Effects of Humac and Alginite Fertilization on Mite Communities (Acari, Mesostigmata) Under Post-Agricultural Land Conditions
Abstract
1. Introduction
2. Materials and Methods
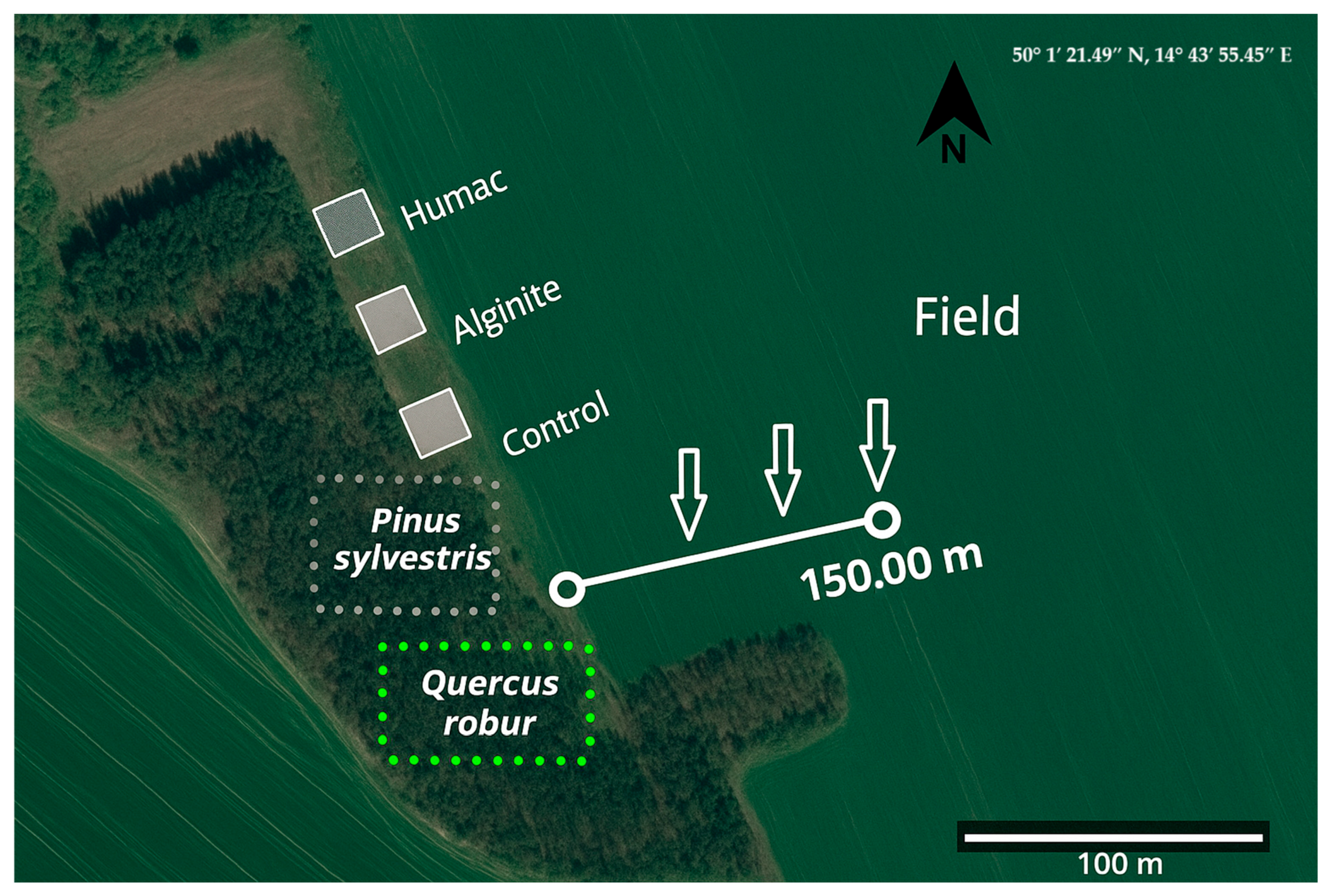
2.1. Site Description and Experimental Design
2.2. Mesostigmata Mites Investigation
2.3. Data Analysis
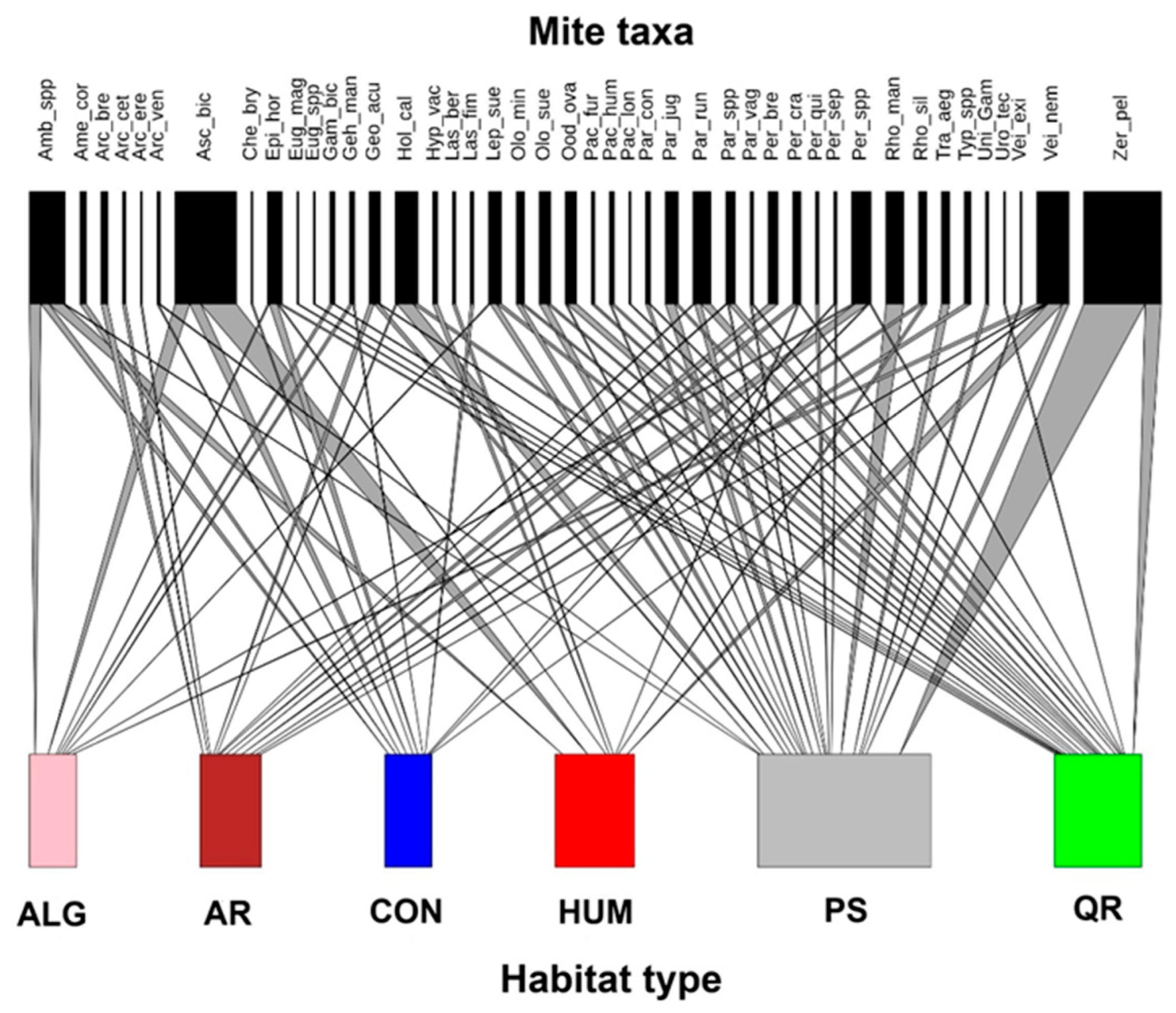
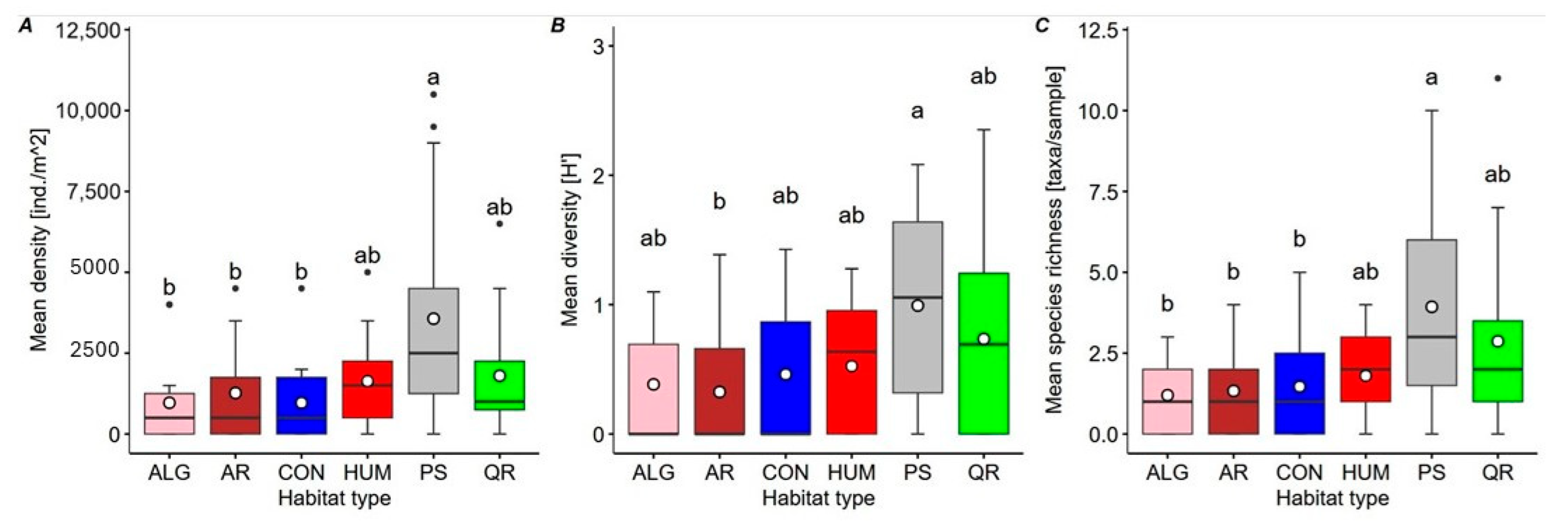
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Mite Taxa | Abbreviation | Family | Control | Humac | Alginite | Quercus robur | Pinus sylvestris | Arable Field |
|---|---|---|---|---|---|---|---|---|
| Amblyseius spp. | Amb_spp | Phytoseiidae | ||||||
| Ameroseius corbiculus (Sowerby, 1806) | Ame_cor | Ameroseiidae | ||||||
| Arctoseius brevichelis Karg, 1969 | Arc_bre | Ascidae | ||||||
| Arctoseius cetratus (Sellnick, 1940) | Arc_cet | Ascidae | ||||||
| Arctoseius eremitus (Berlese, 1918) | Arc_ere | Ascidae | ||||||
| Arctoseius venustulus (Berlese, 1917) | Arc_ven | Ascidae | ||||||
| Asca bicornis (Canestrini & Fanzago, 1887) | Asc_bic | Ascidae | ||||||
| Cheiroseius bryophilus Karg, 1969 | Che_bry | Ascidae | ||||||
| Epicriopsis horridus (Kramer, 1876) | Epi-hor | Ameroseiidae | ||||||
| Eugamasus magnus (Kramer, 1876) | Eug_mag | Parasitidae | ||||||
| Eugamasus spp. | Eug_spp | Parasitidae | ||||||
| Gamasellodes bicolor (Berlese, 1918) | Gam_bic | Ascidae | ||||||
| Geholaspis mandibularis (Berlese, 1904) | Geh_man | Macrochelidae | ||||||
| Holoparasitus calcaratus (C. L. Koch, 1839) | Hol_cal | Parasitidae | ||||||
| Geolaelaps aculeifer (Canestrini, 1883) | Geo_acu | Laelapidae | ||||||
| Hypoaspis vacua (Michael, 1891) | Hyp_vac | Laelapidae | ||||||
| Lasioseius berlesei Oudemans, 1938 | Las_ber | Ascidae | ||||||
| Lasioseius fimetorum Karg, 1971 | Las_fim | Ascidae | ||||||
| Leptogamasus suecicus Trägårdh, 1936 | Lep_sue | Parasitidae | ||||||
| Olodiscus minima (Kramer, 1882) | Olo_min | Uropodidae | ||||||
| Olopachys suecicus Sellnick, 1950 | Olo_sue | Pachylaelapidae | ||||||
| Oodinychus ovalis (C. L. Koch, 1839) | Ood_ova | Trematuridae | ||||||
| Pachylaelaps furcifer Oudemans, 1903 | Pac_fur | Pachylaelapidae | ||||||
| Pachyseius humeralis Berlese, 1910 | Pac_hum | Pachylaelapidae | ||||||
| Pachylaelaps longisetis Halbert, 1915 | Pac_lon | Pachylaelapidae | ||||||
| Paragamasus conus (Karg, 1971) | Par_con | Parasitidae | ||||||
| Paragamasus jugincola Athias-Henriot, 1967 | Par_jug | Parasitidae | ||||||
| Paragamasus runcatellus (Berlese, 1903 sensu Karg, 1971) | Par_run | Parasitidae | ||||||
| Paragamasus spp. | Par_spp | Parasitidae | ||||||
| Paragamasus vagabundus (Karg, 1968) | Par_vag | Parasitidae | ||||||
| Pergamasus brevicornis Berlese, 1903 | Per_bre | Parasitidae | ||||||
| Pergamasus crassipes (Linnaeus, 1758) | Per_cra | Parasitidae | ||||||
| Pergamasus quisquiliarum (Canestrini & Canestrini, 1882) | Per_qui | Parasitidae | ||||||
| Pergamasus septentrionalis (Oudemans, 1902) | Per_sep | Parasitidae | ||||||
| Pergamasus spp. | Per_spp | Parasitidae | ||||||
| Rhodacarus mandibularis Berlese, 1921 | Rho_man | Rhodacaridae | ||||||
| Rhodacarellus silesiacus Willmann, 1936 | Rho_sil | Rhodacaridae | ||||||
| Trachytes aegrota (C. L. Koch, 1841) | Tra_aeg | Trachytidae | ||||||
| Typhlodromus spp. | Typ_spp | Phytoseiidae | ||||||
| Urodiaspis tecta (Kramer, 1876) | Uro_tec | Urodinychidae | ||||||
| Veigaia exigua (Berlese, 1916) | Vei_exi | Veigaiidae | ||||||
| Veigaia nemorensis (C. L. Koch, 1839) | Vei_nem | Veigaiidae | ||||||
| Zercon peltatus C. L. Koch, 1836 | Zer_pel | Zerconidae |
| Mite Taxa | Number of Habitats | Proportion of Habitats | Species Specificity Index d′ |
|---|---|---|---|
| Amb_spp | 4 | 0.667 | 0.432 |
| Ame_cor | 1 | 0.167 | 1.000 |
| Arc_bre | 1 | 0.167 | 1.000 |
| Arc_cet | 1 | 0.167 | 1.000 |
| Arc_ere | 1 | 0.167 | 1.000 |
| Arc_ven | 2 | 0.333 | 0.632 |
| Asc_bic | 3 | 0.500 | 0.557 |
| Che_bry | 1 | 0.167 | 1.000 |
| Epi_hor | 4 | 0.667 | 0.509 |
| Eug_mag | 1 | 0.167 | 1.000 |
| Eug_spp | 1 | 0.167 | 1.000 |
| Gam_bic | 2 | 0.333 | 0.683 |
| Geh_man | 2 | 0.333 | 0.683 |
| Geo_acu | 3 | 0.500 | 0.515 |
| Hol_cal | 3 | 0.500 | 0.688 |
| Hyp_vac | 1 | 0.167 | 1.000 |
| Las_ber | 1 | 0.167 | 1.000 |
| Las_fim | 1 | 0.167 | 1.000 |
| Lep_sue | 3 | 0.500 | 0.536 |
| Olo_min | 2 | 0.333 | 0.651 |
| Olo_sue | 2 | 0.333 | 0.714 |
| Ood_ova | 2 | 0.333 | 0.840 |
| Pac_fur | 1 | 0.167 | 1.000 |
| Pac_hum | 2 | 0.333 | 0.683 |
| Pac_lon | 1 | 0.167 | 1.000 |
| Par_con | 2 | 0.333 | 0.683 |
| Par_jug | 2 | 0.333 | 0.742 |
| Par_run | 3 | 0.500 | 0.571 |
| Par_spp | 3 | 0.500 | 0.632 |
| Par_vag | 2 | 0.333 | 0.632 |
| Per_bre | 1 | 0.167 | 1.000 |
| Per_cra | 3 | 0.500 | 0.573 |
| Per_qui | 1 | 0.167 | 1.000 |
| Per_sep | 2 | 0.333 | 0.632 |
| Per_spp | 4 | 0.667 | 0.619 |
| Rho_man | 1 | 0.167 | 1.000 |
| Rho_sil | 2 | 0.333 | 0.651 |
| Tra_aeg | 1 | 0.167 | 1.000 |
| Typ_spp | 1 | 0.167 | 1.000 |
| Uni_Gam | 1 | 0.167 | 1.000 |
| Uro_tec | 1 | 0.167 | 1.000 |
| Vei_exi | 1 | 0.167 | 1.000 |
| Vei_nem | 5 | 0.833 | 0.415 |
| Zer_pel | 2 | 0.333 | 0.777 |
References
- Otero, I.; Boada, M.; Badia, A.; Pla, E.; Vayreda, J.; Sabaté, S.; Gracia, C.A.; Peñuelas, J. Loss of water availability and stream biodiversity under land abandonment and climate change in a Mediterranean catchment (Olzinelles, NE Spain). Land Use Policy 2011, 28, 207–218. [Google Scholar] [CrossRef]
- Meier, R.; Schwaab, J.; Seneviratne, S.; Sprenger, M.; Lewis, E.; Davin, E. Empirical estimate of forestation-induced precipitation changes in Europe. Nat. Geosci. 2021, 14, 473–478. [Google Scholar] [CrossRef]
- Holátko, J.; Holubík, O.; Hammerschmiedt, T.; Vopravil, J.; Kintl, A.; Brtnický, M. Afforestation of agricultural land affects soil structural stability and related preconditions to resist dought. J. For. Sci. 2022, 68, 496–508. [Google Scholar] [CrossRef]
- Podrázský, V.; Holubík, O.; Vopravil, J.; Khel, T.; Moser, W.; Prknová, H. Effects of afforestation on soil structure formation in two climatic regions of the Czech Republic. J. For. Sci. 2016, 61, 225–234. [Google Scholar] [CrossRef]
- Vopravil, J.; Formánek, P.; Khel, T.; Jacko, K. Water content in soil afforested with a mixture of broadleaves or Scots pine. J. For. Sci. 2024, 70, 91–101. [Google Scholar] [CrossRef]
- Vopravil, J.; Formánek, P.; Heřmanovská, D.; Khel, T.; Jacko, K. The impact of agricultural land afforestation on soil water content in Central Bohemia. J. For. Sci. 2021, 67, 512–521. [Google Scholar] [CrossRef]
- Malica, J.; Urbanowski, C.K.; Turczański, K.; Rączka, G.; Andrzejewska, A.; Skorupski, M.; Kamczyc, J. Environmental role of different-aged pine and oak stands growing on post-agricultural and forest lands in forming the Mesostigmata mites communities. Land Degrad. Dev. 2024, 35, 4887–4906. [Google Scholar] [CrossRef]
- Bernacki, E. Concepts of silviculture on post-agricultural lands. Sylwan 1990, 134, 51–59. [Google Scholar]
- Hammerová, V.; Vacek, S.; Vacek, Z.; Černý, J.; Cukor, J.; Gallo, J.; Kuběnka, M. Forest ecosystem restoration in the Ore Mountains: A review of silvicultural measures addressing environmental degradation. J. For. Sci. 2025, 71, 323–335. [Google Scholar] [CrossRef]
- Rykowski, K. Problems of forest protection on afforested agricultural grounds. Sylwan 1990, 134, 3–12. [Google Scholar]
- Kádár, I.; Ragályi, P.; Murányi, A.; Radimszky, L.; Gajdó, A. Effect of Gérce Alginite on the fertility of an acid sandy soil. Agrokém. Talajt. 2015, 64, 437–452. [Google Scholar] [CrossRef]
- Brindza, J.; Vozár, Ľ.; Miko, M.; Gažo, J.; Kovár, P.; Horčinová Sedláčková, V.; Grygorieva, O. Unique Effects of Alginite as a Bituminous Rock on Soil, Water, Plants and Animal Organisms—Review. Agrobiodivers. Improv. Nutr. Health Life Qual. 2021, 5, 169–184. Available online: https://agrobiodiversity.uniag.sk/scientificpapers/article/view/361 (accessed on 16 October 2025).
- Mackowiak, C.L.; Grossl, P.R.; Bugbee, B.G. Beneficial Effects of Humic Acid on Micronutrient Availability to Wheat. Soil Sci. Soc. Am. J. 2001, 65, 1744–1750. [Google Scholar] [CrossRef]
- Gallo, J.; Záruba, J.; Baláš, M.; Podrázský, V. Výzkumná Plocha Doubek—Introdukované Dřeviny na Zemědělské Půdě; Katedra Pěstování Lesů, Fakulta Lesnická a Dřevařská, Česká Zemědělská Univerzita v Praze: Kostelec nad Černými lesy, Czech Republic, 2022; pp. 45–48. [Google Scholar]
- Dunger, W.; Wanner, M.; Hauser, H.; Hohberg, K.; Schulz, H.J.; Schwalbe, T.; Seifert, B.; Vogel, J.; Voigtländer, K.; Zimdars, B.; et al. Development of soil fauna at mine sites during 46 years after afforestation. Pedobiologia 2001, 45, 243–271. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, X.; Li, T.; Yang, J.; Zhao, L.; Yuan, D.; Guo, Z.; Liu, C.; Duan, C. Changes in soil microbe-mediated carbon, nitrogen and phosphorus cycling during spontaneous succession in abandoned Pb-Zn mining areas. Sci. Total Environ. 2004, 920, 171018. [Google Scholar] [CrossRef]
- Koehler, H.H. Predatory mites (Gamasina, Mesostigmata). In Invertebrate Biodiversity as Bioindicators of Sustainable Landscapes; Elsevier: Amsterdam, The Netherlands, 1999; pp. 395–410. [Google Scholar] [CrossRef]
- Urbanowski, C.K.; Horodecki, P.; Kamczyc, J.; Skorupski, M.; Jagodziński, A.M. Succession of mite assemblages (Acari, Mesostigmata) during decomposition of tree leaves in forest stands growing on reclaimed post-mining spoil heap and adjacent forest habitats. Forests 2018, 9, 718. [Google Scholar] [CrossRef]
- Zhu, Y.; Bian, H.; Ju, C.; Xu, C.; Zhou, Y.; Zhang, H.; Xu, X. Fertilization alters the abundance but not the diversity of soil fauna: A meta-analysis. Glob. Ecol. Biogeogr. 2023, 32, 482–494. [Google Scholar] [CrossRef]
- Burgess, S.; Adams, M.; Turner, N.; Ong, C. The redistribution of soil water by tree root systems. Oecologia 1998, 115, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Pregitzer, K.; King, J.; Burton, A.; Brown, S. Responses of tree fine roots to temperature. New Phytol. 2000, 147, 105–115. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, Z.; Bakker, M.; Kim, J.; Brancheriau, L.; Buatois, B.; Leclerc, R.; Selli, L.; Rey, H.; Jourdan, C.; et al. Linking conifer root growth and production to soil temperature and carbon supply in temperate forests. Plant Soil 2018, 426, 33–50. [Google Scholar] [CrossRef]
- Stover, H.; Henry, H. Soil Homogenization Modifies Productivity, Nitrogen Retention and Decomposition in Restored Grassland. Ecosystems 2019, 23, 264–277. [Google Scholar] [CrossRef]
- Peng, Z.; Qian, X.; Liu, Y.; Li, X.; Gao, H.; An, Y.; Qi, J.; Jiang, L.; Zhang, Y.; Chen, S.; et al. Land conversion to agriculture induces taxonomic homogenization of soil microbial communities globally. Nat. Commun. 2024, 15, 3624. [Google Scholar] [CrossRef]
- Cao, Z.; Han, X.; Hu, C.; Chen, J.; Zhang, D.; Steinberger, Y. Changes in the abundance and structure of a soil mite (Acari) community under long-term organic and chemical fertilizer treatments. Appl. Soil Ecol. 2011, 49, 131–138. [Google Scholar] [CrossRef]
- Tama, A.Y.; Manourova, A.; Mohammad, R.K.; Podrázský, V. Afforestation of Abandoned Agricultural Land: Growth of Non-Native Tree Species and Soil Response in the Czech Republic. Forests 2025, 16, 1113. [Google Scholar] [CrossRef]
- Micherdziński, W. Die Familie Parasitidae Oudemans, 1901 (Acarina, Mesostigmata); PWN: Warsaw, Poland, 1969. [Google Scholar]
- Karg, W. Acari (Acarina) Milben, Unterordnung Anactinochaeta (Parasitiformes). In Die Freilebenden Gamasina (Gamasides), Raubmilben Tierwelt Deutschlands; Gustav Fischer Verlag: Jena, Germany, 1971; Volume 59. [Google Scholar]
- Karg, W. Acari (Acarina) Milben Parasitiformes (Anactinochaeta), Cohors Gamasina Leach Raubmmilben Tierwelt Deutschlands; VEB Gustav Fischer Ver Lag: Jena, Germany, 1993. [Google Scholar]
- Gwiazdowicz, D.J. Ascid mites (Acari, Mesostigmata) from selected forest ecosystems and microhabitats in Poland. In Wydawnictwo Akademii Rolniczej im; Augusta Cieszkowskiego: Bydgoszcz, Poland, 2007; ISBN 978-83-7160-439-3. [Google Scholar]
- Mašán, P.; Halliday, B. Review of the mite family Pachylaelapidae (Acari: Mesostigmata). Zootaxa 2014, 3776, 1–66. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, X.; Liusui, Y.; Sun, X.; Zhao, C.; Liu, H. Diversity and Abundance of Soil Animals as Influenced by Long-Term Fertilization in Grey Desert Soil, China. Sustainability 2015, 7, 10837–10853. [Google Scholar] [CrossRef]
- Faria, L.O.; Souza, A.G.V.; de Mello, M.C.; Berti, M.P.S. Potassium fertilization and bioactivators on the soybean yield and soil microbiota. Pesq. Agropec. Trop. 2023, 53, e74945. [Google Scholar] [CrossRef]
- Aslam, T.J. Impacts of Intensive Agriculture on Soil Fauna and Ecosystem Function: Trading Function for Dependence? Ph.D. Thesis, University of Leeds, Leeds, UK, 2015. Available online: https://etheses.whiterose.ac.uk/id/eprint/10649/1/TJA_thesis.pdf (accessed on 16 October 2025).
- Abo-Shnaf, R.I.; Zaki, A.Y.; Aly, A.I.; Mergawy, M.M. Effect of different fertilization treatments on the biodiversity of mites associated with tomato plants and its yield in Fayoum governorate, Egypt. Acarines JESA 2016, 10, 25–29. [Google Scholar] [CrossRef]
- Amani, M.; Khajehali, J.; Moradi-Faradonbeh, M.; Macchioni, F. Species diversity of soil mites (Acari: Mesostigmata) under different agricultural land use types. Persian J. Acarol. 2020, 9, 353–366. Available online: https://www.biotaxa.org/pja/article/view/202046 (accessed on 16 October 2025).
- Hu, C.; Cao, Z.P. Nematode community structure under compost and chemical fertilizer management practice, in the north China plain. Exp. Agric. 2008, 44, 485–496. [Google Scholar] [CrossRef]
- Walter, D.E.; Stirling, G.R. Microarthropods in Australian sugarcane soils: A survey with emphasis on the mesostigmata as potential regulators of nematode populations. Acarologia 2018, 58, 673–682. [Google Scholar] [CrossRef]
- Feketeova, Z.; Mangova, B.; Čierniková, M. The soil chemical properties influencing the Oribatid mite (Acari; Oribatida) abundance and diversity in coal ash basin vicinage. Appl. Sci. 2021, 11, 3537. [Google Scholar] [CrossRef]
- Lang, B.; Betancur-Corredor, B.; Russell, D.J. Soil mineral nitrogen content is increased by soil mesofauna and nematodes–a meta-analysis. Soil Org. 2023, 95, 117–128. [Google Scholar] [CrossRef]
- Fu, S.; Ferris, H.; Brown, D.; Plant, R. Does the positive feedback effect of nematodes on the biomass and activity of their bacteria prey vary with nematode species and population size? Soil Biol. Biochem. 2005, 37, 1979–1987. [Google Scholar] [CrossRef]
- Liu, J.; Shu, A.; Song, W.; Shi, W.; Li, M.; Zhang, W.; Li, Z.; Liu, G.; Yuan, F.; Zhang, S.; et al. Long-term organic fertilizer substitution increases rice yield by improving soil properties and regulating soil bacteria. Geoderma 2021, 404, 115287. [Google Scholar] [CrossRef]
- Murray, P.J.; Cook, R.; Currie, A.F.; Dawson, L.A.; Gange, A.C.; Grayston, S.J.; Treonis, A.M. Interactions between fertilizer addition, plants and the soil environment: Implications for soil faunal structure and diversity. Soil Biol. Biochem. 2006, 38, 2799–2811. [Google Scholar] [CrossRef]
- Liu, T.; Yang, L.; Hu, Z.; Xue, J.; Lu, Y.; Chen, X.; Griffiths, B.S.; Whalen, J.K.; Liu, M. Biochar exerts negative effects on soil fauna across multiple trophic levels in a cultivated acidic soil. Biol. Fertil. Soils 2020, 56, 597–606. [Google Scholar] [CrossRef]
- Cakir, M.; Makineci, E. Humus characteristics and seasonal changes of soil arthropod communities in a natural sessile oak (Quercus petraea L.) stand and adjacent Austrian pine (Pinus nigra Arnold) plantation. Environ. Monit. Assess. 2013, 185, 8943–8955. [Google Scholar] [CrossRef]
- Malica, J.; Urbanowski, C.K.; Rączka, G.; Skorupski, M.; Pers-Kamczyc, E.; Kamczyc, J. Soil Environment and Fauna Communities in Europe after Afforestation of Post-Agricultural Lands—A Review. Forests 2022, 13, 1713. [Google Scholar] [CrossRef]
- Souto, X.C.; Gonzales, L.; Reigosa, M.J. Comparative analysis of allelopathic effects produced by four forestry species during decomposition process in their soils in Galicia (NW Spain). J. Chem. Ecol. 1994, 20, 3005–3015. [Google Scholar] [CrossRef]
- Malica, J.; Urbanowski, C.K.; Turczański, K.; Rączka, G.; Andrzejewska, A.; Skorupski, M.; Kamczyc, J. Soil mite communities (Acari, Mesostigmata) in pure stands on post-agricultural lands: Does season matter? Exp. Appl. Acarol. 2025, 94, 4. [Google Scholar] [CrossRef] [PubMed]
- Błaszak, C. Mesostigmata. In Fauna Polski—Charakterystyka i Wykaz Gatunków; Muzeum i Instytut Zoologii PAN: Warszawa, Poland, 2008; pp. 51–52. ISBN 978-83-88147-09-847. [Google Scholar]
- Bedano, J.C.; Cantú, M.P.; Doucet, M.E. Influence of three different land management practices on soil mite (Arachnida: Acari) densities in relation to a natural soil. Appl. Soil Ecol. 2006, 32, 293–304. [Google Scholar] [CrossRef]
- Gormsen, D.; Hedlund, K.; Huifu, W. Diversity of soil mite communities when managing plant communities on set-aside arable land. Appl. Soil Ecol. 2006, 31, 147–158. [Google Scholar] [CrossRef]
- Ireson, J.E.; Holloway, R.J.; Chatterton, W.S. An overview of investigations into the use of predatory mites to control the Llucerne flea, Sminthurus viridis (L.). In Proceedings of the 10th International Congress of Acaraology, Canberra, Australia, 5–10 July 1998; CSIRO Publishing: Clayton South, Australia, 2001. [Google Scholar]
- Peachey, R.E.; Moldenke, A.; William, R.D.; Berry, R.; Ingham, E.; Groth, E. Effect of cover crops and tillage system on symphylan (Symphlya: Scutigerella immaculata, Newport) and Pergamasus quisquiliarum Canestrini (Acari: Mesostigmata) populations, and other soil organisms in agricultural soils. Appl. Soil Ecol. 2002, 21, 59–70. [Google Scholar] [CrossRef]
- Manu, M.; Călugăr, A.; Badiu, D. Distribution of the genus Veigaia (Mesostigmata: Veigaiidae) in Romania with notes on the species ecology. Biologia 2017, 72, 628–641. [Google Scholar] [CrossRef]
- Ragusa, S.; Tsolakis, H. Notes on the adaptation of some phytophagous and predacious mites to various ecological parameters in the Mediterranean countries. Web Eco. 2000, 1, 35–47. [Google Scholar] [CrossRef]
- Kalúz, S.; Fenďa, P. Mites (Acari: Mesostigmata) of the Family Ascidae of Slovakia; Institute of Zoology, Slovak Academy of Sciences: Bratislava, Slovak Republic, 2005; pp. 27–28. [Google Scholar]
- Gwiazdowicz, D.J.; Dunlop, J.A. Subfamily Arctoseiinae (Acari, Mesostigmata) in the Museum Für Naturkunde Berlin. Acta Sci. Pol. Silv. Colendar. Ratio Ind. Lignar. 2012, 11, 5–16. [Google Scholar]
- Madej, G.; Kozub, M. Possibilities of using soil microarthropods, with emphasis on mites (Arachnida, Acari, Mesostigmata), in assessment of successional stages in a reclaimed coal mine dump (Pszów, S Poland). Biol. Lett. 2015, 51, 19–36. [Google Scholar] [CrossRef]
- Skorupski, M. Influence of Selected Tree Species on Forest Ecosystem Biodiversity for the Example of Mesostigmata mites in a Common-Garden Experiment; Wydawnictwo Uniwersytetu Przyrodniczego: Poznań, Poland, 2010. [Google Scholar]
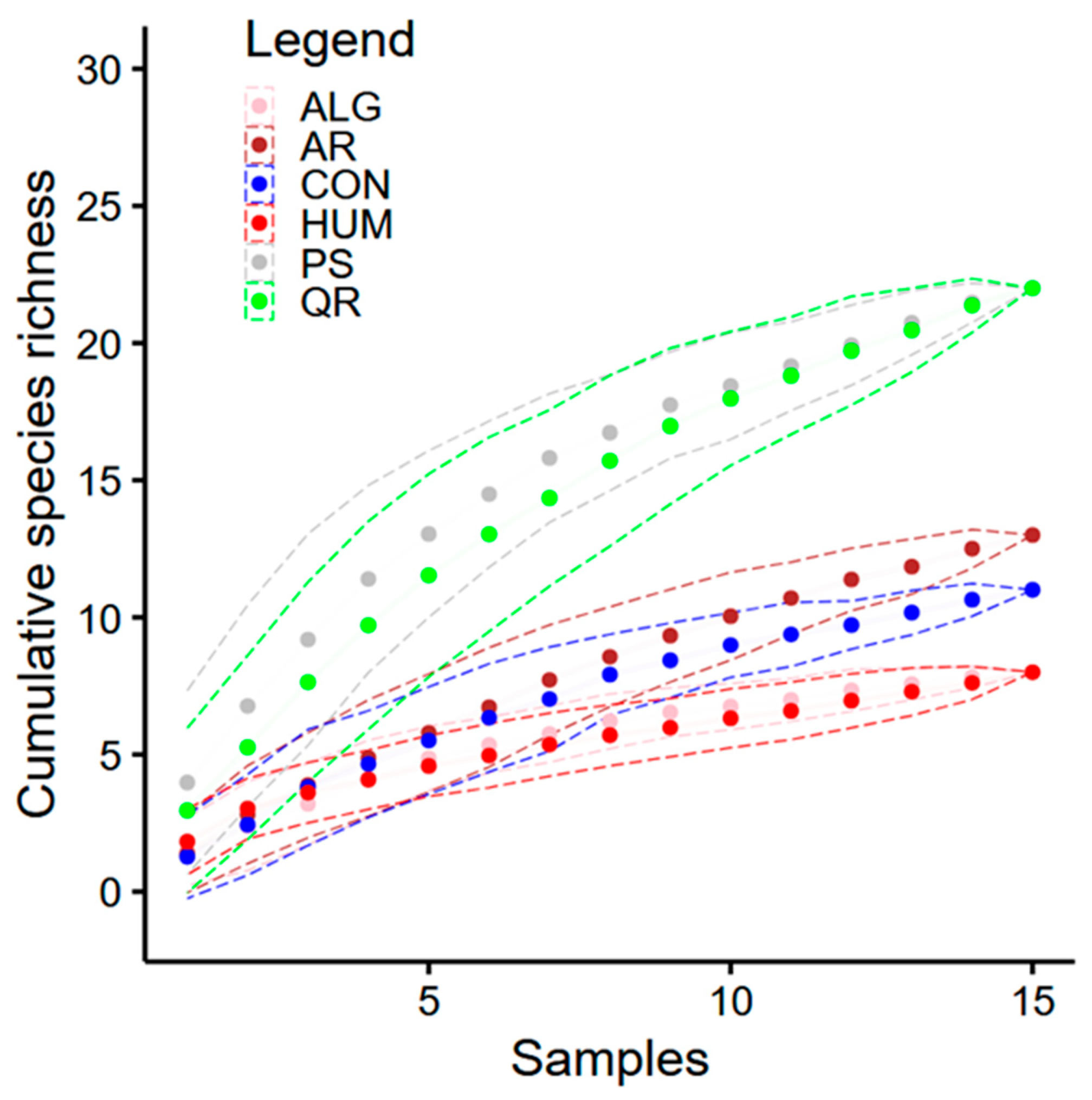
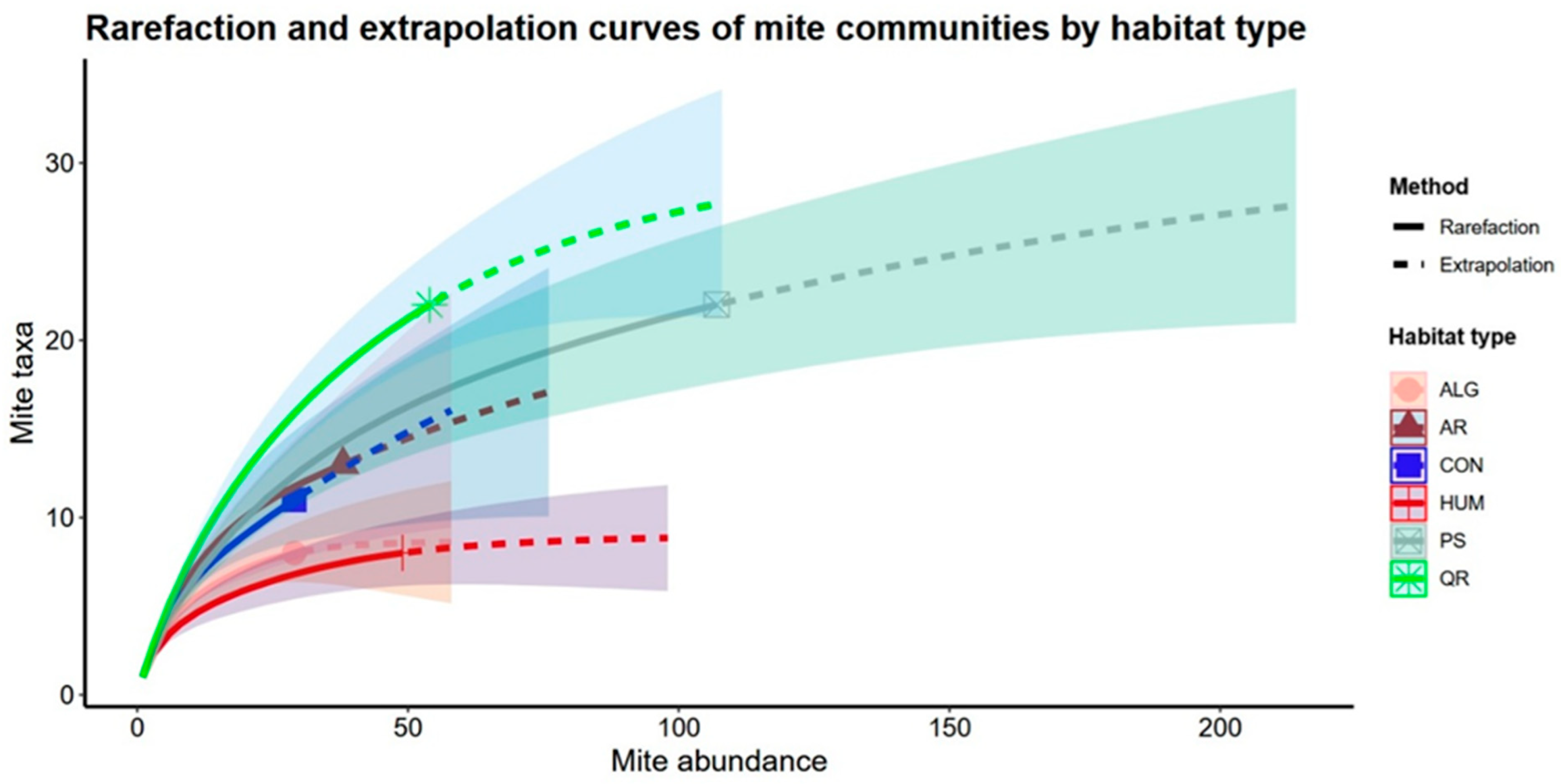
| Assemblage (Habitat Type) | Observed Species Richness | Estimated Species Richness (Asymptotic) | Confidence Interval |
|---|---|---|---|
| ALG | 8 | 8.64 | 8–14.7 |
| AR | 13 | 25.17 | 13–48.2 |
| CON | 11 | 28.38 | 11–57.5 |
| HUM | 8 | 8.98 | 8–14.7 |
| PS | 22 | 32.57 | 22–53.1 |
| QR | 22 | 30.18 | 22–54.4 |
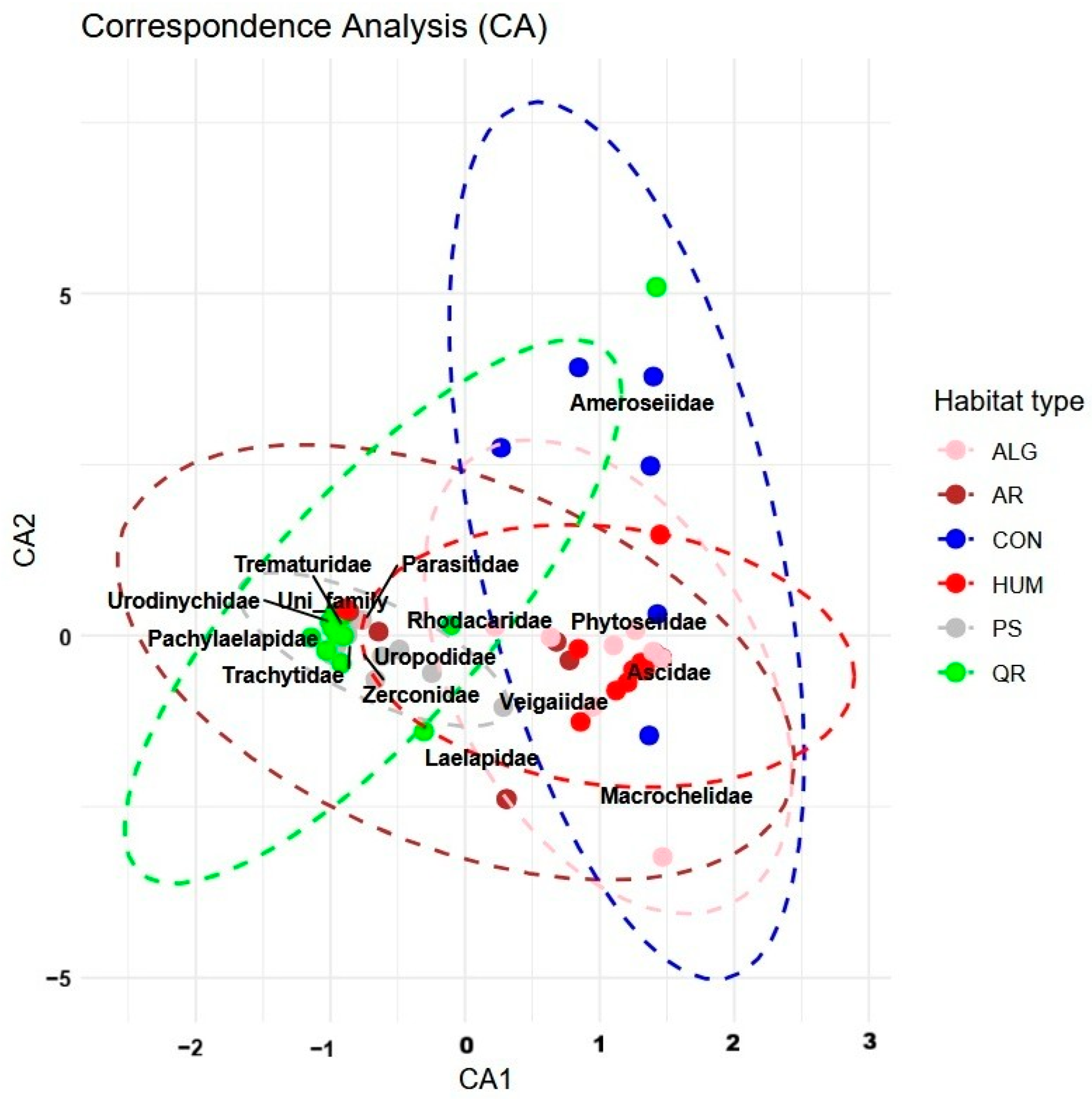
| C2. | F.Model | R2 | p-Value | p.Adjusted | ||
|---|---|---|---|---|---|---|
| PS vs. CON | 6.98 | 0.269 | 0.001 | 0.015 | ||
| PS vs. HUM | 12.20 | 0.347 | 0.001 | 0.015 | ||
| PS vs. ALG | 5.75 | 0.232 | 0.001 | 0.015 | ||
| QR vs. CON | 5.45 | 0.214 | 0.001 | 0.015 | ||
| QR vs. HUM | 11.55 | 0.325 | 0.001 | 0.015 | ||
| QR vs. ALG | 5.21 | 0.207 | 0.001 | 0.015 | ||
| AR vs. HUM | 4.26 | 0.176 | 0.002 | 0.030 | ||
| Term | Df | Sum of Squares | R2 | F | p-value | |
| Model | 5 | 6.5124 | 0.27621 | 4.5031 | 0.001 | |
| Residual | 59 | 17.0654 | 0.72379 | |||
| Total | 64 | 23.5778 | 1.00000 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malica, J.; Urbanowski, C.K.; Kamczyc, J.; Tama, A.Y.; Skorupski, M.; Podrázský, V. Effects of Humac and Alginite Fertilization on Mite Communities (Acari, Mesostigmata) Under Post-Agricultural Land Conditions. Forests 2025, 16, 1596. https://doi.org/10.3390/f16101596
Malica J, Urbanowski CK, Kamczyc J, Tama AY, Skorupski M, Podrázský V. Effects of Humac and Alginite Fertilization on Mite Communities (Acari, Mesostigmata) Under Post-Agricultural Land Conditions. Forests. 2025; 16(10):1596. https://doi.org/10.3390/f16101596
Chicago/Turabian StyleMalica, Jacek, Cezary Krzysztof Urbanowski, Jacek Kamczyc, Abubakar Yahaya Tama, Maciej Skorupski, and Vilém Podrázský. 2025. "Effects of Humac and Alginite Fertilization on Mite Communities (Acari, Mesostigmata) Under Post-Agricultural Land Conditions" Forests 16, no. 10: 1596. https://doi.org/10.3390/f16101596
APA StyleMalica, J., Urbanowski, C. K., Kamczyc, J., Tama, A. Y., Skorupski, M., & Podrázský, V. (2025). Effects of Humac and Alginite Fertilization on Mite Communities (Acari, Mesostigmata) Under Post-Agricultural Land Conditions. Forests, 16(10), 1596. https://doi.org/10.3390/f16101596





