Hydro-Functional Strategies of Sixteen Tree Species in a Mexican Karstic Seasonally Dry Tropical Forest
Abstract
1. Introduction
2. Materials and Methods
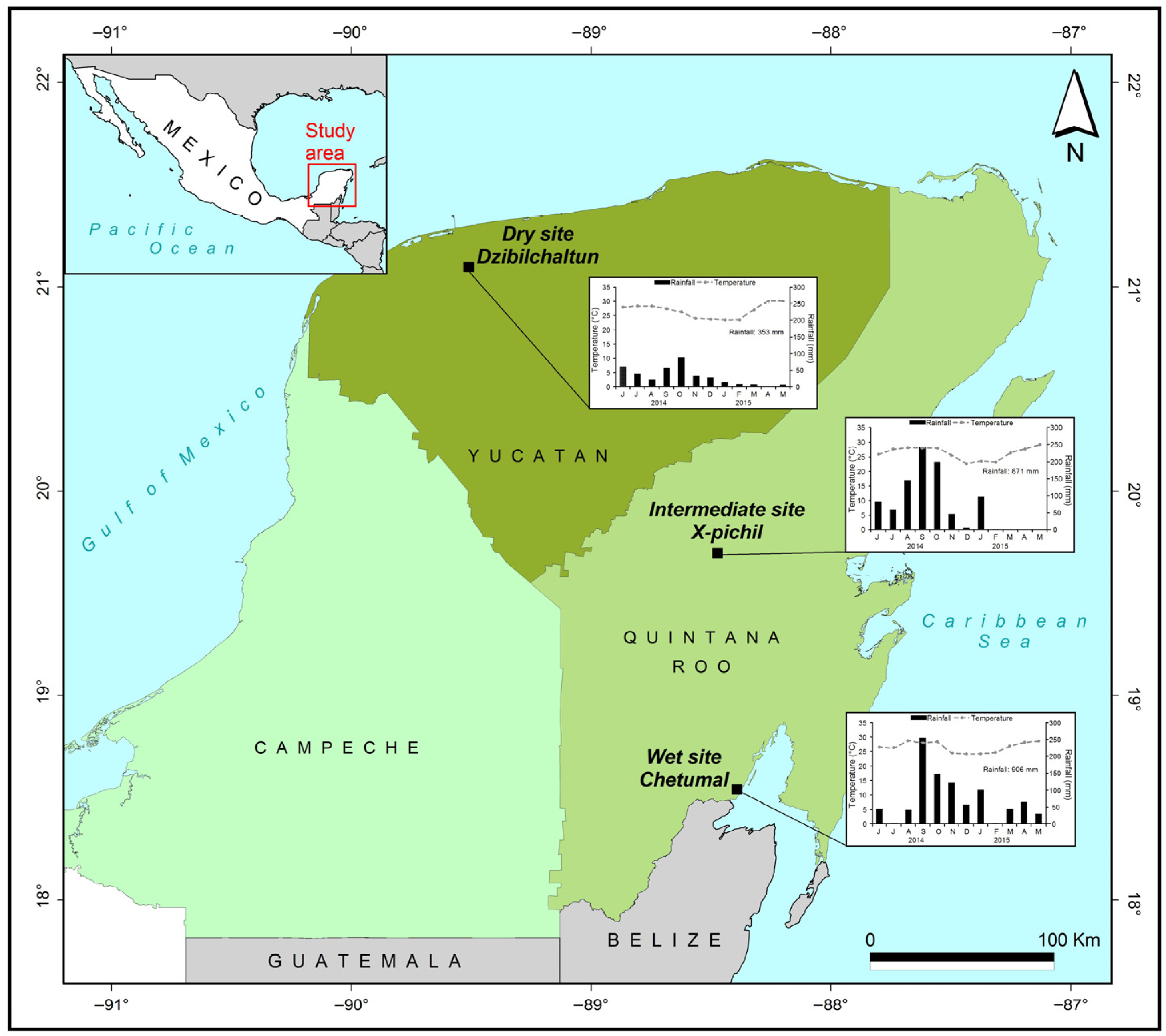
2.1. Study Area
2.2. Species Analyzed
2.3. Sampling Seasons
2.4. Morphophysiological Parameters
2.5. Environmental Parameters
2.6. Statistical Analysis
3. Results
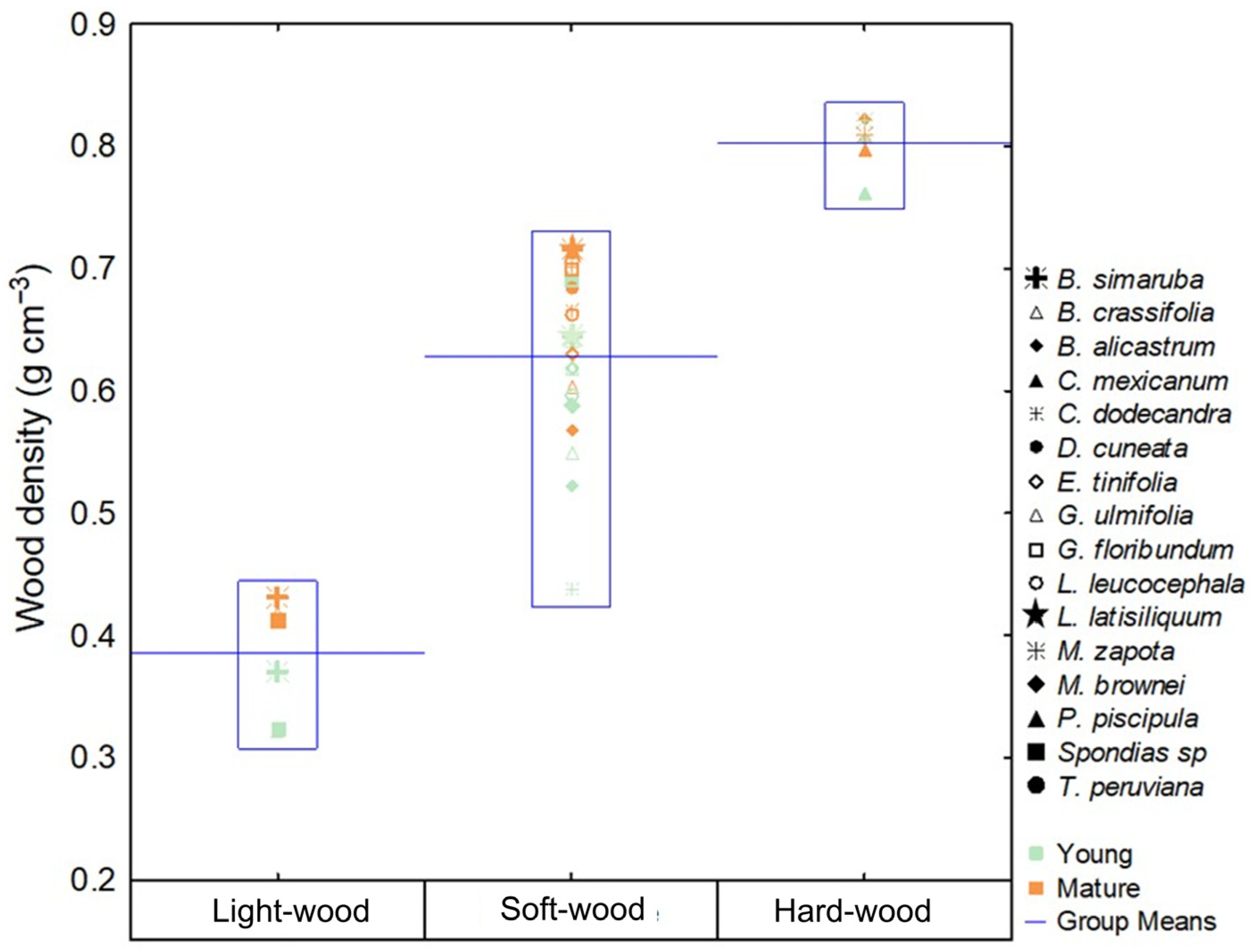
3.1. Wood Density
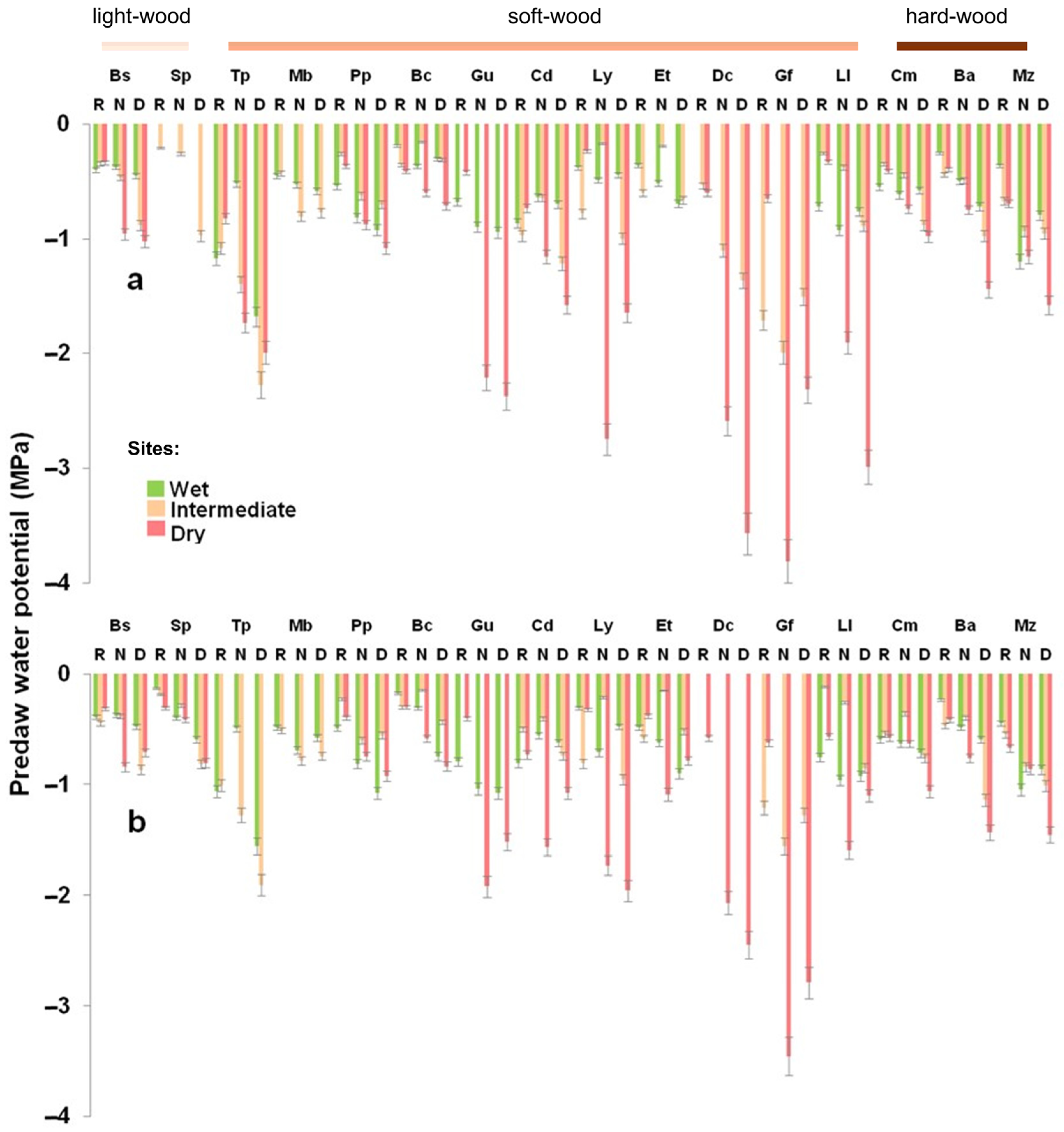
3.2. Water Potential
Comparison Between Natural Conditions and Homegardens
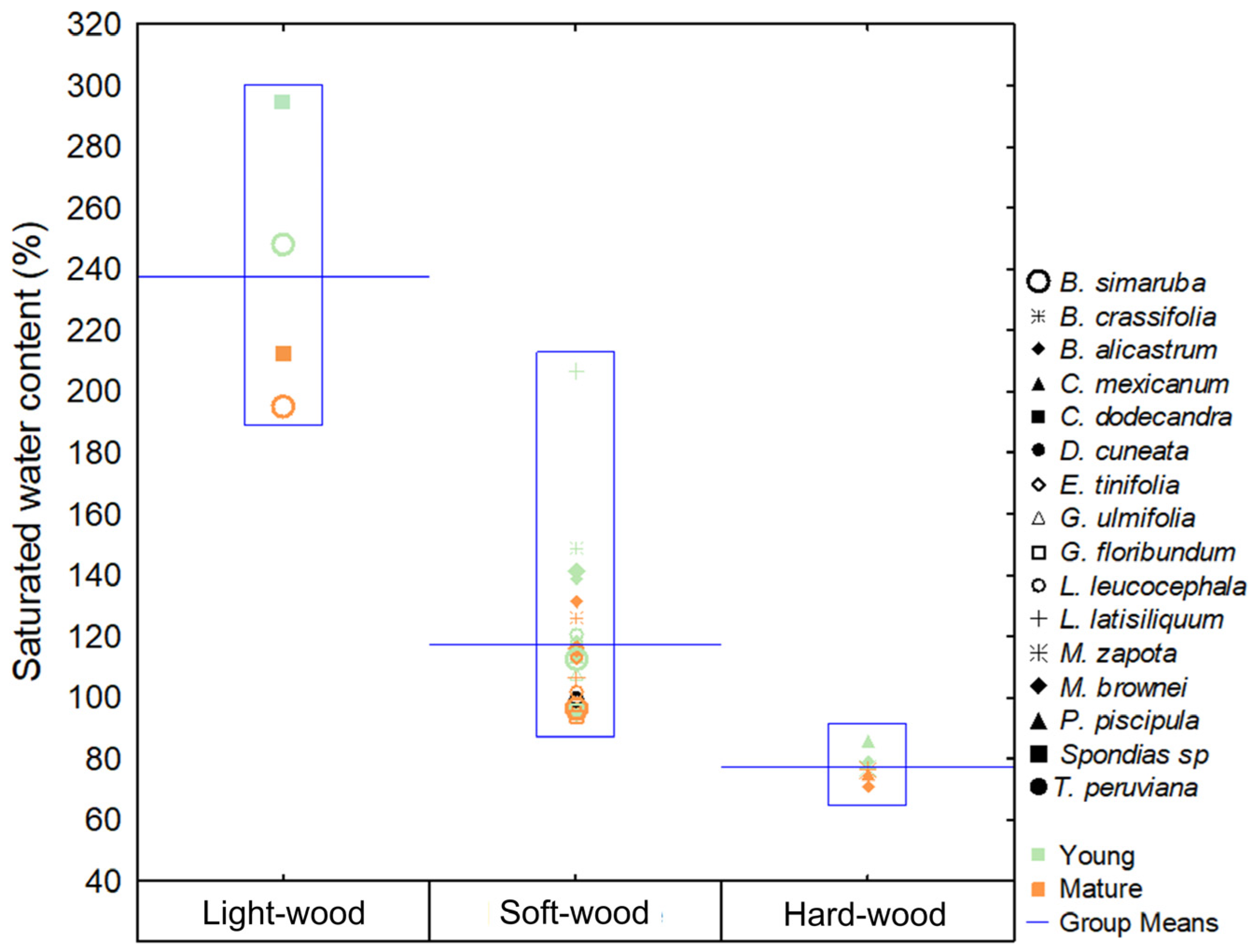
3.3. Saturated Water Content
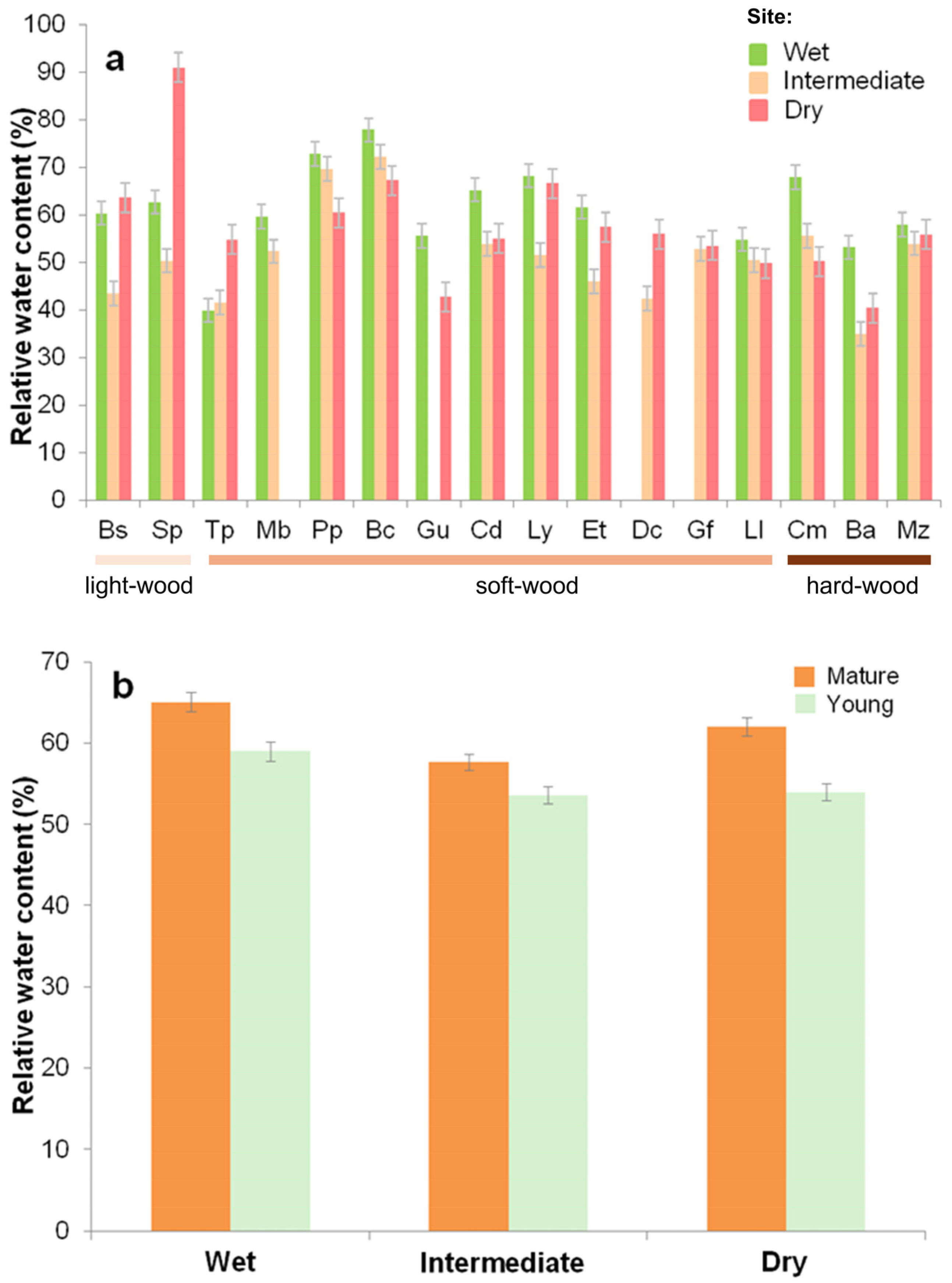
3.4. Relative Water Content
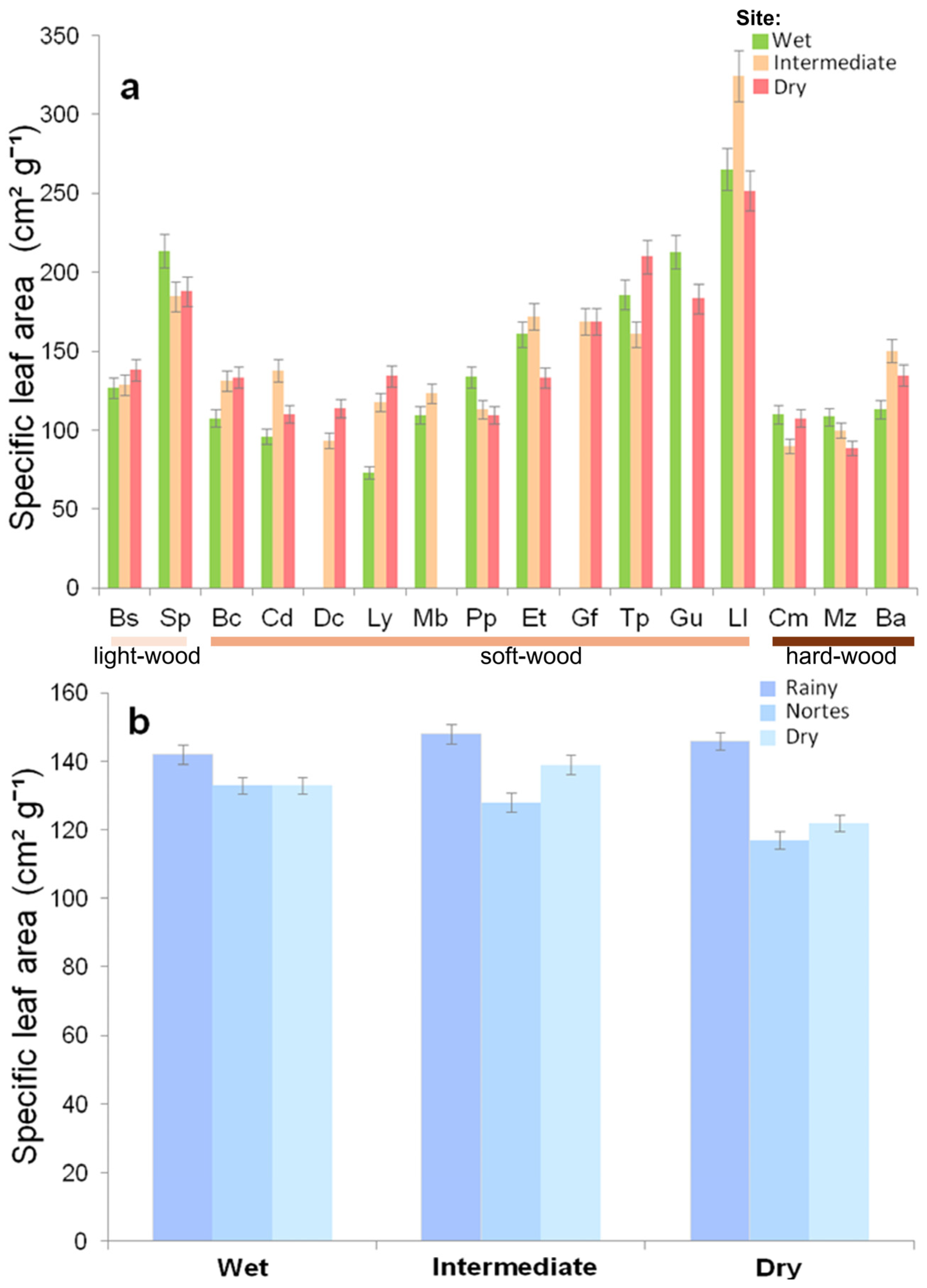
3.5. Specific Leaf Area
3.6. Correlations Between Functional Traits and Environmental Factors
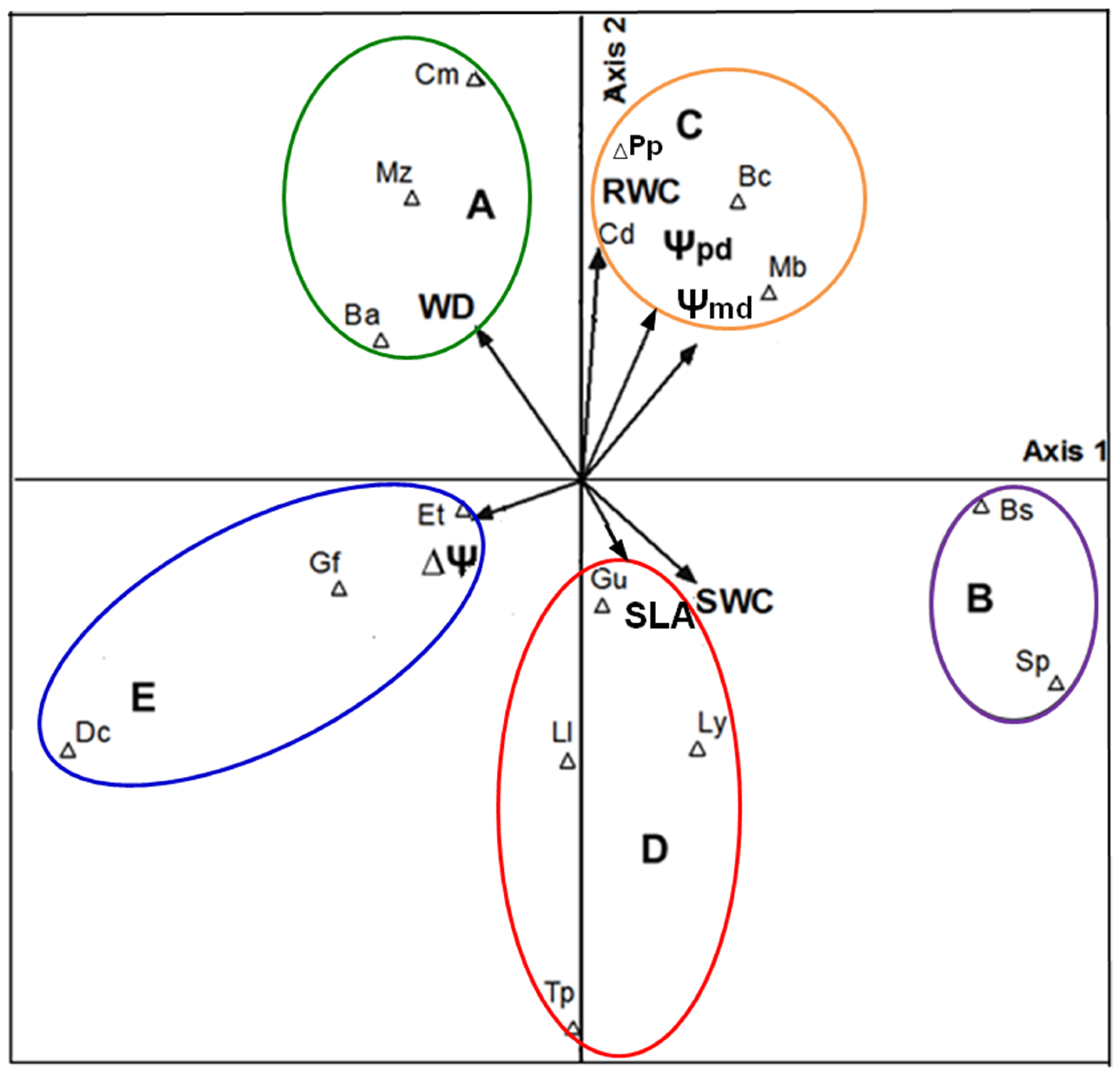
3.7. Functional Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, P.G.; Lugo, A.E. Ecology of Tropical Dry Forest. Annu. Rev. Ecol. Syst. 1986, 17, 67–88. [Google Scholar] [CrossRef]
- IPCC. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2023: Synthesis Report; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 35–115. [Google Scholar] [CrossRef]
- Lopez-Toledo, L.; Prieto-Torres, D.A.; Barros, F.D.V.; Ribeiro, N.; Pennington, R.T. Seasonally dry tropical forests: New insights for their knowledge and conservation. Front. Glob Change 2024, 6, 1350375. [Google Scholar] [CrossRef]
- Hasselquist, N.J.; Allen, M.F.; Santiago, L.S. Water relations of evergreen and drought-deciduous trees along a seasonally dry tropical forest chronosequence. Oecologia 2010, 164, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Gutiérrez, J.; Rifai, S.W.; Deng, X.; Steege, H.t.; Thomson, E.; Corral-Rivas, J.J.; Guimaraes, A.F.; Muller, S.; Klipel, J.; Fauset, S.; et al. Canopy functional trait variation across Earth’s tropical forests. Nature 2025, 641, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Cayuela, L.; Griffith, D.M.; Camacho, A.; Coronado, I.M.; del Castillo, R.F.; Figueroa-Rangel, B.L.; Fonseca, W.; Garibaldi, C.; Kelly, D.L.; et al. Climate Change Increases threat to Plant Diversity in Tropical Forests of Central America and Southern Mexico. PLoS ONE 2024, 19, e0297840. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Konings, A.G.; Trugman, A.T.; Yu, K.; Bowling, D.R.; Gabbitas, R.; Karp, D.S.; Pacala, S.; Sperry, J.S.; Sulman, B.N.; et al. Hydraulic diversity of forests regulates ecosystem resilience during drought. Nature 2018, 561, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Alonzo, R.; Paz, H.; Zuluaga, R.C.; Rosell, J.A.; Olson, M.E. Coordinated Evolution of Leaf and Stem Economics in Tropical Dry Forest Trees. Ecology 2012, 93, 2397–2406. [Google Scholar] [CrossRef]
- De Guzman, M.E.; Acosta-Rangel, A.; Winter, K.; Meinzer, F.C.; Bonal, B.; Santiago, L.S. Hydraulic traits of Neotropical canopy liana and tree species across a broad range of wood density: Implications for predicting drougth mortality with models. Tree Physiol. 2021, 41, 24–34. [Google Scholar] [CrossRef]
- Markesteijn, L.; Poorter, L.; Bongers, F.; Paz, H.; Sack, L. Hydraulics and Life History of Tropical Dry Forest Tree Species: Coordination of Species’ Drought and Shade Tolerance. New Phytol. 2011, 191, 480–495. [Google Scholar] [CrossRef]
- Ávila-Lovera, E.; Urich, R.; Coronel, I.; Tezara, W. Ecophysiological traits change Little along a successional gradient in a tropical dry deciduous woodland from Margarita Island, Venezuela. Front. For. Glob. Change 2023, 6, 1043574. [Google Scholar] [CrossRef]
- Flo, V.; Martínez-Vilalta, J.; Mencuccini, M.; Granda, V.; Anderegg, W.R.; Poyatos, R. Climate and functional traits jointly mediate tree water-use strategies. New Phytol. 2021, 231, 617–630. [Google Scholar] [CrossRef]
- Fagundes, M.V.; Souza, A.F.; Oliveira, R.S.; Ganade, G. Functional traits above and below ground allow species with distinct ecological strategies to coexist in the largest seasonally dry tropical forest in the Americas. Front. For. Glob. Change 2022, 5, 930099. [Google Scholar] [CrossRef]
- Smith-Martin, C.M.; Muscarella, R.; Hammond, W.M.; Jansen, S.; Brodribb, T.J.; Choat, B.; Johnson, D.M.; Vargas-G, G.; Uriarte, M. Hydraulic variability of tropical forests is largely independent of water availability. Ecol. Lett. 2023, 26, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Borchert, R.; Pockman, W.T. Water storage capacitance and xylem tension in isolated branches of temperate and tropical trees. Tree Physiol. 2005, 25, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Lavorel, S.; Garnier, E. Predicting Changes in Community Composition and Ecosystem Functioning from Plant Traits: Revisiting the Holy Grail. Funct. Ecol. 2002, 16, 545–556. [Google Scholar] [CrossRef]
- Valdez-Hernández, M.; Andrade, J.L.; Jackson, P.C.; Rebolledo-Vieyra, M. Phenology of five tree species of a tropical dry forest in Yucatan, Mexico: Effects of environmental and physiological factors. Plant Soil 2010, 329, 155–171. [Google Scholar] [CrossRef]
- Palomo-Kumul, J.; Valdez-Hernández, M.; Islebe, G.A.; Cach-Pérez, M.J.; Andrade, J.L. El Niño-Southern Oscillation affects the water relations of tree species in the Yucatan Peninsula, Mexico. Sci. Rep. 2021, 11, 10451. [Google Scholar] [CrossRef]
- Adams, R.E.; West, J.B. Functional Groups Mask Inter- and Intraspecific Variation in Water Use Strategies in a Seasonally Dry Tropical Forest. Front. Water 2022, 4, 950346. [Google Scholar] [CrossRef]
- Jackson, P.C.; Andrade, J.L.; Reyes-García, C.; Hernández-González, O.; McElroy, T.; Us-Santamaría, R.; Simá, J.L.; Dupuy, J.M. Physiological responses of species to microclimate help explain population dynamics along succession in a tropical dry forest of Yucatan, Mexico. Forests 2018, 9, 411. [Google Scholar] [CrossRef]
- Liu, C.; Huang, Y.; Wu, F.; Liu, W.; Ning, Y.; Huang, Z.; Tang, S.; Liang, Y. Plant adaptability in karst regions. J. Plant Res. 2021, 134, 889–906. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Eller, C.B.; Barros, F.D.V.; Hirota, M.; Brum, M.; Bittencourt, P. Linking plant hydraulics and the fast–slow continuum to understand resilience to drought in tropical ecosystems. New Phytol. 2021, 230, 904–923. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; He, X.; Sun, Y.; Zhang, X.; Tang, X.; Li, Y.; Yi, Y. Distinct endophytes are used by diverse plants for adaptation to karst regions. Sci. Rep. 2019, 9, 5246. [Google Scholar] [CrossRef] [PubMed]
- Ford, D.; Williams, P. Karst Hydrogeology and Geomorphology; John Willey & Sons: Sussex, UK, 2007. [Google Scholar]
- Geekiyanage, N.; Goodale, U.M.; Cao, K.; Kitajima, K. Plant ecology of tropical and subtropical karst ecosystems. Biotropica 2019, 51, 626–640. [Google Scholar] [CrossRef]
- Estrada-Medina, H.; Santiago, L.S.; Graham, R.C.; Allen, M.F.; Jimenez-Osornio, J. Source water, phenology and growth of two tropical dry forest tree species growing on shallow karst soils. Trees 2013, 27, 1297–1307. [Google Scholar] [CrossRef]
- Narvaez-Montoya, C.; Mondragón Bonilla, R.; Goldscheider, N.; Mahlknecht, J. Groundwater salinization patterns in the Yucatan Peninsula reveal contamination and vulnerability of the karst aquifer. Commun. Earth Environ. 2025, 6, 468. [Google Scholar] [CrossRef]
- Hernández-Flores, G.; Gutiérrez-Aguirre, M.A.; Cervantes-Martínez, A.; Marín-Celestino, A.E. Historical analysis of a karst aquifer: Recharge, water extraction, and consumption dynamics on a tourist island (Cozumel, Mexico). In Annales de Limnologie-International Journal of Limnology; EDP Sciences: Les Ulis, France, 2021; Volume 57, p. 16. [Google Scholar] [CrossRef]
- Valdez-Hernández, M.; González-Salvatierra, C.; Reyes-García, C.; Jackson, P.C.; Andrade, J.L. Physiological Ecology of Vascular Plants. In Biodiversity and Conservation of the Yucatán Peninsula; Islebe, G., Calmé, S., León-Cortés, J., Schmook, B., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Thien, L.; Bradburn, A.S.; Welden, A.L. The Woody Vegetation of Dzibilchaltún. A Maya Archeological Site in Northwest Yucatán, México; Middle American Research Institute: New Orleans, LA, USA, 1982; pp. 1–24. [Google Scholar]
- Valdez-Hernández, M.; Sánchez, O.; Islebe, G.A.; Snook, L.K.; Negreros-Castillo, P. Recovery and early succession after experimental disturbance in a seasonally dry tropical forest in Mexico. For. Ecol. Manag. 2014, 334, 331–343. [Google Scholar] [CrossRef]
- Bautista, F.; Palacio, G.; Ortiz-Pérez, M.; Batllori-Sampedro, E.; Castillo-González, M. El origen y el manejo maya de las geoformas, suelos y aguas en la Península de Yucatán. In Caracterización y Manejo de los Sue los de la Península de Yucatán: Implicaciones Agropecuarias, Forestales y Ambientales; Zúñiga, F.B., Ed.; Universidad Autónoma de Campeche: Campeche, Mexico; Universidad Autónoma de Yucatán: Mérida, Mexico, 2005; pp. 21–32. [Google Scholar]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a Worldwide Wood Economics Spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef]
- Mo, L.; Zhang, Y.; Li, X.; Johnson, D.J.; Fortunel, C.; Lin, D.; Swenson, N.G.; Wright, S.J.; Xu, H.; He, N. The Global Distribution and Drivers of Wood Density. Nat. Plants 2024, 10, 1120–1132. [Google Scholar] [CrossRef]
- Koide, R.T.; Robichaux, R.H.; Morse, S.R.; Smith, C.M. Plant water status, hydraulic resistance and capacitance. In Plant Physiological Ecology; Pearcy, R.W., Ehleringer, J.R., Mooney, H.A., Rundel, P.W., Eds.; Springer: Dordrecht, The Netherlands, 1989. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Bucci, S.J.; Scholz, F.G.; Goldstein, G.; Meinzer, F.C.; Arce, M.E. Soil Water Availability and Rooting Depth as Determinants of Hydraulic Architecture of Patagonian Woody Species. Oecologia 2009, 160, 631–641. [Google Scholar] [CrossRef]
- StatSoft, Inc. STATISTICA, Version 12. Data Analysis Software System. StatSoft, Inc.: Tulsa, OK, USA, 2013.
- McCune, B.; Mefford, M.J. PC-ORD, Version 5.10. Multivariate Analysis of Ecological Data. MjM Software: Gleneden Beach, OR, USA, 2006.
- Wei, Z.; Kou, J.; Miao, L.; Hu, F.; Li, L.; Wu, X.; Li, S.; Meng, L. Exploring diurnal variation in soil moisture via sub-daily estimates reconstruction. J. Hydrol. 2025, 662, 134005. [Google Scholar] [CrossRef]
- Hacke, U.G.; Sperry, J.S.; Pockman, W.T.; Davis, S.D.; McCulloh, K.A. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 2001, 126, 457–461. [Google Scholar] [CrossRef]
- Gleason, S.M.; Westoby, M.; Jansen, S.; Choat, B.; Hacke, U.G.; Pratt, R.B.; Bhaskar, R.; Brodribb, T.J.; Bucci, S.J.; Cao, K.-F.; et al. Weak tradeoff between xylem safety and xylem-specific hydraulic efficiency across the world’s woody plant species. New Phytol. 2016, 209, 123–136. [Google Scholar] [CrossRef]
- Rao, P.S.; Saraswathyamma, C.K.; Sethuraj, M.R. Studies on the relationship between yield and meteorological parameters of para rubber tree (Hevea brasiliensis). Agr. Forest Meteorol. 1998, 90, 235–245. [Google Scholar] [CrossRef]
- García, E.G.; Di Stefano, J.F. Fenología de árbol Sideroxylon capiri (Sapotaceae) en el Bosque Seco Tropical de Costa Rica. Rev. Biol. Trop. 2005, 53, 5–14. [Google Scholar] [PubMed]
- Vijayakumar, K.R.; Dey, S.K.; Chandrasekhar, T.R.; Devakumar, A.S.; Mohankrishna, T.; Sanjeeva Rao, P.; Sethuraj, M.R. Irrigation requirement of rubber trees (Hevea brasiliensis) in the subhumid tropics. Agric. Water Manag. 1998, 35, 245–259. [Google Scholar] [CrossRef]
- Dugelby, B. Mecanismos gubernamentales y tradicionales que norman la extracción del látex del chicle en El Petén Guatemala. In La Selva Maya Conservación y Desarrollo; Primack, R.B., Bray, D., Galletti, H.A., Ponciano, I., Eds.; Siglo XXI Editores Mexico: Mexico City, Mexico, 1999; pp. 197–220. [Google Scholar]
- Querejeta, J.I.; Estrada-Medina, H.; Allen, M.F.; Jiménez-Osornio, J.J. Water Source Partitioning Among Trees Growing on Shallow Karst Soils in a Seasonally Dry Tropical Climate. 44. Oecologia 2007, 152, 26–36. [Google Scholar] [CrossRef]
- Reyes-García, C.; Andrade, J.L.; Luis Simá, J.; Us-Santamaría, R.; Jackson, P.C. Sapwood to heartwood ratio affects whole-tree water use in dry forest legume and non-legume trees. Trees 2012, 26, 1317–1330. [Google Scholar] [CrossRef]
- Borchert, R. Soil and Stem Water Storage Determine Phenology and Distribution of Tropical Dry Forest Trees. Ecology 1994, 75, 1437–1449. [Google Scholar] [CrossRef]
- Bucci, S.J.; Goldstein, G.; Meinzer, F.C.; Scholz, F.G.; Franco, A.C.; Bustamante, M. Functional Convergence in Hydraulic Architecture and Water Relations of Tropical Savanna Trees: From Leaf to Whole Plant. Tree Physiol. 2004, 24, 891–899. [Google Scholar] [CrossRef]
- Singh, K.P.; Kushwaha, C.P. Emerging paradigms of tree phenology in dry tropics. Curr. Sci. 2005, 89, 964–975. [Google Scholar]
- Stratton, L.; Goldstein, G.; Meinzer, F.C. Stem water storage capacity and efficiency of water transport: Their functional significance in a Hawaiian dry forest. Plant Cell Environ. 2000, 23, 99–106. [Google Scholar] [CrossRef]
- Olivares, E.; Medina, E. Water and nutrient relations of woody perennials from tropical dry forests. J. Veg. Sci. 1992, 3, 383–392. [Google Scholar] [CrossRef]
- Goldstein, G.; Sarmiento, G.; Meinzer, F. Patrones diarios y estacionales en las relaciones hídricas de árboles siempreverdes de la sabana tropical. Acta Oecologica 1986, 7, 107–119. [Google Scholar]
- Borchert, R. Phenology and Flowering Periodicity of Neotropical Dry Forest Species: Evidence from Herbarium Collections. J. Trop. Ecol. 1996, 12, 65–80. [Google Scholar] [CrossRef]
- Worbes, M.; Blanchart, S.; Fichtler, E. Relations between water balance, wood traits and phenological behavior of tree species from a tropical dry forest in Costa Rica—A multifactorial study. Tree Physiol. 2013, 33, 527–536. [Google Scholar] [CrossRef]
- Johnson, D.M.; Katul, G.; Domec, J.C. Catastrophic hydraulic failure and tipping points in plants. Plant Cell Environ. 2022, 45, 2231–2266. [Google Scholar] [CrossRef]
- Borchert, R. Responses of Tropical Trees to Rainfall Seasonality and Its Long-Term Changes. Clim. Change 1998, 39, 381–393. [Google Scholar] [CrossRef]
- Valladares, F.; Vilagrosa, A.; Peñuelas, J.; Ogaya, R.; Camarero, J.J.; Corcuera, L.; Sisó, S.; Gil-Pelegrín, E. Estrés hídrico: Ecofisiología y escalas de la sequía. In Ecología del Bosque Mediterráneo en un Mundo Cambiante, 2nd ed.; Valladares, F., Ed.; Naturaleza y Parques Nacionales, Ministerio de Medio Ambiente: Madrid, Spain, 2004; pp. 165–192. [Google Scholar]
- Wu, L.; Bai, X.; Li, C.; Li, H.; Cao, Y.; Ran, C.; Zhang, S.; Xiong, L.; Du, C.; Luo, G.; et al. Assessment of carbon sinks caused by the chemical weathering of carbonate rocks under the influence of exogenous acids: Methods, progress, and prospects. Sci. China Earth Sci. 2025, 68, 1785–1804. [Google Scholar] [CrossRef]
- ICRAF 2019. Tree Functional Attributes and Ecological Database. Available online: https://worldagroforestry.org/tree-knowledge/type-of-resource/tree-databases (accessed on 25 June 2025).


| Family | Species | Key | Fruit Type | Leaf Phenology | Individual | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| W | I | D | ||||||||
| Y | M | Y | M | Y | M | |||||
| Light-wood species | ||||||||||
| Burseraceae | Bursera simaruba (L.) Sarg. | Bs | Drupe | D | 4 | 4 | 4 | 4 | 4 | 4 |
| Anacardiaceae | Spondias sp. | Sp | Drupe | D | 0 | 4 | 4 | 4 | 0 | 4 * |
| Soft-wood species | ||||||||||
| Apocynaceae | Thevetia peruviana (Pers.) K. Schum. | Tp | Drupe | E | 4 | 4 | 4 | 4 | 3 * | 0 |
| Boraginaceae | Cordia dodecandra DC. | Cd | Drupe | D | 4 | 4 | 4 * | 3 * | 4 | 2 * |
| Sterculiaceae | Guazuma ulmifolia Lam. | Gu | Capsule | D | 4 | 4 | 0 | 0 | 4 | 4 |
| Boraginaceae | Ehretia tinifolia L. | Et | Drupe | E | 4 | 4 | 4 * | 4 * | 0 | 4 |
| Leguminosae | Piscidia piscipula (L.) Sarg. | Pp | Pod | D | 4 | 4 | 4 | 4 | 4 | 4 |
| Ebenaceae | Diospyros cuneata Standl. | Dc | Drupe | E | 0 | 0 | 4 | 0 | 4 | 4 |
| Anacardiaceae | Metopium brownei (Jacq.) Urb. | Mb | Drupe | D | 4 | 4 | 4 | 4 | 0 | 0 |
| Polygonaceae | Gymnopodium floribundum Rolfe | Gf | Nut | E | 0 | 0 | 4 | 4 | 4 | 4 |
| Fabaceae | Lysiloma latisiliquum (L.) Benth. | Ly | Pod | D | 4 | 4 | 4 | 4 | 4 | 4 |
| Fabaceae | Leucaena leucocephala (Lam.) de Wit | Ll | Pod | D | 4 | 4 | 4 | 4 | 4 | 4 |
| Malpighiaceae | Byrsonima crassifolia (L.) Kunth | Bc | Drupe | D | 4 | 4 | 4 | 4 | 0 | 4 |
| Hard-wood species | ||||||||||
| Moraceae | Brosimum alicastrum Sw. | Ba | Drupe | E | 4 | 4 | 4 * | 4 * | 4 * | 4 * |
| Sapotaceae | Manilkara zapota (L.) P. Royen | Mz | Drupe | E | 4 | 4 | 4 | 4 | 2 * | 3 * |
| Sapotaceae | Chrysophyllum mexicanum Brandegee ex Standl. | Cm | Drupe | E | 4 | 4 | 4 | 4 * | 2 * | 4 * |
| Site | Season | WD | SWC | Ψpd | Ψmd | RWC | SLA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y | M | Y | M | Y | M | Y | M | Y | M | Y | M | |||
| Light-wood species | ||||||||||||||
| B. simaruba | W | rain | 0.34 | 0.44 | 255 | 188 | −0.39 | −0.39 | −0.61 | −0.63 | 54 | 67 | 133 | 120 |
| dry | 0.38 | 0.45 | 242 | 201 | −0.45 | −0.48 | −0.99 | −0.59 | 61 | 68 | 138 | 140 | ||
| nortes | 0.35 | 0.42 | 319 | 230 | −0.37 | −0.37 | −0.5 | −0.52 | 41 | 60 | 127 | 123 | ||
| I | rain | 0.34 | 0.39 | 287 | 227 | −0.34 | −0.45 | −0.57 | −0.7 | 36 | 51 | 129 | 128 | |
| dry | 0.38 | 0.44 | 242 | 173 | −0.88 | −0.87 | −1.1 | −1.08 | 50 | 60 | 122 | 124 | ||
| nortes | 0.34 | 0.43 | 272 | 211 | −0.46 | −0.38 | −0.5 | −0.45 | 48 | 53 | 97 | 102 | ||
| D | rain | 0.39 | 0.46 | 225 | 146 | −0.33 | −0.31 | −0.81 | −0.74 | 58 | 69 | 132 | 144 | |
| dry | 0.43 | 0.42 | 204 | 179 | −1.02 | −0.71 | −1.3 | −1.24 | 55 | 61 | 153 | 153 | ||
| nortes | 0.41 | 0.43 | 188 | 200 | −0.95 | −0.84 | −1.18 | −0.61 | 61 | 63 | * | 131 | ||
| Spondias sp. | W | rain | - | 0.41 | - | 207 | - | −0.13 | - | −0.25 | - | 63 | - | 213 |
| dry | - | 0.45 | - | 201 | - | −0.48 | - | −0.59 | - | 68 | - | 179 | ||
| nortes | - | 0.42 | - | 230 | - | −0.37 | - | −0.52 | - | 60 | - | * | ||
| I | rain | 0.29 | 0.42 | 305 | 196 | −0.2 | −0.18 | −0.99 | −1.05 | 45 | 56 | 184 | 184 | |
| dry | 0.32 | 0.43 | 285 | 175 | −0.97 | −0.59 | −1.6 | −1.5 | 48 | 63 | 239 | 236 | ||
| nortes | 0.35 | 0.45 | 293 | 198 | −0.25 | −0.29 | −0.86 | −0.57 | 35 | 50 | 211 | 161 | ||
| D | rain | - | 0.36 | - | 254 | - | −0.31 | - | −1 | - | 95 | - | 188 | |
| dry | - | 0.38 | - | 282 | - | −0.81 | - | −1.54 | - | 74 | - | 193 | ||
| nortes | - | 0.43 | - | 201 | - | −0.41 | - | −0.75 | - | 89 | - | 199 | ||
| Soft-wood species | ||||||||||||||
| T. peruviana | W | rain | 0.46 | 0.54 | 146 | 145 | −1.16 | −1.06 | −1.75 | −1.53 | 40 | 40 | 184 | 187 |
| dry | 0.48 | 0.54 | 149 | 140 | −1.67 | −1.55 | −2.14 | −2.13 | 36 | 38 | 158 | 145 | ||
| nortes | 0.46 | 0.55 | 176 | 135 | −0.52 | −0.49 | −2.03 | −1.82 | 33 | 42 | 166 | 147 | ||
| I | rain | 0.55 | 0.61 | 134 | 115 | −1.08 | −1.01 | −1.5 | −1.75 | 42 | 41 | 172 | 149 | |
| dry | 0.57 | 0.63 | 112 | 109 | −2.27 | −1.9 | −2.67 | −2.29 | 35 | 39 | 136 | 137 | ||
| nortes | 0.54 | 0.54 | 158 | 145 | −1.39 | −1.27 | −1.66 | −1.8 | 34 | 34 | 148 | 122 | ||
| D | rain | 0.52 | - | 121 | - | −0.82 | - | −1.49 | - | 54 | - | 209 | - | |
| dry | 0.53 | - | 140 | - | −1.9 | - | −2.47 | - | 36 | - | 115 | - | ||
| nortes | 0.56 | - | 116 | - | −1.73 | - | −2.19 | - | 48 | - | 150 | - | ||
| L. leucocephala | W | rain | 0.55 | 0.67 | 115 | 94.8 | −0.71 | −0.75 | −1.12 | −1.33 | 46 | 64 | 256 | 273 |
| dry | 0.54 | 0.67 | 142 | 104 | −0.76 | −0.93 | −1.63 | −1.61 | 46 | 59 | 182 | 174 | ||
| nortes | 0.6 | 0.66 | 129 | 107 | −0.92 | −0.96 | −1.83 | −1.69 | 49 | 71 | 197 | 203 | ||
| I | rain | 0.6 | 0.64 | 110 | 97.2 | −0.25 | −0.12 | −1.66 | −1.64 | 48 | 53 | 355 | 293 | |
| dry | 0.61 | 0.66 | 116 | 97.6 | −0.88 | −0.85 | −2.16 | −2.16 | 38 | 45 | 179 | 172 | ||
| nortes | 0.57 | 0.62 | 140 | 121 | −0.37 | −0.26 | −1.09 | −0.64 | 40 | 43 | 171 | 196 | ||
| D | rain | 0.63 | 0.7 | 118 | 99 | −0.32 | −0.57 | −0.96 | −1.49 | 43 | 56 | 256 | 246 | |
| dry | 0.65 | 0.68 | 99.2 | 92.1 | −2.98 | −1.1 | −3.78 | −2.82 | 41 | 52 | 147 | 152 | ||
| nortes | 0.61 | 0.67 | 115 | 104 | −1.9 | −1.59 | −3.29 | −3.31 | 39 | 55 | 167 | 147 | ||
| P. piscipula | W | rain | 0.66 | 0.71 | 108 | 81.3 | −0.54 | −0.49 | −0.63 | −0.57 | 72 | 74 | 132 | 134 |
| dry | 0.64 | 0.72 | 114 | 105 | −0.92 | −1.08 | −2.04 | −1.8 | 67 | 72 | 88 | 111 | ||
| nortes | 0.63 | 0.7 | 131 | 109 | −0.81 | −0.81 | −1.36 | −1.25 | 61 | 64 | 119 | 99 | ||
| I | rain | 0.61 | 0.75 | 107 | 82.3 | −0.25 | −0.23 | −0.86 | −0.83 | 68 | 71 | 121 | 104 | |
| dry | 0.65 | 0.74 | 103 | 92.1 | −0.7 | −0.55 | −2.15 | −1.01 | 65 | 68 | 116 | 79 | ||
| nortes | 0.64 | 0.68 | 124 | 122 | −0.62 | −0.6 | −1 | −0.84 | 63 | 68 | 112 | 94 | ||
| D | rain | 0.63 | 0.7 | 109 | 87.8 | −0.36 | −0.39 | −1.04 | −0.92 | 65 | 56 | 117 | 101 | |
| dry | 0.67 | 0.73 | 108 | 96.1 | −1.08 | −0.93 | −1.61 | −1.7 | 60 | 65 | 109 | 109 | ||
| nortes | 0.67 | 0.7 | 110 | 90.9 | −0.87 | −0.75 | −1.9 | −1.33 | 64 | 73 | 97 | 86 | ||
| E. tinifolia | W | rain | 0.63 | 0.65 | 111 | 111 | −0.35 | −0.48 | −0.99 | −0.89 | 61 | 63 | 173 | 148 |
| dry | 0.64 | 0.67 | 112 | 106 | −0.69 | −0.9 | −1.8 | −1.56 | 67 | 67 | 152 | 145 | ||
| nortes | 0.67 | 0.67 | 118 | 117 | −0.51 | −0.62 | −1.13 | −1.37 | 62 | 62 | 163 | 139 | ||
| I | rain | 0.57 | 0.58 | 119 | 129 | −0.6 | −0.58 | −1.68 | −1.48 | 49 | 43 | 171 | 172 | |
| dry | 0.59 | 0.61 | 121 | 121 | −0.66 | −0.52 | −2.01 | −2.05 | 66 | 67 | 177 | 191 | ||
| nortes | 0.61 | 0.61 | 125 | 123 | −0.19 | −0.15 | −0.25 | −0.28 | 68 | 66 | 141 | 151 | ||
| D | rain | - | 0.63 | - | 105 | - | −0.38 | - | −1.15 | - | 57 | - | 133 | |
| dry | - | 0.6 | - | 107 | - | −0.78 | - | −1.62 | - | 64 | - | 160 | ||
| nortes | - | 0.66 | - | 97.4 | - | −1.09 | - | −1.44 | - | 74 | - | 132 | ||
| C. dodecandra | W | rain | 0.69 | 0.7 | 105 | 104 | −0.86 | −0.81 | −1.23 | −1.25 | 65 | 65 | 104 | 87 |
| dry | 0.69 | 0.7 | 104 | 98.2 | −0.69 | −0.62 | −1.17 | −1.07 | 65 | 66 | 148 | 125 | ||
| nortes | 0.67 | 0.66 | 109 | 104 | −0.63 | −0.56 | −1.09 | −1 | 70 | 71 | 115 | 146 | ||
| I | rain | 0.58 | 0.7 | 92.5 | 90.5 | −0.97 | −0.5 | −1.95 | −1.9 | 52 | 55 | 141 | 132 | |
| dry | 0.62 | 0.72 | 115 | 92.7 | −1.21 | −0.74 | −2.47 | −2.08 | 64 | 68 | 82 | 95 | ||
| nortes | 0.57 | 0.68 | 146 | 94 | −0.64 | −0.43 | −0.75 | −0.65 | 65 | 73 | 150 | 187 | ||
| D | rain | 0.62 | 0.72 | 107 | 86.2 | −0.73 | −0.73 | −1.54 | −1.97 | 61 | 52 | 100 | 114 | |
| dry | 0.61 | 0.72 | 127 | 98.5 | −1.57 | −1.08 | −2.18 | −1.58 | 54 | 60 | 153 | 99 | ||
| nortes | 0.6 | 0.69 | 129 | 98.3 | −1.28 | −1.56 | −2.03 | −1.95 | 51 | 65 | 125 | 102 | ||
| G. floribundum | W | rain | 0.67 | 0.69 | 107 | 101 | −1.71 | −1.21 | −3.19 | −2.81 | 54 | 52 | 180 | 157 |
| dry | 0.7 | 0.71 | 98.4 | 92.7 | −1.5 | −1.27 | −3.99 | −3.08 | 59 | 63 | 126 | 113 | ||
| nortes | 0.69 | 0.70 | 111 | 96.7 | −1.99 | −1.55 | −3.5 | −3.1 | 65 | 67 | 161 | 130 | ||
| D | rain | 0.71 | 0.72 | 81 | 87.5 | −0.65 | −0.63 | −2.33 | −2.27 | 49 | 58 | 176 | 161 | |
| dry | 0.70 | 0.70 | 87.3 | 90.8 | −2.31 | −2.79 | −4.5 | −4.72 | 54 | 59 | 123 | 92 | ||
| nortes | 0.70 | 0.71 | 88.7 | 92 | −4.01 | −3.45 | −5.41 | −4.33 | 59 | 66 | 144 | 159 | ||
| L. latisiliquum | W | rain | 0.41 | 0.55 | 205 | 130 | −0.37 | −0.31 | −1.95 | −2.43 | 65 | 70 | 74 | 72 |
| dry | 0.36 | 0.63 | 254 | 111 | −0.44 | −0.47 | −1.93 | −1.71 | 56 | 76 | 154 | 145 | ||
| nortes | 0.42 | 0.66 | 218 | 111 | −0.48 | −0.71 | −2.23 | −2.64 | 67 | 74 | 77 | 72 | ||
| I | rain | 0.44 | 0.66 | 214 | 109 | −0.77 | −0.81 | −2.65 | −2.68 | 45 | 58 | 117 | 117 | |
| dry | 0.43 | 0.66 | 199 | 112 | −0.99 | −0.95 | −2.19 | −2.12 | 35 | 56 | 266 | 198 | ||
| nortes | 0.40 | 0.66 | 248 | 116 | −0.16 | −0.21 | −1.76 | −0.82 | 42 | 61 | 147 | 94 | ||
| D | rain | 0.43 | 0.73 | 198 | 92 | −0.23 | −0.32 | −1.94 | −2.37 | 65 | 67 | 156 | 112 | |
| dry | 0.47 | 0.72 | 161 | 86 | −1.64 | −1.95 | −2.30 | −2.94 | 32 | 55 | 183 | 87 | ||
| nortes | 0.46 | 0.69 | 158 | 86 | −2.74 | −1.72 | −3.92 | −2.44 | 42 | 66 | 124 | 95 | ||
| M. brownei | W | rain | 0.59 | 0.67 | 143 | 97.4 | −0.45 | −0.48 | −0.54 | −0.49 | 57 | 62 | 107 | 112 |
| dry | 0.62 | 0.67 | 123 | 111 | −0.58 | −0.58 | −0.96 | −0.81 | 57 | 66 | 93 | 93 | ||
| nortes | 0.58 | 0.64 | 150 | 114 | −0.52 | −0.69 | −0.84 | −1.22 | 59 | 65 | 93 | 104 | ||
| I | rain | 0.53 | 0.61 | 149 | 120 | −0.43 | −0.51 | −0.62 | −0.59 | 44 | 60 | 128 | 117 | |
| dry | 0.57 | 0.63 | 149 | 124 | −0.77 | −0.74 | −1.86 | −1.35 | 57 | 63 | 174 | 217 | ||
| nortes | 0.63 | 0.65 | 137 | 127 | −0.8 | −0.78 | −0.9 | −0.85 | 53 | 60 | 116 | 102 | ||
| G. ulmifolia | W | rain | 0.62 | 0.64 | 102 | 97.3 | −0.67 | −0.79 | −0.9 | −0.93 | 53 | 59 | 205 | 220 |
| dry | 0.57 | 0.66 | 126 | 95.2 | −0.94 | −1.07 | −1.86 | −2.25 | 52 | 64 | 166 | 195 | ||
| nortes | 0.59 | 0.64 | 124 | 104 | −0.89 | −1.04 | −1.46 | −1.58 | 54 | 59 | 160 | 192 | ||
| D | rain | 0.64 | 0.63 | 97.2 | 97.9 | −0.42 | −0.4 | −1.27 | −1.25 | 47 | 39 | 182 | 184 | |
| dry | 0.65 | 0.65 | 98.6 | 103 | −2.37 | −1.51 | −2.5 | −1.66 | 53 | 53 | 149 | 105 | ||
| nortes | 0.65 | 0.65 | 101 | 97.9 | −2.2 | −1.92 | −3.16 | −3.23 | 57 | 57 | 161 | 151 | ||
| D. cuneata | W | rain | 0.69 | - | 91.8 | - | −0.53 | - | −2.11 | - | 42 | - | 93 | - |
| dry | 0.70 | - | 88 | - | −1.36 | - | −3.62 | - | 56 | - | 76 | - | ||
| nortes | 0.70 | - | 91.4 | - | −1.1 | - | −2.8 | - | 62 | - | 67 | - | ||
| D | rain | 0.66 | 0.68 | 103 | 99.3 | −0.59 | −0.58 | −1.96 | −2.13 | 55 | 57 | 126 | 101 | |
| dry | 0.69 | 0.7 | 95 | 96.7 | −3.57 | −2.44 | −5.3 | −4.83 | 56 | 61 | 78 | 66 | ||
| nortes | 0.70 | 0.69 | 97.5 | 104 | −2.58 | −2.07 | −3.41 | −3.06 | 62 | 63 | 84 | 76 | ||
| B. crassifolia | W | rain | 0.51 | 0.64 | 157 | 99.2 | −0.18 | −0.17 | −1.11 | −1 | 81 | 75 | 89 | 125 |
| dry | 0.54 | 0.6 | 154 | 118 | −0.3 | −0.74 | −1.26 | −1.36 | 78 | 73 | 94 | 117 | ||
| nortes | 0.51 | 0.59 | 161 | 120 | −0.36 | −0.3 | −1.33 | −1.09 | 84 | 77 | 96 | 134 | ||
| I | rain | 0.5 | 0.66 | 166 | 114 | −0.35 | −0.3 | −1.27 | −1.37 | 70 | 75 | 132 | 130 | |
| dry | 0.53 | 0.64 | 155 | 116 | −0.3 | −0.43 | −1.52 | −1.41 | 59 | 65 | 111 | 127 | ||
| nortes | 0.59 | 0.58 | 150 | 150 | −0.15 | −0.15 | −0.71 | −0.48 | 67 | 54 | 109 | 112 | ||
| D | rain | 0.59 | 0.58 | 123 | 133 | −0.41 | −0.3 | −1.32 | −1.33 | 69 | 63 | 134 | 132 | |
| dry | 0.57 | 0.56 | 146 | 153 | −0.71 | −0.83 | −1.56 | −1.53 | 55 | 61 | 115 | 122 | ||
| nortes | 0.6 | 0.57 | 128 | 132 | −0.59 | −0.58 | −1.48 | −1.19 | 67 | 72 | 115 | 114 | ||
| Hard-wood species | ||||||||||||||
| B. alicastrum | W | rain | 0.78 | 0.83 | 84.9 | 74.9 | −0.25 | −0.23 | −0.32 | −0.35 | 54 | 52 | 111 | 115 |
| dry | 0.77 | 0.85 | 89.6 | 66.3 | −0.7 | −0.59 | −1.93 | −1.55 | 56 | 54 | 112 | 113 | ||
| nortes | 0.79 | 0.81 | 93.6 | 77.4 | −0.49 | −0.49 | −0.6 | −0.6 | 56 | 54 | 119 | 122 | ||
| I | rain | 0.8 | 0.77 | 72.6 | 65.1 | −0.44 | −0.47 | −0.56 | −0.59 | 31 | 39 | 140 | 159 | |
| dry | 0.83 | 0.84 | 76.8 | 71.5 | −0.97 | −1.14 | −2.27 | −1.95 | 56 | 52 | 112 | 112 | ||
| nortes | 0.8 | 0.81 | 83.8 | 73.6 | −0.49 | −0.39 | −0.49 | −0.45 | 64 | 66 | 116 | 120 | ||
| D | rain | 0.82 | 0.82 | 66.6 | 64.1 | −0.39 | −0.41 | −1.66 | −1.58 | 42 | 39 | 135 | 134 | |
| dry | 0.83 | 0.84 | 67.8 | 70.3 | −1.43 | −1.43 | −2.85 | −3.23 | 47 | 49 | 100 | 104 | ||
| nortes | 0.83 | 0.83 | 72.2 | 76.5 | −0.75 | −0.76 | −0.79 | −1 | 55 | 55 | 106 | 106 | ||
| M. zapota | W | rain | 0.79 | 0.73 | 80 | 80 | −0.36 | −0.45 | −0.56 | −0.44 | 58 | 58 | 106 | 110 |
| dry | 0.8 | 0.77 | 91 | 97.3 | −0.79 | −0.86 | −1.22 | −1.38 | 62 | 60 | 98 | 103 | ||
| nortes | 0.81 | 0.79 | 87.2 | 85.4 | −1.19 | −1.05 | −2.04 | −1.66 | 63 | 64 | 104 | 103 | ||
| I | rain | 0.81 | 0.83 | 76.2 | 71 | −0.67 | −0.55 | −1.3 | −1.08 | 54 | 54 | 105 | 94 | |
| dry | 0.84 | 0.86 | 70.5 | 68.6 | −0.95 | −1.01 | −1.92 | −2.17 | 64 | 59 | 99 | 86 | ||
| nortes | 0.85 | 0.86 | 75 | 71.9 | −0.93 | −0.84 | −1.34 | −1.49 | 69 | 67 | 92 | 91 | ||
| D | rain | 0.84 | 0.82 | 66.6 | 63.5 | −0.69 | −0.67 | −1.55 | −1.64 | 57 | 55 | 89 | 87 | |
| dry | 0.83 | 0.82 | 69.8 | 73 | −1.57 | −1.45 | −2.71 | −3.17 | 56 | 56 | 53 | 84 | ||
| nortes | 0.82 | 0.81 | 74.8 | 77.5 | −1.15 | −0.86 | −1.92 | −1.68 | 61 | 68 | 88 | 84 | ||
| C. mexicanum | W | rain | 0.79 | 0.81 | 75.5 | 67.5 | −0.54 | −0.59 | −1.45 | −1.12 | 68 | 67 | 109 | 110 |
| dry | 0.75 | 0.79 | 90.5 | 79.5 | −0.57 | −0.72 | −1.5 | −1.7 | 65 | 66 | 104 | 98 | ||
| nortes | 0.77 | 0.76 | 92.8 | 97.4 | −0.61 | −0.63 | −1.43 | −1.09 | 61 | 60 | 142 | 107 | ||
| I | rain | 0.77 | 0.74 | 79.2 | 57.1 | −0.34 | −0.54 | −1.04 | −1.1 | 64 | 48 | 74 | 104 | |
| dry | 0.76 | 0.82 | 83.9 | 70.1 | −0.87 | −0.76 | −1.56 | −1.85 | 65 | 58 | 70 | 90 | ||
| nortes | 0.73 | 0.83 | 96.7 | 68.7 | −0.44 | −0.36 | −1.01 | −0.47 | 69 | 71 | 72 | 108 | ||
| D | rain | 0.73 | 0.83 | 74.9 | 80.7 | −0.41 | −0.58 | −1.7 | −1.44 | 38 | 56 | 98 | 112 | |
| dry | 0.78 | 0.81 | 85.1 | 61.6 | −0.98 | −1.06 | −2.02 | −2.11 | 61 | 59 | 82 | 80 | ||
| nortes | 0.78 | 0.78 | 90.7 | 89.8 | −0.74 | −0.63 | −1.45 | −1.87 | 68 | 69 | 89 | 86 | ||
| Environmental Variables | Biological Variables | |||||||
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Rainfall (mm) | VPD (kPa) | Ψpd (MPa) | Ψmd (MPa) | SWC (%) | RWC (%) | WD (g cm−3) | |
| Ψpd (MPa) | −0.1151 c | 0.4919 c | −0.2572 c | |||||
| Ψmd (MPa) | −0.2195 c | 0.4151 c | −0.2136 c | 0.6754 c | ||||
| SWC (%) | −0.0804 a | 0.0522 | −0.0966 b | 0.2190 c | 0.1966 c | |||
| RWC (%) | −0.0708 a | −0.0420 | 0.0384 | 0.1613 c | 0.1533 c | −0.0861 b | ||
| WD (g cm−3) | 0.0357 | −0.0767 a | 0.0447 | −0.2009 c | −0.1535 c | −0.9231 c | 0.1578 c | |
| SLA (cm2 g−1) | 0.0357 | 0.1080 c | −0.0337 | 0.0603 | 0.0513 | 0.3153 c | −0.2934 c | −0.4078 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palomo-Kumul, J.; Valdez-Hernández, M.; Islebe, G.A.; Osorio-de-la-Rosa, E.; Cruz-Piñon, G.; López-Huerta, F.; Juárez-Aguirre, R. Hydro-Functional Strategies of Sixteen Tree Species in a Mexican Karstic Seasonally Dry Tropical Forest. Forests 2025, 16, 1535. https://doi.org/10.3390/f16101535
Palomo-Kumul J, Valdez-Hernández M, Islebe GA, Osorio-de-la-Rosa E, Cruz-Piñon G, López-Huerta F, Juárez-Aguirre R. Hydro-Functional Strategies of Sixteen Tree Species in a Mexican Karstic Seasonally Dry Tropical Forest. Forests. 2025; 16(10):1535. https://doi.org/10.3390/f16101535
Chicago/Turabian StylePalomo-Kumul, Jorge, Mirna Valdez-Hernández, Gerald A. Islebe, Edith Osorio-de-la-Rosa, Gabriela Cruz-Piñon, Francisco López-Huerta, and Raúl Juárez-Aguirre. 2025. "Hydro-Functional Strategies of Sixteen Tree Species in a Mexican Karstic Seasonally Dry Tropical Forest" Forests 16, no. 10: 1535. https://doi.org/10.3390/f16101535
APA StylePalomo-Kumul, J., Valdez-Hernández, M., Islebe, G. A., Osorio-de-la-Rosa, E., Cruz-Piñon, G., López-Huerta, F., & Juárez-Aguirre, R. (2025). Hydro-Functional Strategies of Sixteen Tree Species in a Mexican Karstic Seasonally Dry Tropical Forest. Forests, 16(10), 1535. https://doi.org/10.3390/f16101535









