Abstract
Atmospheric nitrogen deposition affects the health of forest ecosystems by altering soil microbial activity. However, the effects of nitrogen addition levels, morphology and ecosystem type on whether nitrogen addition is beneficial or detrimental to soil health is controver-sial, and most studies have focused on the negative effects on microbial structure. Based on this, this study conducted a four-year experiment of nitrogen (NaNO3) addition at two levels (10 and 20 kg N hm−2·yr−1) in the understory soil of Larix olgensis in northeastern China to study soil microbial properties, soil enzyme activities, and to analyze soil physi-cochemical properties and the correlation between them. The results showed that nitrogen addition reduced soil pH and increased soil NH4+-N and NO3−-N contents, thus promoting the activities of Urease (Ure), Acid phosphatase (ACP) and N-Acetamidoglucosidase (NAG) and inhibiting the activity of Leucine aminopeptidase (LAP) in soil, further improving the diversity and richness of soil microorganisms and increasing the dominant taxa of beneficial microorganisms. This may be due to soil acidification caused by the addition of nitrogen, which increases the effectiveness of nitrogen in the soil, improving soil properties, moving soil health in a beneficial direction, promoting beneficial microbial activity, and making the soil more suitable for the growth of the acid-loving tree species L. olgensis. In general, N addition favored the development of soil bacterial communities and the maintenance of soil nutrient status, and had a positive effect on the soil nutrient status of L. olgensis. The results of this study may provide an important scientific basis for adaptive management of forest ecosystems in the context of global nitrogen deposition.
1. Introduction
Since the industrial revolution, the use of chemical fuels and nitro-gen fertilizers have led to a rapid increase in nitrogen deposition worldwide [1]. Over the last 150 years, atmospheric nitrogen deposition has increased 4 to 5 times [2] and is expected to continue to increase 2.5 times by the end of this century [3]. In the context of the continued increase in nitrogen deposition, soil health has become a prominent research area in ecology. Numerous studies have shown that soil biological properties can sensitively reflect changes in soil health [4]. Therefore, soil microbial biomass, microbial community structure composition, and microbial diversity have become essential biological indicators for characterizing changes in soil health. However, the results of current studies on the response of soil nutrients to nitrogen deposition are inconsistent, and based on the above understanding, exploring the effects of nitrogen addition on soil enzyme activity and microbial community structure diversity can provide data to support the predictions regarding the future impact of increased nitrogen deposition on soil health in forest ecosystems.
A large number of studies worldwide have investigated the effects of nitrogen addition on soil microbial communities and have shown that a surge in nitrogen deposition can have serious effects on ecosystem health. The risk of accelerated soil acidification and nitrate leaching directly or indirectly affects soil enzyme activity, soil microbial diversity and community structure composition by altering environmental factors [5]. For instance, nitrogen addition can reduce the content of total phospholipid fatty acids (TPLFAs), bacteria and fungi in the soil due to nitrogen addition [6]. It also decreases soil pH and causes soil acidification, which in turn leads to changes in microbial community structure [7]. Most studies have shown that increased N additions inhibit the growth of some bacterial groups and reduce species richness and community composition [8,9]. In turn, changes in microbial community structure can further affect soil nutrient health and lead to ecosystem degradation or loss of ecosystem function [10]. However, it has also been shown [11,12] that nitrogen deposition within a certain range may have a positive effect on biodiversity. These differences may be due to different nitrogen addition patterns, levels, topography, and timing of nitrogen addition. Further research is needed to determine whether the effects of N addition on forest soil health are beneficial or detrimental.
L. olgensis is one of the major silvicultural species widely distributed in northeastern China and. Notably, northeastern China has become an area with high nitrogen deposition in China due to the high consumption of energy. Therefore, it is important to study the effect of N addition on L. olgensist soil health. In our study, we conducted experiments with different levels of nitrogen addition in L. olgensis and measured soil nutrient, soil enzyme activity and soil microbial community structure diversity after four years of fertilization. We hypothesized that 1. nitrogen addition has adverse effects on L. olgensis and 2. a low level of nitrogen addition is beneficial to soil nutrient health of L. olgensis, while a high level of nitrogen addition can damage soil health. This study elucidates the effects of nitrogen addition on soil health and provides data to support the sustainable management of L. olgensis in the context of nitrogen deposition.
2. Materials and Methods
2.1. Experimental Area and Design
The experimental area was located in the back of Jilin Agricultural University, in L. olgensis forest. (43°05′–45°15′ N; 124°18′–127°05′ E) at an altitude of 250–350 m above sea level on the top of the mountain. The average annual temperature is 4.8 °C, while the hottest month (July) averages 23 °C. Annual precipitation varies from 522 to 615 mm, with summer precipitation accounting for more than 60% of the annual precipitation. The soil in the study area is loamy, and the main vegetation in the understory is Larix olgensis, Bromus inermis, Viola prionantha, and Chelidonium majus.
The nitrogen addition test was set up in 2018 in a completely randomized grouping design with sample sites in the understory plot of L. olgensis in the back of Jilin Agricultural University, each with an area of 5 m × 5 m and relatively flat, with a buffer zone of at least 2 m width between any two sample sites to avoid mutual interference between sites. The N treatments consisted of three levels of N addition (0, 10, 20 kg N hm−2·yr−1) with the applied N fertilizer in the form of NaNO3, while the control plots received no N. There were three treatments in the experiment, and each group of treatments was replicated five times. The nitrogen addition treatments were applied twice a year, in May and October [13,14]. Nitrogen was applied by mixing the desired fertilizer in each treatment sample with 1 kg of sand (sand passed through a 4 mm sieve and applied after seed removal) and applying it to the soil surface.
In August 2021, soil samples were collected, and apoplastic material and weeds were removed from the soil surface before sampling. Each test sample site had five sampling locations, and soil samples were collected from the 0–10 cm soil layer using a 5 cm soil auger. The five samples were mixed into one sample in a self-sealing bag and transported back to the laboratory on dry ice for microbial community diversity testing. At the same time, soil pretreatment was performed, and fresh samples were passed through a 2 mm sieve to remove residues, and then divided into two parts. One part of the samples was placed in a ventilated area for air-drying treatment for the determination of soil pH, total nitrogen, total carbon, and total phosphorus; one part of the samples was placed in a refrigerator at −20 °C for the determination of soil ammonium nitrogen, nitrate nitrogen, microbial carbon, microbial nitrogen, microbial phosphorus, soil enzyme activity, and other indicators The samples were refrigerated at −20 °C for the determination of soil ammonium nitrogen, nitrate nitrogen, microbial carbon, microbial nitrogen, microbial phosphorus and soil enzyme activity.
2.2. Soil Index Determination Method
2.2.1. Chemical Test Methods of Soil Properties
Determination of soil pH. Using a 50 mL small beaker, weigh 10 g of air-dried soil sample passing through a 2 mm sieve and add 25 mL of distilled water (soil-water ratio of 1:2.5). Stir thoroughly with a glass rod and let it stand for 2 h, then determine the pH value of the soil supernatant with a pH meter (PHS-25, Shanghai, China).
Determination of soil ammonium nitrogen (NH4+-N) and nitrate nitrogen (NO3-N): In a 100 mL shaker, weigh 20 g of fresh soil sample after passing through a 2 mm sieve. Add 50 g of 2 mol/L KCl solution, shake on a shaker for 30 min at 175 r/min, remove and filter (filtrate should be clarified), and determine with a continuous flow analyzer (QC8500, Shanghai, China).
Determination of soil total carbon (TC), total nitrogen (TN) and total phosphorus (TP): Determined by using j200 laser elemental analyzer [15]. Elemental analysis mainly includes Axiom software operation and Data analysis data analysis software operation.
Determination of soil microbial biomass [16]: Microbial carbon (MBC) was determined by chloroform fumigation-K2SO4 extraction-carbon analyzer [17], microbial nitrogen (MBN) was determined by chloroform fumigation-K2SO4 extraction-flow injection nitrogen analyzer [17], and microbial phosphorus (MBP) was determined by chloroform fumigation-NaHCO3 extraction-Pi correction method.
2.2.2. Soil Enzyme Activity Test Methods
Four types of enzyme activities were determined as follows: Urease (Ure) by phenol-sodium hypochlorite colorimetric method for urease [18], Acid phosphatase (ACP) by sodium benzene phosphate colorimetric method for acid phosphatase (with acetate buffer pH = 5.0) [18], N-Acetamidoglucosidase (NAG) by microplate fluorescence method, and Leucine aminopeptidase (LAP) by double antibody sandwich method using Shanghai Enzyme Link reagent kit.
2.2.3. Methods for Determination of Soil Microbial Community Structure
Extraction of genomic DNA and PCR amplification: The genomic DNA was extracted by CTAB or SDS method, and the purity and concentration of DNA were checked by agarose gel electrophoresis, and the sample was diluted to 1 ng/μL with sterile water in a centrifuge tube. Phusion® High-Fidelity PCR Master Mix with GC Buffer from England Biolabs, and high efficiency high fidelity enzymes were used for PCR to ensure amplification efficiency and accuracy, depending on the selection of the sequencing region.
Primers corresponding to regions: 16S V4 region primers (515F and 806R): identification of bacterial diversity. 18S V4 region primers (528F and 706R): identification of eukaryotic microbial diversity. ITS1 region primers (ITS5-1737F and ITS2-2043R): identification of fungal diversity. In addition, the amplified regions include: 16S V3-V4/16S V4-V5/16SV5-V7; Archaea 16S V4-V5/Archaea 16S V8; 18S V9 and ITS2 region.
Mixing and purification of PCR products: The PCR products were detected by electrophoresis using 2% agarose gel; the PCR products that passed the test were purified by magnetic beads, quantified by enzyme labeling, mixed in equal amounts according to the concentration of PCR products, mixed thoroughly, and then detected by electrophoresis using 2% agarose gel, The target bands were recovered using the gel recovery kit provided by Qiagen, Hilden, Germany.
Library construction and sequencing: The library was constructed using TruSeq® DNA PCR-Free Sample Preparation Kit, and the library was quantified by Qubit and Q-PCR, and then sequenced using NovaSeq6000. The sequencing was entrusted to Tianjin Novozyme Biotechnology Co., Tianjin, China.
2.3. Statistical Analysis
Graphs were prepared using GraphPad Prism 9, and data were statistically and analytically analyzed using SPSS 26.0. The effects of different nitrogen application patterns and nitrogen application levels on soil environmental factors, enzyme activity, and soil bacterial diversity in L. olgensis understory were analyzed using one-way ANOVA. The significance of differences between treatments was compared multiplexed using the LSD method (p < 0.05). Pearson correlation analysis was used to correlate soil enzyme activity, soil environmental factors, and bacterial diversity. The main factors influencing the addition of nitrogen on soil bacterial diversity were studied by using Canoco 5 for redundancy analysis, with bacterial diversity as a sample and soil environmental factors and soil enzyme activity as variables.
3. Results
3.1. Effect of Nitrogen Addition on Soil Chemistry
In our study, all treatments significantly reduced soil pH, TC, and C/N values regardless of the amount of N added. Compared to the control group, the low and high N additions decreased pH by 2.9% and 4.4%, respectively, TC content by 6.7% and 17.3%, and C/N values by 14.3% and 24.8%. High levels of N addition to the soil significantly reduced pH, TC, and C/N values (p < 0.01) more than low levels of N addition (Table 1 and Table 2). In most of the findings, N addition leads to soil acidification [5], which is consistent with our findings. This is due to the fact that nitrogen addition increases NH4+ and NO3− in the soil, and the main acidification mechanisms in the soil are nitrification of NH4+ and leaching of NO3−. However, existing studies [19] showed that soil carbon content increased gradually with increasing N addition. In the present study, soil TC content showed a decreasing trend with increasing N addition. This may be attributed to the reduction in soil pH following N addition. It has been shown [20,21] that soil acidification leads to reduced mineral sorption by microbial residues, easing the decomposition of organic matter and reducing soil TC levels. The reduction in soil total carbon content may also be related to soil invertebrates. Soil invertebrates can strongly fragment and break down soil organic matter, converting it into compounds that are readily available to plants and easily mineralized, which leads to a reduction in soil TC content [22]. C/N ratio is an important indicator to characterize soil quality, and the stability of the C/N ratio is important in relation to soil health as well as plant growth. This study showed that the range of soil C/N ratio after individual N additions was 11.41–13.39, which was basically within the range of the mean C/N values (10–12) of Chinese soils. This implies that although N addition significantly affected the content of total soil carbon and nitrogen, relatively stable ratios existed for them as structural components.

Table 1.
Results of repeated measures ANOVA: Effect of nitrogen addition on soil environmental factors.

Table 2.
Analysis of variance of nitrogen addition on soil environmental factors, enzyme activity, and soil microbial properties.
Our results showed that different levels of N addition significantly increased NH4+-N, NO3−-N, and TN contents of the soil (p < 0.01). Compared to the control group, low and high N additions increased NH4+-N by 29.5% and 50.5%, respectively; increased NO3−-N by 137.4% and 157.0%; and increased TN by 8.9% and 11.4%. High N addition increased NH4+-N, NO3−-N, and TN contents more (p < 0.01) (Table 1 and Table 2). The results of many studies also indicate this trend: soil NH4+-N, NO3−-N and TN contents increase with N addition [23,24,25]. The increase in inorganic N content indicates that N addition promotes the mineralization rate of organic N and converts nitrogenous organic matter in the soil into inorganic N, thus increasing the effective N content, which is available to plants and microorganisms. The increase in N content in the soil also indicated that the amount of N addition met the N demand of plants and microorganisms, which in turn weakened the rate of nutrient cycling and increased N aggregation.
3.2. Effect of Nitrogen Addition on Soil Microbial Biomass
In this study, the MBC content in the soil decreased significantly when different levels of N were added. Compared to the control group, the low and high nitrogen additions reduced the MBC content by 3.9% and 5.7% respectively, and the effect of high N addition on the MBC content was greater (p < 0.05). However, most of the studies found that the MBC content increased with the increase of nitrogen addition [26,27]. One possible reason for this discrepancy is that N addition leads to soil acidification, which not only leads to inhibition of microbial growth, but also produces an “aluminum toxicity effect” that reduces the MBC content in the soil. Another possible reason is that plants take up and use a large amount of carbon in the soil, thus reducing the organic carbon content and causing microorganisms to release carbon from their bodies for plant growth, resulting in a decrease in soil MBC content.
The results of our study revealed a general trend of low level promotion and high-level inhibition of MBN content. The MBN content was lower for high nitrogen addition than for the control, and the highest MBN content was found for low nitrogen addition (p < 0.05). Such a pattern was also found in the study of others [28], where low levels of nitrogen addition had a positive effect on MBN, while high levels had a negative effect. However, it has also been shown [29] that the level of MBN increases with increasing nitrogen addition. This is similar to our findings. It is possible that at the beginning of nitrogen addition, the nitrogen content in the soil increased to meet the microbial demand for nitrogen, which promoted the decomposition and utilization of soil organic matter by microorganisms, thus increasing the MBN content. However, as the nitrogen addition continued, it reduced the microbial demand for N, which led to a decrease in MBN content [30].
According to Table 2 and Figure 1, MBP content increased significantly (p < 0.05), and compared to the control, low and high N additions increased the MBP content by 13.9% and 26.2%, respectively, with the highest rate of increase for high nitrogen additions. However, one study found that the MBP content decreased with increasing nitrogen addition [31]. This difference may be due to the fact that high nitrogen addition increased phosphorus limitation, prompting microorganisms to secrete more phosphorus hydrolases to meet nutrient uptake, so MBP content continued to increase.
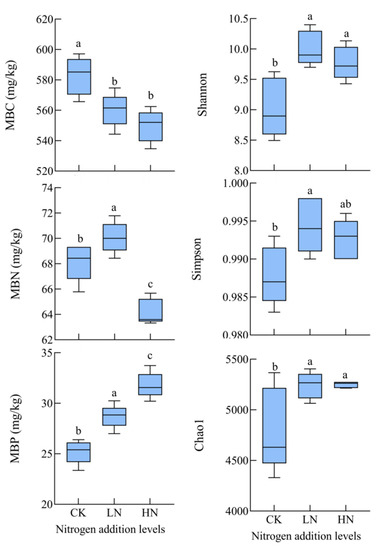
Figure 1.
Effect of different N treatments on soil microbial properties CK: 0 kg N hm−2·yr−1, LN: 10 kg N hm−2·yr−1, HN: 20 kg N hm−2·yr−1. Different lowercase letters indicate differences in different levels of nitrogen addition (p < 0.05).
3.3. Effect of Nitrogen Addition on Soil Enzyme Activities
Our results showed that different levels of nitrogen addition enhanced soil urease (Ure), acid phosphatase (ACP), and N-acetylaminoglucosidase (NAG) activities and inhibited leucine aminopeptidase (LAP) activities (Table 2 and Figure 1). Low and high nitrogen additions increased Ure activity by 19.9% and 27.9%, increased ACP activity by 2.0% and 14.9%, increased NAG activity by 5.2% and 10.0%, and reduced LAP activity by 6.8% and 10.7%, respectively. The promotion of Ure, ACP and NAG activities was significantly higher with high nitrogen addition than with low nitrogen addition (p < 0.05), and the inhibition of LAP activity was significantly enhanced with high nitrogen addition (p < 0.05) (Table 2 and Figure 2).
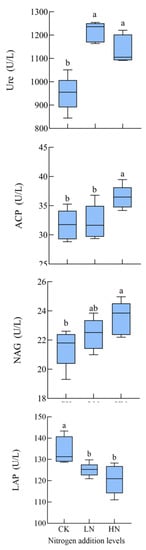
Figure 2.
Effects of different nitrogen treatments on soil enzyme activities. CK: 0 kg N hm−2·yr−1, LN: 10 kg N hm−2·yr−1, HN: 20 kg N hm−2·yr−1. Different lowercase letters indicate differences in different levels of nitrogen addition (p < 0.05).
Enzyme production by soil microorganisms follows the economic principle that nitrogen addition decreases the microbial demand for nitrogen and increases the demand for carbon and phosphorus, leading to a decrease in nitrogen hydrolase activity [32,33], which is consistent with the results of most studies. In the present study, nitrogen addition limited the activity of the nitrogen acquisition enzyme LAP due to the increase in soil effective nitrogen content under nitrogen addition conditions, which resulted in a decrease in the activity of enzymes associated with nitrogen acquisition. The results of correlation analysis indicated that the decrease in soil pH after nitrogen addition was the main reason for the decrease in LAP activity. However, contrary to the results of some studies [34], in our study, NAG activity increased in all treatments due to the increased level of nitrogen addition. This difference may be due to the increase in plant biomass after nitrogen addition, which leads to an increase in organic N in the soil and induces NAG secretion.
Different levels of nitrogen addition promoted the activity of soil urea, ACP, which has been confirmed by numerous studies [35,36,37], due to the fact that nitrogen deposition increases the effectiveness of nitrogen in the soil to some extent, promoting the demand of microorganisms for carbon and nitrogen nutrients, and the increased uptake of nitrogen by microorganisms and plants stimulates the activity of soil urease, hence the promotion effect of nitrogen addition [38]. The increase in N effectiveness shifted the microbial extracellular enzymes from N-limiting to phosphorus-limiting, stimulating the microbial demand for phosphorus, contributes to the consequent increase in the activity of enzymes associated with the phosphorus cycle, which also promoted acid phosphatase activity. RDA analysis showed (Figure 3 and Figure 4) that the main environmental factor affecting enzyme activity was NO3−-N. This is consistent with the resource allocation model formulation [34], where the variation in soil enzyme activity depends mainly on the effective N content of the soil.
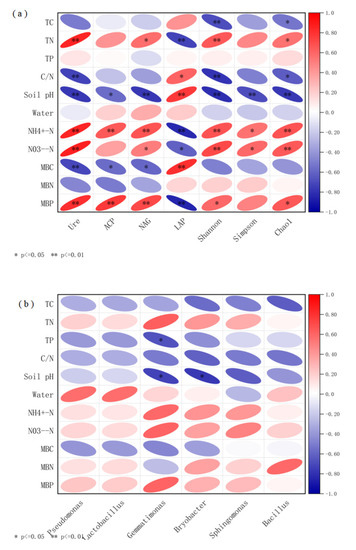
Figure 3.
Correlation heat map. (a) Correlation between soil environmental factors and soil biological properties. (b) Correlation between soil environmental factors and soil microbial community structure. * significantly correlated at the 0.05 level, ** highly significantly correlated at the 0.01 level.
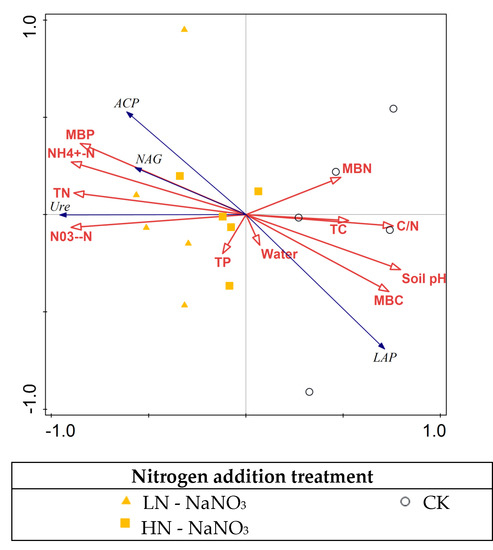
Figure 4.
Redundancy analysis (RDA) of various environmental factors associated with soil enzyme activity under nitrogen addition conditions.
3.4. Effect of Different Nitrogen Addition Treatments on Soil Bacterial Diversity
Soil microbial diversity can reflect the overall dynamics of the microbial community to some extent. Different nitrogen addition treatments had different effects on soil bacterial abundance and diversity indices (Figure 1), and both nitrogen addition levels significantly increased the Shannon, Simpson, and Chao1 indices compared to the control (p < 0.05), with the magnitude of increase showing low nitrogen addition > high nitrogen addition. It has been shown [39] that nitrogen addition led to a decrease in soil bacterial richness index in forests in southern China. The results of a study in an experimental field of grassland fertilization in China [40] found that nitrogen addition led to a decrease in the bacterial diversity index. Many studies [41] found that N application had a negative effect on soil microbial diversity. In the present study, different levels of nitrogen addition treatments increased the soil bacterial diversity index (Shannon and Simpson) and soil bacterial richness (Chao1), which is not consistent with the results of previous studies. This difference may be due to the fact that soils in the study area are N-deficient, while N addition favors plant growth and the growth of a more diverse bacterial community. In this study, soil bacterial diversity index and soil bacterial richness were positively correlated with soil effectiveness, so nitrogen addition would increase bacterial diversity by increasing soil effectiveness.
3.5. Effect of Different Nitrogen Addition Treatments on Soil Bacterial Structure
High-throughput sequencing technology is an efficient sequencing method for analyzing the composition of soil microbial communities. According to the OUT classification and taxonomic status as shown in Figure 5, the average relative abundance of bacterial phyla in soil samples in all treatments was greater than 1% at the phylum level: Proteobacteria, Acidobacteria, Cyanobacteria, Firmicutes, Verrucomicrobiota, Bacteroidota, Chloroflexi, Planctomycetes, Actinobacteria, and Myxococcota, and the above ten phyla together accounted for 61%–66% of the total bacterial abundance. The effects of different N addition treatments on the community structure of soil bacterial dominant bacteria were different; Nitrogen addition increased the relative abundance of Proteobacteria, Acidobacteriota, Verrucomicrobiota, Actinobacteriota, Myxococcota, decreased the relative abundance of Cyanobacteria, Bacteroidota, Chloroflexi, Planctomycetes, and the effect on the relative abundance of Firmicutes showed a trend of low-level promotion and high-level inhibition.
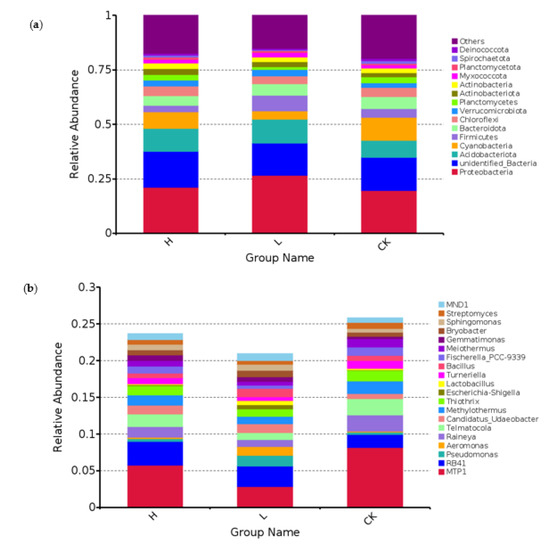
Figure 5.
Effect of nitrogen addition on the abundance of bacterial. (a) Bacteria phylum Level; (b) Bacteria genus level. Note: “Others” indicates the sum of the relative abundances of all gates other than those shown in the figure. H: high N addition, L: low N addition, CK: no N addition.
It has been shown [42] that although the structural composition of microbial communities may differ in different ecosystems, the dominant bacterial groups in the communities are basically the same. In the present study, there were ten dominant bacterial taxa after nitrogen addition treatment, and the top five dominant taxa in terms of abundance were Proteobacteria, Acidobacteria, Cyanobacteria, Stachybotrys, and Vertebrates, which were basically consistent with the results of previous studies. Different studies have shown [43] that Acidobacteria, Proteobacteria, and Actinobacteria phylum prefer soils with low soil pH. The abundance of some eutrophic groups, such as Proteobacteria phylum, increases when the nitrogen content in the soil increases [44]. In the present study, the addition of nitrogen increased the relative abundance of Proteobacteria, which may be due to the wide range of adaptation of Proteobacteria to soil pH, from poor acidic soils to alkaline environments. Previous studies have suggested that Actinomycetes are eutrophic bacteria [40], and many genera are drought and salinity tolerant and are the dominant flora in arid and saline soil types, capable of degrading cellulose and chitin, producing a variety of extracellular enzymes and secondary metabolites, and being a source of soil nutrient supply. In our study, nitrogen addition resulted in an increasing trend in the relative abundance of Actinomycetes, which may be related to the increased effectiveness of soil nitrogen under nitrogen addition conditions in this study. Nitrogen addition also increased the relative abundance of acidic flora in our study, which may be due to the significant negative correlation between acidic flora and soil pH in this study. The nitrogen addition treatment significantly reduced the relative abundance of green algae. The results of the study so far [45] show that they are involved in the second step of nitrification, i.e., nitrite oxidizing bacteria were first identified in Chloroflexi by Sorokin et al. [45], and the reduction in the relative abundance of Chloroflexi phylum in the present study may imply a weakening of soil nitrification, reducing Verrucomicrobiota is a common class of bacterial phylum in soil. Microorganisms of this bacterial phylum are associated with the degradation of polysaccharides in soil and are therefore important players in the carbon cycle in soil ecosystems [46]. The decrease in the relative abundance of the Verrucomicrobiota phylum in this study may be related to the decrease in soil C.
We further investigated the differences between two levels of nitrogen addition on bacterial communities at the genus level. We extracted the 20 most abundant genera to study the changes of nitrogen addition on bacterial community structure at the genus level (Figure 5). Different levels of nitrogen addition increased the relative abundance of Pseudomonas, Lactobacillus, Gemmatimonas, Bryobacter, and Sphingomonas, and low nitrogen addition compared to the control increased the relative abundance of the five taxa; except for Gemmatimonas, the relative abundance of all the other four taxa increased with the increase of nitrogen addition. The overall effect of nitrogen addition on the relative abundance of Bacillus showed a trend of “low promotion and high suppression”, decreasing the relative abundance of Aeromonas, Candidatus_Udaeobacter, Methylothermus, Turneriella, and Bacillus. Meiothermus, and Streptomyces.
Studies have shown [47,48] that Gemmatimonas belonging to Gemmatimonadetes can be involved in soil organic matter fixation. In our study, both levels of N addition increased the relative abundance of Gemmatimonas, suggesting that N addition may promote the growth and development of Larix olgensis plants, improve the physical properties of the soil, and promote the activity of microorganisms and soil organisms. Pseudomonas belongs to Proteobacteria [49]. Both horizontal N additions increased the abundance of Pseudomonas and this promotion may enhance soil N fixation [49]. Our study also found that nitrogen addition increased the abundance of Bacillus medium, a common bacterial group in soil with high abundance and phosphorus solubilizing ability. It secretes secondary metabolites that induce disease resistance in plants and have a strong probiotic effect [50]. It can form spores and are highly adaptable to the environment; therefore, it can grow and reproduce well when the nutrient content in the subsoil is low [51]. In addition, nitrogen addition promotes the beneficial bacteria Bryobacter, which has the ability to decompose lignin and cellulose, and Sphingomonas, which has the ability to fix nitrogen or promote growth in crop roots. According to correlation analysis, soil pH and soil TP were significantly correlated with the beneficial microflora whose abundance increased after nitrogen addition, and the environmental factors that mainly affected the change in bacterial community structure were soil water content and C/N (Figure 6), which indicated that N addition did change the nutrient status of the soil by affecting the physicochemical properties of the soil.
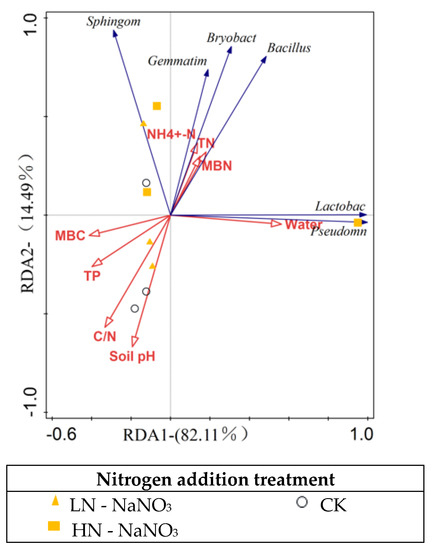
Figure 6.
Redundancy analysis (RDA) of various environmental factors associated with dominant taxa of soil bacteria under nitrogen addition.
4. Conclusions
Based on the above findings, nitrogen addition had an overall positive effect on the soil nutrient status of Larix olgensis in a long-term nitrogen-deficient state because the nitrogen-deficient state inhibited the nutrient cycling of the soil, thus limiting the growth of aboveground species and disrupting the ecological balance. In contrast, nitrogen addition lifted the nitrogen-limited status of L. olgensis by increasing the effectiveness of soil nitrogen, which led to an increase in soil microbial biomass and diversity indices, and the relative abundance of beneficial microbial taxa increased significantly after nitrogen addition. The main environmental factor affecting soil enzyme activity was NO3−-N, and the main environmental factors affecting soil microbial community structure were C/N and water content. This indicates that TC, TN, C/N, soil pH, and NH4+-N play important roles in the changes of soil bacterial community abundance, diversity, and relative abundance of dominant taxa in the study area. In general, moderate N application or atmospheric deposition favored soil nutrient cycling and microbial growth in L. olgensis. The results of this experiment provide data to further elucidate the response mechanisms of soil microbial community structure to nitrogen deposition in the boreal forests of China in the context of global environmental change. In this paper, we studied only the microbial diversity and community structure composition; following research should focus on the functional dynamic of microorganisms and study functional microorganisms and functional genes to make them develop in a direction that is beneficial to the ecosystem.
Author Contributions
Conceptualization, T.Q., L.Z. and M.L.; methodology, T.Q., M.L. and X.Z.; software, M.L. and H.L.; validation, T.Q. and M.L.; formal analysis, L.Z.; investigation, L.Z.; resources, L.Z. and T.Q.; data curation, M.L. and X.Z.; writing—original draft preparation, T.Q. and M.L.; writing—review and editing, all; visualization, H.L.; supervision, L.Z.; project administration, L.Z.; funding acquisition, L.Z. and T.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Jilin Provincial Science and Technology Department project (20220101180JC).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ma, M.; Jiang, X.; Guan, D.; Wei, D.; Zhao, B.; Li, J. Impact of 36 years of nitrogen fertilization on microbial community composition and soil carbon cycling-related enzyme activities in rhizospheres and bulk soils in northeast China. Appl. Soil Ecol. 2019, 136, 148–157. [Google Scholar] [CrossRef]
- Bedison, J.E.; McNeil, B.E. Is the growth of temperate forest trees enhanced along an ambient nitrogen deposition gradient? Ecology 2009, 90, 1736–1742. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G.; Angers, D.A.; Beare, M.H.; Sparling, G.P.; Wardle, D.A.; Voroney, R.P. Interpretation of microbial biomass measurements for soil quality assessment in humid temperate regions. Can. J. Soil Sci. 1999, 79, 507–520. [Google Scholar] [CrossRef]
- Du, Y.; Guo, P.; Liu, J.; Wang, C.; Yang, N.; Jiao, Z. Different types of nitrogen deposition show variable effects on the soil carbon cycle process of temperate forests. Glob. Chang. Biol. 2014, 20, 3222–3228. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Zhang, X.; Wang, H.; Yang, H.; Zhao, W.; Li, S. Nitrogen additions inhibit nitrification in acidic soils in a subtropical pine plantation: Effects of soil pH and compositional shifts in microbial groups. J. For. Res. 2019, 30, 669–678. [Google Scholar] [CrossRef]
- Shen, F.; Wu, J.; Fan, H.; Liu, W.; Guo, X.; Duan, H.; Wei, X. Soil N/P and C/P ratio regulate the responses of soil microbial community composition and enzyme activities in a long-term nitrogen loaded Chinese fir forest. Plant Soil 2019, 436, 91–107. [Google Scholar] [CrossRef]
- Nie, Y.; Wang, M.; Zhang, W.; Ni, Z.; Hashidoko, Y.; Shen, W. Ammonium nitrogen content is a dominant predictor of bacterial community composition in an acidic forest soil with exogenous nitrogen enrichment. Sci. Total Environ. 2018, 624, 407–415. [Google Scholar] [CrossRef]
- Wang, C.; Lu, X.; Mori, T.; Mao, Q.; Zhou, K.; Zhou, G.; Mo, J. Responses of soil microbial community to continuous experimental nitrogen additions for 13 years in a nitrogen-rich tropical forest. Soil Biol. Biochem. 2018, 121, 103–112. [Google Scholar] [CrossRef]
- Zhao, Q.; Zeng, D.H. Nitrogen addition effects on tree growth and soil properties mediated by soil phosphorus availability and tree species identity. For. Ecol. Manag. 2019, 449, 117478. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, G.; Li, P.; Xue, S. Direct and indirect effects of elevated CO2 and nitrogen addition on soil microbial communities in the rhizosphere of Bothriochloa ischaemum. J. Soils Sediments 2019, 19, 3679–3687. [Google Scholar] [CrossRef]
- Allison, S.D.; LeBauer, D.S.; Ofrecio, M.R.; Reyes, R.; Ta, A.M.; Tran, T.M. Low levels of nitrogen addition stimulate decomposition by boreal forest fungi. Soil Biol. Biochem. 2009, 41, 293–302. [Google Scholar] [CrossRef]
- Chen, Z.; Geng, S.; Zhou, X.; Gui, H.; Zhang, L.; Huang, Z.; Han, S. Nitrogen addition decreases soil aggregation but enhances soil organic carbon stability in a temperate forest. Geoderma 2022, 426, 116112. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Yan, B.; Liu, G. The responses of soil nitrogen transformation to nitrogen addition are mainly related to the changes in functional gene relative abundance in artificial Pinus tabulaeformis forests. Sci. Total Environ. 2020, 723, 137679. [Google Scholar] [CrossRef]
- Jain, J.; Quarles, C.D., Jr.; Moore, J.; Hartzler, D.A.; McIntyre, D.; Crandall, D. Elemental mapping and geochemical characterization of gas producing shales by laser induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2018, 150, 1–8. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Fang, H.J.; Cheng, S.L.; Yu, G.R.; Yang, X.M.; Xu, M.J.; Wang, Y.S.; Li, Y.N. Nitrogen deposition impacts on the amount and stability of soil organic matter in an alpine meadow ecosystem depend on the form and rate of applied nitrogen. Eur. J. Soil Sci. 2014, 65, 510–519. [Google Scholar] [CrossRef]
- Du, Z.; Wang, W.; Zeng, W.; Zeng, H. Nitrogen deposition enhances carbon sequestration by plantations in northern China. PLoS ONE 2014, 9, e87975. [Google Scholar] [CrossRef]
- Ye, C.; Chen, D.; Hall, S.J.; Pan, S.; Yan, X.; Bai, T.; Guo, H.; Zhang, Y.; Bai, Y.; Hu, S. Reconciling multiple impacts of nitrogen enrichment on soil carbon: Plant, microbial and geochemical controls. Ecol. Lett. 2018, 21, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Shutenko, G.S.; Andriuzzi, W.S.; Dyckmans, J.; Luo, Y.; Wilkinson, T.L.; Schmidt, O. Rapid transfer of C and N excreted by decomposer soil animals to plants and above-ground herbivores. Soil Biol. Biochem. 2022, 166, 108582. [Google Scholar] [CrossRef]
- Yan, B.; Sun, Y.; He, G.; He, R.; Zhang, M.; Fang, H.; Shi, L. Nitrogen enrichment affects soil enzymatic stoichiometry via soil acidification in arid and hot land. Pedobiologia 2020, 81, 150663. [Google Scholar] [CrossRef]
- Sun, Z.C.; Ma, T.Y.; Xu, S.Q.; Guo, H.R.; Hu, C.C.; Chen, C.J.; Liu, X.Y. Levels and variations of soil bioavailable nitrogen among forests under high atmospheric nitrogen deposition. Sci. Total Environ. 2022, 838, 156405. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.C.; Zabowski, D.; Rochefort, R.M.; Edmonds, R.L. Increased microbial uptake and plant nitrogen availability in response to simulated nitrogen deposition in alpine meadows. Geoderma 2019, 336, 68–80. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, X.; Wu, J.; Zhao, J.; Zhao, M.; Chu, G.; Zhang, D. Changes in soil microbial biomass, community composition, and enzyme activities after half-century forest restoration in degraded tropical lands. Forests 2019, 10, 1124. [Google Scholar] [CrossRef]
- Qu, W.; Xie, B.; Hua, H.; Bohrer, G.; Penuelas, J.; Wu, C.; Han, G. Long-term nitrogen enrichment accelerates soil respiration by boosting microbial biomass in coastal wetlands. Soil Biol. Biochem. 2022, 175, 108864. [Google Scholar] [CrossRef]
- Jia, X.; Zhong, Y.; Liu, J.; Zhu, G.; Shangguan, Z.; Yan, W. Effects of nitrogen enrichment on soil microbial characteristics: From biomass to enzyme activities. Geoderma 2020, 366, 114256. [Google Scholar] [CrossRef]
- Xing, T.T.; Cai, A.D.; Lu, C.A.; Ye, H.L.; Wu, H.L.; Huai, S.C.; Lin, Q.M. Increasing soil microbial biomass nitrogen in crop rotation systems by improving nitrogen resources under nitrogen application. J. Integr. Agric. 2022, 21, 1488–1500. [Google Scholar] [CrossRef]
- Zhang, N.; Wan, S.; Li, L.; Bi, J.; Zhao, M.; Ma, K. Impacts of urea N addition on soil microbial community in a semi-arid temperate steppe in northern China. Plant Soil 2008, 311, 19–28. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Q.; Sun, Q.-Y.; Mao, B.; Zeng, D.-H. Understory vegetation interacts with nitrogen addition to affect soil phosphorus transformations in a nutrient-poor Pinus sylvestris var. mongolica plantation. For. Ecol. Manag. 2022, 507, 120026. [Google Scholar] [CrossRef]
- Dong, C.; Wang, W.; Liu, H.; Xu, X.; Zeng, H. Temperate grassland shifted from nitrogen to phosphorus limitation induced by degradation and nitrogen deposition: Evidence from soil extracellular enzyme stoichiometry. Ecol. Indic. 2019, 101, 453–464. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Allison, S.D.; Vitousek, P.M. Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Jian, S.; Li, J.; Chen, J.I.; Wang, G.; Mayes, M.A.; Dzantor, K.E.; Luo, Y. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef]
- Marklein, A.R.; Houlton, B.Z. Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol. 2012, 193, 696–704. [Google Scholar] [CrossRef]
- Li, Y.; Nie, C.; Liu, Y.; Du, W.; He, P. Soil microbial community composition closely associates with specific enzyme activities and soil carbon chemistry in a long-term nitrogen fertilized grassland. Sci. Total Environ. 2019, 654, 264–274. [Google Scholar] [CrossRef]
- Wang, Q.K.; Wang, S.L.; Liu, Y.X. Responses to N and P fertilization in a young Eucalyptus dunnii plantation: Microbial properties, enzyme activities and dissolved organic matter. Appl. Soil Ecol. 2008, 40, 484–490. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Zhang, X.; Mao, Q.; Li, X.; You, Y.; Mo, J. Nitrogen addition reduces soil bacterial richness, while phosphorus addition alters community composition in an old-growth N-rich tropical forest in southern China. Soil Biol. Biochem. 2018, 127, 22–30. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, X.; Song, L.; Lin, X.; Zhang, H.; Shen, C.; Chu, H. Nitrogen fertilization directly affects soil bacterial diversity and indirectly affects bacterial community composition. Soil Biol. Biochem. 2016, 92, 41–49. [Google Scholar] [CrossRef]
- Jia, M.; Gao, Z.; Gu, H.; Zhao, C.; Liu, M.; Liu, F.; Han, G. Effects of precipitation change and nitrogen addition on the composition, diversity, and molecular ecological network of soil bacterial communities in a desert steppe. PLoS ONE 2021, 16, e0248194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cong, J.; Lu, H.; Yang, C.; Yang, Y.; Zhou, J.; Li, D. An integrated study to analyze soil microbial community structure and metabolic potential in two forest types. PLoS ONE 2014, 9, e93773. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Terhonen, E.; Koskinen, K.; Paulin, L.; Kasanen, R.; Asiegbu, F.O. Bacterial diversity and community structure along different peat soils in boreal forest. Appl. Soil Ecol. 2014, 74, 37–45. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, D.Y.; Lücker, S.; Vejmelkova, D.; Kostrikina, N.A.; Kleerebezem, R.; Rijpstra, W.I.C.; Daims, H. Nitrification expanded: Discovery, physiology and genomics of a nitrite-oxidizing bacterium from the phylum Chloroflexi. ISME J. 2012, 6, 2245–2256. [Google Scholar] [CrossRef] [PubMed]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef]
- Guo, L.; Zheng, S.; Cao, C.; Li, C. Tillage practices and straw-returning methods affect topsoil bacterial community and organic C under a rice-wheat cropping system in central China. Sci. Rep. 2016, 6, 33155. [Google Scholar] [CrossRef] [PubMed]
- McIlroy, S.J.; Starnawska, A.; Starnawski, P.; Saunders, A.M.; Nierychlo, M.; Nielsen, P.H.; Nielsen, J.L. Identification of active denitrifiers in full-scale nutrient removal wastewater treatment systems. Environ. Microbiol. 2016, 18, 50–64. [Google Scholar] [CrossRef]
- Lee, H.; Oh, S.-Y.; Lee, Y.M.; Jang, Y.; Jang, S.; Kim, C.; Lim, Y.W.; Kim, J.-J. Successional Variation in the Soil Microbial Community in Odaesan National Park, Korea. Sustainability 2020, 12, 4795. [Google Scholar] [CrossRef]
- Gaur, A.C.; Gaind, S. Role of phosphorus solubilizing microorganisms in crop productivity and enriched organic manure. In National Seminar on Organic Farming Held During; Indian Agricultural Research Institute: New Delhi, India, 1992; pp. 28–29. [Google Scholar]
- Yan, S.; Zhao, J.; Ren, T.; Liu, G. Correlation between soil microbial communities and tobacco aroma in the presence of different fertilizers. Ind. Crops Prod. 2020, 151, 112454. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).