; Liu, C.-J.; Weigel, D.; Wang, J.-W. Negative Regulation of Anthocyanin Biosynthesis in Arabidopsis by a miR156-Targeted SPL Transcription Factor. Plant Cell 2011, 23, 1512–1522. [Google Scholar] [CrossRef]
- Petroni, K.; Tonelli, C. Recent Advances on the Regulation of Anthocyanin Synthesis in Reproductive Organs. Plant Sci. 2011, 181, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, T.; Saito, M.; Yamada, E.; Fujita, K.; Kakizaki, Y.; Nishihara, M. Isolation and Characterization of GtMYBP3 and GtMYBP4, Orthologues of R2R3-MYB Transcription Factors That Regulate Early Flavonoid Biosynthesis, in Gentian Flowers. J. Exp. Bot. 2012, 63, 6505–6517. [Google Scholar] [CrossRef]
- Nakatsuka, T.; Yamada, E.; Saito, M.; Fujita, K.; Nishihara, M. Heterologous Expression of Gentian MYB1R Transcription Factors Suppresses Anthocyanin Pigmentation in Tobacco Flowers. Plant Cell Rep. 2013, 32, 1925–1937. [Google Scholar] [CrossRef]
- Albert, N.W.; Davies, K.M.; Schwinn, K.E. Gene Regulation Networks Generate Diverse Pigmentation Patterns in Plants. Plant Signal. Behav. 2014, 9, e29526. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, Y.; Dong, Q.; Wang, F.; Kong, F.; Liu, G.; Lei, Y.; Yang, H.; Zhou, Y.; Li, C. Weighted Gene Co-Expression Network Analysis Reveals Key Module and Hub Genes Associated with the Anthocyanin Biosynthesis in Maize Pericarp. Front. Plant Sci. 2022, 13, 1013412. [Google Scholar] [CrossRef]
- Piatkowski, B.T.; Imwattana, K.; Tripp, E.A.; Weston, D.J.; Healey, A.; Schmutz, J.; Shaw, A.J. Phylogenomics Reveals Convergent Evolution of Red-Violet Coloration in Land Plants and the Origins of the Anthocyanin Biosynthetic Pathway. Mol. Phylogenet. Evol. 2020, 151, 106904. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.T.; Verwoerd, W.S. A Systems Approach to Identifying Correlated Gene Targets for the Loss of Colour Pigmentation in Plants. BMC Bioinf. 2011, 12, 343. [Google Scholar] [CrossRef]
- Sintupachee, S.; Ngamrojanavanich, N.; Sitthithaworn, W.; De-Eknamkul, W. Molecular Cloning, Bacterial Expression and Functional Characterisation of Cytochrome P450 Monooxygenase, CYP97C27, and NADPH-Cytochrome P450 Reductase, CPR I, from Croton stellatopilosus Ohba. Plant Sci. 2014, 229, 131–141. [Google Scholar] [CrossRef]
- Kaspera, R.; Naraharisetti, S.B.; Evangelista, E.A.; Marciante, K.D.; Psaty, B.M.; Totah, R.A. Drug Metabolism by CYP2C8.3 Is Determined by Substrate Dependent Interactions with Cytochrome P450 Reductase and Cytochrome B5. Biochem. Pharmacol. 2011, 82, 681–691. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 Enzymes in Drug Metabolism: Regulation of Gene Expression, Enzyme Activities, and Impact of Genetic Variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Wulandari, R.; Budiyanto, M.A.K.; Waluyo, L. The Influence of Various Concentration of Red Roses (Rosa damascena Mill) Flower Extract to Anthocyanin Color Stability Jelly as Biology Learning Source. JPBI J. Pendidik. Biol. Indones. 2016, 2, 48–56. [Google Scholar] [CrossRef]
- Yudiono, K. Effect of Maltodextrin Concentrations and Drying Temperature on the Physico-Chemical Characteristics and Color Measurements of Butterfly Pea Flowers (Clitoria ternatea L) Powder. Turk. J. Agric. Food Sci. Technol. 2024, 12, 657–665. [Google Scholar] [CrossRef]
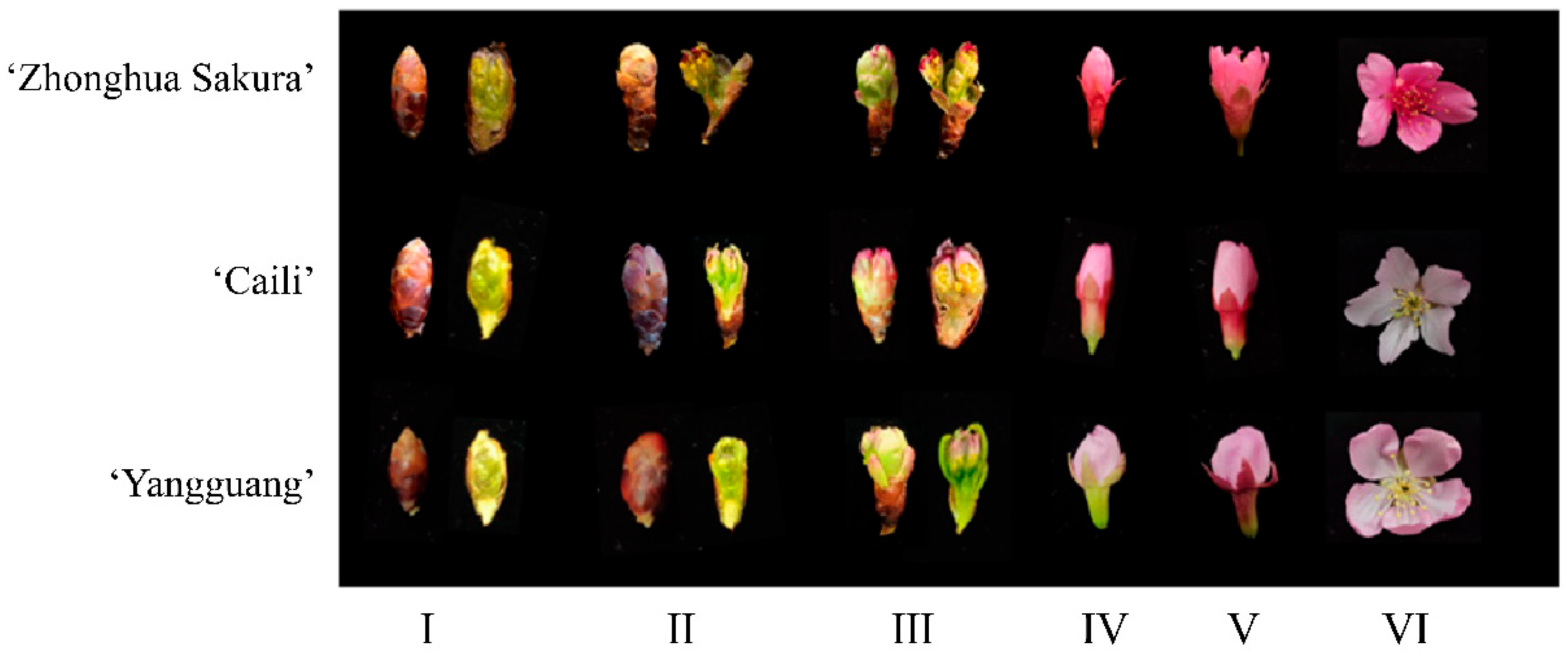
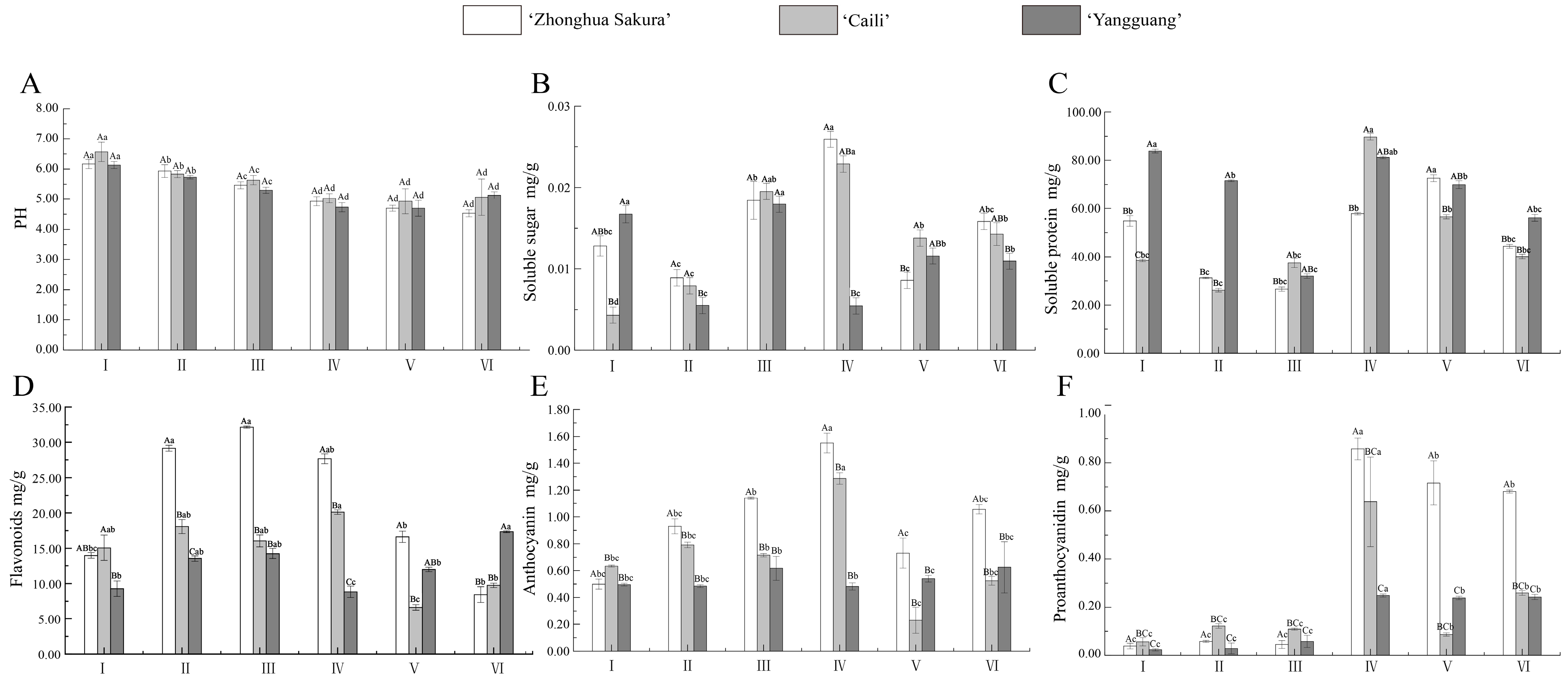

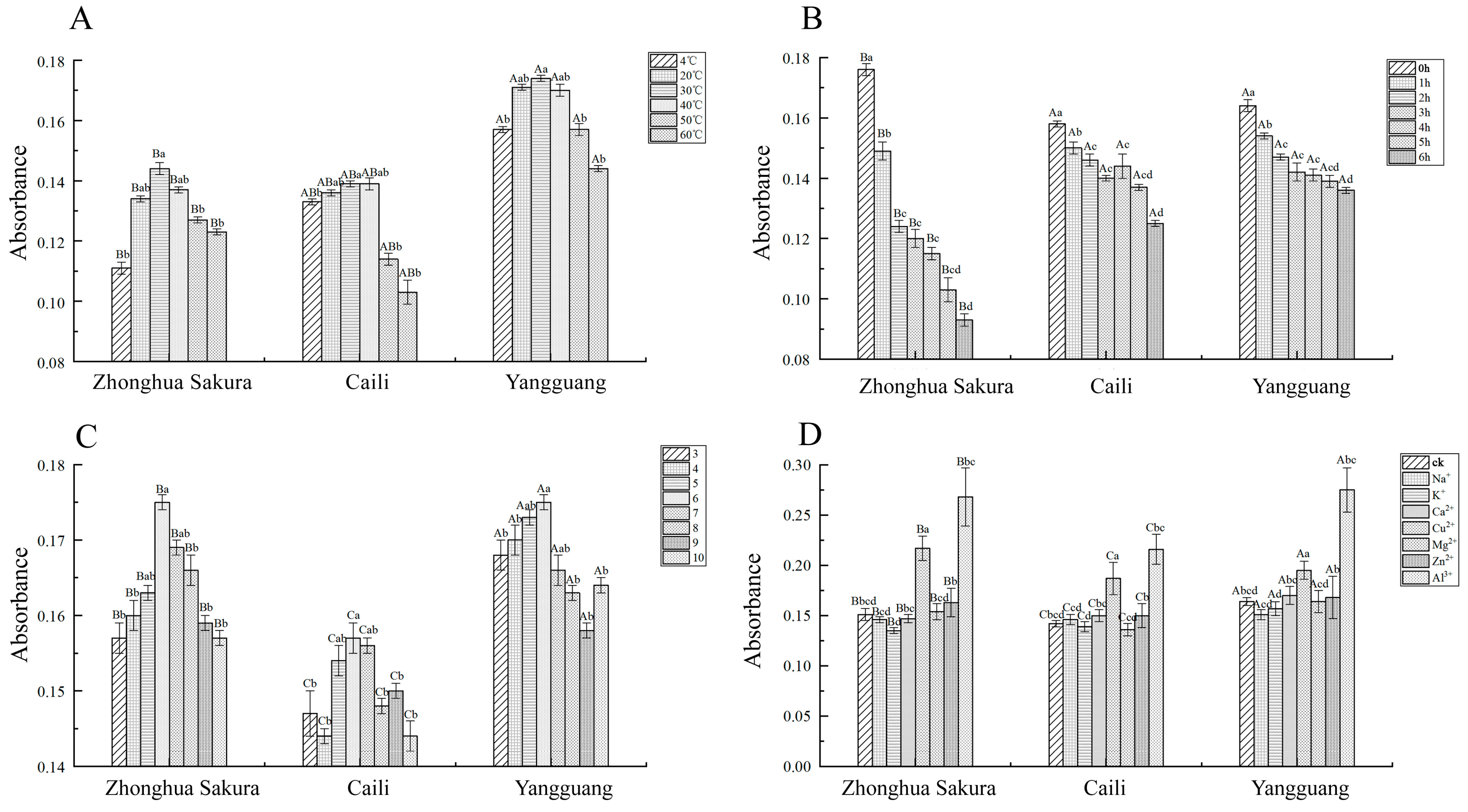
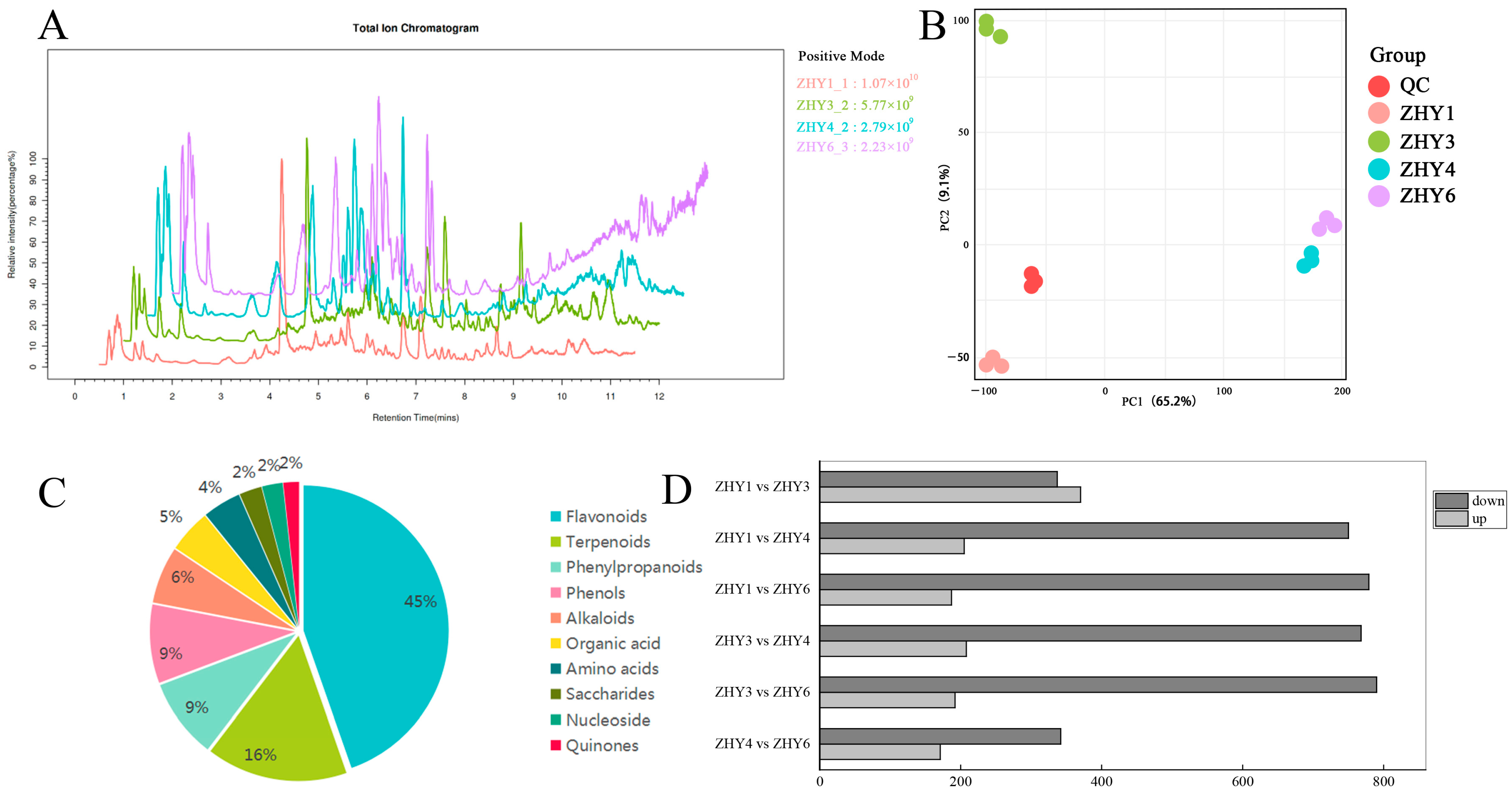
| Cultivars | Growth Indicator | Times | |||||
|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | ||
| ‘Zhonghua Sakura’ | Flower length | 5.80 ± 0.76 Be | 8.37 ± 0.73 Bd | 12.22 ± 1.40 Bc | 14.48 ± 1.26 Bb | 17.07 ± 0.71 Ba | 12.67 ± 0.99 Bc |
| Flower width | 1.94 ± 0.31 Be | 2.84 ± 0.82 Bd | 5.75 ± 1.01 Bc | 5.76 ± 0.55 Bc | 7.94 ± 1.68 Bb | 8.78 ± 0.84 Ba | |
| Flower Crown | 21.99 ± 2.75 B | ||||||
| ‘Caili’ | Flower length | 4.50 ± 0.45 Be | 6.63 ± 0.78 Bd | 8.16 ± 0.95 Bc | 11.21 ± 1.09 Bb | 17.21 ± 1.31 Ba | 11.51 ± 1.11 Bb |
| Flower width | 1.87 ± 0.33 Be | 2.36 ± 0.47 Bd | 3.52 ± 0.42 Bc | 3.91 ± 0.65 Bc | 6.02 ± 0.76 Bb | 7.45 ± 1.81 Ba | |
| Flower Crown | 21.19 ± 4.40 B | ||||||
| ‘Yangguang’ | Flower length | 7.88 ± 0.66 Be | 9.77 ± 0.62 Bd | 16.23 ± 1.71 Bc | 16.41 ± 1.67 Bc | 21.26 ± 1.70 Ba | 19.01 ± 1.00 Bb |
| Flower width | 3.28 ± 0.34 Be | 4.33 ± 0.34 Bd | 6.33 ± 0.79 Bc | 6.56 ± 0.83 Bc | 9.17 ± 2.33 Bb | 16.87 ± 1.43 Ba | |
| Flower Crown | 40.91 ± 2.34 A | ||||||
| Lightness | Redness | Blueness | pH | Soluble Sugar | Soluble Protein | Total Flavonoids | Proanthocyanidins | Anthocyanins | Chlorophyll | Carotenoids | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lightness | 1 | ||||||||||
| Redness | −0.976 ** | 1 | |||||||||
| Blueness | 0.692 ** | −0.672 ** | 1 | ||||||||
| pH | 0.489 * | −0.477 * | 0.408 | 1 | |||||||
| Soluble Sugar | 0.481 * | −0.550 * | 0.039 | −0.011 | 1 | ||||||
| Soluble protein | −0.048 | 0.114 | 0.123 | −0.170 | −0.808 ** | 1 | |||||
| Total flavonoids | 0.162 | −0.177 | −0.019 | 0.119 | −0.798 ** | 0.529 * | 1 | ||||
| Proanthocyanidins | −0.484 * | 0.446 | −0.833 ** | −0.232 | 0.105 | −0.157 | 0.218 | 1 | |||
| Anthocyanins | −0.636 ** | 0.612 ** | −0.865 ** | −0.416 | 0.062 | −0.108 | 0.134 | 0.877 ** | 1 | ||
| Chlorophyll | −0.418 | 0.421 | −0.424 | −0.092 | −0.267 | 0.007 | 0.398 | 0.400 | 0.480 * | 1 | |
| Carotenoids | −0.640 ** | 0.604 ** | −0.658 ** | −0.310 | 0.198 | −0.223 | −0.056 | 0.438 | 0.653 ** | 0.399 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Y.; Cao, S.; Wang, Y.; Zhu, J.; Fang, X.; Ou, G.; Shu, M.; Zhou, W.; Yang, W.; Yu, L.; et al. Integrative Transcriptomic and Metabolomic Approaches to Deep Pink Flower Color in Prunus campanulata and Insights into Anthocyanin Biosynthesis. Forests 2025, 16, 1633. https://doi.org/10.3390/f16111633
Wen Y, Cao S, Wang Y, Zhu J, Fang X, Ou G, Shu M, Zhou W, Yang W, Yu L, et al. Integrative Transcriptomic and Metabolomic Approaches to Deep Pink Flower Color in Prunus campanulata and Insights into Anthocyanin Biosynthesis. Forests. 2025; 16(11):1633. https://doi.org/10.3390/f16111633
Chicago/Turabian StyleWen, Yuxing, Shoujin Cao, Yuxin Wang, Jianchao Zhu, Xudong Fang, Guangmei Ou, Man Shu, Wei Zhou, Wenhai Yang, Lin Yu, and et al. 2025. "Integrative Transcriptomic and Metabolomic Approaches to Deep Pink Flower Color in Prunus campanulata and Insights into Anthocyanin Biosynthesis" Forests 16, no. 11: 1633. https://doi.org/10.3390/f16111633
APA StyleWen, Y., Cao, S., Wang, Y., Zhu, J., Fang, X., Ou, G., Shu, M., Zhou, W., Yang, W., Yu, L., & Yang, Y. (2025). Integrative Transcriptomic and Metabolomic Approaches to Deep Pink Flower Color in Prunus campanulata and Insights into Anthocyanin Biosynthesis. Forests, 16(11), 1633. https://doi.org/10.3390/f16111633





